Abstract
PURPOSE
Bacterial adhesion on provisional crown materials retained for a long time can influence the duration for which permanent prosthetic restorations can be healthily worn in the oral cavity. The aim of this study was to compare seven different commonly used provisional crown materials with regard to Streptococcus mutans and Candida albicans surface adhesion.
MATERIALS AND METHODS
For each group, twenty specimens of the provisional fixed prosthodontic materials TemDent (Schütz), Imıdent (Imıcryl), Tab 2000 (Kerr), Structur Premium (Voco), Systemp (Ivoclar Vivadent), Acrytemp (Zhermack), and Takilon-BBF (Takilon) were prepared (diameter, 10.0 mm; height, 2.0 mm). Surface roughness was assessed by atomic force microscopy. Each group was then divided into 2 subgroups (n=10) according to the microbial suspensions used: S. mutans and C. albicans. The specimens were incubated at 37℃ with S. mutans or C. albicans for seven days. Bacterial adherence on surfaces was assessed using the 2,3-bis[2-methyloxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide (XTT) assay.
RESULTS
S. mutans showed maximum adhesion to Structur, followed by Systemp, Acrytemp, Takilon, Tab 2000, Imident, and TemDent (P<.05). The highest vital C. albicans adhesion was noted on Takilon, followed by Imident and Tab 2000; the lowest adhesion was noted on Systemp (P<.05).
CONCLUSION
The materials showed significant differences in the degree of bacterial adhesion. C. albicans showed higher surface adhesion than S. mutans on provisional crown and fixed partial denture denture materials.
Keywords: Candida albicans, Streptococcus mutans, Provisional prosthetic materials, Microbial Adhesion
INTRODUCTION
Provisional prosthetic restorations prevent leakage into dentinal tubules by covering the prepared dental tissue until the preparation of permanent prostheses. These materials play a major role in prosthetic treatment by providing thermal isolation and an appropriate fit with the prepared dental tissue.1,2 Provisional restorations also have diagnostic and preprosthetic functions, such as arranging irregular occlusal planes, increasing vertical dimension, and shaping or localizing gingiva.3,4
Provisional crown and fixed partial denture materials are classified according to the resin composition. The oldest group of these provisional materials is acrylic polymethyl methacrylates (PMMAs). PMMAs are available in fine powder form obtained by mixing polymerized methyl methacrylate to liquid monomer. Higher-strength PMMAs contain high-molecular-weight acrylate monomers. More recent prosthetic materials including bis-acrylate composite resins are more commonly used in producing direct provisional prosthetics for the oral cavity. Irrespective of the provisional prosthetic restoration materials used, the length of time for which the provisional restorations are worn before permanent restoration is a substantial factor in determining the life span of permanent prostheses and the health of the supporting teeth and periodontal tissues.5
Provisional restorations worn for prolonged durations allow bacterial colonization on their surfaces as an important factor effecting successful restoration. Owing to the high surface roughness on provisional prosthetic materials and their low marginal adaptation, bacterial colonization on provisional prosthetic materials is higher than that on permanent prosthetic materials.5 Various surface characteristics may affect the quantity and quality of bacterial accumulation on the surfaces.6 An increase in surface roughness facilitates microbial adhesion, which is difficult to eliminate from pits and grooves.7
Another major factor for bacterial adhesion is the species of the bacteria. Streptococci are one of the most common “early colonizing bacteria”8 and are known as the primary pathogenesis of tooth caries.9 Candida albicans, is the most frequent opportunistic intraoral pathogen.10,11,12 C. albicans was isolated from oral cavities of 25% of healthy individuals in previous studies, and the percentage increased to 50 – 90% in cases of immunosuppression.11,12
Previous studies have used various methods, such as scanning electron microscopy, radiolabeling, antibody assay, and direct plate counting for quantifying the adhesion of specific bacterial species to defined dental substrates.5,7,12,13,14 However, these methods are expensive, tedious, and time-consuming. Quantitative colorimetric methods are more efficient and cost-effective for evaluating the adhesion of microorganisms on non-biological materials. The XTT assay estimates metabolic activity by measuring the oxidation of 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide, a tetrazolium salt, to 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide. This is a simple, rapid, and reliable colorimetric method for quantitative determination of bacterial adhesion on the surfaces of various materials.12,15,16,17
Several studies have tested microbial adhesion, mostly on permanent restoration materials such as amalgam, glass ionomer, or composite resins.12 However, there are limited data on microbial adhesion of prosthodontic provisional materials. Therefore the aim of this study is to compare surface adhesion of Streptococcus mutans (RSKK 676) and Candida albicans (ATCC 90028, Refik Saydam Institute, Ankara, Turkey) on seven different provisional crown and fixed partial denture materials by using the XTT colometric assay.
