Abstract
Developmental and epileptic encephalopathy (DEE) is a group of conditions characterized by the co-occurrence of epilepsy and intellectual disability (ID), typically with developmental plateauing or regression associated with frequent epileptiform activity. The cause of DEE remains unknown in the majority of cases. We performed whole-genome sequencing (WGS) in 197 individuals with unexplained DEE and pharmaco-resistant seizures and in their unaffected parents. We focused our attention on de novo mutations (DNMs) and identified candidate genes containing such variants. We sought to identify additional subjects with DNMs in these genes by performing targeted sequencing in another series of individuals with DEE and by mining various sequencing datasets. We also performed meta-analyses to document enrichment of DNMs in candidate genes by leveraging our WGS dataset with those of several DEE and ID series. By combining these strategies, we were able to provide a causal link between DEE and the following genes: NTRK2, GABRB2, CLTC, DHDDS, NUS1, RAB11A, GABBR2, and SNAP25. Overall, we established a molecular diagnosis in 63/197 (32%) individuals in our WGS series. The main cause of DEE in these individuals was de novo point mutations (53/63 solved cases), followed by inherited mutations (6/63 solved cases) and de novo CNVs (4/63 solved cases). De novo missense variants explained a larger proportion of individuals in our series than in other series that were primarily ascertained because of ID. Moreover, these DNMs were more frequently recurrent than those identified in ID series. These observations indicate that the genetic landscape of DEE might be different from that of ID without epilepsy.
Keywords: epileptic encephalopathy, NTRK2, GABRB2, GABBR2, DHDDS, NUS1, CLTC, RAB11, SNAP25
Introduction
Epilepsy is often associated with major comorbidities, most frequently intellectual disability (ID), which affects 25% of children with epilepsy.1, 2 Conversely, the frequency of lifetime history of epilepsy ranges from 7%–15% for individuals with mild to moderate ID to 45%–82% for those with severe ID.3 The co-occurrence of epilepsy and ID can involve at least two non-exclusive mechanisms. In some cases, uncontrolled seizures can be detrimental to developing cortical networks and can lead to regression and poor cognitive outcomes in children.4 The term epileptic encephalopathy (EE) has been used to designate disorders where the epileptic activity itself contributes to cognitive slowing or regression, and EE can occur in a child with or without preexisting developmental delay.5 In other cases, a single genetic or environmental process is sufficient to induce both seizures and cognitive impairment.6 For instance, mutations that induce specific synaptic defects might result in aberrant connectivity and seizures, as well as alter synaptic plasticity and cause learning disabilities. The term developmental encephalopathy (DE) has been proposed to designate disorders where developmental delay emerges before the presence of epileptic activity or in the presence of infrequent epileptic activity.5 Because it is not always easy to dissect the contribution of each of these mechanisms and because some genetic disorders can involve both mechanisms in the same or in different individuals, the term developmental and epileptic encephalopathy (DEE) has been coined to refer to conditions characterized by ID and epilepsy where both mechanisms might play a role.5
Recently, parent-child exome sequencing studies in cases of sporadic DEE have shown that de novo mutations (DNMs) are an important cause of DEE. However, only a minority of the studied cases were solved by these approaches, thus underlining the genetic heterogeneity of DEE and the need to sequence large cohorts to increase the power to identify genes associated with DEE.7, 8 With an average of ∼1 DNM affecting the coding sequence of an individual, one of the challenges has been to determine whether the candidate DNMs are pathogenic or coincidental. To address this, Samocha et al. published a statistical framework that determines the rate of de novo variants per gene per class of variant (e.g., missense, nonsense, frameshift, or canonical splice site [CSS]) in order to determine whether there is gene enrichment for a particular variant class in the studied cohort and thus provide evidence that the observed DNMs are most likely implicated in the disease.9 This strategy was recently successfully employed in meta-analyses of DNMs identified from various sequenced trios affected by ID and/or developmental disorders for the identification of genes enriched in DNMs in these cohorts.10, 11
In this study, we performed whole-genome sequencing (WGS) on 197 DEE individuals and their unaffected parents. We focused our analyses on DNMs (single-nucleotide variations [SNVs], small insertions or deletions [indels]), and copy-number variations (CNVs) affecting coding or splice-site regions. To identify genes implicated in DEE, we performed meta-analyses of the DNMs identified in our series and those found in other studies of DEE or ID trios and looked for genes statistically enriched in DNMs. We also performed targeted sequencing and leveraged our network of collaborators and gene-matching tools to find additional similarly affected individuals with DNMs in some of our prioritized genes and thus provide additional support for their implication in the disease. On the basis of these collective approaches, we provide herein evidence implicating DNMs in eight genes in DEE.
Subjects and Methods
Subjects
The DEE series screened by WGS (n = 197 trios) was recruited at three centers in Canada—the Sainte-Justine University Hospital Center in Montreal (HSJ; 99 trios), the Toronto Western Hospital (TWH; 35 trios), and the Hospital for Sick Children in Toronto (HSC; 63 trios)—after the study was approved by the research ethics boards and informed consent was obtained from each participant or legal guardian. This series, referred to as the Canadian Epilepsy Network (CENet) DEE cohort, included subjects with diverse DEE phenotypes. The criteria used for the selection of these individuals were as follows: (1) intractable epilepsy defined as an absence of response to two appropriate and well-tolerated anti-epileptic therapies (AEDs) over a 6-month period and an average of at least one focal, generalized tonic-clonic, myoclonic, tonic, atonic, or absence seizure or epileptic spasm per month during the period of poor control; (2) ID or global developmental delay (GDD); (3) absence of malformations or focal and multifocal structural abnormalities on brain MRI; and (4) absence of parental consanguinity and family history of epilepsy, ID, or autism in first-degree relatives. Each individual was classified into a specific epilepsy syndrome when possible (Table S1). The majority (∼90%) had had array comparative genome hybridization performed on a clinical basis, and only those with no pathogenic or possibly pathogenic CNVs were included. Many of the individuals were previously screened and found to be negative for mutations in various DEE gene-panel tests. A subset of candidate genes identified in the course of this study were sequenced in a cohort composed of 595 individuals with DEE of unknown cause (Table S2), most of whom had been tested for mutations in genes previously associated with DEE, as well as for pathogenic CNVs, as previously described.12 We were also able to recruit, through various collaborations, additional subjects with DNMs in candidate genes; these DNMs were identified by clinical or research exomes. Informed consent was similarly obtained from these individuals or their legal guardians.
WGS
WGS was performed at the McGill University and Genome Quebec Innovation Center as part of the Illumina Genome Network (IGN) according to the IGN standard procedure. In brief, genomic DNA extracted from blood samples was subjected to an additional cleaning step with the ZR-96 DNA Clean & Concentrator-5 Kit (Zymo) and then used for generating sequencing libraries with the TruSeq DNA PCR-Free Library Preparation Kit according to the manufacturer’s procedure. Sequencing was done either on the HiSeq 2000 (100 bp paired-end reads; one genome per three lanes) or on the HiSeq 2500 (125 bp paired-end reads; one genome per two lanes) such that a minimum final coverage of 30× was attained after data processing.
WGS Data Processing, Variant Calling, and Analyses
The Illumina sequencing reads were generated with bcl2fastq v.1.8.4. Trimmomatic v.0.32 was used to remove bad-quality reads and to trim the read edges with a lower quality. The filtered reads were aligned to reference Homo sapiens assembly b37 (GRCh37) with BWA-mem v.0.7.10 for the creation of a binary alignment map (BAM) file. Read-set BAM files from different sequencing lanes for the same sample were merged into a single global BAM file with Picard v.1.123. Regions containing multiple base mismatches were realigned locally with Picard. Once local regions were realigned, Picard was also used to recalculate the coordinates of read mates and to mark duplicates for removal. Individual base-quality values were recalibrated with the Genome Analysis Toolkit (GATK) v.3.3-0. Genotypes were called with the GATK HaplotypeCaller, and all variant calls were merged and recalibrated in three different sensitivity tranches with GATK according to its recommended best practices. All variant sites were annotated with a custom version of ANNOVAR.13 Only variants with positions covered at ≥10× and supported by at least four variant reads constituting ≥25% of the total reads for each called position were considered. Rare variants included those present with a minor allele frequency (MAF) of ≤0.005 in 1000 Genomes, GoNL, ExAC Browser v.0.3, or the NHLBI Exome Sequencing Project Exome Variant Server (EVS) or ≤2% in the unaffected parents from the entire trio dataset. Variant segregation (in child-parent trios) was analyzed with an in-house script. We identified putative DNMs by excluding those present in the genomes of the parents and those with a MAF ≥ 0.001 in the ExAC Browser. Potential de novo variants outside the exonic and splice consensus regions were further excluded if they were present in small-repeat regions (for SNVs and indels), present in Alu regions (for indels), or had an SNV variant quality-score recalibration (VQSRT) from ≥99.90 to 100.00 or an indel VQSRT different than PASS. We visually inspected the sequencing reads carrying putative DNMs in each trio by using the Integrative Genomics Viewer (IGV)14 to exclude obvious false positives or inherited variants. Putative DNMs affecting the coding and consensus splice regions were validated by Sanger sequencing in the corresponding trio.
CNV Analyses
CNVs were identified by two algorithms: Lumpy, whose calls integrate multiple breakpoint signals, and PopSV, whose calls rely on deviation from normalized read depths across samples.15, 16 Default parameters were used unless otherwise specified. For PopSV, 5 kb bin scans of the genome were used. We filtered CNV calls to exclude those with a size < 1 kb and with a quality-value (PopSV) or evidence-set (Lumpy) score ≤ 0.1%. CNVs falling in regions of segmental duplications were also excluded. To identify de novo CNVs, we excluded those present in any of the parents’ samples from the entire dataset, those in population controls from 1000 Genomes, and those from the CNV map of high-quality datasets of common variants.17 De novo CNVs called by both Lumpy and PopSV were prioritized for validation. Potential de novo CNVs detected by only one algorithm, and thus likely to be enriched with false positives, were considered for validation only if they affected exonic regions and if they could not be ruled out as inherited or false positives upon visual inspection by IGV of the reads near the breakpoints. CNVs were validated in the trio by standard qPCR (TaqMan assay) and/or by Sanger sequencing.
Targeted Sequencing Using the Molecular Inversion Probe (MIP) Technique
Seven of our initially prioritized genes (DHDDS [OMIM: 608172], RYR2 [OMIM: 180902], HECW2 [OMIM: 617245], GABRB2 [OMIM: 600232], NUS1 [OMIM: 610463], NTRK2 [OMIM: 600456], and CLTC [OMIM: 118955]) were selected for MIP sequencing in a cohort of 595 individuals with DEE. We used a multiplex targeted-capture strategy to target the coding exons and intron-exon boundaries (a minimum of 5 bp of flanking sequence) in each of the seven genes. Single-molecule MIPs (smMIPs) were used as previously described18 with minor modifications detailed below. The molecular tag within the probe consisted of five random nucleotides that allowed for distinction of genomic molecules and a high-confidence consensus call. Library preparation remained the same as described by O’Roak et al.,19 except the ratio of probe to genomic DNA was adjusted to 2,000:1, a 10-fold increase from the previously reported ratio. We performed sequencing on an Illumina HiSeq 2500 to generate 100 bp paired-end reads. Raw read mapping and processing were performed as previously described.12 Private variants (absent from SNP public databases: ExAC Browser v.0.3, EVS, and 1000 Genomes) predicted to affect the protein sequence (missense variants, nonsense variants, indels, and CSSs) were validated by Sanger sequencing in the proband and the parents.
Gene-Specific DNM Enrichment
We used the DenovolyzeR open-access program to assess whether a specific gene is enriched in DNMs in subjects with DEE, GDD, and/or ID.20 This R package program is based on gene-specific mutation rates.9 DNM gene-specific p values calculated by DenovolyzeR for loss-of-function (LoF) variants (nonsense variants, CSSs, and frameshift indels) and functional variants (missense and LoF variants) were further corrected for multiple testing (Bonferroni correction) on the basis of the 19,618 genes with available mutation rates on which DenovolyzeR based its calculation and the number of tests (two; for LoF and functional categories; i.e., 2 × 19,618 = 39,236). A corrected p value (c.p value) < 0.05 was considered statistically significant. To increase statistical power, we performed a meta-analysis to combine DNMs identified herein with those previously reported from trio whole-exome sequencing (WES) done on other DEE cohorts.8, 21, 22 We also performed another meta-analysis to combine DNMs from the DEE cohorts with those from exome or genome sequencing from published ID cohorts.10, 11, 23, 24, 25, 26, 27, 28 Only studies consisting of more than ten trios were included in these meta-analyses (Table S3). To further increase the power to detect gene-specific DNM enrichment in genes whose mutations are not yet an established cause of DEE, we applied a strategy similar to that of Lelieveld et al.,11 who excluded from their meta-analysis trios with DNMs found in their curated list of genes previously associated with ID. Therefore, we performed a meta-analysis after excluding trios with DNMs affecting the autosomal-dominant or X-linked genes mentioned in this list (n = 572), which also includes genes associated with DEE, or trios with such mutations in 21 genes not reported in this list but subsequently found to be enriched with DNMs by Lelieveld et al. and/or by the recent Deciphering Developmental Disorders (DDD) trio sequencing study.10, 11
Clustering of De Novo Missense Variants
We used the open-source program Denovonear, which had been used in the DDD study,10, 29 to calculate the probability of the proximity of de novo missense variants in genes of interest on the basis of one million simulations weighted by the context trinucleotide rates. We considered a p value < 0.01 to be statistically significant.
