Summary
Background
Non-communicable diseases are the leading global cause of death and disproportionately afflict those living in low-income and lower-middle-income countries (LLMICs). The association between socioeconomic status and non-communicable disease behavioural risk factors is well established in high-income countries, but it is not clear how behavioural risk factors are distributed within LLMICs. We aimed to systematically review evidence on the association between socioeconomic status and harmful use of alcohol, tobacco use, unhealthy diets, and physical inactivity within LLMICs.
Methods
We searched 13 electronic databases, including Embase and MEDLINE, grey literature, and reference lists for primary research published between Jan 1, 1990, and June 30, 2015. We included studies from LLMICs presenting data on multiple measures of socioeconomic status and tobacco use, alcohol use, diet, and physical activity. No age or language restrictions were applied. We excluded studies that did not allow comparison between more or less advantaged groups. We used a piloted version of the Cochrane Effective Practice and Organisation of Care Group data collection checklist to extract relevant data at the household and individual level from the included full text studies including study type, methods, outcomes, and results. Due to high heterogeneity, we used a narrative approach for data synthesis. We used descriptive statistics to assess whether the prevalence of each risk factor varied significantly between members of different socioeconomic groups. The study protocol is registered with PROSPERO, number CRD42015026604.
Findings
After reviewing 4242 records, 75 studies met our inclusion criteria, representing 2 135 314 individuals older than 10 years from 39 LLMICs. Low socioeconomic groups were found to have a significantly higher prevalence of tobacco and alcohol use than did high socioeconomic groups. These groups also consumed less fruit, vegetables, fish, and fibre than those of high socioeconomic status. High socioeconomic groups were found to be less physically active and consume more fats, salt, and processed food than individuals of low socioeconomic status. While the included studies presented clear patterns for tobacco use and physical activity, heterogeneity between dietary outcome measures and a paucity of evidence around harmful alcohol use limit the certainty of these findings.
Interpretation
Despite significant heterogeneity in exposure and outcome measures, clear evidence shows that the burden of behavioural risk factors is affected by socioeconomic position within LLMICs. Governments seeking to meet Sustainable Development Goal (SDG) 3.4—reducing premature non-communicable disease mortality by a third by 2030—should leverage their development budgets to address the poverty-health nexus in these settings. Our findings also have significance for health workers serving these populations and policy makers tasked with preventing and controlling the rise of non-communicable diseases.
Funding
WHO.
Introduction
Non-communicable diseases account for 70% of global deaths,1 and the disproportionate concentration of premature deaths from these diseases in lower-income countries has been described as “the social justice issue of our generation”.2, 3 The Sustainable Development Goals (SDGs) include the target of reducing premature deaths from non-communicable diseases by a third over the next 15 years.4 The disconnect between non-communicable disease prevention, development, and poverty reduction strategies was mentioned by WHO in the first Global Action plan in 2008,5, 6, 7 with calls for improved coordination culminating in two “Non-communicable diseases and Development Cooperation” dialogues in 2015.8
Development agencies—mainly working with the poorest members of low-income and lower-middle-income countries (LLMICs)—might be more likely to realign their activities to address non-communicable disease prevention if there was clear evidence that non-communicable diseases and their risk factors affect these populations.8 The distribution of diseases and risk factors between nations is well established, but little evidence for the socioeconomic distribution of risk factors within LLMICs has been published.9 The urgent need for disaggregated data was underlined at the 2011 UN High Level Meeting on non-communicable diseases.10
Research in context.
Evidence before this study
We searched PubMed and Google scholar on July 28, 2015, with no language restrictions. Our search terms were a list of World Bank-defined low-income and lower-middle income countries (LLMICs); MeSH and free-text terms for tobacco use, alcohol use, diet, and physical inactivity; and socioeconomic status. Studies published before 1990 were excluded. There was a moderate risk of bias among the included studies.
From this search, we identified a 2005 non-systematic review of surveys from 11 low-income and middle-income country (LMIC) WHO subregions and a meta-analysis of studies examining tobacco use and income. These studies report higher prevalence of tobacco use (odds ratio 1·48, 95%CI 1·38–1·59) and lower alcohol use in the poorest strata of LMICs compared with more affluent groups. The most comprehensive analysis to date comes from an analysis of LMIC World Health Survey data from 2002–04. Self-reports from 232 056 participants from 48 countries—of which 25 were upper-middle-income and 23 were low-income or lower-middle income (LLMICs)—suggested that those with more education and assets were more likely to be physically inactive and consume insufficient fruit and vegetables and less likely to smoke daily. The socioeconomic patterning of heavy episodic drinking was mixed and inequalities were more pronounced in the least developed countries. The findings from that study are now 10 years old, and largely drawn from upper-middle or high-income countries.