MATERIALS AND METHODS
The protocols shown below were used for preparation of solutions for XTT colorimetric analysis.
The XTT (SERVA Electrophoresis, Heidelberg, Germany) protocol was adapted for this study according to the manufacturer's recommendations. XTT powder (100 mg) was dissolved in 100 mL of sterile phosphate-buffered saline (PBS) solution and aliquoted into sterile Eppendorf tubes. Phenazine methosulfate (PMS) was used as the reducing agent, and 38.3 mg of PMS powder was dissolved in a 100 mL solution of sterile PBS and aliquoted into Eppendorf tubes. The reagents are photo-sensitive; therefore, the tubes were wrapped in aluminum foils and were stored at −20℃ until they were used.
The provisional fixed prosthodontic materials -Temdent Classic, Imident, Tab 2000, Structur Premium, Systemp c&b II, Acrytemp and Takilon BBF (Table 1)- were prepared (10.0-mm diameter, 2.0-mm height) using a special PVC mold (n = 20).
Table 1. Materials used in this study.
| Product | Manufacturer | MR* | Main Components of monomer mixture | LOT Numbers |
|---|---|---|---|---|
| TemDent Classic | Schütz Dental GmbH/Rosbach, Germany | 2:01 | Polymethyl methacrylate | 2009002782 |
| Imident | Imicryl Dis Malzemeleri/Konya, Turkey | 1:2.4 | Polymethyl methacrylate | A100-016 |
| Tab 2000 | Kerr Italy/Scafati, Italy | 3:2 | Polymethyl methacrylate | 61565 |
| Structur Premium | VOCO GmbH/Cuxhaven, Germany | 1:1 | Bisacrylic composite resin | 1150157 |
| Systemp c&b ll | Ivoclar Vivadent AG/Schaan, Liechtenstein | 4:1 | Polyurethane polymethacrylate | F55051 |
| Acrytemp | Zhermack spA/Via Bovazecchino, Italy | 4:01 | Bisacrylic composite resin | C700200 |
| Takilon BBF | WP GmbH/Barmstedt, Germany | 2.1:10 | Polymethyl methacrylate | - |
*MR: Mixing ratio, dimethacrylate-base: catalyst [by volume] or monomethacrylate-liquid : powder [volume : mass].
All the surfaces of the specimens were sterilized with ultraviolet light for 24 hours. Each group was then divided into 2 subgroups (n = 10) according to the microbial suspensions used: Streptococcus mutans (RSKK 676) and Candida albicans (ATCC 90028, Refik Saydam Institute, Ankara, Turkey). One milliliter of microbial suspension was used to measure optical density (OD) values using a spectrophotometer set at 600 nm. The specimens were placed in 24-well cell culture plates using sterile instruments. The microbial suspension was added to plate and incubated at 37℃ for seven days. The microbial culture with the same OD was refreshed for 7 days infection period. The specimens were rinsed with 600 mL PBS to remove the loosely adherent microorganisms from the specimen surface. The specimens were then placed in 96-well cell culture plates using sterile tweezers.
XTT and PMS aliquots were thawed at room temperature. After stirring at low speed in vortex, XTT and PMS solutions were mixed in a sterile glass beaker at a ratio of 20:1 by volume. To avoid any contamination, all experiments were performed in a safety cabinet (Biosafety Level 2: BSL 2), with lights turned off.
The specimens were placed in a 96-well cell culture plates, and 158 µL of PBS and 42 µL of XTT-PMS mixture were added to each well, and incubated at 37℃ for 3 hours. After 1 hour, the plates were vortexed for 30 seconds at a low speed. Colorimetric change was then measured using a microtiter plate reader at 492 nm. 100 µL of a PBS and XTT-PMS mixture added to another 96-well cell culture plate was used as a blank for the assay. Surface roughness of tested materials was assessed by atomic force microscopy (AFM). Data were statistically analyzed by ANOVA, Kruskal-Wallis, Mann-Whitney U and Tukey HSD tests (α = .05). Correlation between the OD obtained using the microplate reader and color of the formazan products was confirmed. For example, a low OD, indicating a low bacterial number, corresponded to yellow color and orange color was observed in dense groups.