Results
We performed WGS on 197 individuals with DEE and their unaffected parents. The average coverage of the genomes was 37.9×, and 99% of the genome (GRCh37) bases were covered at ≥ 10× (Figure S1). The average number of SNVs and indels per genome was ∼4,182,490 and ∼23,532, respectively (Table S4). The average number of CNVs per subject, excluding those in segmentally duplicated regions, varied between 275 (PopSV) and 400 (Lumpy). In total, we detected an average of 66 high-quality DNMs (61 SNVs and 5 indels) that passed IGV inspection (∼75% of total DNMs calls) per individual, translating into a mutation rate of ∼1.2 × 10−8 DNMs per diploid genome per generation, which is in the range reported from other WGS trio studies.24, 30, 31, 32
We next focused our attention on the putative DNMs that affect coding and CSS regions and that passed IGV inspection. We were able to validate by Sanger sequencing 95% of these calls. In total, 288 DNMs were validated (1.46 DNMs/trio), representing an average of ∼1.37 de novo SNVs and 0.09 indels per individual, which is in the range of what was observed in a previous WES study of DEE trios (Table S5).8 We did not detect any DNMs in the coding or CSS regions of 39 probands (20%) (Figure S2A). Of only de novo SNVs, 7.8% (nonsense and CSS variants) are predicted to cause a loss of function, whereas 72% are predicted to cause a missense change (Figure S2B). We compared the de novo SNV rates observed in our DEE individuals with those observed in unaffected siblings of individuals with an autism spectrum disorder (ASD) (66.5% missense and 4.8% LoF)33 or in Icelandic control individuals (82% missense and 2.7% LoF).32 We found more LoF SNVs in our EE subjects than in the exomes of control siblings (p = 0.03, binomial exact test) or Icelandic genomes (p = 0.00002, binomial exact test), suggesting that a subset of these variants contributes to the disease.
We also searched for de novo CNVs. In total, 12 CNVs were called as de novo by both Lumpy and PopSV, and all were successfully validated by qPCR and/or Sanger sequencing. In addition, 35 putative de novo CNVs encompassing exonic regions were identified by only one of the algorithms; six of these putative CNVs were confirmed to be de novo by qPCR, 17 were inherited, and 12 were false positives. In total, 10/18 validated de novo CNVs, including five deletions and five duplications, affected exonic regions (Table S6).
Likely Pathogenic Variants Identified in the CENet Series
For all DNMs and rare recessive (bi-allelic and X-linked hemizygous) variants affecting the coding regions or CSSs, we assessed the involvement of the corresponding genes in epilepsy or related neurodevelopmental disorders by searching PubMed (gene name and “epileptic encephalopathy,” “epilepsy,” “seizure,” “mental retardation,” or “intellectual disability”) and verifying the gene’s OMIM description. Using the American College of Medical Genetics and Genomics 2015 guidelines for interpreting sequence variants,34 we initially identified pathogenic or likely pathogenic variants in 50/197 (25%) subjects in genes that, when mutated, have been shown to cause DEE and/or ID. Of these, 88% were explained by DNMs, and 12% were caused by inherited recessive mutations (Tables 1, S5, and S7).
Table 1.
Genes Affected by Pathogenic or Likely Pathogenic Variants in the CENet Cohort
| Variant Type | Genes Whose Mutations Are an Established Cause of DEE and/or ID | Candidate Genes |
|---|---|---|
| DNMs (n = 53) | (n = 44) | (n = 9) |
| Missense | SCN1A (3), SCN2A (3), SCN8A (4), KCNT1 (3), CACNA1A (2), GNAO1 (2), ATP1A3 (1), CDKL5 (1), COL4A1 (1), DDX3X (1), DNM1 (1), FGF12 (1), GABRG2 (1), HECW2 (1), KCNA2 (1), KCNQ2 (1), MED13L (1), MEF2C (1), NAA10 (1), PPP2R1A (1) | NTRK2 (2), DHDDS (1), GABBR2 (1), GABRB2 (1), RAB11A (1), SNAP25 (1) |
| Nonsense | SCN1A (2), ANKRD11 (1), HIVEP2 (1), IQSEC2 (2), NF1 (1), SYNGAP1 (1) | – |
| Frameshift | ARID1B (1), CDKL5 (1), IQSEC2 (1), KIAA2022 (1) | CLTC (1), NUS1 (1) |
| Canonical splice site | SCN1A (1), SCN8A (1) | – |
| De Novo CNVs (n = 4) | (n = 3) | (n = 1) |
| Deletions | deletion (exons 21–23) of DNMT3A (1), deletion encompassing PCDH19 (1) | deletion (exon 2) of NUS1 (1) |
| Duplications | duplication encompassing UBE3A (1) | – |
| Inherited Recessive SNVs or Indels (n = 6) | (n = 6) | (n = 0) |
| Bi-allelic | WWOX (1), SZT2 (1), NAGA (1), TBC1D24 (1) | – |
| Hemizygous | SLC9A6 (1), IQSEC2 (1) | – |
The number of individuals affected by pathogenic or likely pathogenic variants in the specified genes is indicated in parentheses.
We also identified pathogenic de novo CNVs in three individuals, including an 8 Mb deletion encompassing PCDH19 (OMIM: 300460) in a female individual, a 5.2 Mb duplication corresponding to the 15q11–q13 region located between the recurrent breakpoints BP2 and BP3, and a 3.4 kb exonic deletion of DNMT3A (OMIM: 615879), all of which have been previously associated with ID and/or epilepsy.
Targeted MIP Sequencing
From the WGS results of our first 120 DEE trios, we prioritized seven of our best candidate genes (CLTC, DHDDS, GABRB2, HECW2, NTRK2, RYR2, and NUS1) for targeted resequencing in 595 unsolved cases of DEE. These genes were selected on the basis of the documentation of predicted-damaging DNMs in at least two individuals with unsolved DEE from the CENet series or in one individual with unsolved DEE from the CENet series and in at least one previously reported individual with DEE and/or ID. Exon 1 of NUS1 was excluded from the analysis because it was poorly covered across the samples (18% of the target bases at ≥10×), possibly because of its high GC content. On average, 90% of the target bases were covered at ≥10× in 476 samples. Reduced coverage was obtained in the remaining 119 cases such that only 70% of the target bases reached ≥10×, probably as a result of poor DNA quality. Four predicted-damaging missense variants absent from the ExAC Browser were identified, each in a single DEE subject, in NTRK2 (c.1301A>G [p.Tyr434Cys] [GenBank: NM_006180.4]), GABRB2 (c.730T>C [p.Tyr244His] [GenBank: NM_021911.2] and c.911C>T [p.Ala304Val]), and HECW2 (c.4484G>A [p.Arg1495Lys] [GenBank: NM_020760.1]). These variants were validated to be de novo by Sanger sequencing. Interestingly, two of the DNMs affecting GABRB2 (p.Tyr244His) and NTRK2 (p.Tyr434Cys) were also recurrent in the CENet series. The missense variant (p.Arg1495Lys) in HECW2 was also recurrent given that it was previously reported as a de novo variant in a DDD case.10 Recently, DNMs in HECW2 have been shown to cause DEE.25, 35
Involvement of NTRK2, GABRB2, CLTC, DHDDS, and NUS1 in DEE
We next sought to identify additional DEE or ID individuals who carry DNMs in the candidate genes that were prioritized for MIP sequencing by mining GeneMatcher36 and DDD research variants in DECIPHER37 and by contacting our network of collaborators. Through this approach, we were able to obtain additional supporting evidence for the involvement of the following genes in DEE.
NTRK2
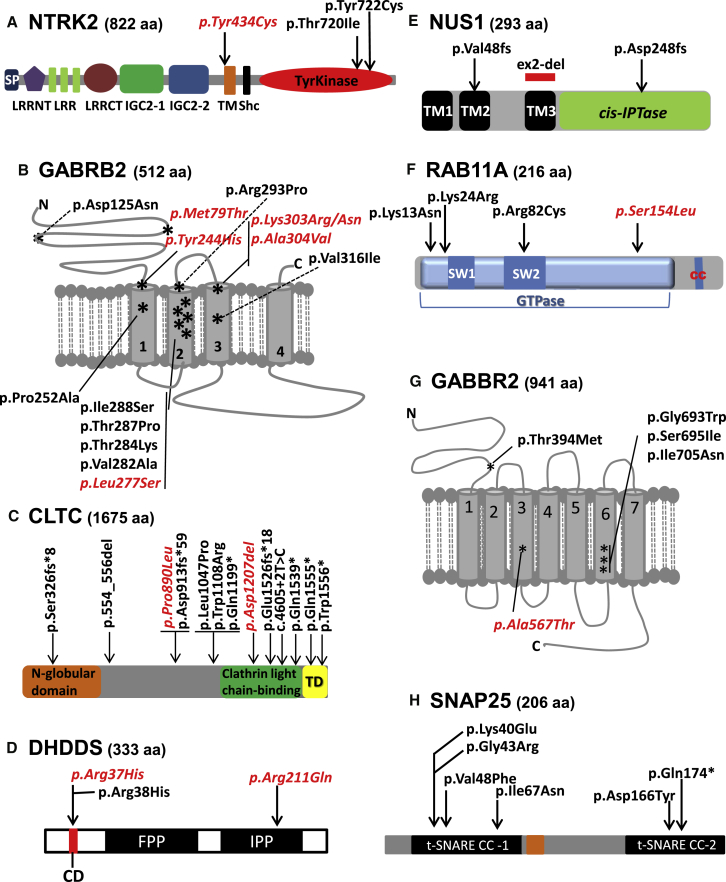
Our strategy involving trio WGS and targeted sequencing led to the identification of three DEE individuals carrying de novo predicted-damaging missense variants in NTRK2 (GenBank: NM_006180.4); these included an individual with the c.2159C>T (p.Thr720Ile) variant and two unrelated individuals with the same c.1301A>G (p.Tyr434Cys) variant. In addition, we identified two other individuals with the de novo p.Tyr434Cys missense variant through clinical WES.
In total, we identified four individuals with the p.Tyr434Cys missense variant. All subjects with this missense variant had severe GDD or ID and optic nerve hypoplasia with visual impairment, and three had significant feeding impairment (Table 2 and Supplemental Note). Three of them presented with epileptic spasms in the first few months of life and subsequently developed intractable seizures of various types in association with multifocal epileptic activity on electroencephalography (EEG), whereas the remaining individual had startle-like myoclonic events at 12 hr of life and developed, at 5 years of age, focal seizures that caused impaired awareness and occasionally evolved into bilateral tonic-clonic seizures. Clustering analysis using the Denovonear algorithm indicated that the presence of the p.Tyr434Cys variant in four individuals with similar phenotypes is statistically significant (p = 0.0001).
Table 2.
Summary of the Clinical Features in Cases with DNMs in NTRK2 (GenBank: NM_006180.4)
| Individual | Gender | Age at Last Examination | DNM (Detection) | Cognitive and Behavioral Features | Epilepsy Diagnosis | Age at Seizure Onset | Seizure Types | AEDs | EEG | Brain MRI | Associated Neurological Features and Seizure Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HSC0103 | male | 2 years, 9 months | c.1301A>G (p.Tyr434Cys) (WGSa) | severe GDD | IS | 3 days | ES, Fo | VGB, ACTH, LEV, CLB, TPM, VPA | modified hyps. | optic nerve hypoplasia | limb hypertonia and hyperreflexia, acquired microcephaly, visual impairment, swallowing difficulties, intractable seizures |
| indvSLIJ | male | 6 years, 3 months | c.1301A>G (p.Tyr434Cys) (cWESb) | severe ID, ASD | DEE | 12 hr with recurrence at 5 years | M, FIA | OXBZ, DZP | DS, TIRDA | optic nerve hypoplasia | hypotonia, lower-limb spasticity, visual impairment, seizures controlled on OXBZ for 1 month |
| T25821 | female | 4 years, 7 months | c.1301A>G (p.Tyr434Cys) (MIPS) | severe GDD, severe ID | IS | 4 months | ES, To | prednisolone, VGB, B6, LEV, CLB, TPM, LCM, KD, VPA, RFN, ZNS, CBD, DZP, PHT | MF, hyps. | optic nerve hypoplasia, myelination delay | acquired microcephaly, hypotonia, subtle choreoathetosis, visual impairment, feeding difficulties, intractable seizures, high tolerance to painful stimuli (parents report) |
| HF303 | male | 4 years, 3 months | c.1301A>G (p.Tyr434Cys) (cWESb, WGSc) | severe GDD, suspected severe ID, ASD | IS | 4 months | ES, FIA | PB, LEV, ACTH, VGB, CLB, ZNS, DZP, CBD | DS, MF | optic nerve hypoplasia | limb hypotonia, visual impairment, swallowing difficulties, intractable seizures, high tolerance to painful stimuli (parents report) |
| HSJ0335 | female | 9 years | c.2159C>T (p.Thr720Ile) (WGSa) | GDD, moderate to severe ID, ASD | DEE | 2.5 years (febrile size at 23 months) | febrile, FIA, GTC, SE | CLB, LEV, TPM, VPA, CBZ | normal, DS after SE | delayed myelination, reduced WM, ventriculomegaly, thin CC | swallowing difficulties, hyperphagia after 3 years of age, no seizures for 2 years under CBZ |
Underlining indicates treatment with clinical response (decreased seizure frequency or severity), and italics indicates a negative response (aggravation of seizure frequency and/or severity). Abbreviations are as follows: WGS, whole-genome sequencing; cWES, clinical whole-exome sequencing; MIPS, molecular inversion probe sequencing; GDD, global developmental delay; ID, intellectual disability; ASD, autism spectrum disorder; IS, infantile spasms; DEE, developmental and epileptic encephalopathy; Fo, focal; FIA, focal impaired awareness; ES, epileptic spasm; M, myoclonic; To, tonic; GTC, generalized tonic-clonic; SE, status epilepticus; AED, anti-epileptic therapy; ACTH, adrenocorticotropin; B6, vitamin B6; CBD, cannabidiol; CLB, clobazam; CBZ, carbamazepine; DZP, diazepam; KD, ketogenic diet; LCM, lacosamide; LEV, levetiracetam; OXBZ, oxcarbazepine; PB, phenobarbital; PHT, phenytoin; RFN, rufinamide; TPM, topiramate; VGB, vigabatrin; VPA, valproic acid; ZNS, zonisamide; EEG, electroencephalography; hyps., hypsarrhythmia; DS, diffuse slowing; MF, multifocal; TIRDA, temporal intermittent rhythmic delta frequency activity; MRI, magnetic resonance imaging; WM, white-matter tracts; and CC, corpus callosum.