Added value of this study
To our knowledge, this study is the first systematic review to examine the distribution of the main non-communicable behavioural risk factors across different socioeconomic groups within LLMICs and the first study to report on physical activity and socioeconomic status in developing countries. This work supports ongoing efforts to link non-communicable disease prevention with the global development agenda and provides evidence for development agencies on how to engage with non-communicable diseases. Our study shows that lower socioeconomic groups are more likely to drink alcohol, use tobacco, and consume insufficient fruit and vegetables than more advantaged groups. Higher socioeconomic groups were found to be more inactive and might consume more fats, salt, and processed food. Our findings substantially augment the scant evidence from previous LLMIC-based reviews on individual risk factors. With the use of broader measures of socioeconomic status, we found significant differences between castes, classes, sexes, and occupational groups with the widest differences observed across different educational strata.
Implications of all the available evidence
Combined with previous work, the association between non-communicable disease risk factors and socioeconomic status seems to be dependent on setting, population, and exposure definitions. Tobacco use seems to be almost universally more prevalent in low socioeconomic groups than in high socioeconomic groups, whereas alcohol and diet require further investigation. Our findings have importance for the development community that have a part to play in ensuring that their projects do not promote environments that promote non-communicable diseases in low-income settings. This study shows that there is a clear socioeconomic gradient of non-communicable disease risk behaviours within most LLMICs. Education was strongly correlated with healthier behaviour in most settings and might be an important tool in controlling the epidemic. Other interventions should be focused on social groups that are most at risk.
Only a few studies on the intranational distribution of behavioural risk factors have been published: a 2005 non-systematic review of surveys from 11 low-income and-middle-income country (LMIC) WHO subregions11 and a meta-analysis of studies examining tobacco use and income.12 These studies report a higher prevalence of tobacco use (odds ratio [OR] 1·48, 95% CI 1·38–1·59) and lower alcohol use in the poorest strata of LMICs than in more affluent strata. The most comprehensive analysis to date comes from an analysis of LMIC World Health Survey data from 2002–04.13 Self-reports from 232 056 participants from 48 countries (23 of which were LLMICs) suggested that those with more education and assets were more likely to be physically inactive and consume insufficient fruit and vegetables, and less likely to smoke daily, than were those with a lower level of education. The socioeconomic patterning of heavy episodic drinking was mixed and inequalities were more pronounced in the least developed countries. The findings from this study are now 10 years old and largely drawn from non-LLMICs. These reviews were limited by indirect estimates of behaviour and narrow definitions of socioeconomic status.
Non-communicable diseases are the leading cause of death and individuals living in LLMICs are 1·5 times more likely to die prematurely from these conditions than those living in high-income countries.14 With increasing international attention being paid to the epidemic, international and intranational health inequalities, and the potential role for development agencies in combatting non-communicable diseases, it is important that we have up to date information about the socioeconomic patterning of the most important non-communicable disease risk factors in lower-income settings.
We aimed to systematically review current evidence on the association between socioeconomic status and harmful use of alcohol, tobacco use, unhealthy diets, and physical inactivity within LLMICs.
Methods
Search strategy and selection criteria
We did a systematic review following a registered protocol and PRISMA guidelines15 (appendix). We searched Embase, MEDLINE, Web of Science, Global Health, and TRoPHI for all studies that included primary data published between Jan 1, 1990, and July 30, 2015. We also searched grey literature in Digital Dissertations (Global full-text plus), WHOLIS (WHO Library), and the WHO regional databases AIM (AFRO), LILACS (AMRO/PAHO), IMEMR (EMRO), IMSEAR (SEARO), and WPRIM (WPRO). We reviewed the first 30 results from Google Scholar and searched MEDLINE In-process and other non-indexed citations, the websites of the World Bank, DFID, USAID, and WHO, and scrutinised the reference lists of included papers and contacted key authors to uncover additional or forthcoming work.
