Abstract
Although the deleterious influence of protein deficiency on fetal programming is well documented, the impact of a Western diet on epigenetic mechanisms is less clear. We hypothesized that high-fat high-sucrose diet (HFHSD) consumption during pregnancy leads to epigenetic modifications within the progeny’s compensatory renin–angiotensin system (RAS), affecting autonomic and metabolic functions. Dams were fed HFHSD (45% fat and 30% sucrose) or regular chow (RD) from mating until weaning of the pups (~7 weeks). Offspring from both groups were then maintained on chow and studied in adulthood (3–7 months). Offspring from HFHSD-exposed dams (OH) exhibited no difference in body weight or fasting blood glucose compared to controls (OR). In 3-month-old offspring, DNA methylation was significantly lower for the ACE2 gene (P < 0.05) in the brainstem, kidney and cecum. Moreover, ACE2 activity in the hypothalamus was increased at 7 months (OH: 91 ± 1 vs. OR: 74 ± 4 AFU/mg/min, P < 0.05). Although baseline blood pressure was not different between groups, vagal tone in OH was significantly impaired compared to OR. At the same time, OH offspring had a 1.7-fold increase in AT1a receptor expression and a 1.3-fold increase in ADAM17 mRNA. DOCA-salt treatment further revealed and exacerbated hypertensive response in the OH progeny (OH: 130 ± 6 vs. OR: 108 ± 3 mmHg, P < 0.05). Taken together, our data suggest that perinatal exposure to HFHSD resulted in epigenetic modifications of the compensatory brain RAS, potentially affecting plasticity of neuronal networks leading to autonomic dysfunction in the male offspring.
Keywords: High-fat diet, Epigenetic, ACE2, Renin-angiotensin system
Introduction
Brain development is a process that starts in utero and takes years in humans, with data suggesting that maturity of the central nervous system (CNS) is not reached until the early twenties. Therefore, the environment during pregnancy and early life is critical in shaping adult cardiovascular (CV) fitness and CNS plasticity. Early studies, soon after World War II, have shown the long-term impact of food deprivation on CV function, suggesting that an adverse perinatal environment can direct the offspring for a programmed sensitivity to CV diseases later in life (Barker 1990, 1995; Stein et al. 1996). While the effects of nutrient restriction have been well documented in experimental models and humans, the role of a hyper-caloric diet is less understood.
It is well established that the autonomic nervous system is involved in the regulation of systemic glucose and blood pressure (BP). Clinical investigations have revealed a high risk of developing type 2 diabetes if autonomic dysfunction is present, indicating the importance of autonomic regulation in glucose homeostasis; however, the underlying mechanisms are poorly understood. Pre-autonomic command neurons within the paraventricular nucleus (PVN) of the hypothalamus are key parts of many of these regulatory pathways (O’Hare and Zsombok 2016; Wulsin et al. 2015). Chemical stimulation or lesions within the PVN alter plasma glucose level, indicating that the activity of pre-autonomic PVN neurons pivotally controls glucose homeostasis (Zelena et al. 2006). Within the PVN, the importance of the brain renin-angiotensin system (RAS) is also well established in the maintenance of normal BP and in neuro-cardiovascular dysregulation, leading to increased sympathetic activity and hypertension (Xu et al. 2011). Angiotensin (Ang)-II, by means of its type 1 receptor (AT1R), promotes increased sympathetic activity, enhanced glutamatergic activity, salt and water reabsorption, vasoconstriction, aldosterone and vasopressin release, and inflammation, all contributing to high BP. On the other hand, ACE2 (Angiotensin Converting Enzyme type 2) cleaves Ang-II into the vasodilator peptide Ang-(1–7) and has been identified as a pivotal player in the ACE2/Ang-(1–7)/Mas receptor compensatory axis of the RAS (Xu et al. 2011). While numerous overexpression studies (Der Sarkissian et al. 2008; Diez-Freire et al. 2006; Huentelman et al. 2005; Yamazato et al. 2007, 2009; Feng et al. 2008, 2010; Xiao et al. 2011; Sriramula et al. 2011; Zheng et al. 2011; Xia et al. 2009) have established the benefits of ACE2 in preventing the progression and improving the treatment of hypertension in experimental models, our group was the first to show post-translational impairment of endogenous ACE2 in the PVN during hypertension (Xia et al. 2013; Deshotels et al. 2014).
High-fat feeding up-regulates components of the RAS in several tissues including heart, brain, adipose, pancreas, and blood (Gupte et al. 2008). ACE2 plays a significant role in maintaining BP and glucose homeostasis (Xia and Lazartigues 2010; Chhabra et al. 2013). Knocking out ACE2 leads to exacerbated neurogenic hypertension, up-regulation in the classical RAS pathway, and increased inflammation within the brain (Xia et al. 2013). The classical RAS is also up-regulated by high-fat feeding and animal models of type 2 diabetes mellitus have elevated level of circulating Ang-II. Not surprisingly, this can be countered by AT1R blockade, which also attenuates obesity-associated hypertension in rats (Boustany et al. 2005). An overactive RAS deteriorates the insulin signaling pathway and promotes hyperglycemia (Folli et al. 1997; Henriksen 2007). The molecular basis underlying the epigenetic control of autonomic function is largely unknown and this uncertainty greatly hampers the ability to implement effective strategies for reducing type 2 diabetes, neurogenic hypertension, and their complications.