RESULTS
C. albicans adhered to provisional test materials significantly more than S. mutans in all test groups (P < .05). The negative control groups showed no bacterial growth on material surfaces, confirming no microbial contamination during the testing procedures.
Table 2 presents mean, standard deviation, standard error values of S. mutans adhesion on provisional materials and summarizes the results of One-way ANOVA and Tukey HSD tests. Acrytemp, Systemp, and Structur showed the highest OD values of adhered S. mutans more than the other groups (P < .05). However, TemDent and Imident showed the lowest adhesion values of S. mutans (P < .05). No statistically significant difference was determined between Tab2000, Takilon and Acrytemp (P > .05) (Table 2).
Table 2. Results of statistical analysis of OD values for S. mutans adhesion on provisional materials.
| ANOVA | ||||
|---|---|---|---|---|
| Mean | Std. Deviation | Std. Eror | Sig. | |
| Structur d | 0.0197 | 0.00819 | 0.00259 | |
| Systemp cd | 0.0190 | 0.00706 | 0.00223 | |
| Acrytemp bcd | 0.0163 | 0.00595 | 0.00188 | 0.000 |
| Tab2000 ab | 0.0089 | 0.00513 | 0.00162 | |
| Temdent a | 0.0046 | 0.00398 | 0.00126 | |
| Imident a | 0.0068 | 0.00621 | 0.00197 | |
| Takilon abc | 0.0107 | 0.00818 | 0.00192 | |
Table 3 presents mean ranks, standard deviation of OD values of C. albicans adhesion on provisional materials and summarizes the results of Kruskall-Wallis and Mann-Whitney U tests. The results showed that the highest OD values for C. albicans were observed in Takilon, followed by Imident (P < .05). However, Systemp had the lowest OD values when compared to other tested materials (P < .05). There were no significantly differences between the other test groups (P < .05) (Table 3).
Table 3. Results of statistical analysis of OD values for C. albicans adhesion on provisional materials.
| Kruskall-Wallis | ||||
|---|---|---|---|---|
| Mean | Std. Deviation | Std. Eror | P values | |
| Structur ab | 31.65 | 8.29 | ||
| Systemp a | 20.10 | 7.09 | ||
| Acrytemp ab | 30.45 | 5.34 | 20.887 | .002 |
| Tab2000 ab | 35.45 | 5.13 | ||
| Temdent ab | 30.60 | 8.93 | ||
| Imident b | 41.90 | 6.31 | ||
| Takilon c | 58.35 | 9.03 | ||
DISCUSSION
In this study, S. mutans and C. albicans were selected to determine the microbial adhesion on different provisional prosthetic crown and fixed partial denture materials. S. mutans is the primary progenitor pathogen in the formation of intraoral caries, and C. albicans is the most common opportunistic intraoral pathogen isolated from the oral cavity.9,18 Many different methods have been employed in previous studies to determine microbial adhesion; XTT method, used in this study, is a novel cost-effective method that can detect observable and surviving microorganisms.19,20 XTT is a colorimetric microbiological tool used for evaluation of the metabolic activity of adherent aerobic bacteria and a measure of cell viability. This approach depends on the ability of metabolically active cells to convert the yellow water-soluble XTT into orange colored soluble compounds of formazan, measured by their absorbance at 480 nm with a micro plate UV/Vis spectrophotometer.