We used English search terms (appendix) but did not restrict results by language or age of participants. Records were included if they presented primary data from one or more of the 84 LLMICs, as defined by the 2013 World Bank analytic classifications16 or on one or more non-communicable behavioural risk factor (defined by WHO as tobacco use, unhealthy diet, harmful alcohol use, and physical inactivity)17 and if the data were stratified by at least one socioeconomic indicator.
To accommodate differing views, capture all relevant studies, and broaden the systematic review, we included household or individual-level data measures of income, wealth, assets, socioeconomic status, education, caste, and occupation (where categories were ordinal). We excluded studies that did not allow comparison between more or less advantaged groups. Authors were contacted for additional data where socioeconomic status and behavioural risk factors were measured but reported independently. We used the same dates and strategy for all searches, tailored to specific databases by LA and an experienced medical librarian (NR).
With the use of a piloted form (appendix), LA and JW independently screened titles and abstracts, calculating percentage agreement and Cohen's κ statistic at 10% intervals (every 424 records). Once inter-rater agreement exceeded 95% and Cohen's κ was higher than 0·75 (excellent agreement18), LA screened all remaining records, bringing uncertainties to JW and KW, with disagreements resolved by group consensus. The same protocol was used for full-text systematic review. If data from included studies were unclear or if more information was required, the authors were contacted by email. If this information was not available, the study was excluded.
Data analysis
LA used a piloted version of the Cochrane Effective Practice and Organisation of Care Group data collection checklist19 to extract relevant data from the included full-text studies including study type, methods, outcomes, and results (appendix). JW independently cross-checked a random 10% sample of included papers. Disagreements and ambiguities were resolved by full group consensus. Authors were contacted by email if more information was required.
We assessed data quality using a modified Newcastle-Ottawa scale,20 as recommended by the Cochrane Collaboration (appendix).21 Appropriate versions of the scoring rubric were used for randomised controlled trials, case-control studies, and cross-sectional studies. Scores were based on design-specific sources of bias, methods for selecting participants, exposure measures, outcome variables, and methods to control confounding. The source of funding was recorded for each study.
The main outcome was differences in prevalence or relative risk of non-communicable disease behavioural risk factors between different socioeconomic groups. We also planned to examine how age, sex, urban or rural location, and study quality affected findings. We assessed variability within studies in our quality scoring. This included considering the uniformity of training for those conducting the study and the instruments used to gather data. Significant heterogeneity between studies, particularly in the exposure and outcome measures precluded quantitative synthesis and meta-analysis. We used a narrative approach, grouping studies by outcome measure and WHO region. We analysed differences between outcomes, geographic regions, age groups, and sex. We also present sensitivity analyses for each risk factor having removed all medium and low-quality studies. The protocol of this study is registered with PROSPERO, number CRD42015026604. We used Excel to generate simple descriptive statistics.
Role of the funding source
An employee of the funder (BM) contributed to the study design and review of draft manuscripts. The funder did not have any involvement in data collection, data analysis, or data interpretation. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
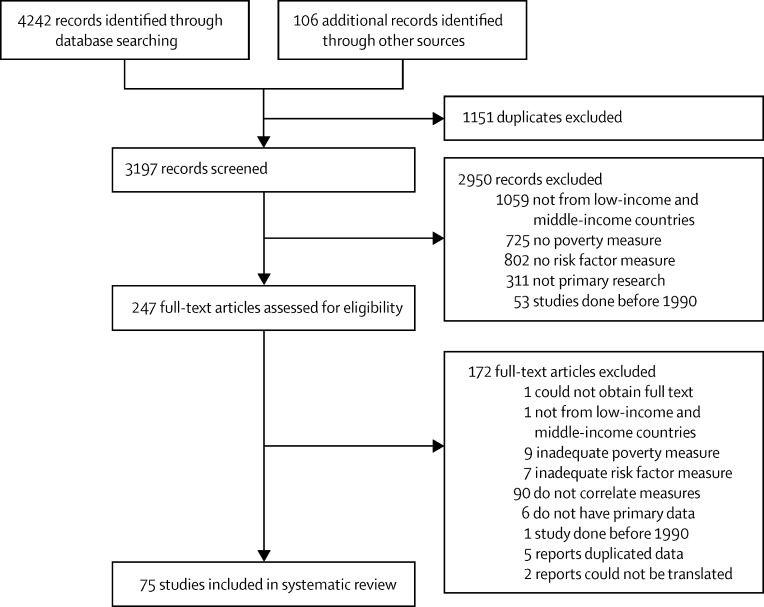
Our literature search returned 4242 records and 106 additional records were retrieved from other sources (figure 1). Over 1000 studies were from higher-income or upper-middle-income countries. We assessed 247 full-text articles, of which 75 met our inclusion criteria. These articles covered 39 countries and presented data for 2 135 314 individuals aged older than 10 years. The median sample size (individuals for whom data of interest were reported) was 1984 (range 66–471 143).