Several studies highlighted how maternal nutrition dictates tissue-specific gene expression and the level of methylation of those genes in the progeny (Gluckman et al. 2007; Burdge et al. 2007). Perinatal exposure to high-fat diet has been shown to program the vascular RAS, resulting in aortic stiffness and renal renin activity, ultimately making the progeny prone to hypertension (Armitage et al. 2005). It was shown that a first hit treatment with maternal high-fat feeding induces high BP in female offspring rats but not males, while, vascular endothelium-mediated relaxation was deteriorated in both sexes of offspring (Khan et al. 2003, 2004). Prenatal exposure to high sucrose increases Ang-II levels and AT1R expression within major arteries, which leads to increased baseline BP in aged offspring (Wu et al. 2016). Moreover, data about diet-induced programming indicate predictive adaptive responses to maternal hyper-caloric diet which can either speed the development of the offspring in order to account for a shorter lifespan or slow development in order to maintain low energy requirements (Berghänel et al. 2016).
There is a need to understand the mechanisms by which epigenetic programming of the cardiovascular system occurs. DNA methylation is the most widely studied epigenetic mechanism, which alters gene expression without changing the DNA sequence. Most existing epidemiological studies rely on peripheral blood DNA, whereas epigenetic alterations are tissue-specific and it is unclear whether and to what extent methylation variation in peripheral blood could reflect the epigenetic profile in specific tissues. DNA methylation events may occur during the lifetime of the parental generation and then passed onto the progeny. It can occur right before implantation of the fetus and can have a direct impact on its development. Finally, it can also occur within the fetus, which is affected by the perinatal environment (Reik et al. 2001; Bird 2002; Lillycrop et al. 2007). DNA methylation is often linked to silencing of a gene; however, it really depends on the location of methylation on the DNA. For example, the glucocorticoid receptor gene promoter is susceptible to methylation induced by stress or restricted prenatal nutrition. This leads to increased glucocorticoid receptor gene expression which entails behavioral and CV dysfunction in the offspring (Meaney and Szyf 2005; Jin et al. 2011).
In the present study, we hypothesized that high-fat high sucrose diet (HFHSD) consumption during pregnancy would lead to DNA methylation modifications within the progeny’s compensatory RAS, affecting autonomic and metabolic functions. Our data show reduced ACE2 methylation in various tissues, associated with enhanced ACE2 activity in the brain and hyper-sensitivity to hypertension among the offspring. To the best of our knowledge, this is the first evidence for DNA methylation modifications within the compensatory RAS following perinatal exposure to a hyper-caloric diet.
Methods
Animals and Diet
Mice were housed in a temperature- and humidity-controlled facility under a 12-h dark/light cycle. Wild-type males and females C57Bl/6 J mice (10–12 week-old) were housed together and fed either regular diet (RD; Harlan Laboratories, 2019S) and autoclaved tap water or 45% kcal high-fat diet (OpenSource Diets®, D12451) and 30% sucrose in the drinking water (HFHSD) until mating. Pregnant females were further maintained on RD or HFHSD until the pups were weaned. Post weaning, the offspring from dams on RD (OR) and those from HFHSD-fed dams (OH) were given regular diet (Harlan Laboratories, 2019S). All the following procedures were performed in the male progeny, as per the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee at the Louisiana State University Health Sciences Center (#3116).
DNA Methylation Bisulfite Sequencing
DNA extraction, Methyl-Seq library preparation, and sequencing were carried out by a service provider (Omega Bioservices, Norcross, GA). DNA was extracted from various samples using the E.Z.N.A. HP Tissue DNA Kit (Omega Bio-tek, Norcross, GA) according to the manufacturer’s protocol. DNA concentration was measured using the QuantiFluor dsDNA System on a Quantus Fluorometer (Promega, Madison, WI). An Agilent SureSelectXT Methyl-Seq Target Enrichment kit (Agilent Technologies, Santa Clara, CA) was used for targeted methylation sequencing. Briefly, 3 µg of genomic DNA was fragmented using a Bioruptor sonicator (Diagenode, Denville, NJ). DNA fragment ends were repaired, 3′ adenylated, and ligated to methylated adapters. The resulting adapter-ligated libraries were hybridized with a custom designed SureSelect Methyl-Seq capture probe pool to enrich target sequences. The captured target DNA libraries were eluted and underwent bisulfite conversion to modify unmethylated cytosine residues to uracil residues using an EZ DNA Methylation-Gold Kit (Zymo Research, Irvine, CA). The libraries were PCR-amplified, Illumina indexed added, and pooled for multiplexed sequencing on an Illumina HiSeq 2500 sequencer (Illumina, San Diego, CA) using the pair-end 100 bp run format.
BP Recording and Autonomic Function
For each surgery, mice were anesthetized with isoflurane (2%) in an oxygen flow (1 L/min) and placed on a heating pad to maintain body temperature. Post-operative care included an injection of Buprenorphine-SR (1.2 mg/Kg, sc) for sustained relief of pain. Before the DOCA-salt paradigm, mice underwent uni-nephrectomy and after a week of recovery, they were implanted with telemetry probes (TA11PA-C10, Data Sciences International) for conscious BP monitoring, as described previously (Xia et al. 2013; Sriramula et al. 2015). A week later, baseline BP was recorded for 3 days. Mice (n = 5) were then implanted subcutaneously with a DOCA-silicone sheet for delivery of 0.75 mg of DOCA per gram of body weight). Drinking water was then replaced with 1% NaCl. BP was continuously recorded for an additional 2 weeks. At the end of the protocol, mice were euthanized under anesthesia and the brain and plasma were collected and stored at −80 °C until used.
Autonomic function was assessed, in conscious freely moving mice, prior to DOCA-salt treatment, using a pharmacological method involving ip injection of propranolol (β-blocker, 4 mg/kg), atropine (muscarinic receptor antagonist, 1 mg/kg) and chlorisondamine (ganglionic blocker, 5 mg/kg). Each injection was separated by at least a 3-h recovery period. Changes in HR (ΔHR) or mean arterial pressure (ΔMAP) were calculated following administration of these blockers. For each mouse, BP was recorded continuously for one to 2 h using a higher sampling rate of 2000 Hz for baroreceptor reflex analysis. Spontaneous baroreceptor reflex sensitivity (SBRS), reflecting the baroreflex control of HR, was calculated by the sequence method using HemoLab software, (http://www.haraldstauss.com/HaraldStaussScientific/products/default.html), as previously (Xia et al. 2013; Stauss et al. 2006).