CENet.
GeneDx.
HudsonAlpha study.
The subject with the p.Thr720Ile missense variant had moderate to severe ID, ASD, and intractable generalized tonic-clonic and focal seizures with impaired awareness starting at the age of 2.5 years. Unlike the individuals with the p.Tyr434Cys missense variant, she had hyperphagia and early-onset obesity from the age of 3 years. Interestingly, Yeo et al. reported that an individual carrying the de novo c.2165A>G (p.Tyr722Cys) variant, affecting an amino acid residue adjacent to Thr720, presented with a phenotype similar to that of our subject; characteristics of this phenotype included excessive weight gain, moderate ID, language delay, autistic features, hypotonia, and seizures.38
NTRK2 encodes the TRKB receptor, a member of the neurotrophin receptor tyrosine kinase family.39 TRKB has high affinity for brain-derived neurotrophic factor (BDNF) and for neurotrophin-4. BDNF-TRKB signaling is a critical regulator of neuronal development and function.40 The p.Tyr434Cys variant is located at the beginning of the transmembrane domain of NTRK2 (Figure 1A). The fact that this de novo variant has been identified in four individuals with a similar phenotype suggests that it confers a specific property to the protein, possibly via a gain-of-function or a dominant-negative mechanism. The p.Thr720Ile and p.Tyr722Cys variants cluster in the catalytic domain of NTRK2 (Figure 1A). In vitro studies indicate that p.Tyr722Cys impairs BDNF-induced TRKB receptor autophosphorylation and downstream signaling.38 It is currently unknown whether p.Thr720Ile affects NTRK2’s function in a similar way, but its proximity to p.Tyr722Cys and the similar phenotypes of both individuals carrying these variants suggest that this could be the case. Interestingly, mice expressing 25% of normal TRKB levels are hyperphagic and overweight.41 Altogether, our findings unequivocally show that DNMs in NTRK2 cause DEE.
Figure 1.
Localization of De Novo Variants in Protein Domains Encoded by Genes of Interest
GABRB2 (A), CLTC (B), NUS1 (C), NTRK2 (D), and DHDDS, SNAP25, GABBR2, and RAB11A (E). Recurrent de novo variants are in italics and red font. The transmembrane domains of GABRB2 and GABBR2 are labeled 1–4 and 1–7, respectively. Abbreviations are as follows: TM, transmembrane domain; TD, trimerization domain; SP, signal peptide; LRRNT, leucine-rich repeat N-terminal domain; LRR, leucine-rich repeat; LRRCT, leucine-rich repeat C-terminal domain; IGC2, immunoglobulin C-2 type 1 domain; IGC2-2, immunoglobulin C-2 type 2 domain; Shc, SHC1 interaction domain; IPP, isopentenyl diphosphate binding site; CD, catalytic domain; FPP, farnesyl diphosphate binding site; SW, switch domain; and CC, prenylation residue.
GABRB2
Our WGS and MIP screens identified three DEE individuals carrying DNMs in GABRB2 (GenBank: NM_021911.2), including the c.911C>T (p.Ala304Val) variant in one subject and the recurrent c.730T>C (p.Tyr244His) variant in two subjects. Two other individuals with DNMs in GABRB2, one with the c.830T>C (p.Leu277Ser) variant and another with the c.373G>A (p.Asp125Asn) variant, were identified by the DDD study.10 We also identified from WES and targeted gene-panel sequencing six individuals with de novo missense variants in GABRB2, including one with the same c.830T>C (p.Leu277Ser) variant found in the DDD subject, one with c.851C>A (p.Thr284Lys), one with c.878G>C (p.Arg293Pro), one with a missense c.908A>G (p.Lys303Arg) variant adjacent to c.911C>T (p.Ala304Val) (which was identified in our MIP screen), one with c.946G>A (p.Val316Ile), and one with a de novo c.236T>C (p.Met79Thr) variant. This latter individual was previously reported to have a de novo frameshift in CHAMP1 (c.1876_1877delAG [p.Ser626Leufs] [GenBank: NM_032436.2]), which also most likely contributes to the cognitive impairment of the subject (F3-II.1 in Isidor et al.42). All of these de novo missense variants are predicted to be damaging (PolyPhen-2, SIFT, and CADD). Their localization in GABRB2 is shown in Figure 1B.
We were able to obtain detailed clinical information for all of these 11 individuals (Table 3 and Supplemental Note). They all displayed moderate to severe ID (or severe GDD), with the exception of the individual with the p.Val316Ile variant, who achieved normal milestones at 21 months of age. Most individuals had microcephaly (n = 7/11), which was acquired in six individuals and congenital in the seventh. Within the first year of life, most individuals developed refractory seizures (predominantly myoclonic seizures and absences), which sometimes evolved into myoclonic status epilepticus or non-convulsive status epilepticus. Some individuals developed focal seizures with impaired awareness or autonomic seizures, tonic seizures, atonic seizures, and/or rarely generalized tonic-clonic seizures. In five of the individuals, the epilepsy remained refractory despite multiple drug trials. Two individuals were given a trial of vigabatrin and showed marked deterioration. Responses to lamotrigine, valproate, levetiracetam, or high-dose steroids were observed in five individuals. Axial hypotonia, spasticity, dystonia, and choreoathetosis appeared to be common features. Cortical visual impairment was present in 3/11 individuals. Brain MRI was usually normal, although delayed myelination or diffuse T2 hypersignal in the subcortical white matter was noted in three individuals.
Table 3.
Summary of the Clinical Features of Individuals with DNMs in GABRB2 (GenBank: NM_021911.2)
| Individual | Gender | Age at Last Examination | DNM (Detection) | Cognitive and Behavioral Features | Epilepsy Diagnosis | Age at Seizure Onset | Seizure Types | AEDs | EEG | Brain MRI | Associated Neurological Features and Seizure Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1242500 | female | 9.3 years | c.236T>C (p.Met79Thr) (cWESa) | GDD, severe ID | DEE | 11 months | A or FIA | LEV | normal | arachnoid cyst | acquired microcephaly, axial hypotonia, spasticity, ataxia, minor dysmorphic traits (short perineum, tapered fingers, short broad great toes), seizures controlled with LEV |
| K.02591 | female | 10 years | c.373G>A (p.Asp125Asn) (WESb) | GDD, moderate ID | DEE | 6 years | febrile, GTC | VPA | ND | normal | acquired microcephaly, no seizures (responded to VPA; off medication) |
| indvLB | female | 1.5 years | c.878G>C (p.Arg293Pro) (WES) | GDD | no seizures | NA | NA | NA | normal | normal | severe psychomotor delay, generalized dyskinesia, dystonia, cortical visual impairment |
| CNSA01M | male | 4 years | c.908A>G (p.Lys303Arg) (targeted gene panel) | GDD, severe ID | EOEE | 1 day | Fo, MF, To | VPA, LEV, TPM, LTG | MF, slow background | diffuse T2 hypersignal in white matter at birth and 18 months | acquired microcephaly, neonatal feeding difficulties, nonambulation, hypotonia, spasticity, dystonia, rare seizures under TPM |
| T21213B | female | 14 years, 6 months | c.911C>T (p.Ala304Val) (MIPS) | GDD, severe ID | DEE | 4 years | M, A, At, non-convulsive SE | CLB, VGB, pred., TPM, HCT, VPA, LTG, LEV, CZP, SULTH | biF SW or sharp SW | normal | acquired microcephaly, nonambulation, hypotonia, intractable seizures |
| HSJ0753 | female | 4 years | c.730T>C (p.Tyr244His) (WGSc) | severe GDD | DEE | 4 months | M, GTC, MSE | LEV, VPA, TPM, B6, DZP, CLB, PB, PHT, CBD, KD | biF SW, hyps., continuous diffuse SW | normal (at 9 days and at 1 year) | acquired microcephaly, nonambulation, axial hypotonia, spasticity, nystagmus, cortical visual impairment, intractable seizures |
| T23211 | female | 5 years, 1 month | c.730T>C (p.Tyr244His) (MIPS) | GDD, severe ID | DEE | <5 months | To, Fo, autonomic, M, SE | PB, LEV, CZP, P5P, B6, FOL, VGB, TPM, CBZ, NZP, OXBZ, VPA | MF (biF predominant), slow background | delay in myelination, reduction of white matter | congenital microcephaly, axial hypotonia, peripheral hypertonia, cortical visual impairment, choreoathetosis, dystonia, failure to thrive, intractable seizures |
| HA076 | male | 15 years, 8 months | c.830T>C (p.Leu277Ser) (WES) | GDD, severe ID | DEE | 4 years, 8 months | M, At, A, GTC | VPA, TPM, CZP, CLB, LEV, LTG | slow, rhythmic notched slow waves | MF T2 hypersignal in white matter at 2 years, normal at 4 and 9 years | spasticity, poor coordination, broad-base gait, seizure control with LVT and LTG |
| G64518 | female | 10 years | c.830T>C (p.Leu277Ser) (WESb) | GDD, severe ID | DEE | 2 years | GTC, A, febrile | VPA, LTG | high-amplitude rhythmic slow waves | mild increase in LVs at 2 years; normal at 3 years | acquired microcephaly, brisk reflexes, seizure control with LTG |
| 31841 | male | 17 days | c.851C>A (p.Thr284Lys) (WES) | severe GDD | EME | 7 days | M, To | PB, LEV, MDZ, biotin, FOL, B6 | BS | normal | hypotonia, jitteriness, back arching, apneas, intractable seizures, deceased at age 17 days |
| 3001866 | female | 21 months | c.946G>A (p.Val316Ile) (cWESa) | language delay | DEE | 12 months | A or Fo, GTC | LEV, OXBZ, CNZ, ZNS | normal | normal | apneas, neuroendocrine cell hyperplasia of infancy, intractable seizures |
Individual 1242500 was also previously identified with a pathogenic de novo mutation in CHAMP1 (Isidor et al.42). Underlining indicates treatment with clinical response (decreased seizure frequency or severity), and italics indicates a negative response (aggravation of seizure frequency and/or severity). Abbreviations are as follows: NA, not applicable; ND, not done; cWES, clinical whole-exome sequencing; WGS, whole-genome sequencing; MIPS, molecular inversion probe sequencing; GDD, global developmental delay; ID, intellectual disability; DEE, developmental and epileptic encephalopathy; EOEE, early-onset epileptic encephalopathy; EME, early myoclonic encephalopathy; A, absence; At, atonic; GTC, generalized tonic-clonic; SE, status epilepticus; MSE, myoclonic status epilepticus; To, tonic; M, myoclonic; FIA, focal impaired awareness; Fo, focal; MF, multifocal; CZP, clonazepam; FOL, folinic acid; HCT, hydrocortisone; LTG, lamotrigine; MDZ, midazolam; NZP, nitrazepam; P5P, pyridoxal 5-phosphate; pred., prednisone; SULTH, sulthiam; AED, anti-epileptic therapy; LEV, levetiracetam; VPA, valproic acid; TPM, topiramate; CLB, clobazam; VGB, vigabatrin; B6, vitamin B6; DZP, diazepam; PB, phenobarbital; PHT, phenytoin; CBD, cannabidiol; KD, ketogenic diet; OXBZ, oxcarbazepine; ZNS, zonisamide; EEG, electroencephalography; biF, bi-frontal predominance; BS, burst suppression; SW, spike-wave; hyps., hypsarrhythmia; MRI, magnetic resonance imaging; WM, white-matter tracts; and LV, lateral ventricle.