Figure 1.
Study selection
Five articles presented data for all risk factors, 41 articles reported on a single risk factor, and the remaining 29 articles reported data for two or three risk factors. Ten different socioeconomic indicators were used (income, wealth or assets, state-defined poverty, literacy, education, occupational class, occupational status [employed or unemployed], job seniority, caste, researcher-defined socioeconomic status).
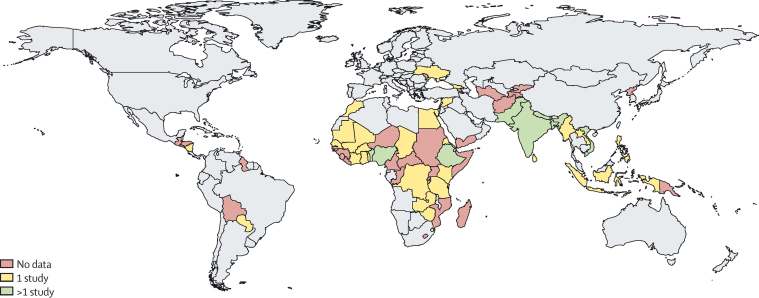
One article presented Global Adult Tobacco Survey data from two LLMICs,22 another reported World Health Survey data for smoking rates in 28 LLMICs,23 and the remaining 73 articles reported data from one country each. 44 studies were done in southeast Asian populations, with 35 pertaining to India. Data were presented for 20 African LLMICs countries, whereas the Americas and Europe had the lowest representation with two apiece. There were no data for 45 of the 84 LLMICs (figure 2).
Figure 2.
Sources of data from low-income and lower-middle-income countries
Over half of the included studies had been published since 2010 and seven were published before 2000.24, 25, 26, 27, 28, 29, 30 70 studies were cross-sectional, two were prospective longitudinal cohort studies,31, 32 two were case control studies,33, 34 and one study was a randomised controlled trial.35 Five studies were not peer reviewed; all were WHO STEPS surveys.36, 37, 38, 39, 40
Overall, 13 studies were of low quality, 33 were moderate, and 29 were of high quality, leading to a low risk of bias across studies. The most common study weaknesses were loss to follow-up and failure to control for confounding factors. Studies in African populations were more likely to be non-peer reviewed, to be of a lower quality, and have smaller sample sizes. Studies reporting data on Indian populations and tobacco use tended to be larger and of a higher quality than those for other populations and risk factors. Most studies were funded by governments, public health agencies, development agencies, and non-governmental or non-profit organisations. A summary of all high quality studies is presented in the table. Details of all included studies are available in the appendix.
Table.