One of the factors that influence the microbial adhesion to materials is the organic and inorganic composition of the materials, which determines surface roughness and wettability, thereby affecting the microbial adhesion.21 Physicochemical properties of some materials may favor bacterial colonization and plaque formation. In a previous study, it was reported that S. mutans adhesion was significantly higher on composite materials among different dental materials. It was also concluded that the increased adhesion of S. mutans on composite material was due to the effect of fillers and monomers.22 In our study, we observed that S. mutans adhesion was the highest in Structur Premium, which is a the bis-acrylic resin material, and the lowest in the TemDent that belongs to a PMMA group. This can be explained by the variation in molecular weights and number of methacrylate monomers in the organic matrix of the adhesive. Rosentritt et al. reported that bacterial growth was inhibited on the PMMA materials suspended in distilled water for six days. This result confirms the possible detrimental effects of the residual monomers present in PMMA materials on cell viabilities.22 Due to the possible long term detrimental effects of PMMA materials on microbial cells might explain the lowest microbial adhesion of these groups. Also, the chemical products and heat released after polymerization may affect the vitability of the microbial cells. Therefore, we considered that groups with methacrylate content may have a similar unfavorable effect on the viability of S. mutans cells. However, further definitive studies are required to confirm this hypothesis.
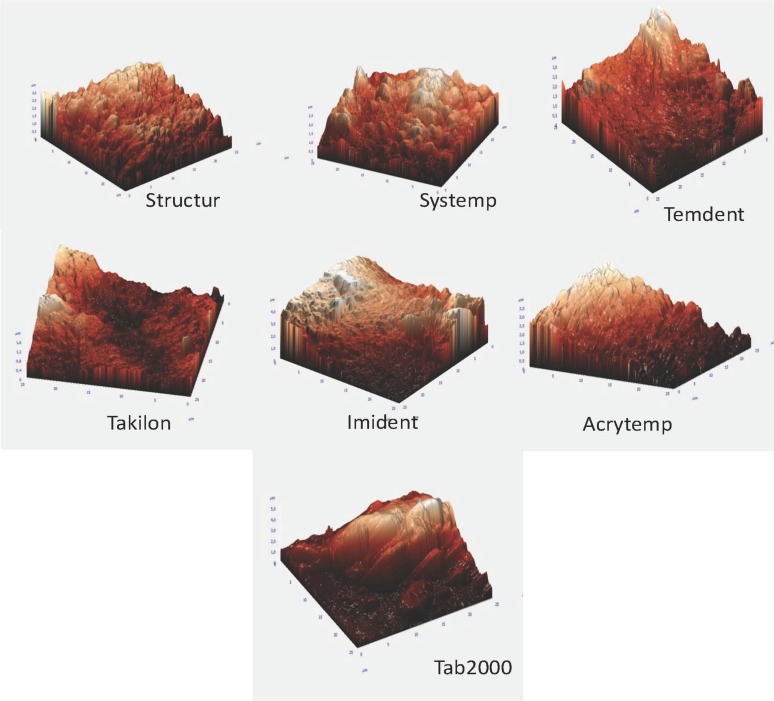
Surface-free energy and roughness are two factors that affect bacterial adhesion the most.23,24 Microscopic examination has revealed that microbial colonization initializes in the crevices, grooves, or pits on the surface.25 In our study, the samples were examined by AFM for evaluating surface roughness. In the AFM images, more prominent peaks and grooves were observed in groups with PMMA content, whereas the bis-acrylic groups had more shallow pits and bulges. The roughness of Tab 2000 was the highest (995.813 nm), whereas that of Structur was the lowest (344.821 nm) (Fig. 1).
Fig. 1. Atomic force micrographs for provisional materials.
We observed that S. mutans adhesion was less on the PMMA group; adhesion exhibited negative correlation with surface roughness (Pearson Correlation: −2.73, P (sig.): 0.022 <.05), which was consistent with a previous study by Buegers et al..5 Another study also reported that when the surface roughness was below 0.2 µm, there was no correlation between the amount of bacterial involvement and surface roughness.24 In our study, mean roughness values were between 0.04 µm and 0.0756 µm, and this can explain the lack of correlation between surface roughness and S. mutans adhesion. Surface-free energy has strong effect on bacterial adhesion; the hydrophobicity of PMMA groups is higher than the bis-acrylate groups5,15 and therefore S. mutans adhesion may be lower in PMMA groups.