Plasma Insulin Levels
Blood was collected following decapitation and plasma insulin concentrations were measured using ELISA kits (Crystal Chem Inc, Cat# 90080; ALPCO Diagnostics, 80-CPTMS-E01) according to the manufacturer’s protocol.
Plasma Glucose Levels
Blood was collected following decapitation and plasma glucose concentrations were measured using a glucose assay kits (Sigma-Aldrich, Product Code GAGO-20) according to the manufacturer’s protocol. Fasting blood glucose was measured, after 6 h of fasting, with a TRUEtrack blood glucose monitoring system (Nipro Diagnostics).
Plasma Cholesterol Levels
Blood was collected following decapitation and total cholesterol level was measured using colorimetric analysis provided in the kit (WAKO, pure Chemical Industries, Ltd, Cat 431-52501, CA).
Quantitative Real-Time PCR (qRT-PCR)
RNA was isolated from hypothalamus and liver using Qiagen RNeasy® Mini kit (Qiagen, Valencia, CA). One-Step qRT-PCR was performed using Power SYBR Green RNA-to-CT 1-Step Kit (Life Technologies, USA). A total of 5 ng target mRNA was added per well and assayed on a LightCycler® 480 II (Roche, Indianapolis, IN) using βactin mRNA as an internal control. Fold changes in target genes were determined by the 2−∆∆Ct method. The primer sequences are listed in Table 1.
Table 1.
Primers used for qRT-PCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| mACE2 | gag gat aag cct aaa atc agc tct tg | tcg gaa cag gaa cat ttc gtt |
| mAT1aR | tca cca gat caa gtg cat ttt ga | aga gtt aag ggc cat ttt gct tt |
| mADAM17 | tgc agg gtt taa agg gta tgg a | att gaa gtg tct ttc acc agg ttt t |
| mβ-Actin | cat cct ctt cct ccc tgg aga aga | aca gga ttc cat acc caa gaa gga ag |
ACE2 Activity
ACE2 activity from hypothalamus homogenates was measured using a fluorogenic substrate Mca-APK(Dnp), as described previously (Pedersen et al. 2011; Sriramula et al. 2017). Measurements were performed in duplicate for each sample, in the presence and absence of DX600 (ACE2 inhibitor). Specific activity from each sample was then normalized to protein content and presented as Fluorescence Units (FU)/min/µg protein.
Statistics
Data are presented as mean ± SEM. Unless otherwise stated, data were analyzed by Student’s t test, one-way ANOVA, followed by Tukey’s post hoc test for multiple comparisons between means, two-way ANOVA followed by bonferroni’s post hoc test for multiple comparisons between means or repeated-measures ANOVA followed by Bonferroni’s post hoc test for multiple comparisons, as appropriate, using Prism 5 (GraphPad Software, San Diego, CA). Differences were considered statistically significant at P < 0.05.
Results
Impact of HFHSD on Metabolic Parameters in Dams and Offspring
For this study, after being housed with their male counterpart, dams were fed HFHSD, or chow, during gestation (3 weeks) and remained on this diet until weaning of the pups at 21 days.
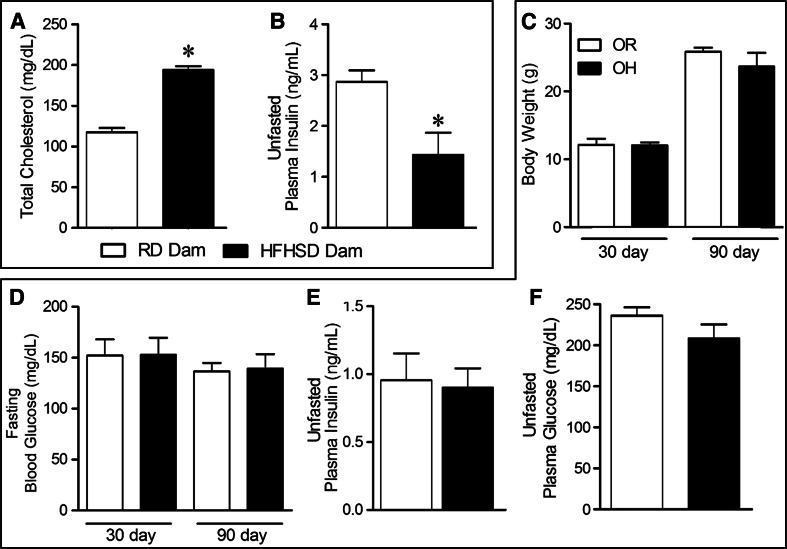
C57Bl6/J females (3-month-old), exposed to a HFHSD, supplemented with 30% sucrose in the drinking water, for 7 weeks exhibited a significant increase in plasma cholesterol levels (Fig. 1a) compared to the RD group (HFHSD: 194 ± 5 vs. RD: 117 ± 5 mg/dL, P < 0.05). Therefore, direct exposure to the hyper-caloric diet increased total calorie intake within the HFHSD-fed dams leading to a change in lipid profile. Interestingly, non-fasted insulin levels were significantly lower in dams exposed to HFHSD (Fig. 1b), while unfasted plasma glucose was not different between the groups (HFHSD: 200 ± 27 vs. RD: 219 ± 13 mg/dL, P > 0.05). Together these data suggest that HFHSD intake led to hyperlipidemia and insulin secretory deficiency in the mothers.
Fig. 1.