Characteristics of included high-quality studies
| Site | Study design | Number of participants | Population | Age (years) | Exposure | Outcome | |
|---|---|---|---|---|---|---|---|
| Physical activity | |||||||
| Kinra, 2010 | India | Cross-sectional | 1983 | 1600 villages in 18 states | 20–69 | Socioeconomic status | Low physical activity; <1·69 MET |
| Gupta, 2003 | India | Cross-sectional | 573 | General population in Jaipur | NA | Education | Low physical activity; <30 min leisure time physical activity 3 times a week |
| Oanh, 2008 | Vietnam | Cross-sectional | 1776 | STEPS survey in Ho Chi Minh | 25–64 | Assets/education/income | Low physical activity; <600 MET min per week |
| Gupta, 2012 | India | Cross-sectional | 6198 | Middle-class areas of 11 cities | 18–75 | Education/occupation/socioeconomic status | Low physical activity; no regular work or leisure time physical activity |
| Dhungana, 2014 | Nepal | Cross-sectional | 406 | Rural community in Sindhuli | 20–50 | Education/socioeconomic status/caste | Low physical activity; <150 minutes moderate physical activity/week |
| Zeba, 2014 | Burkina Faso | Cross-sectional | 330 | Ouagadougou residents | 25–60 | Assets/education | Physical activity and sedentary time; means >3 h and <3 h MET, respectively |
| Reddy, 2007 | India | Cross-sectional | 19 969 | Industrial workers from 10 cities | 20–69 | Education | Leisure time physical activity |
| Singh, 1997 | India | Cross-sectional | 1767 | Two villages in rural north India | 25–64 | Socioeconomic status | Sedentary* |
| Alcohol | |||||||
| Bonu, 2005 | India | Cross-sectional | 22 685 | Inpatients from 1995 National Survey | >10 | Alcohol use | Poverty; borrowing or financial distress during hospital admission |
| Gupta, 2012 | India | Cross-sectional | 6198 | Middle-class areas of 11 cities | 18–75 | Education/occupation/socioeconomic status | Alcohol abuse |
| Samuel, 2012 | India | Cross-sectional | 2218 | Rural and urban southern India | 26–32 | Assets/education | Alcohol use |
| Hashibe, 2003 | India | Case-control | 47 773 | Adults in Kerala | >35 | Income/education/occupation | Alcohol use |
| Neufeld, 2005 | India | Cross-sectional | 471 143 | 1995 National Sample Survey | >10 | Poverty/caste/education | Alcohol use; regular use of any alcoholic beverage |
| Kinra, 2010 | India | Cross-sectional | 1983 | 1600 villages in 18 states | 20–69 | Socioeconomic status | Alcohol use; consumed >10 days per month over last 6 months |
| Dhungana, 2014 | Nepal | Cross-sectional | 406 | Rural community in Sindhuli | 20–50 | Education/socioeconomic status/caste | Alcohol use; used alcohol up to 30 days before interview |
| Subramanian, 2005 | India | Cross-sectional | 301 984 | 1998 National Family Health Survey | >18 | Assets/caste/education | Household member drinks alcohol |
| Diet | |||||||
| Hashibe, 2003 | India | Case-control | 47 773 | Adults in Kerala | >35 | Income/education/occupation | Daily vegetables, high intake of fruit |
| Gupta, 2012 | India | Cross-sectional | 6198 | Middle-class areas of 11 cities | 18–75 | Education/occupation/socioeconomic status | Less than two servings fruit and vegetables per day, more than 20 g fat per day |
| Ganesan, 2012 | India | Cross-sectional | 1261 | Urban diabetics from Chennai | >40 | Socioeconomic status | Low or high fibre diet; scored using a questionnaire |
| Kinra, 2010 | India | Cross-sectional | 1983 | 1600 villages in 18 states | 20–69 | Socioeconomic status | Low fruit and vegetable intake; <400 g/day |
| Dhungana, 2014 | Nepal | Cross-sectional | 406 | Rural community in Sindhuli | 20–50 | Education/socioeconomic status/caste | Low fruit and vegetable intake; <400 g/day |
| Zeba, 2014 | Burkina Faso | Cross-sectional | 330 | Ouagadougou residents | 25–60 | Assets/education | Unhealthy diet; fat/sugar/fibre/plant protein/complex carbohydrates |
| Agrawal, 2014a | India | Cross-sectional | 156 317 | National Family Health Survey | 20–49 | Caste/socioeconomic status | Non-vegetarian; eats meat, fish, milk, eggs, curd, dairy |
| Agrawal, 2014b | India | Cross-sectional | 156 317 | National Family Health Survey | 20–49 | Caste/wealth | Daily fish consumption |
| Tobacco | |||||||
| Bonu, 2005 | India | Cross-sectional | 22 685 | Inpatients from 1995 Nat. Survey | >10 | Tobacco use | Poverty; borrowing or financial distress during hospitalisation |