Baylor College of Medicine and Miraca.
DDD study.
CENet.
GABRB2 encodes the β2 subunit of the GABAA receptor, a neuronal pentameric ionotropic ligand-gated chloride channel that induces synaptic inhibition when activated by its agonist GABA.43 Variants in other GABAA receptor subunits encoded by GABRA1 (OMIM: 137160), GABRB1 (OMIM: 137190), GABRB3 (OMIM: 137192), and GABRG2 (OMIM: 137164) are established causes of DEE. Three individuals with DNMs in GABRB2 and detailed phenotypic information have been previously published: one of these subjects, who carries the DNM c.236T>C (p.Met79Thr), also found in one of our subjects, showed generalized seizures and moderate ID; another one displayed ID, seizures (of unspecified type), and cortical visual impairment (c.754C>G [p.Pro252Ala]); and the last one was found to have early-onset myoclonic encephalopathy (c.859A>C [p.Thr287Pro]).44, 45, 46 Four additional subjects with de novo missense mutations in GABRB2, including c.845T>C (p.Val282Ala), c.863T>G (p.Ile288Ser), c.909G>T (p.Lys303Asn), and c.911C>T (p.Ala304Val), have been reported.47, 48, 49 The amino acid residues affected by the latter two of these DNMs (p.Lys303Asn and p.Ala304Val) were also found to be mutated in our series. These four individuals appear to show ID or GDD and epilepsy, but no detailed clinical information was available.
Out of the 13 GABRB2 DNMs previously reported or described herein, ten encode variants clustered within a stretch of 60 amino acids (positions 244–304) encompassing three transmembrane domains and/or their boundaries (p value = 0.000002, Denovonear) (Figure 1B). These clustering variants appear to be mostly associated with severe GDD or ID and, with the exception of p.Arg293Pro, intractable generalized seizures and DEE. So far, only one of these de novo missense variants, p.Thr287Pro, has been functionally tested in transfected HEK293 cells and found to reduce cell-surface expression and peak current amplitudes of GABAA channels.46 It is currently unknown whether the other de novo missense variants in GABRB2 behave similarly to p.Thr287Pro, especially the closely clustering or recurrent ones (p.Tyr244His, p.Leu277Ser, p.Lys303Leu, and p.Ala304Val), which might confer specific properties to the protein such as gain-of-function or dominant-negative effects. Collectively, the previously and presently reported individuals with DNMs in GABRB2 confirm that de novo missense mutations in GABRB2 can cause a DEE phenotype.
CLTC
Our WGS trio screen identified a de novo frameshift variant (c.4575dupA [p.Glu1526fs∗18]) in CLTC (GenBank: NM_004859.3) in an individual with moderate ID associated with severe refractory seizures (absence, myoclonic, tonic, generalized tonic-clonic, and focal seizures). We obtained detailed clinical information on 11 additional individuals with DNMs in CLTC, four of whom were identified by the DDD study10 and seven of whom we identified through clinical WES (Table 4 and Supplemental Note). We were able to obtain detailed clinical information for all 12 of these individuals. Most of these individuals presented with early-onset hypotonia and GDD, which evolved over time into mild to severe ID (or borderline intelligence). Four individuals also developed ataxia. When performed, neuromuscular investigations (electromyography and biopsy) were negative. Two individuals had pharmaco-resistant epilepsy with a preponderance of myoclonic and generalized tonic-clonic seizures. One individual had one isolated seizure and is now seizure free after being weaned from medication. Two other individuals had severe GDD or ID with seizures, starting between the ages of 1 and 2 years, that were well controlled with valproate or levetiracetam. Interestingly, three of the ID subjects (one sequenced by the CAUSES [Clinical Assessment of the Utility of Sequencing and Evaluation as a Service] study and another sequenced in the context of the Undiagnosed Patient Program at the Ospedale Pediatrico Bambino Gesù in Rome) had a recurrent de novo missense mutation (c.2669C>T [p.Pro890Leu]), which was also reported in a DDD trio for which we were not able to obtain phenotypic information. The presence of the same DNM in CLTC in four independent subjects was statistically significant for missense clustering (p = 0.0000001, Denovonear). The positions of these various DNMs in CLTC are shown in Figure 1C.
Table 4.
Summary of the Clinical Features of Individuals with DNMs in CLTC (GenBank: NM_004859.3)
| Individual | Gender | Age at Last Examination | DNM (Detection) | Cognitive and Behavioral Features | Epilepsy Diagnosis | Age at Seizure Onset | Seizure Types | AEDs | EEG | Brain MRI | Associated Neurological Features and Seizure Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PBSD | female | 11 years, 2 months | c.977_980delCAGT (p.Ser326Cysfs∗8) (cWESa) | GDD, borderline IQ at 5 years | no seizures | NA | NA | NA | NA | T2 hypersignal in white matter (hypomyelination) | ADHD, impulsivity, poor socialization skills, mild hypotonia, wide-based gait |
| 5289183 | male | 20 years, 5 months | c.1660_1668del (p.Met554_Tyr556del) (cWESa) | borderline IQ, learning disabilities | NA | 14 years | one seizure | LEV | normal | normal | progressive paraparesis with lower-limb spasticity, ataxia, myoclonus, one seizure without recurrence under LEV, no seizures for 4 years off meds |
| indvAA | male | 3 years, 2 months | c.2669C>T (p.Pro890Leu) (cWESb) | GDD | no seizures | NA | NA | NA | normal | normal | mild ataxia, possible myoclonus |
| CAUSES1 | male | 4 years, 7 months | c.2669C>T (p.Pro890Leu) (WES) | GDD, suspected ID | no seizures | NA | NA | NA | NA | normal | mild hypotonia, oral and motor apraxia, suspected ADHD |
| 18052017 | female | 30 years | c.2669C>T (p.Pro890Leu) (WESc) | moderate ID | no seizures | NA | NA | NA | normal | normal | bradykinesia, bradypsychism, hypomimia, hypokinesia, clumsiness, attention instability |
| indvPAR | male | 16 years | c.3140T>C (p.Leu1047Pro) (trio cWES) | severe ID | DEE | 1 year | suspected FIA | VPA | non-specific irritative pattern, no foci | thin and short CC with hypoplasia of its posterior part, wide Virchow-Robin spaces | neonatal-onset hypotonia, no speech, acquired microcephaly, severe gastroinstestinal reflux, no seizures under VPA |
| 273692 | male | 4 years | c.3322T>C (p.Trp1108Arg) (WESd) | severe GDD, suspected severe ID | DEE | 2 years | M, GTC, possible gelastic seizures | LEV | abnormal | pontocerebellar atrophy, delayed myelination | nonambulation, spasticity, dystonia, myoclonus, neonatal feeding difficulties, visual impairment, seizure control with LEV |
| DDD261801 | male | 10 years, 7 months | c.3595C>T (p.Gln1199∗) (WESd) | mild GDD, mild ID | no seizures | NA | NA | NA | NA | normal | neonatal-onset hypotonia, congenital ptosis, poor social skills |
| indvMB | female | 7.5 years | c.3621_3623del (p.Asp1207del) (WES) | GDD, severe ID | DEE | 3 years | febrile GTC, M, To | VPA, LTG, CLB, CZP, LEV, TPM, LCM | MSW, biF | thin CC, T2 hypersignal in white matter, enlarged LVs | acquired microcephaly, severe hypotonia, ataxia, oral and motor apraxia, intractable seizures |
| HSC0054 | female | 23 years | c.4575dupA (p.Glu1526Argfs∗18) (WGSe) | GDD, moderate ID | DEE | 5 months | A, M, To, GTC, Fo | CLB, VPA,HCTZ, LEV, LTG, KD | gen. SW and PSW | delayed myelination, normal at 20 years | neonatal hypotonia, scoliosis, intractable seizures until puberty, no seizures under LEV and LTG |
| LDKQS | male | 12 years, 10 months | c.4605+2T>C (cWESa) | GDD, moderate ID | no seizures | NA | NA | NA | NA | normal | hypotonia, neonatal feeding difficulties, sensorineural hearing loss |
| DDD0280 | female | 6 years | c.4663C>T (p.Gln1555∗) (WESd) | GDD, moderate to severe ID | no seizures | NA | NA | NA | NA | ND | hypotonia |
| 281177 | male | 11 years | c.4667G>A (p.Trp1556∗) (WESd) | moderate ID | no seizures | NA | NA | NA | NA | ND | neonatal hypotonia |
Underlining indicates treatment with clinical response (decreased seizure frequency or severity). Abbreviations are as follows: NA, not applicable; ND, not done; cWES, clinical whole-exome sequencing; WGS, whole-genome sequencing; GDD, global developmental delay; IQ, intelligence quotient; ID, intellectual disability; DEE, developmental and epileptic encephalopathy; FIA, focal impaired awareness; M, myoclonic; GTC, generalized tonic-clonic; To, tonic; A, absence; Fo, focal; AED, anti-epileptic therapy; LEV, levetiracetam; VPA, valproic acid; LTG, lamotrigine; CLB, clobazam; CZP, clonazepam; TPM, topiramate; LCM, lacosamide; KD, ketogenic diet; HCTZ, hydrochlorothiazide; EEG, electroencephalography; MSW, multifocal spike-wave; biF, bi-frontal predominance; gen. SW, generalized spike-wave; PSW, poly-spike and wave; MRI, magnetic resonance imaging; WM, white-matter tracts; CC, corpus callosum; LV, lateral ventricle; and ADHD, attention-deficit hyperactivity disorder.
GeneDx.
Radboud University Medical Center.
Ospedale Pediatrico Bambino Gesù.
DDD study.
CENet.
CLTC encodes the widely expressed clathrin heavy chain 1, which is involved in endocytosis, intracellular trafficking, and synaptic recycling.50, 51 Recently, a de novo frameshift in CLTC (c.2737_2738dupGA [p.Asp913Glufs∗59]) was reported in a subject with GDD, unclassified epilepsy, and dysmorphic features.52, 53 In their study of 800 probands with ID, Leliveld et al.11 reported two additional DNMs, c.4615C>T (p.Glu1539∗) and c.3621_3623del (p.Asp1207del), the latter of which was also identified in one of our DEE subjects. CLTC is predicted to be intolerant of LoF mutations and has a pLi score of 1.00 according to the ExAC Browser.54 The phenotypic spectrum associated with these individuals is heterogeneous and ranges from mild ID or learning disability to severe ID or DEE. Interestingly, individuals with refractory epilepsy were found to carry variants in the first section of the clathrin light-chain binding domain, whereas truncating DNMs affecting the C terminus of CLTC tended to be associated with hypotonia, GDD, and ID (Figure 1C).
DHDDS
WGS identified a de novo missense variant (c.110G>A [p.Arg37His]) in DHDDS (GenBank: NM_024887.3) in one of our DEE individuals (HSJ0762). We identified, by clinical WES, another DEE individual who carries the same de novo p.Arg37His. Interestingly, this missense variant lies adjacent to p.Arg38His, which was reported in a DEE subject from the Epi4K study.7 Clustering analysis indicated that the presence of these two de novo variants in three individuals is statistically significant (p = 0.0005, Denovonear). In addition, we identified by clinical or research WES two other individuals with DEE and the de novo missense mutation c.632G>A (p.Arg211Gln) in DHDDS and obtained detailed clinical information on a third subject, also with the same de novo p.Arg211Gln (indvNCJ herein), who was previously reported in a recent WES study of ID trios.11 The positions of these various identified de novo variants in DHDDS are shown in Figure 1D, and their associated phenotypes are summarized in Table 5 and detailed in the Supplemental Note.
Table 5.