Effect of HFHSD on metabolic parameters in dams and their offspring: Direct exposure to HFHSD led to a significant increase in total plasma cholesterol levels (a), while plasma insulin levels in unfasted dams (n = 4–5) was significantly lowered (b). Next, among the offspring, body weight (n = 13) increased uniformly between the OR and OH mice (c) and fasting blood glucose levels (d) at 30 or 90 days were not different. Unfasted plasma insulin (e) and plasma glucose (f) levels were unchanged between the 2 groups (n = 3–6). Statistical significance: *P < 0.05 and **P < 0.001 versus RD (student’s t test)
Following weaning, all pups were fed RD. Perinatal exposure to HFHSD (OH) or RD (OR) led to similar growth curves and there were no significant differences in body weight (Fig. 1c) or fasting blood glucose (Fig. 1d) in pups. Similarly, 6-month-old offspring showed no differences in plasma insulin (Fig. 1e) and plasma glucose (Fig. 1f) levels.
Impact of HFHSD on BP and Autonomic Regulation in Offspring
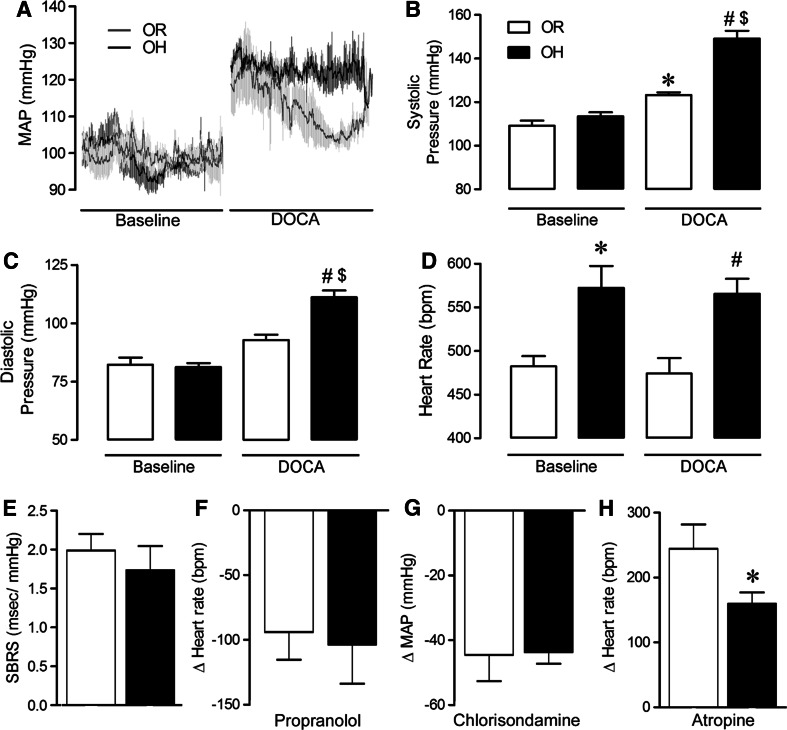
Figure 2a shows averaged BP recordings in baseline conditions and following DOCA-salt treatment. Perinatal exposure to HFHSD did not significantly alter baseline BP parameters (Fig. 2b–c) but resulted in a significant tachycardia in OH pups (Fig. 2d). As expected, baseline SBRS was not altered by HFHSD perinatal exposure (Fig. 2e) and sympathetic drive to the heart (Fig. 2f) and the vasculature (Fig. 2g) were also similar between progenies. However, there was a distinct deterioration in vagal tone (Fig. 2h), as evidenced by a reduced tachycardic response to atropine, in these young OH mice that may have contributed to the persistent tachycardia in this group.
Fig. 2.
Perinatal exposure to HFHSD sensitizes mice to neurogenic hypertension. Average (n = 4–5) 30 min BP traces (a) before and after DOCA-salt treatment (12 days) in OR (gray line) and OH (black line) progeny. DOCA-salt treatment led to significantly increased systolic (b), and diastolic (c) BP in OH mice compared to OR (n = 4). In addition, OH mice exhibited a persistent tachycardia compared to OR mice (d). Baseline spontaneous baroreceptor sensitivity (SBRS; e) as well as sympathetic tone to the heart (f) and vasculature (g) were unchanged between groups while vagal tone (Student’s t test) (h) was significantly reduced in OH mice (n = 4–7). Statistical significance: *P < 0.05 versus OR mice, # P < 0.05 versus OR + DOCA mice and $ P < 0.05 versus OH mice using one-way ANOVA followed by Tukey’s multiple comparison test
To determine whether exposure of HFHSD may have programmed neuronal networks to respond differently to cardiovascular challenges, 3-month-old mice were subjected to a low-dose DOCA-salt treatment to induce neurogenic hypertension (Sriramula et al. 2015; Xia et al. 2013). Interestingly, this mild DOCA-salt paradigm produced only a small increase in systolic BP (+14 ± 4 mmHg, P < 0.05, Fig. 2b) and diastolic BP (+11 ± 2 mmHg, P < 0.05, Fig. 2c) in OR pups. However, the OH progeny exhibited a more pronounced elevation of both systolic (+35 ± 3 mmHg, P < 0.05, Fig. 2b) diastolic BP (+29 ± 2 mmHg, P < 0.05, Fig. 2c). HR was not affected by DOCA-salt treatment in the OR group and the tachycardia previously observed at baseline, persisted in OH mice undergoing DOCA-salt treatment (Fig. 2d).