In the evaluation of C. albicans adhesion, PMMA groups showed more adhesion compared to bis-acrylic groups. In a previous study, similar to our results, PMMA groups exhibited higher C. albicans adhesion, and even application of a different surface coating on the PMMA surface did not alter initial C. albicans adhesion.26 C. albicans adhesion did not show a statistically significant correlation with surface roughness,27 which was also confirmed by our results. C. albicans showed highest adhesion in PMMA groups with high hydrophobicity. Cell surface hydrophobicity, which facilitates the relation between the cells and the surfaces, is an important factor in the adhesion of C. albicans. There are statistically significant correlations between cell hydrophobicity and the adhesion of C. albicans to the epithelial cells of the mouth and acrylic resin surfaces.28 However, there is no comparison of composite-based materials and PMMA materials. In parallel with previous studies, our study showed that C. albicans adhesion was high on PMMA surfaces with the highest roughness, no statistically significant correlation between surface roughness and C. albicans adhesion could be deduced (Pearson Correlation: 0.076, P (sig.): 0.533>.05).
Some previous in vitro studies used flow chambers, or a precoating procedure, to mimic intraoral conditions, such as bacterial adhesion on surfaces in the oral cavity subjected to salivary wash, formation of pellicle by salivary proteins, and the co-adhesion effect of fungi.11 In this study, these techniques were not used because the intent was to evaluate the effects of material composition alone on microbial adhesion. However, in future studies, these techniques can be used in addition to our method for evaluating and developing materials that prevent microbial adhesion.
CONCLUSION
According to the findings of this study, the quantity of bacterial adhesion differed significantly among the assessed provisional materials. Average adherence value for C. albicans was higher than S. mutans. C. albicans showed higher adhesion on PMMA-based provisional material groups, but S. mutans showed higher adhesion on bisacrylic-based groups. Further research is needed to determine the mechanism of interaction between microbial cells and provisional materials.
References
- 1.Johnston JF, Dykema RW, Phillips RW, Goodacre J. Johnston's modern practice in fixed prosthodontics. 4th ed. Philadelphia: WB Saunders; 1986. pp. 77–90. [Google Scholar]
- 2.Bayindir F, Kürklü D, Yanikoğlu ND. The effect of staining solutions on the color stability of provisional prosthodontic materials. J Dent. 2012;40:e41–e46. doi: 10.1016/j.jdent.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Gegauff AG, Holloway JA. Provisional restorations. Contemporary fixed prosthodontics. 3rd ed. Missouri: Mobsy Inc.; 2001. pp. 380–416. [Google Scholar]
- 4.Gough M. A review of temporary crowns and bridges. Dent Update. 1994;21:203–207. [PubMed] [Google Scholar]
- 5.Buergers R, Rosentritt M, Handel G. Bacterial adhesion of Streptococcus mutans to provisional fixed prosthodontic material. J Prosthet Dent. 2007;98:461–469. doi: 10.1016/S0022-3913(07)60146-2. [DOI] [PubMed] [Google Scholar]
- 6.An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res. 1998;43:338–348. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Morgan TD, Wilson M. The effects of surface roughness and type of denture acrylic on biofilm formation by Streptococcus oralis in a constant depth film fermentor. J Appl Microbiol. 2001;91:47–53. doi: 10.1046/j.1365-2672.2001.01338.x. [DOI] [PubMed] [Google Scholar]
- 8.Whittaker CJ, Klier CM, Kolenbrander PE. Mechanisms of adhesion by oral bacteria. Annu Rev Microbiol. 1996;50:513–552. doi: 10.1146/annurev.micro.50.1.513. [DOI] [PubMed] [Google Scholar]
- 9.Gaines S, James TC, Folan M, Baird AW, O'Farrelly C. A novel spectrofluorometric microassay for Streptococcus mutans adherence to hydroxylapatite. J Microbiol Methods. 2003;54:315–323. doi: 10.1016/s0167-7012(03)00015-0. [DOI] [PubMed] [Google Scholar]