Summary of the Clinical Features of Individuals with DNMs in DHDDS (GenBank: NM_024887.3) and NUS1 (GenBank: NM_138459.4)
| ID | Gender | Age at Last Examination | Gene | DNM (Detection) | Cognitive and Behavioral Features | Epilepsy Diagnosis | Age at Seizure Onset | Seizure Types | AEDs | EEG | Brain MRI | Associated Neurological Features and Seizure Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| indvSG | female | 5 years, 1 month | DHDDS | c.110G>A (p.Arg37His) (cWESa) | GDD, severe ID | DEE | 18 months | MA photo+, GTC, febrile Fo | VPA, LTG, LEV, ETH, VPA | gen. SW, photo+ | normal | hypotonia, short stature, intractable seizures |
| HSJ0762 | male | 5 years, 6 months | DHDDS | c.110G>A (p.Arg37His) (WGSb) | GDD | DEE | 1 years | M, A, At, fever sensitive | LEV, VPA | gen. SW, diffuse slowing | normal | hypotonia, tremor, wide-based gate, ataxia, no seizures for 1 year on VPA |
| indvEF | female | 5 years, 6 months | DHDDS | c.632G>A (p.Arg211Gln) (cWESc) | GDD, borderline IQ | DEE | 4 years | MA | LEV, LTG, OXBZ | epileptiform | normal, Chiari I malformation | hypotonia, tremor, ataxia, inattention, obesity, seizures controlled with OXBZ |
| MDB31882 | male | 35 years | DHDDS | c.632G>A (p.Arg211Gln) (WESd) | GDD, severe ID | DEE | 6–9 years | M | VPA, benzodiazepines | gen. PSW | normal | gen. tremor, facial myokimia, bradykinesia, hypomimia, rigidity, freezing and impaired postural reactions, frontal lobe impairment features, no seizures since the age of 9 years, normal glycosylation assay, current therapy: VPA, clonazepam, tetrabenazine |
| indvNCJ | female | 7 years, 1 month | DHDDS | c.632G>A (p.Arg211Gln) (cWESe) | GDD, moderate to severe ID | NA | 7 years | M | none | normal | normal | ataxia, myoclonus, tremor, dystonia, short stature, no treatment initiated yet for cortical myoclonus, normal glycosylation assay |
| indvKW | male | 7 years, 11 months | NUS1 | c.743delA (p.Asp248Alafs) (cWESc) | GDD, severe ID | DEE | 12 months | M, GTC | LEV | biF epileptiform | normal | ataxia with LEV, lack of coordination, seizures controlled with LEV |
| HSJ0623 | male | 15 years | NUS1 | c.128_141dup (p.Val48Profs)∗7 (WGSb) | GDD, moderate ID, ASD | DEE | 10 months | MA, At, febrile GTC | VPA, LTG, LEV, ETH, CZP, CBZ, Stiri., CLB | diffuse slowing, biF or gen spikes | normal | ADHD, tremor, seizures controlled under VPA and CLB |
| HSJ0627 | female | 20 years | NUS1 | exon 2 deletion (WGSb) | motor delay, mild ID | DEE | 2.5 years | M status, MA, At | VPA, LEV, CLB, FEL, LTG, CZP | gen. SW and PSW | normal | tremor, dysarthria, seizures controlled on VPA, LTG, and CZP |
Underlining indicates treatment with clinical response (decreased seizure frequency or severity), and italics indicates a negative response (aggravation of seizure frequency and/or severity). Abbreviations are as follows: NA, not applicable; cWES, clinical whole-exome sequencing; WGS, whole-genome sequencing; GDD, global developmental delay; ID, intellectual disability; IQ, intelligence quotient; ASD, autism spectrum disorder; DEE, developmental and epileptic encephalopathy; MA, myoclonic absence; MA photo+, myoclonic absences with photosensitivity; GTC, generalized tonic-clonic; Fo, focal; M, myoclonic; A, absence; At, atonic; AED, anti-epileptic therapy; FEL, felbamate; VPA, valproic acid; LTG, lamotrigine; LEV, levetiracetam; ETH, ethosuximide; CZP, clonazepam; CBD, cannabidiol; CLB, clobazam; LTG, lamotrigine; OXBZ, oxcarbazepine; Stiri, stiripentol; EEG, electroencephalography; gen., generalized; SW, spike-wave; photo+, photosensitive; PSW, poly-spike and wave; biF, bi-frontal predominance; MRI, magnetic resonance imaging; and ADHD, attention-deficit hyperactivity disorder.
Baylor College of Medicine.
CENet.
GeneDx.
Ospedale Pediatrico Bambino Gesù.
Radboud University Medical Center.
These five individuals with DNMs in DHDDS presented with a generalized epilepsy disorder with myoclonic seizures, either as myoclonic absences or as isolated cortical myoclonus, and sometimes with light sensitivity or fever susceptibility. Two of these individuals also presented with other generalized seizure types, including atonic seizures or generalized tonic-clonic seizures. In three individuals, EEG revealed clear generalized spike-wave discharges (and additional photosensitivity in one individual). The seizures were aggravated by levetiracetam in two individuals, but favorable responses to valproic acid were observed. Interestingly, all individuals presented with marked hypotonia, and four had mixed movement disorders including ataxia, tremors, and dystonia.
DHDDS encodes dehydrodolichyl diphosphate synthase (also known as hCIT), which is essential for dolichol monophosphate (Dol-P) synthesis and global N-linked glycosylation.55 The Arg37 and Arg38 residues fall into an evolutionarily conserved stretch of five amino acids (positions 34–38) corresponding to the catalytic domain of the enzyme (Figure 1D). The crystal structure and mutagenesis studies done on the bacterial Dhdds enzyme (M. luteus undecaprenyl diphosphate synthase [UDPS]) show that the Arg203 residue, which is equivalent to Arg211 in the human DHDDS, is critical for the homoallylic binding to the substrate isopentenyl diphosphate.56, 57 The identification of recurrent or clustering DNMs in individuals with a similar phenotype in DHDDS is highly suggestive of pathogenicity.
A homozygous missense variant (c.124A>G [p.Lys42Glu]) was previously found in DHDDS in consanguineous families affected by retinitis pigmentosa.58, 59 In addition, bi-allelic truncating or splicing variants in DHDDS were reported in a case of type 1 congenital disorder of glycosylation with severe GDD and refractory seizures.60 We hypothesize that null alleles of DHDDS disrupt brain development only in the context of a recessive genotype, whereas the DNMs documented in our study cause DEE via a dominant-negative or gain-of-function mechanism.
NUS1
In NUS1 (GenBank: NM_138459.3), we identified two DNMs in individuals from our WGS trio study; these included a frameshift variant in exon 1 (c.128_141dup [p.Val48Profs∗7]) in one individual and a ∼1.3 kb deletion encompassing the entire exon 2 in the other. In addition, we identified a de novo truncating variant in NUS1 (c.743delA [p.Asp248Alafs∗4]) by clinical WES in an individual with DEE (Table 5, Supplemental Note, and Figure 1E). These individuals with DNMs in NUS1 all presented with GDD (or isolated motor delay) which eventually evolved into mild to severe ID. Furthermore, they all presented with generalized myoclonic seizures (with myoclonic status epilepticus in one individual and with myoclonic absences in two individuals). All individuals showed other generalized seizure types, including atonic seizures (drop attacks) or generalized tonic-clonic seizures. EEG revealed either generalized epileptic activity or bi-frontal epileptic discharges. Movement disorders, including tremor (in two individuals) and ataxia (in one individual), were also common. Altogether, this clinical phenotype is highly reminiscent of the one we observed in individuals with DNMs in DHDDS.
NUS1 encodes Nogo-B receptor (NgBR), which physically interacts with DHDDS to stabilize the dehydrodolichyl diphosphate synthase complex and potentiate its enzymatic activity.55, 61 Both indel mutations identified in this study affect upstream exons and thus have the potential to induce nonsense-mediated decay of the transcript. In addition, both variants are predicted to abolish the conserved C-terminal domain, which is required for the interaction with DHDDS.61 The deletion of exon 2 causes an in-frame deletion of amino acids 139–180, leading to the loss of transmembrane domain 3 (TM3), which is critical for the proper topology of NUS1. Previously, a homozygous missense mutation affecting its C terminus (c.869G>A [p.Arg290His]) was identified in two siblings with type 1a congenital disorder of glycosylation and a severe phenotype of early-onset refractory epilepsy, congenital scoliosis, developmental delay with hypotonia, microcephaly, hearing and visual impairment, and severe cortical atrophy.62 This mutation was found to decrease cis-PTase activity when expressed with hCIT (DHDDS) in yeast. In addition, Szafrans et al. reported individuals with early-onset seizures and 6q22.1 microdeletions centered on a 250 kb critical region that includes only NUS1 and the promoter of SLC35F1.63
Collectively, our finding of DEE individuals with two truncating DNMs and one de novo whole-exon deletion in NUS1, the reported DEE individuals with NUS1 microdeletions, and the fact that NUS1 is a functional direct interactor of DHDDS suggest that heterozygous mutations in NUS1 can cause DEE, possibly via a mechanism of haploinsufficiency. This is in agreement with the fact that NUS1 does not tolerate LoF variants, as suggested by the ExAC Browser, in which no such LoF mutations have been reported (pLi = 0.87).54 The more severe phenotype previously observed in the siblings with the homozygous p.Arg290His variant could be due to a more dramatic reduction in NUS1 activity as a result of the recessive nature of a potentially hypomorphic mutation. Failure to identify other individuals with NUS1 truncating mutations from the MIP screen or other published EE trios could be due in part to reduced capture efficiency of exon 1, which encodes 137/293 (∼47%) amino acids of NUS1. Indeed, in the ExAC Browser, exon 1 of NUS1 is, on average, poorly covered by WES in comparison with the rest of the exons of the gene.
Meta-analyses of DNMs from DEE and DEE-ID Cohorts
In order to further assess the involvement of various candidate genes in DEE, we sought to determine whether DNMs were enriched in certain genes in a series of affected individuals by taking advantage of a statistical framework that is based on the use of gene-specific mutation rates.9 To increase power, we meta-analyzed DNMs from our DEE cohort along with DNMs from published WES studies of DEE trios (combined DEE trios = 624; Table S3). In total, 12 genes were found to be statistically enriched with LoF and/or functional DNMs (Table 6); mutations in all of these genes are now considered causative of DEE.
Table 6.
Genes Enriched with DNMs in the DEE Cohorts
| Gene |
De Novo LoF Variants |
De Novo Functional Variants |
||||||
|---|---|---|---|---|---|---|---|---|
| Observed | Expected | p Value | c.p Value | Observed | Expected | p Value | c.p Value | |
| CDKL5 | 3 | 0 | 8.57E−9 | 0.0003∗ | 5 | 0 | 8.90E−10 | 3.49E−5∗ |
| DNM1 | 0 | 0 | 1 | 1 | 6 | 0 | 1.03E−11 | 4.04E−7∗ |
| GABRB3 | 0 | 0 | 1 | 1 | 4 | 0 | 7.58E−9 | 0.0003∗ |
| GNAO1 | 0 | 0 | 1 | 1 | 4 | 0 | 4.47E−9 | 0.0002∗ |
| IQSEC2 | 3 | 0 | 6.76E−9 | 0.00026∗ | 3 | 0 | 1.14E−5 | 0.45 |
| KCNQ2 | 0 | 0 | 1 | 1 | 4 | 0 | 1.69E−7 | 0.007∗ |
| KCNT1 | 0 | 0 | 1 | 1 | 4 | 0.1 | 9.12E−7 | 0.036∗ |
| SCN1A | 7 | 0 | 1.84E−17 | 7.2E−13∗ | 14 | 0.1 | 6.37E−27 | 2.5E−22∗ |
| SCN2A | 0 | 0 | 1 | 1 | 7 | 0.1 | 3.81E−12 | 1.49E−7∗ |
| SCN8A | 1 | 0 | 0.007 | 1 | 7 | 0.1 | 3.08E−12 | 1.21E−7∗ |
| SLC35A2 | 2 | 0 | 4.74E−7 | 0.018∗ | 3 | 0 | 4.89E−7 | 0.019∗ |
| STXBP1 | 1 | 0 | 0.006 | 1 | 6 | 0 | 1.27E−12 | 4.98E−8∗ |
c.p value is the corrected p value = p value × 2 × 19,618 (significant < 0.05, indicated by an asterisk). LoF variants are nonsense, frameshift, and CSS de novo variants. Functional variants are LoF and missense variants.
Given that epilepsy is a frequent comorbidity of ID, we performed a second meta-analysis combining the DNMs from published ID trios with those from the DEE cohorts used above (DEE-ID cohorts: 5,948 trios and 7,778 DNMs). In total, 111 genes were found to be enriched with functional and/or LoF DNMs, and 37 of these were found to be mutated in at least one DEE individual (Table S8). Interestingly, DNM enrichment has not been previously documented for 22/111 genes, including nine genes that have either not been directly associated with ID or DEE (BTF3 [OMIM: 602542], CHD3 [OMIM: 602120], FBXO11 [OMIM: 607871], PLK5, SETD1B [OMIM: 611055], and SF1 [OMIM: 601516]) or been described only in single or few individuals and therefore represent candidates pending additional evidence (CLTC,52 GABBR2 [OMIM: 607340],12, 27 and PHIP [OMIM: 612870]).64 Among these, only GABBR2, PHIP, and CLTC had some DNMs in DEE individuals, whereas the rest had DNMs only in the ID cohorts (Table S8).
Lelieveld et al. recently showed increased power to detect ID-associated genes in a meta-analysis after excluding individuals with DNMs in genes previously found to be causally linked to ID.11 We applied a similar strategy here to both the DEE-ID cohorts and excluded individuals with DNMs in any of the genes mentioned in the list established by these authors.11 We also removed the individuals with DNMs in genes that showed DNM enrichment from the recent meta-analyses done on trios with ID or developmental disorders.10, 11 This retained 4,424 trios from the combined DEE-ID cohorts. As a result, three additional genes from the DEE-ID cohort showed modest but significant enrichment of functional DNMs; these included GABRB2 (c.p value = 0.036), RAB11A (OMIM: 605570; c.p value = 0.036), and SNAP25 (OMIM: 600322; c.p value = 0.042), all of which were found with predicted-damaging DNMs in individuals from our CENet DEE cohort, as well as in individuals from the ID cohorts.