HFHSD-Induced Epigenetic Effect on the Compensatory RAS
To determine the impact of HFHSD on epigenetic changes within the compensatory RAS, we examined gene methylation of its major component, ACE2. While brain and kidney are involved in BP regulation, the gut microbiota has recently been shown to modulate BP and metabolic homeostasis (Gomez-Guzman et al. 2015; Mell et al. 2015; Patten et al. 2004). We observed a consistent decrease in the levels of mACE2 methylation in the brainstem, kidney, and cecum of 3-month-old OH mice (Fig. 3a–c, P < 0.05), supporting a role for HFHSD in regulating this epigenetic process. To determine whether this reduced methylation was linked to altered gene expression, we assessed gene expression for AT1aR, ADAM17 and ACE2 in the hypothalamus, another brain region critical for BP regulation. The 3-month-old OH progeny exhibited significantly higher levels of AT1aR mRNA (Fig. 3e), as well as an increase in ADAM17 mRNA (OH: 1.3 ± 0.1 vs. OR: 1.0 ± 0.04, P < 0.05). In-spite of decreased ACE2 gene methylation, ACE2 activity did not increase within the 3-month OH offspring (Fig. 3f), possibly due to the negative regulation exerted by AT1aR and ADAM17 on this enzyme (Xia et al. 2013; Pedersen et al. 2015). However, at 7 months, the putative inhibitory effect of AT1aR (Fig. 3e) and ADAM17 (OH: 1.1 ± 0.2 vs. OR: 1.0 ± 0.1) was lost and this was associated with increased ACE2 activity within OH offspring compared to the OR controls (Fig. 3f). Together, our data suggest that perinatal exposure to HFHSD shaped the expression of the brain RAS, most notably resulting in an increase in ACE2 expression and activity within the CNS.
Fig. 3.
HFHSD-induced methylation changes in ACE2 gene within the offspring. DNA methylation of the mACE2 gene was consistently downregulated in brainstem (a), kidney (b) and cecum (c) of OH mice (Student’s t test; n = 6). Gene expression was transiently increased for ACE2 (d) and AT1aR (e) in 3-month-old OH offspring but normalized in 7-month-old animals. At that later time point, ACE2 activity was significantly increased (two-way ANOVA with Bonferroni post hoc test, n = 3–6/group) in OH mice (f). Statistical significance: *P < 0.05, **P < 0.01 and ***P < 0.001 versus OR mice, # P < 0.05 versus OH mice
Discussion
Recent evidence suggests that epigenetic mechanisms, especially DNA methylation, play a critical role in the development of cardio-metabolic diseases (Wahl et al. 2017; Ligthart et al. 2016). In addition, gene methylations are most commonly thought to be associated with impaired gene expression. In this study, we investigated the effects of perinatal HFHSD exposure on the main component of the RAS compensatory axis, ACE2. The main finding is that a 7-week exposure of gestating and nursing dams to HFHSD unexpectedly led to a reduction of ACE2 methylation in major organs such as the brain, kidney, and cecum. Moreover, within the brain, these epigenetic changes were associated with temporary changes in ACE2 and AT1aR gene expression and ultimately translated into enhanced ACE2 activity, associated with alterations of autonomic function.
ACE2 has been reported to play a critical role within the central nervous system in modulating autonomic function and BP regulation (Mendoza and Lazartigues 2015). Despite its compensatory role in alleviating the deleterious effects of the classical RAS (i.e., ACE/Ang-II/AT1R) activation, endogenous expression of this enzyme has been shown to fluctuate with the development of hypertension, often with a rise in activity early on, followed by a reduction as the disease is established (Burrell et al. 2005).
We observed a significant decrease in ACE2 gene methylation in 3-month-old OH pups which should have led to an increased ACE2 activity. However, at that age, it was accompanied by an increased AT1aR and ADAM17 gene expression which could have potentially downregulated the compensatory activity of ACE2 (Sriramula et al. 2015; Xia et al. 2013). This hypothesis is supported by the observation that reduction of AT1aR and ADAM17 gene expression in the 7-month-old OH offspring was associated with an increased hypothalamic ACE2 activity. It remains to be determined whether this elevated ACE2 activity could have impacted the impaired parasympathetic drive observed in 3-month-old offspring. Furthermore, the predictive adaptive mechanism found in 7-month-old mice, where ACE2 activity is increased and AT1a receptor expression is decreased must be put to test. A second hit test with DOCA- salt treatment in 7-month-old mice, could reveal, if these changes are adequately robust to protect these mice from a cardiovascular insult.
Due to experimental constraints and tissue availability, ACE2 gene expression was studied within the hypothalamus, while ACE2 gene methylation was measured in the brainstem. However, both regions are closely connected in the regulation of autonomic control of BP and our observation that ACE2 methylation is also reduced in the kidney and cecum (Fig. 3a–c) strongly suggests that ACE2 methylation was uniformly reduced all over the body.
The “second hit” test is often used in epigenetic studies to assess the impact of system plasticity in the face of a future challenge. Here, DOCA-salt treatment unmasked enhanced sensitivity to hypertension in the offspring exposed to HFHSD, suggesting that epigenetic modifications of ACE2 and other genes may have lowered the threshold for the development of hypertension. To the best of our knowledge, this is the first report showing alterations in ACE2 activity due to epigenetic modifications. Other groups have reported reduction in ACE2 protein expression following hyper-cholesterolemic diets (Tikoo et al. 2015) or trends to reduced expression after high fructose diets (Tain et al. 2016). Interestingly, in the later, an inhibitor of soluble epoxide hydrolase was shown to increase ACE2 expression and attenuate the high fructose-induced hypertension, supporting the critical role of ACE2 in maintaining a normal BP. In this study, we did not assess ACE2 activity following the development of DOCA-salt hypertension in the OH progeny. However, in RD-fed mice, we have previously reported a reduction in ACE2 activity in the brain and a parallel rise in AT1aR. Further work is needed to determine how ACE2 activity is affected by hypertension in HFHSD-exposed pups.
Our group previously reported that ACE2 expression within the pancreas can regulate glucose homeostasis and prevent the development of diabetes in experimental models associated with obesity, including high-fat diet (Bindom et al. 2010; Chodavarapu et al. 2016). To the best of our knowledge, no study has previously investigated the impact of a hyper-caloric diet on epigenomic modifications within the compensatory RAS. The fact that a similar reduction was observed in the brain, kidney, and cecum strongly supports the global character of this mechanism, likely affecting organs that contribute to BP regulation and possibly other physiological functions. We previously reported that Ang-II signaling activation leads to ADAM17 up-regulation, promoting ACE2 shedding from the plasma membrane, a process that is thought to be deleterious through impairment of the enzyme’s compensatory activity (Xia et al. 2013). However, although ADAM17 gene expression was increased in the brain, and possibly other tissues, by HFHSD exposure, it did not result in a reduction of ACE2 activity.