- 10.Scully C, Flint SR, Porter SR, Moos KF. Oral and maxillofacial diseases. 3rd ed. New York: Mosby Co.; 2004. pp. 114–115. [Google Scholar]
- 11.Odds FC. Candida and Candidosis. 2nd ed. London: Bailliere Tindalll; 1988. pp. 42–59. [Google Scholar]
- 12.Karayazgan B, Atay A, Saracli MA, Gunay Y. Evaluation of Candida albicans formation on feldspathic porcelain subjected to four surface treatment methods. Dent Mater J. 2010;29:147–153. doi: 10.4012/dmj.2009-016. [DOI] [PubMed] [Google Scholar]
- 13.Taylor RL, Verran J, Lees GC, Ward AJ. The influence of substratum topography on bacterial adhesion to polymethyl methacrylate. J Mater Sci Mater Med. 1998;9:17–22. doi: 10.1023/a:1008874326324. [DOI] [PubMed] [Google Scholar]
- 14.Okte E, Sultan N, Doğan B, Asikainen S. Bacterial adhesion of Actinobacillus actinomycetemcomitans serotypes to titanium implants: SEM evaluation. A preliminary report. J Periodontol. 1999;70:1376–1382. doi: 10.1902/jop.1999.70.11.1376. [DOI] [PubMed] [Google Scholar]
- 15.Grivet M, Morrier JJ, Benay G, Barsotti O. Effect of hydrophobicity on in vitro streptococcal adhesion to dental alloys. J Mater Sci Mater Med. 2000;11:637–642. doi: 10.1023/a:1008913915399. [DOI] [PubMed] [Google Scholar]
- 16.Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL. An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J Immunol Methods. 1991;142:257–265. doi: 10.1016/0022-1759(91)90114-u. [DOI] [PubMed] [Google Scholar]
- 17.Paull KD, Shoemaker RH, Boyd MR, Parsons JL, Risbood PA, Barbera WA, Sharma MN, Baker DC, Hand E, Scudiero DA, Monks A, Alley MC, Grote M. The synthesis of XTT: A new tetrazolium reagent that is bioreducible to a water-soluble formazan. J Heterocycl Chem. 1988;25:911–914. [Google Scholar]
- 18.Blankenship JR, Mitchell AP. How to build a biofilm: a fungal perspective. Curr Opin Microbiol. 2006;9:588–594. doi: 10.1016/j.mib.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Tsang CS, Ng H, McMillan AS. Antifungal susceptibility of Candida albicans biofilms on titanium discs with different surface roughness. Clin Oral Investig. 2007;11:361–368. doi: 10.1007/s00784-007-0122-3. [DOI] [PubMed] [Google Scholar]
- 20.De-Deus G, Canabarro A, Alves G, Linhares A, Senne MI, Granjeiro JM. Optimal cytocompatibility of a bioceramic nanoparticulate cement in primary human mesenchymal cells. J Endod. 2009;35:1387–1390. doi: 10.1016/j.joen.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Zortuk M, Kesim S, Kaya E, Ozbilge H, Kiliç K, Cölgeçen O. Bacterial adhesion of porphyromonas gingivalis on provisional fixed prosthetic materials. Dent Res J (Isfahan) 2010;7:35–40. [PMC free article] [PubMed] [Google Scholar]
- 22.Rosentritt M, Hahnel S, Gröger G, Mühlfriedel B, Bürgers R, Handel G. Adhesion of Streptococcus mutans to various dental materials in a laminar flow chamber system. J Biomed Mater Res B Appl Biomater. 2008;86:36–44. doi: 10.1002/jbm.b.30985. [DOI] [PubMed] [Google Scholar]
- 23.Sardin S, Morrier JJ, Benay G, Barsotti O. In vitro streptococcal adherence on prosthetic and implant materials. Interactions with physicochemical surface properties. J Oral Rehabil. 2004;31:140–148. doi: 10.1046/j.0305-182x.2003.01136.x. [DOI] [PubMed] [Google Scholar]
- 24.Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 25.Nyvad B, Fejerskov O. Scanning electron microscopy of early microbial colonization of human enamel and root surfaces in vivo. Scand J Dent Res. 1987;95:287–296. doi: 10.1111/j.1600-0722.1987.tb01844.x. [DOI] [PubMed] [Google Scholar]
- 26.Mıura T, Hayakawa T, Okumorı N, Iohara K, Yoshinari M. Antifungal activity against Candida albicans on PMMA coated with protamine derivatives. J Oral Tissue Engin. 2010;8:30–38. [Google Scholar]
- 27.Bürgers R, Schneider-Brachert W, Rosentritt M, Handel G, Hahnel S. Candida albicans adhesion to composite resin materials. Clin Oral Investig. 2009;13:293–299. doi: 10.1007/s00784-008-0226-4. [DOI] [PubMed] [Google Scholar]
- 28.Anil S, Ellepola AN, Samaranayake LP. The impact of chlorhexidine gluconate on the relative cell surface hydrophobicity of oral Candida albicans. Oral Dis. 2001;7:119–122. [PubMed] [Google Scholar]