Additional Supporting Evidence for the Involvement of RAB11A, GABBR2, and SNAP25 in DEE
Our meta-analyses of the DEE-ID trios showed significant enrichment of DNMs in GABBR2, PHIP, CLTC, RAB11A, SNAP25, and GABRB2, whose mutations have not yet been confirmed as causes of DEE. With the exception of PHIP, we found predicted-damaging DNMs in all of these genes in individuals from the CENet series. We further validated the involvement of GABRB2 and CLTC in DEE by identifying additional individuals in the context of our MIP screen or other WGS or WES studies (see above). As shown below, we also provide additional evidence for the involvement of RAB11A, GABBR2, and SNAP25 in DEE.
RAB11A
We found a de novo predicted-damaging missense variant in RAB11A (c.244C>T [p.Arg82Cys] [(GenBank: NM_004663.3]) in a CENet individual with refractory epileptic spasms and erratic myoclonus with developmental regression. She subsequently developed focal seizures and severe ID. Using WES, we also identified another predicted-damaging de novo missense mutation in RAB11A (c.71A>G [p.Lys24Arg]) in an individual with moderate GDD and abnormal EEG but with no seizures reported so far. Three additional individuals with DNMs in RAB11A, including two individuals with the same variant (c.461C>T [p.Ser154Leu]) and another individual with a different variant (c.39A>C [p.Lys13Asn]), were identified in the context of the DDD study.10 We were able to obtain detailed clinical information on the individuals who had the p.Ser154Leu variant and showed moderate GDD without epilepsy. The other individual from the DDD study had abnormalities of the nervous system according to DECIPHER, but we could not get additional clinical information. Brain atrophy and/or abnormalities of the corpus callosum were noted for three of the individuals with available MRI information (Table 7 and Supplemental Note).
Table 7.
Summary of the Clinical Features of Individuals with DNMs in RAB11A (GenBank: NM_004663.4), GABBR2 (GenBank: NM_005458.7), and SNAP25 (GenBank: NM_003081.3)
| Individual | Gender | Age at Last Examination | Gene | DNM (Detection) | Cognitive and Behavioral Features | Epilepsy Diagnosis | Age at Seizure Onset | Seizure Types | AEDs | EEG | Brain MRI | Associated Neurological Features and Seizure Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HK055 | male | 5.5 years | RAB11A | c.71A>G (p.Lys24Arg) (WES) | GDD, moderate ID | no seizures | NA | NA | NA | abnormal background activity, no epileptic charges | central brain atrophy, bilateral periventricular white-matter damage, thin CC | acquired microcephaly, axial hypotonia, obesity, aggressive behavior |
| HSJ0637 | female | 9.5 years | RAB11A | c.244C>T (p.Arg82Cys) (WGSa) | GDD, severe ID | IS | 4 months | M, ES, Fo | NZP, CLB, VGB, TPM, VPA, LEV | modified hyps., M, diffuse slowing with M spikes | atrophy, partial agenesis of CC, delayed myelination, decreased NAA | acquired microcephaly, axial hypotonia |
| 24631 | male | 4 years | RAB11A | c.461C>T (p.Ser154Leu) (WESb) | moderate GDD | no seizures | NA | NA | NA | NA | partial agenesis of the CC | distractible, possible ADHD |
| 84049 | female | 9 years, 11 months | RAB11A | c.461C>T (p.Ser154Leu) (WESb) | moderate ID | no seizures | NA | NA | NA | NA | ND | possible hyperactivity, obesity |
| HSJ0048 | male | 14 years | GABBR2 | c.2077G>T (p.Gly693Trp) (WGSa) | severe GDD, severe ID | DEE, IS | 11 months | FIA, ES, GTC | CBZ, VGB, VPA, TPM, CLB, PHT, LEV, LCM, LTG | modified hyps. | increased sub-arachnoid spaces | axial and limb hypotonia, hyporeflexia, scoliosis, hypersalivation |
| HSJ0745 | male | 23 years | SNAP25 | c.496G>T (p.Asp166Tyr) (WGSa) | GDD, moderate ID | DEE | 18 months | GTC, FIA | VPA, CLB | gen. SW, CSWS | mild diffuse cortical atrophy | apneas, bradycardia, severe constipation, minor dysmorphic traits, no seizures for 2 years on VPA |
Underlining indicates treatment with clinical response (decreased seizure frequency or severity). Abbreviations are as follows: NA, not applicable; ND, not done; WES, whole-exome sequencing; WGS, whole-genome sequencing; GDD, global developmental delay; ID, intellectual disability; IS, infantile spasms; DEE, developmental and epileptic encephalopathy; M, myoclonic; ES, epileptic spasm; Fo, focal; FIA, focal impaired awareness; GTC, generalized tonic-clonic; AED, anti-epileptic therapy; NZP, nitrazepam; CLB, clobazam; VGB, vigabatrin; TPM, topiramate; VPA, valproic acid; LEV, levetiracetam; CBZ, carbamazepine; PHT, phenytoin; LCM, lacosamide; LTG, lamotrigine; EEG, electroencephalography; hyps., hypsarrhythmia; gen. SW, generalized spike-wave; MRI, magnetic resonance imaging; CC, corpus callosum; ADHD, attention-deficit hyperactivity disorder; CSWS, continuous spike and wave during sleep; and NAA, N-acetylaspartate.
CENet.
DDD study.
RAB11A encodes a GTPase that regulates the recycling of a wide range of receptors at the cell surface.65 Interestingly, RAB11A regulates synaptic plasticity by modulating the endocytic recycling of NTRK2 and AMPA receptors at the post-synaptic membrane of neurons.66, 67, 68 The highly conserved Arg82 residue is located in the nucleotide-sensitive switch domain II of RAB11A and is involved in binding to the RAB11A effector FIP3.69, 70 The p.Lys24Arg, p.Lys13Asn, and p.Ser154Leu variants do not affect any of the nucleotide-sensitive switch domains of RAB11A (Figure 1F). The fact that RAB11A is enriched with DNMs in the DEE-ID cohorts and was found to have a recurrent de novo missense in two individuals of the DDD cohort suggests that DNMs in this gene can cause a DEE or ID phenotype.
GABBR2
We identified from our WGS a de novo missense mutation in GABBR2 (c.2077G>T [p.Gly693Trp] [GenBank: NM_005458.7]) in one CENet subject who presented with focal seizures with impaired awareness and later developed epileptic spasms while on carbamazepine. He remains with refractory focal and generalized tonic-clonic seizures, severe ID, severe limb and axial hypotonia, and hyporeflexia (Table 7 and Supplemental Note). The Epi4K Consortium reported two de novo predicted-damaging missense variants (c.2114T>A [p.Ile705Asn] and c.2084G>T [p.Ser695Ile]) in GABBR2 in two individuals with unsolved infantile spasms.8 Lopes et al. recently reported a de novo missense mutation in GABBR2 (c.1699G>A [p.Ala567Thr]) in an individual with severe ID and Rett-syndrome-like features but no seizures.27
GABBR2 encodes a γ-aminobutyric acid type B receptor that inhibits neuronal activity through G-protein-coupled second-messenger signaling at both the presynaptic and post-synaptic membranes, where it regulates neurotransmitter release and the activity of ion channels.71 This receptor is the target of baclofen, a medication often used to treat spasticity. The hypotonia and hyporeflexia observed in our subject might therefore reflect underactivation at the neuromuscular junction or in spinal motor control centers. Interestingly, unlike the missense mutation identified by Lopes et al., which affects TM3 of GABBR2, the three DNMs in the individuals with infantile spasms affect TM6 of the protein (p = 0.001, Denovonear), suggesting that these TM6 variants are specific to DEE. Meta-analysis of DNMs from DEE-ID cohorts showed an enrichment of functional DNMs in GABBR2. In addition to the subject with the c.1699G>A (p.Ala567Thr) variant from Lopes et al., the DDD cohort contained two other individuals with the p.Ala567Thr variant and one individual with a c.1181C>T (p.Thr394Met) variant that affects the N-terminal extracellular region of the receptor.10 We conclude that de novo missense mutations in GABBR2 have the potential to cause DEE or ID with no seizures, depending perhaps on where they affect the protein.
SNAP25
We identified from our WGS a de novo missense mutation in SNAP25 (c.496G>T [p.Asp166Tyr] [GenBank: NM_003081.3 and NM_130811.2]) in a male with DEE. He presented with apneas, GDD, nocturnal generalized tonic-clonic seizures, and focal seizures with impaired awareness and progressively developed moderate ID (Table 7 and Supplemental Note). SNAP25 is a member of the SNARE complex and is required for the exocytosis of neurotransmitters during synaptic transmission by mediating synaptic vesicle fusion.72, 73 Developmentally regulated alternative splicing of two similar exon 5 sequences of SNAP25 generates two isoforms (a and b), which differ only by nine residues in this exon 5. Various mutant Snap25 mouse lines displayed cognitive deficit and seizures or susceptibility to seizures.74, 75 SNAP25 interacts with STXBP1, another SNARE synaptic protein in which variants are known to cause DEE.26, 76 So far, only two de novo mutations have been reported in SNAP25: a missense mutation affecting both isoforms (c.142G>T [Val48p.Phe] [GenBank: NM_003081.3 and NM_130811.2]) in an individual with DEE77 and a missense mutation affecting a conserved residue in exon 5 of only SNAP25b (c.200T>A [p.Ile67Asn] [GenBank: NM_130811.2]) in a girl showing congenital myasthenia, cerebellar ataxia, and ID.78 Both mutations affect the N-terminal t-SNARE coiled-coil homology domain of SNAP25. The de novo missense variant identified in our cohort (p.Asp166Tyr) is predicted to be damaging (by SIFT, PolyPhen-2, and CADD) and alters a conserved residue in the second t-SNARE coiled-coil homology domain common to both isoforms (Figure 1H). In addition, three DNMs in SNAP25 (c.118A>G [p.Lys40Glu] [GenBank: NM_130811.2], c.127G>C [p.Gly43Arg], and c.520C>T [p.Gln174∗]) were recently reported in the DDD study, but no detailed clinical information was available on these.10 Collectively, these findings support the involvement of SNAP25 mutations in DEE.
Pattern of DNMs Associated with DEE
Out of the 53 pathogenic or likely pathogenic de novo point variants identified in our CENet series, 35 are missense and 15 are LoF, resulting in a missense/LoF ratio of 2.5 (Table S5). We examined the list of DNMs identified in the Epi4K series of individuals with DEE and found a similar ratio of pathogenic or likely pathogenic missense variants to LoF variants (n = 56 DNMs; missense/LoF ratio = 43/13 = 3.3).8 Interestingly, these observed missense-to-LoF ratios of de novo pathogenic or likely pathogenic variants in both the CENet (p = 0.004, two-tailed Fischer’s exact test) and Epi4K (p = 0.0004, two-tailed Fischer’s Exact test) series were significantly higher than those similarly observed in 192 published trios with moderate to severe ID (WES and WGS) and detailed phenotypic and pathogenic variant information (missense/LoF ratio = 36/42 = 0.85).23, 24, 26, 28 Remarkably, out of all the pathogenic or likely pathogenic DNMs identified in the CENet series (n = 53), ∼45% were also independently reported in ClinVar (n = 24 [19 missense and 5 LoF]) (Table S5). This rate of recurrent pathogenic and likely pathogenic DNMs was significantly higher in the CENet DEE series than in the exomes or genomes of the 192 previously published trios with moderate to severe ID (out of 80 pathogenic or likely pathogenic variants, only 19 [13 missense and 6 LoF] were also reported independently in ClinVar) (p = 0.0012, two-tailed Fischer’s exact test).23, 24, 26, 28
Discussion
In this study, we performed WGS on 197 individuals with DEE and their unaffected parents. We initially identified pathogenic variants in 53/197 (27%) individuals, including 50 with point mutations in genes previously found to be causally linked to DEE or ID, one with a recurrent pathogenic CNV (15q11–q13 duplication), and two with CNVs encompassing genes previously associated with ID or DEE (PCDH19 and DNMT3A). Moreover, we were able to explain DEE in ten additional individuals from the series by identifying DNMs in candidate genes for which we provide additional evidence for their involvement in DEE (NTRK2, GABRB2, CLTC, DHDDS, NUS1, RAB11A, GABBR2, and SNAP25). Overall, our approach allowed us to obtain a molecular diagnosis in 63/197 (32%) individuals. It is important to note that the diagnostic yield of WGS would have most likely been higher in an unbiased series given that many of our subjects had previously been screened by targeted sequencing and/or array genomic hybridization. Interestingly, two of the four pathogenic de novo CNVs identified in our series would have been missed by clinical array genomic hybridization because of their size (<5 kb), providing some support for the added value of WGS.
The main cause of DEE in our series was de novo point mutations (53/63 solved cases), whereas the remaining cases could be explained by inherited mutations (6/63 solved cases) or de novo CNVs (4/63 solved cases). De novo missense variants explained a larger proportion of individuals with DEE in our series than of individuals ascertained because of ID in other series. Interestingly, more than half of these pathogenic missense mutations were recurrent, suggesting that at least a subset of them confer a specific property to the protein, such as dominant-negative or gain-of-function effects. Shohat et al. recently showed that, compared with genes with missense mutations, genes with LoF mutations were associated with different pathways across neuro-developmental disorders such as ID, ASD, and schizophrenia.79 For instance, genes with missense variants involved in neuro-developmental disorders code for proteins that show a higher number of protein interactions than those encoded by genes with LoF variants. Together, these data raise the possibility that the genetic landscape of DEE is enriched with gene products that act as protein hubs. It would be important to understand why these hubs are specifically associated with DEE.