The goal of this study was to determine whether perinatal exposure to a HFHSD could induce epigenomic modifications within the compensatory RAS that could be translated into impaired autonomic and metabolic functions. While our data are encouraging and support this hypothesis, we acknowledge several limitations, the main one being the lack of protein expression for several of our targets. More work is warranted in this direction and towards the reversal of these epigenomic modifications. In conclusion, we provided evidence for perinatal HFHSD to trigger epigenomic modifications within the compensatory RAS, potentially altering autonomic function and exposing the male progeny to higher sensitivity to CV and possibly metabolic diseases.
Acknowledgements
The authors would like to thank Dr. Weining Tang (Omega Bio-tek, Norcross, GA) for providing technical expertise regarding the design of the methylation array.
Funding
This work was supported by an Established Investigator Award from the American Heart Association (12EIA8030004 to E.L.), the National Center for Research Resources (RR018766), and the National Institute of General Medical Sciences (GM103514 and GM106392).
Compliance with Ethical Standards
Conflict of interest
The authors declare no potential conflict of interest.
References
- Armitage JA, Lakasing L, Taylor PD, Balachandran AA, Jensen RI, Dekou V, Ashton N, Nyengaard JR, Poston L (2005) Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J Physiol 565(Pt 1):171–184. doi:10.1113/jphysiol.2005.084947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ (1990) The fetal and infant origins of adult disease. BMJ: Brit Med J 301(6761):1111–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ (1995) Fetal origins of coronary heart disease. BMJ 311(6998):171–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghänel A, Heistermann M, Schülke O, Ostner J (2016) Prenatal stress effects in a wild, long-lived primate: predictive adaptive responses in an unpredictable environment. Proc Biol Sci 283(1839). doi:10.1098/rspb.2016.1304 [DOI] [PMC free article] [PubMed]
- Bindom SM, Hans CP, Xia H, Boulares AH, Lazartigues E (2010) Angiotensin-I Converting Enzyme Type 2 (ACE2) gene therapy improves glycemic control in diabetic mice. Diabetes 59(10):2540–2548. doi:10.2337/db09-0782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A (2002) DNA methylation patterns and epigenetic memory. Genes Dev 16(1):6–21. doi:10.1101/gad.947102 [DOI] [PubMed] [Google Scholar]
- Boustany CM, Brown DR, Randall DC, Cassis LA (2005) AT1-receptor antagonism reverses the blood pressure elevation associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 289(1):R181–R186. doi:10.1152/ajpregu.00507.2004 [DOI] [PubMed] [Google Scholar]
- Burdge GC, Slater-Jefferies J, Torrens C, Phillips ES, Hanson MA, Lillycrop KA (2007) Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br J Nutr 97(3):435–439. doi:10.1017/S0007114507352392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell LM, Risvanis J, Kubota E, Dean RG, MacDonald PS, Lu S, Tikellis C, Grant SL, Lew RA, Smith AI, Cooper ME, Johnston CI (2005) Myocardial infarction increases ACE2 expression in rat and humans. Eur Heart J 26(4):369–375 [DOI] [PubMed] [Google Scholar]
- Chhabra KH, Chodavarapu H, Lazartigues E (2013) Angiotensin converting enzyme 2: a new important player in the regulation of glycemia. IUBMB Life 65(9):731–738. doi:10.1002/iub.1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodavarapu H, Chhabra KH, Xia H, Shenoy V, Yue X, Lazartigues E (2016) High-fat diet-induced glucose dysregulation is independent of changes in islet ACE2 in mice. Am J Physiol - Regul Integr Comp Physiol 311(6):R1223–R1233. doi:10.1152/ajpregu.00362.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der Sarkissian S, Grobe JL, Yuan L, Narielwala DR, Walter GA, Katovich MJ, Raizada MK (2008) Cardiac overexpression of angiotensin converting enzyme 2 protects the heart from ischemia-induced pathophysiology. Hypertension 51(3):712–718. doi:10.1161/hypertensionaha.107.100693 [DOI] [PubMed] [Google Scholar]
- Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM (2014) Angiotensin II mediates angiotensin converting enzyme Type 2 internalization and degradation through an Angiotensin II Type I receptor-dependent mechanism. Hypertension 64(6):1368–1375. doi:10.1161/hypertensionaha.114.03743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Freire C, Vazquez J, Correa de Adjounian MF, Ferrari MFR, Yuan L, Silver X, Torres R, Raizada MK (2006) ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics 27(1):12–19 [DOI] [PubMed] [Google Scholar]
- Feng Y, Yue X, Xia H, Bindom SM, Hickman PJ, Filipeanu CM, Wu G, Lazartigues E (2008) Angiotensin-converting enzyme 2 overexpression in the subfornical organ prevents the angiotensin ii-mediated pressor and drinking responses and is associated with Angiotensin II Type 1 receptor downregulation. Circ Res 102(6):729–736. doi:10.1161/circresaha.107.169110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RAS, Speth RC, Sigmund CD, Lazartigues E (2010) Brain-selective overexpression of human angiotensin-converting enzyme Type 2 attenuates neurogenic hypertension. Circ Res 106(2):373–382. doi:10.1161/circresaha.109.208645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folli F, Kahn CR, Hansen H, Bouchie JL, Feener EP (1997) Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Investig 100(9):2158–2169. doi:10.1172/JCI119752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Lillycrop KA, Vickers MH, Pleasants AB, Phillips ES, Beedle AS, Burdge GC, Hanson MA (2007) Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc Natl Acad Sci USA 104(31):12796–12800. doi:10.1073/pnas.0705667104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Guzman M, Toral M, Romero M, Jimenez R, Galindo P, Sanchez M, Zarzuelo MJ, Olivares M, Galvez J, Duarte J (2015) Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol Nutr Food Res 59(11):2326–2336. doi:10.1002/mnfr.201500290 [DOI] [PubMed] [Google Scholar]