Of the eight genes highlighted herein for their involvement in DEE, we were not able to show de novo gene enrichment for three of them: NTRK2, DHDDS, and NUS1. However, the multiple occurrences of DNMs affecting the same conserved amino acid residues in NTRK2 and DHDDS in individuals with a similar phenotype nonetheless represent strong evidence implicating the encoding genes in DEE. Indeed, other DEE-related genes with site-specific recurrent DNMs, such as GRIN2D (OMIM: 300776) and FGF12 (OMIM: 601513), did not also show DNM enrichment in our meta-analyses. Genetic forms of neuro-developmental disorders that are caused by recurrent DNMs associated with gain-of-function or dominant-negative effects tend to be rare because there are typically a smaller number of variants that can confer such effects than of variants that can induce haploinsufficiency. It is thus likely that meta-analyses involving larger numbers of subjects will be necessary for identifying these rare forms of DEE. No DNM enrichment was observed for NUS1 in our meta-analysis, possibly because of the poor capture of its exon 1, which represents almost half of the entire coding sequence of this gene. However, the identification of three DNMs in NUS1 (including two truncating variants and a microdeletion) in DEE individuals with similar phenotypes and the fact that NUS1 is a functional direct interactor of DHDDS strongly support the involvement of this gene in DEE.
Several of the DEE-related genes highlighted in this study code for proteins that interact directly or indirectly with other proteins encoded by genes associated with epilepsy. Such proteins include (1) DHDDS and NUS1, which form a complex for the synthesis of dolichol monophosphate;55, 61 (2) SNAP25, which interacts with the DEE-associated STXBP1 for the docking of neurotransmitter vesicles; and (3) RAB11A, which is involved in the endocytosis of NTRK2.68 In addition, both GABRB2 and GABBR2 belong to the family of GABAergic receptors, which include other members involved in epilepsy (encoded by GABRA1 [OMIM: 137160], GABRB1 [OMIM: 137190], GABRB3 [OMIM: 137192], and GABRG2 [OMIM: 137164]). The identification of multiple genes acting along pathways or playing biological functions that have already been linked to epilepsy raises the possibility that many of the major pathways involved in DEE have been identified. Stratifying genetic forms of DEE on the basis of the involvement of these pathways could facilitate the development of tailored therapies.
Acknowledgments
We thank the individuals participating in this study and their families for their contributions. This study was funded by grants from Genome Canada and Génome Québec, the Jeanne and Jean-Louis Lévesque Foundation (to J.L.M.), the Michael Bahen Chair in Epilepsy Research (to B.A.M.), the Ontario Brain Institute (EpLink), the McLaughlin Foundation and the University of Toronto (to D.M.A. and B.A.M.), and the National Institute of Neurological Disorders and Stroke (RO1 NS069605 to H.C.M.). We thank the members of the massive parallel sequencing and bioinformatics teams at the McGill University and Genome Quebec Innovation Center for their services. S.M.-M. was supported by the University of Toronto McLaughlin Accelerator Grant in Genomic Medicine (MC-2013-08). See Supplemental Data for additional acknowledgements.
Published: November 2, 2017
Footnotes
Supplemental Data include Supplemental Acknowledgments, a Supplemental Note, two figures, and eight tables and can be found with this article online at https://doi.org/10.1016/j.ajhg.2017.09.008.
Contributor Information
Berge A. Minassian, Email: berge.minassian@sickkids.ca.
Jacques L. Michaud, Email: jacques.michaud@recherche-ste-justine.qc.ca.
Web Resources
1000 Genomes Project, http://browser.1000genomes.org/index.html
Denovonear, https://github.com/jeremymcrae/denovonear
DenovolyzeR, http://denovolyzer.org/
DDD Research Variants, https://decipher.sanger.ac.uk/ddd#research-variants
ExAC Browser, http://exac.broadinstitute.org/
GATK Best Practices, https://software.broadinstitute.org/gatk/best-practices
NHLBI Exome Sequencing Project (ESP) Exome Variant Server, http://evs.gs.washington.edu/EVS/
OMIM, http://www.omim.org
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/
SIFT, http://sift.jcvi.org/
Supplemental Data
References
- 1.Berg A.T., Langfitt J.T., Testa F.M., Levy S.R., DiMario F., Westerveld M., Kulas J. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008;49:608–614. doi: 10.1111/j.1528-1167.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- 2.Tuchman R., Cuccaro M. Epilepsy and autism: neurodevelopmental perspective. Curr. Neurol. Neurosci. Rep. 2011;11:428–434. doi: 10.1007/s11910-011-0195-x. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd C., Hosking G. Epilepsy in school children with intellectual impairments in Sheffield: the size and nature of the problem and the implications for service provision. J. Ment. Defic. Res. 1989;33:511–514. doi: 10.1111/j.1365-2788.1989.tb01508.x. [DOI] [PubMed] [Google Scholar]
- 4.Ben-Ari Y., Holmes G.L. Effects of seizures on developmental processes in the immature brain. Lancet Neurol. 2006;5:1055–1063. doi: 10.1016/S1474-4422(06)70626-3. [DOI] [PubMed] [Google Scholar]
- 5.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., Hirsch E., Jain S., Mathern G.W., Moshé S.L. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks-Kayal A. Molecular mechanisms of cognitive and behavioral comorbidities of epilepsy in children. Epilepsia. 2011;52(Suppl 1):13–20. doi: 10.1111/j.1528-1167.2010.02906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen A.S., Berkovic S.F., Cossette P., Delanty N., Dlugos D., Eichler E.E., Epstein M.P., Glauser T., Goldstein D.B., Han Y., Epi4K Consortium. Epilepsy Phenome/Genome Project De novo mutations in epileptic encephalopathies. Nature. 2013;501:217–221. doi: 10.1038/nature12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EuroEPINOMICS-RES Consortium. Epilepsy Phenome/Genome Project. Epi4K Consortium De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies. Am. J. Hum. Genet. 2014;95:360–370. doi: 10.1016/j.ajhg.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samocha K.E., Robinson E.B., Sanders S.J., Stevens C., Sabo A., McGrath L.M., Kosmicki J.A., Rehnström K., Mallick S., Kirby A. A framework for the interpretation of de novo mutation in human disease. Nat. Genet. 2014;46:944–950. doi: 10.1038/ng.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deciphering Developmental Disorders Study Prevalence and architecture of de novo mutations in developmental disorders. Nature. 2017;542:433–438. doi: 10.1038/nature21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lelieveld S.H., Reijnders M.R., Pfundt R., Yntema H.G., Kamsteeg E.J., de Vries P., de Vries B.B., Willemsen M.H., Kleefstra T., Löhner K. Meta-analysis of 2,104 trios provides support for 10 new genes for intellectual disability. Nat. Neurosci. 2016;19:1194–1196. doi: 10.1038/nn.4352. [DOI] [PubMed] [Google Scholar]
- 12.Epi4K Consortium De Novo Mutations in SLC1A2 and CACNA1A Are Important Causes of Epileptic Encephalopathies. Am. J. Hum. Genet. 2016;99:287–298. doi: 10.1016/j.ajhg.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H., Wang K. Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat. Protoc. 2015;10:1556–1566. doi: 10.1038/nprot.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinson J.T., Thorvaldsdóttir H., Winckler W., Guttman M., Lander E.S., Getz G., Mesirov J.P. Integrative genomics viewer. Nat. Biotechnol. 2011;29:24–26. doi: 10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Layer R.M., Chiang C., Quinlan A.R., Hall I.M. LUMPY: a probabilistic framework for structural variant discovery. Genome Biol. 2014;15:R84. doi: 10.1186/gb-2014-15-6-r84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monlong J., Meloche C., Rouleau G.A., Cossette P., Girard S.L., G B. Human copy number variants are enriched in regions of low-mappability. BioRxiv. 2016 doi: 10.1093/nar/gky538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarrei M., MacDonald J.R., Merico D., Scherer S.W. A copy number variation map of the human genome. Nat. Rev. Genet. 2015;16:172–183. doi: 10.1038/nrg3871. [DOI] [PubMed] [Google Scholar]
- 18.Hiatt J.B., Pritchard C.C., Salipante S.J., O’Roak B.J., Shendure J. Single molecule molecular inversion probes for targeted, high-accuracy detection of low-frequency variation. Genome Res. 2013;23:843–854. doi: 10.1101/gr.147686.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Roak B.J., Vives L., Fu W., Egertson J.D., Stanaway I.B., Phelps I.G., Carvill G., Kumar A., Lee C., Ankenman K. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ware J.S., Samocha K.E., Homsy J., Daly M.J. Interpreting de novo Variation in Human Disease Using denovolyzeR. Curr. Protoc. Hum. Genet. 2015;87:1–15. doi: 10.1002/0471142905.hg0725s87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hino-Fukuyo N., Kikuchi A., Arai-Ichinoi N., Niihori T., Sato R., Suzuki T., Kudo H., Sato Y., Nakayama T., Kakisaka Y. Genomic analysis identifies candidate pathogenic variants in 9 of 18 patients with unexplained West syndrome. Hum. Genet. 2015;134:649–658. doi: 10.1007/s00439-015-1553-6. [DOI] [PubMed] [Google Scholar]
- 22.Michaud J.L., Lachance M., Hamdan F.F., Carmant L., Lortie A., Diadori P., Major P., Meijer I.A., Lemyre E., Cossette P. The genetic landscape of infantile spasms. Hum. Mol. Genet. 2014;23:4846–4858. doi: 10.1093/hmg/ddu199. [DOI] [PubMed] [Google Scholar]
- 23.de Ligt J., Willemsen M.H., van Bon B.W., Kleefstra T., Yntema H.G., Kroes T., Vulto-van Silfhout A.T., Koolen D.A., de Vries P., Gilissen C. Diagnostic exome sequencing in persons with severe intellectual disability. N. Engl. J. Med. 2012;367:1921–1929. doi: 10.1056/NEJMoa1206524. [DOI] [PubMed] [Google Scholar]
- 24.Gilissen C., Hehir-Kwa J.Y., Thung D.T., van de Vorst M., van Bon B.W., Willemsen M.H., Kwint M., Janssen I.M., Hoischen A., Schenck A. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. doi: 10.1038/nature13394. [DOI] [PubMed] [Google Scholar]
- 25.Halvardson J., Zhao J.J., Zaghlool A., Wentzel C., Georgii-Hemming P., Månsson E., Ederth Sävmarker H., Brandberg G., Soussi Zander C., Thuresson A.C., Feuk L. Mutations in HECW2 are associated with intellectual disability and epilepsy. J. Med. Genet. 2016;53:697–704. doi: 10.1136/jmedgenet-2016-103814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamdan F.F., Srour M., Capo-Chichi J.M., Daoud H., Nassif C., Patry L., Massicotte C., Ambalavanan A., Spiegelman D., Diallo O. De novo mutations in moderate or severe intellectual disability. PLoS Genet. 2014;10:e1004772. doi: 10.1371/journal.pgen.1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes F., Barbosa M., Ameur A., Soares G., de Sá J., Dias A.I., Oliveira G., Cabral P., Temudo T., Calado E. Identification of novel genetic causes of Rett syndrome-like phenotypes. J. Med. Genet. 2016;53:190–199. doi: 10.1136/jmedgenet-2015-103568. [DOI] [PubMed] [Google Scholar]
- 28.Rauch A., Wieczorek D., Graf E., Wieland T., Endele S., Schwarzmayr T., Albrecht B., Bartholdi D., Beygo J., Di Donato N. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–1682. doi: 10.1016/S0140-6736(12)61480-9. [DOI] [PubMed] [Google Scholar]
- 29.Deciphering Developmental Disorders Study Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519:223–228. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Francioli L.C., Polak P.P., Koren A., Menelaou A., Chun S., Renkens I., van Duijn C.M., Swertz M., Wijmenga C., van Ommen G., Genome of the Netherlands Consortium Genome-wide patterns and properties of de novo mutations in humans. Nat. Genet. 2015;47:822–826. doi: 10.1038/ng.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldmann J.M., Wong W.S., Pinelli M., Farrah T., Bodian D., Stittrich A.B., Glusman G., Vissers L.E., Hoischen A., Roach J.C. Parent-of-origin-specific signatures of de novo mutations. Nat. Genet. 2016;48:935–939. doi: 10.1038/ng.3597. [DOI] [PubMed] [Google Scholar]