- Gupte M, Boustany-Kari CM, Bharadwaj K, Police S, Thatcher S, Gong MC, English VL, Cassis LA (2008) ACE2 is expressed in mouse adipocytes and regulated by a high-fat diet. Am J Physiol Regul Integr Comp Physiol 295(3):R781–R788. doi:10.1152/ajpregu.00183.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen EJ (2007) Improvement of insulin sensitivity by antagonism of the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 293(3):R974–R980. doi:10.1152/ajpregu.00147.2007 [DOI] [PubMed] [Google Scholar]
- Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK (2005) Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol 90(5):783–790 [DOI] [PubMed] [Google Scholar]
- Jin B, Li Y, Robertson KD (2011) DNA methylation: superior or subordinate in the epigenetic hierarchy? Genes Cancer 2(6):607–617. doi:10.1177/1947601910393957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L (2003) Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension 41(1):168–175. doi:10.1161/01.hyp.0000047511.97879.fc [DOI] [PubMed] [Google Scholar]
- Khan I, Dekou V, Hanson M, Poston L, Taylor P (2004) Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation 110(9):1097–1102. doi:10.1161/01.cir.0000139843.05436.a0 [DOI] [PubMed] [Google Scholar]
- Ligthart S, Marzi C, Aslibekyan S, Mendelson MM, Conneely KN, Tanaka T, Colicino E, Waite LL, Joehanes R, Guan W, Brody JA, Elks C, Marioni R, Jhun MA, Agha G, Bressler J, Ward-Caviness CK, Chen BH, Huan T, Bakulski K, Salfati EL, Fiorito G, Wahl S, Schramm K, Sha J, Hernandez DG, Just AC, Smith JA, Sotoodehnia N, Pilling LC, Pankow JS, Tsao PS, Liu C, Zhao W, Guarrera S, Michopoulos VJ, Smith AK, Peters MJ, Melzer D, Vokonas P, Fornage M, Prokisch H, Bis JC, Chu AY, Herder C, Grallert H, Yao C, Shah S, McRae AF, Lin H, Horvath S, Fallin D, Hofman A, Wareham NJ, Wiggins KL, Feinberg AP, Starr JM, Visscher PM, Murabito JM, Kardia SLR, Absher DM, Binder EB, Singleton AB, Bandinelli S, Peters A, Waldenberger M, Matullo G, Schwartz JD, Demerath EW, Uitterlinden AG, van Meurs JBJ, Franco OH, Chen Y-DI, Levy D, Turner ST, Deary IJ, Ressler KJ, Dupuis J, Ferrucci L, Ong KK, Assimes TL, Boerwinkle E, Koenig W, Arnett DK, Baccarelli AA, Benjamin EJ, Dehghan A (2016) DNA methylation signatures of chronic low-grade inflammation are associated with complex diseases. Genome Biol 17(1):255. doi:10.1186/s13059-016-1119-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC (2007) Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Brit J Nutr 97(6):1064–1073. doi:10.1017/s000711450769196x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M (2005) Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci 28(9):456–463. doi:10.1016/j.tins.2005.07.006 [DOI] [PubMed] [Google Scholar]
- Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B (2015) Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genom 47(6):187–197. doi:10.1152/physiolgenomics.00136.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza A, Lazartigues E (2015) The compensatory renin-angiotensin system in the central regulation of arterial pressure: new avenues and new challenges. Therapeutic Adv Cardiovasc Disease 9(4):201–208. doi:10.1177/1753944715578056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare JD, Zsombok A (2016) Brain-liver connections: role of the preautonomic PVN neurons. Am J Physiol Endocrinol Metab 310(3):E183–E189. doi:10.1152/ajpendo.00302.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten GS, Adams MJ, Dallimore JA, Abeywardena MY (2004) Depressed prostanoid-induced contractility of the gut in spontaneously hypertensive rats (SHR) is not affected by the level of dietary fat. J Nutr 134(11):2924–2929 [DOI] [PubMed] [Google Scholar]
- Pedersen KB, Sriramula S, Chhabra K, Xia H, Lazartigues E (2011) Species-specific inhibitor sensitivity of angiotensin-converting enzyme 2 (ACE2) and its implication for ACE2 activity assays. Am J Physiol - Regul Integr Comp Physiol 301(5):1293–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen KB, Chodavarapu H, Porretta C, Robinson LK, Lazartigues E (2015) Dynamics of ADAM17-mediated shedding of ACE2 applied to pancreatic islets of male db/db mice. Endocrinology 156(12):4411–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Dean W, Walter J (2001) Epigenetic reprogramming in mammalian development. Science 293(5532):1089–1093. doi:10.1126/science.1063443 [DOI] [PubMed] [Google Scholar]
- Sriramula S, Cardinale JP, Lazartigues E, Francis J (2011) ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc Res 92(3):401–408. doi:10.1093/cvr/cvr242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriramula S, Xia H, Xu P, Lazartigues E (2015) Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension 65(3):577–586. doi:10.1161/hypertensionaha.114.04691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriramula S, Pedersen KB, Xia H, Lazartigues E (2017) Determining the enzymatic activity of angiotensin-converting enzyme 2 (ACE2) in brain tissue and cerebrospinal fluid using a quenched fluorescent substrate. Methods Mol Biol 1527:117–126 [DOI] [PubMed] [Google Scholar]