- 32.Kong A., Frigge M.L., Masson G., Besenbacher S., Sulem P., Magnusson G., Gudjonsson S.A., Sigurdsson A., Jonasdottir A., Jonasdottir A. Rate of de novo mutations and the importance of father’s age to disease risk. Nature. 2012;488:471–475. doi: 10.1038/nature11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iossifov I., O’Roak B.J., Sanders S.J., Ronemus M., Krumm N., Levy D., Stessman H.A., Witherspoon K.T., Vives L., Patterson K.E. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., ACMG Laboratory Quality Assurance Committee Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berko E.R., Cho M.T., Eng C., Shao Y., Sweetser D.A., Waxler J., Robin N.H., Brewer F., Donkervoort S., Mohassel P. De novo missense variants in HECW2 are associated with neurodevelopmental delay and hypotonia. J. Med. Genet. 2017;54:84–86. doi: 10.1136/jmedgenet-2016-103943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sobreira N., Schiettecatte F., Valle D., Hamosh A. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum. Mutat. 2015;36:928–930. doi: 10.1002/humu.22844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Firth H.V., Richards S.M., Bevan A.P., Clayton S., Corpas M., Rajan D., Van Vooren S., Moreau Y., Pettett R.M., Carter N.P. DECIPHER: Database of Chromosomal Imbalance and Phenotype in Humans Using Ensembl Resources. Am. J. Hum. Genet. 2009;84:524–533. doi: 10.1016/j.ajhg.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeo G.S., Connie Hung C.C., Rochford J., Keogh J., Gray J., Sivaramakrishnan S., O’Rahilly S., Farooqi I.S. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 39.Andero R., Choi D.C., Ressler K.J. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog. Mol. Biol. Transl. Sci. 2014;122:169–192. doi: 10.1016/B978-0-12-420170-5.00006-4. [DOI] [PubMed] [Google Scholar]
- 40.Yoshii A., Constantine-Paton M. Postsynaptic BDNF-TrkB signaling in synapse maturation, plasticity, and disease. Dev. Neurobiol. 2010;70:304–322. doi: 10.1002/dneu.20765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu B., Goulding E.H., Zang K., Cepoi D., Cone R.D., Jones K.R., Tecott L.H., Reichardt L.F. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003;6:736–742. doi: 10.1038/nn1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isidor B., Küry S., Rosenfeld J.A., Besnard T., Schmitt S., Joss S., Davies S.J., Lebel R.R., Henderson A., Schaaf C.P. De Novo Truncating Mutations in the Kinetochore-Microtubules Attachment Gene CHAMP1 Cause Syndromic Intellectual Disability. Hum. Mutat. 2016;37:354–358. doi: 10.1002/humu.22952. [DOI] [PubMed] [Google Scholar]
- 43.Jacob T.C., Moss S.J., Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srivastava S., Cohen J., Pevsner J., Aradhya S., McKnight D., Butler E., Johnston M., Fatemi A. A novel variant in GABRB2 associated with intellectual disability and epilepsy. Am. J. Med. Genet. A. 2014;164A:2914–2921. doi: 10.1002/ajmg.a.36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bosch D.G., Boonstra F.N., de Leeuw N., Pfundt R., Nillesen W.M., de Ligt J., Gilissen C., Jhangiani S., Lupski J.R., Cremers F.P., de Vries B.B. Novel genetic causes for cerebral visual impairment. Eur. J. Hum. Genet. 2016;24:660–665. doi: 10.1038/ejhg.2015.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishii A., Kang J.Q., Schornak C.C., Hernandez C.C., Shen W., Watkins J.C., Macdonald R.L., Hirose S. A de novo missense mutation of GABRB2 causes early myoclonic encephalopathy. J. Med. Genet. 2017;54:202–211. doi: 10.1136/jmedgenet-2016-104083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baldridge D., Heeley J., Vineyard M., Manwaring L., Toler T.L., Fassi E., Fiala E., Brown S., Goss C.W., Willing M. The Exome Clinic and the role of medical genetics expertise in the interpretation of exome sequencing results. Genet. Med. 2017;19:1040–1048. doi: 10.1038/gim.2016.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Retterer K., Juusola J., Cho M.T., Vitazka P., Millan F., Gibellini F., Vertino-Bell A., Smaoui N., Neidich J., Monaghan K.G. Clinical application of whole-exome sequencing across clinical indications. Genet. Med. 2016;18:696–704. doi: 10.1038/gim.2015.148. [DOI] [PubMed] [Google Scholar]
- 49.Sajan S.A., Jhangiani S.N., Muzny D.M., Gibbs R.A., Lupski J.R., Glaze D.G., Kaufmann W.E., Skinner S.A., Annese F., Friez M.J. Enrichment of mutations in chromatin regulators in people with Rett syndrome lacking mutations in MECP2. Genet. Med. 2017;19:13–19. doi: 10.1038/gim.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kasprowicz J., Kuenen S., Miskiewicz K., Habets R.L., Smitz L., Verstreken P. Inactivation of clathrin heavy chain inhibits synaptic recycling but allows bulk membrane uptake. J. Cell Biol. 2008;182:1007–1016. doi: 10.1083/jcb.200804162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson M.S. Forty Years of Clathrin-coated Vesicles. Traffic. 2015;16:1210–1238. doi: 10.1111/tra.12335. [DOI] [PubMed] [Google Scholar]
- 52.DeMari J., Mroske C., Tang S., Nimeh J., Miller R., Lebel R.R. CLTC as a clinically novel gene associated with multiple malformations and developmental delay. Am. J. Med. Genet. A. 2016;170A:958–966. doi: 10.1002/ajmg.a.37506. [DOI] [PubMed] [Google Scholar]
- 53.Helbig K.L., Farwell Hagman K.D., Shinde D.N., Mroske C., Powis Z., Li S., Tang S., Helbig I. Diagnostic exome sequencing provides a molecular diagnosis for a significant proportion of patients with epilepsy. Genet. Med. 2016;18:898–905. doi: 10.1038/gim.2015.186. [DOI] [PubMed] [Google Scholar]
- 54.Lek M., Karczewski K.J., Minikel E.V., Samocha K.E., Banks E., Fennell T., O’Donnell-Luria A.H., Ware J.S., Hill A.J., Cummings B.B., Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grabińska K.A., Park E.J., Sessa W.C. cis-Prenyltransferase: New Insights into Protein Glycosylation, Rubber Synthesis, and Human Diseases. J. Biol. Chem. 2016;291:18582–18590. doi: 10.1074/jbc.R116.739490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fujihashi M., Zhang Y.W., Higuchi Y., Li X.Y., Koyama T., Miki K. Crystal structure of cis-prenyl chain elongating enzyme, undecaprenyl diphosphate synthase. Proc. Natl. Acad. Sci. USA. 2001;98:4337–4342. doi: 10.1073/pnas.071514398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rebl A., Anders E., Wimmers K., Goldammer T. Characterization of dehydrodolichyl diphosphate synthase gene in rainbow trout (Oncorhynchus mykiss) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2009;152:260–265. doi: 10.1016/j.cbpb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 58.Zelinger L., Banin E., Obolensky A., Mizrahi-Meissonnier L., Beryozkin A., Bandah-Rozenfeld D., Frenkel S., Ben-Yosef T., Merin S., Schwartz S.B. A missense mutation in DHDDS, encoding dehydrodolichyl diphosphate synthase, is associated with autosomal-recessive retinitis pigmentosa in Ashkenazi Jews. Am. J. Hum. Genet. 2011;88:207–215. doi: 10.1016/j.ajhg.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Züchner S., Dallman J., Wen R., Beecham G., Naj A., Farooq A., Kohli M.A., Whitehead P.L., Hulme W., Konidari I. Whole-exome sequencing links a variant in DHDDS to retinitis pigmentosa. Am. J. Hum. Genet. 2011;88:201–206. doi: 10.1016/j.ajhg.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sabry S., Vuillaumier-Barrot S., Mintet E., Fasseu M., Valayannopoulos V., Héron D., Dorison N., Mignot C., Seta N., Chantret I. A case of fatal Type I congenital disorders of glycosylation (CDG I) associated with low dehydrodolichol diphosphate synthase (DHDDS) activity. Orphanet J. Rare Dis. 2016;11:84. doi: 10.1186/s13023-016-0468-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harrison K.D., Park E.J., Gao N., Kuo A., Rush J.S., Waechter C.J., Lehrman M.A., Sessa W.C. Nogo-B receptor is necessary for cellular dolichol biosynthesis and protein N-glycosylation. EMBO J. 2011;30:2490–2500. doi: 10.1038/emboj.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park E.J., Grabińska K.A., Guan Z., Stránecký V., Hartmannová H., Hodaňová K., Barešová V., Sovová J., Jozsef L., Ondrušková N. Mutation of Nogo-B receptor, a subunit of cis-prenyltransferase, causes a congenital disorder of glycosylation. Cell Metab. 2014;20:448–457. doi: 10.1016/j.cmet.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Szafranski P., Von Allmen G.K., Graham B.H., Wilfong A.A., Kang S.H., Ferreira J.A., Upton S.J., Moeschler J.B., Bi W., Rosenfeld J.A. 6q22.1 microdeletion and susceptibility to pediatric epilepsy. Eur. J. Hum. Genet. 2015;23:173–179. doi: 10.1038/ejhg.2014.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webster E., Cho M.T., Alexander N., Desai S., Naidu S., Bekheirnia M.R., Lewis A., Retterer K., Juusola J., Chung W.K. De novo PHIP-predicted deleterious variants are associated with developmental delay, intellectual disability, obesity, and dysmorphic features. Cold Spring Harb Mol Case Stud. 2016;2:a001172. doi: 10.1101/mcs.a001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelly E.E., Horgan C.P., McCaffrey M.W. Rab11 proteins in health and disease. Biochem. Soc. Trans. 2012;40:1360–1367. doi: 10.1042/BST20120157. [DOI] [PubMed] [Google Scholar]
- 66.Bodrikov V., Pauschert A., Kochlamazashvili G., Stuermer C.A.O. Reggie-1 and reggie-2 (flotillins) participate in Rab11a-dependent cargo trafficking, spine synapse formation and LTP-related AMPA receptor (GluA1) surface exposure in mouse hippocampal neurons. Exp. Neurol. 2017;289:31–45. doi: 10.1016/j.expneurol.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 67.Correia S.S., Bassani S., Brown T.C., Lisé M.F., Backos D.S., El-Husseini A., Passafaro M., Esteban J.A. Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat. Neurosci. 2008;11:457–466. doi: 10.1038/nn2063. [DOI] [PubMed] [Google Scholar]
- 68.Huang S.H., Wang J., Sui W.H., Chen B., Zhang X.Y., Yan J., Geng Z., Chen Z.Y. BDNF-dependent recycling facilitates TrkB translocation to postsynaptic density during LTP via a Rab11-dependent pathway. J. Neurosci. 2013;33:9214–9230. doi: 10.1523/JNEUROSCI.3256-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eathiraj S., Mishra A., Prekeris R., Lambright D.G. Structural basis for Rab11-mediated recruitment of FIP3 to recycling endosomes. J. Mol. Biol. 2006;364:121–135. doi: 10.1016/j.jmb.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 70.Shiba T., Koga H., Shin H.W., Kawasaki M., Kato R., Nakayama K., Wakatsuki S. Structural basis for Rab11-dependent membrane recruitment of a family of Rab11-interacting protein 3 (FIP3)/Arfophilin-1. Proc. Natl. Acad. Sci. USA. 2006;103:15416–15421. doi: 10.1073/pnas.0605357103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Blein S., Hawrot E., Barlow P. The metabotropic GABA receptor: molecular insights and their functional consequences. Cell. Mol. Life Sci. 2000;57:635–650. doi: 10.1007/PL00000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Antonucci F., Corradini I., Fossati G., Tomasoni R., Menna E., Matteoli M. SNAP-25, a Known Presynaptic Protein with Emerging Postsynaptic Functions. Front. Synaptic Neurosci. 2016;8:7. doi: 10.3389/fnsyn.2016.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Südhof T.C. Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron. 2013;80:675–690. doi: 10.1016/j.neuron.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Corradini I., Donzelli A., Antonucci F., Welzl H., Loos M., Martucci R., De Astis S., Pattini L., Inverardi F., Wolfer D. Epileptiform activity and cognitive deficits in SNAP-25(+/-) mice are normalized by antiepileptic drugs. Cereb. Cortex. 2014;24:364–376. doi: 10.1093/cercor/bhs316. [DOI] [PubMed] [Google Scholar]
- 75.Watanabe S., Yamamori S., Otsuka S., Saito M., Suzuki E., Kataoka M., Miyaoka H., Takahashi M. Epileptogenesis and epileptic maturation in phosphorylation site-specific SNAP-25 mutant mice. Epilepsy Res. 2015;115:30–44. doi: 10.1016/j.eplepsyres.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Dawidowski D., Cafiso D.S. Munc18-1 and the Syntaxin-1 N Terminus Regulate Open-Closed States in a t-SNARE Complex. Structure. 2016;24:392–400. doi: 10.1016/j.str.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rohena L., Neidich J., Truitt Cho M., Gonzalez K.D., Tang S., Devinsky O., Chung W.K. Mutation in SNAP25 as a novel genetic cause of epilepsy and intellectual disability. Rare Dis. 2013;1:e26314. doi: 10.4161/rdis.26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen X.M., Selcen D., Brengman J., Engel A.G. Mutant SNAP25B causes myasthenia, cortical hyperexcitability, ataxia, and intellectual disability. Neurology. 2014;83:2247–2255. doi: 10.1212/WNL.0000000000001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shohat S., Ben-David E., Shifman S. Varying Intolerance of Gene Pathways to Mutational Classes Explain Genetic Convergence across Neuropsychiatric Disorders. Cell Rep. 2017;18:2217–2227. doi: 10.1016/j.celrep.2017.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.