- Stauss HM, Moffitt JA, Chapleau MW, Abboud FM, Johnson AK (2006) Baroreceptor reflex sensitivity estimated by the sequence technique is reliable in rats. Am J Physiol - Heart Circ Physiol 291(1):H482–H483 [DOI] [PubMed] [Google Scholar]
- Stein CE, Fall CHD, Kumaran K, Osmond C, Barker DJP, Cox V (1996) Fetal growth and coronary heart disease in South India. Lancet 348(9037):1269–1273. doi:10.1016/S0140-6736(96)04547-3 [DOI] [PubMed] [Google Scholar]
- Tain Y-L, Lee W-C, Wu KLH, Leu S, Chan JYH (2016) Targeting arachidonic acid pathway to prevent programmed hypertension in maternal fructose-fed male adult rat offspring. J Nutr Biochem 38:86–92. doi:10.1016/j.jnutbio.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Tikoo K, Patel G, Kumar S, Karpe PA, Sanghavi M, Malek V, Srinivasan K (2015) Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol 93(3):343–351. doi:10.1016/j.bcp.2014.11.013 [DOI] [PubMed] [Google Scholar]
- Wahl S, Drong A, Lehne B, Loh M, Scott WR, Kunze S, Tsai P-C, Ried JS, Zhang W, Yang Y, Tan S, Fiorito G, Franke L, Guarrera S, Kasela S, Kriebel J, Richmond RC, Adamo M, Afzal U, Ala-Korpela M, Albetti B, Ammerpohl O, Apperley JF, Beekman M, Bertazzi PA, Black SL, Blancher C, Bonder M-J, Brosch M, Carstensen-Kirberg M, de Craen AJM, de Lusignan S, Dehghan A, Elkalaawy M, Fischer K, Franco OH, Gaunt TR, Hampe J, Hashemi M, Isaacs A, Jenkinson A, Jha S, Kato N, Krogh V, Laffan M, Meisinger C, Meitinger T, Mok ZY, Motta V, Ng HK, Nikolakopoulou Z, Nteliopoulos G, Panico S, Pervjakova N, Prokisch H, Rathmann W, Roden M, Rota F, Rozario MA, Sandling JK, Schafmayer C, Schramm K, Siebert R, Slagboom PE, Soininen P, Stolk L, Strauch K, Tai ES, Tarantini L, Thorand B, Tigchelaar EF, Tumino R, Uitterlinden AG, van Duijn C, van Meurs JBJ, Vineis P, Wickremasinghe AR, Wijmenga C, Yang T-P, Yuan W, Zhernakova A, Batterham RL, Smith GD, Deloukas P, Heijmans BT, Herder C, Hofman A, Lindgren CM, Milani L, van der Harst P, Peters A, Illig T, Relton CL, Waldenberger M, Järvelin M-R, Bollati V, Soong R, Spector TD, Scott J, McCarthy MI, Elliott P, Bell JT, Matullo G, Gieger C, Kooner JS, Grallert H, Chambers JC (2017) Epigenome-wide association study of body mass index, and the adverse outcomes of adiposity. Nature 541(7635):81–86. doi:10.1038/nature20784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Shi A, Zhu D, Bo L, Zhong Y, Wang J, Xu Z, Mao C (2016) High sucrose intake during gestation increases angiotensin II type 1 receptor-mediated vascular contractility associated with epigenetic alterations in aged offspring rats. Peptides 86:133–144. doi:10.1016/j.peptides.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Wulsin LR, Horn PS, Perry JL, Massaro JM, D’Agostino RB (2015) Autonomic Imbalance as a predictor of metabolic risks, cardiovascular disease, diabetes, and mortality. J Clin Endocrinol Metab 100(6):2443–2448. doi:10.1210/jc.2015-1748 [DOI] [PubMed] [Google Scholar]
- Xia H, Lazartigues E (2010) Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep 12(3):170–175. doi:10.1007/s11906-010-0105-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E (2009) Angiotensin II Type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension 53:210–216. doi:10.1161/hypertensionaha.108.123844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Sriramula S, Chhabra K, Lazartigues E (2013) Brain ACE2 shedding contributes to the development of neurogenic hypertension. Circ Res 113:1087–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Gao L, Lazartigues E, Zucker IH (2011) Brain-selective overexpression of angiotensin-converting enzyme 2 attenuates sympathetic nerve activity and enhances baroreflex function in chronic heart failure. Hypertension 58(6):1057–1065. doi:10.1161/hypertensionaha.111.176636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Sriramula S, Lazartigues E (2011) ACE2/Ang-(1-7)/Mas pathway in the brain: the axis of good. Am J Physiol - Regul Integr Comp Physiol 300(4):R804–817. doi:10.1152/ajpregu.00222.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK (2007) Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 49(4):926–931 [DOI] [PubMed] [Google Scholar]
- Yamazato Y, Ferreira AJ, Hong K-H, Sriramula S, Francis J, Yamazato M, Yuan L, Bradford CN, Shenoy V, Oh SP, Katovich MJ, Raizada MK (2009) Prevention of pulmonary hypertension by angiotensin-converting enzyme 2 gene transfer. Hypertension 54(2):365–371. doi:10.1161/hypertensionaha.108.125468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelena D, Filaretova L, Mergl Z, Barna I, Tóth ZE, Makara GB (2006) Hypothalamic paraventricular nucleus, but not vasopressin, participates in chronic hyperactivity of the HPA axis in diabetic rats. Am J Physiol Endocrinol Metab 290(2):E243–E250. doi:10.1152/ajpendo.00118.2005 [DOI] [PubMed] [Google Scholar]
- Zheng H, Liu X, Patel KP (2011) Angiotensin-converting enzyme 2 overexpression improves central nitric oxide mediated sympathetic outflow in chronic heart failure. Am J Physiol Heart Circ Physiol. doi:10.1152/ajpheart.00330.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]