| Hashibe, 2003 | India | Case-control | 47 773 | Adults in Kerala | >35 | Income/education/occupation | Smoking, tobacco chewing |
| Corsi, 2014 | India | Cross-sectional | 4534 | 20 villages in Andhra Pradesh | >20 | Income/education | Current smoker, ever smoker |
| Kinra, 2010 | India | Cross-sectional | 1983 | 1600 villages in 18 states | 20–69 | Socioeconomic status | Daily smoker at any time in the last 6 months |
| Neufeld, 2005 | India | Cross-sectional | 471 143 | 1995 National Sample Survey | >10 | Poverty/caste/education | Regular smoker, regularly chews tobacco |
| Gupta, 2003 | India | Cross-sectional | 573 | General population in Jaipur | NA | Education | Past or present use of any tobacco product |
| Singh, 2000 | India | Cross-sectional | 1767 | Two villages in rural north India | 25–64 | Socioeconomic status | Uses tobacco more than once per week |
| Gupta, 2012 | India | Cross-sectional | 6198 | Middle-class areas of 11 cities | 18–75 | Education/occupation/socioeconomic status | Daily use of a tobacco product |
| Reddy, 2007 | India | Cross-sectional | 19 969 | Industrial workers from 10 cities | 20–69 | Education | Use of any tobacco product in previous 30 days |
| Singh, 2007 | India | Cross-sectional | 2222 | Residents of Moradabad | 25–64 | Socioeconomic status | Use of any tobacco product |
| Samuel, 2012 | India | Cross-sectional | 2218 | Rural and urban southern India | 26–32 | Assets/education | Current tobacco user |
| Gupta, 2015 | India | Cross-sectional | 6198 | Middle-class areas of 11 cities | >20 | Education | Quit for >1 year having used tobacco for >1 year previously |
| Narayan, 1996 | India | Cross-sectional | 13 558 | Residents of Delhi | 25–64 | Education/occupation | Current smoker or has smoked >100 times in the past |
| Rani, 2003 | India | Cross-sectional | 334 553 | 1998 National Family Health Survey | >15 | Wealth/education/caste | Smokes, chews tobacco |
| Heck, 2012 | Bangladesh | Cross-sectional | 19 934 | Married Bangladeshi adults | 18–75 | Education | Betel quid use |
| Dhungana, 2014 | Nepal | Cross-sectional | 406 | Rural community in Sindhuli | 20–50 | Education/socioeconomic status/caste | Smoking until last 30 days before interview |
| Bovet, 2002 | Tanzania | Cross-sectional | 9254 | Residents of Dar es Salaam | 25–64 | Wealth/education | Smokes one or more cigarettes per day |
| Minh, 2007 | Vietnam | Cross-sectional | 1984 | 2005 STEPS survey of Bavi district | 25–64 | Education/socioeconomic status | Smoker |
| Tonstad, 2013 | Cambodia | Cross-sectional | 5592 | 2006 National Tobacco Survey | >18 | Education/income/occupation | Quit; not used tobacco for >2 years among ever users |
| Ali, 2006 | Pakistan | Cross-sectional | 411 | Men from rural Sindh province | >18 | Education/Income | Has smoked >100 cigarettes |
| Hosseinpoor, 2012 | 28 LLMICs | Cross-sectional | 213 807 | 2003 World Health Survey | >18 | Socioeconomic status | Daily or occasional tobacco smoker |
| Jena, 2012 | India | Cross-sectional | 69 296 | 2009 Global Tobacco Survey data | >15 | Occupation/education | Hardcore smoker† |
| Kishore, 2013 | India, Thailand, and Bangladesh | Cross-sectional | 92 491 | 2009 Global Adult Tobacco Survey | >15 | Education | Hardcore smoker† |
MET=Metabolic Equivalent of Task. LLMIC=low-income and lower-middle-income countries.
Walks less than 14·5 km, less than 20 flights of stairs, or does no moderate activity 5 days per week.
Hardcore smoker is defined as someone who currently smokes daily, with no quit attempt in last 12 months or whose last quit was for less than 24 h; no intention to quit in next 12 months or not interested in quitting first smoke within 30 min of waking; and who has knowledge of harms. High-quality survey findings and findings for physical activity, alcohol, diet, and tobacco are in the appendix.
29 studies from 15 countries reported measures of physical activity.25, 28, 31, 32, 36, 37, 38, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60 Three WHO STEPS surveys from India, Eritrea, and Côte d'Ivoire had not been peer-reviewed, but the remaining 26 were published in peer-reviewed journals. Three studies were low quality, 18 were moderate (including the WHO surveys), and eight were of high quality. Nine studies37, 38, 48, 52, 53, 54, 55, 57, 61 reported outcomes based on WHO recommendations and results from the International and Global Physical Activity Questionnaires,62, 63, 64 five studies used definitions derived from other sources,32, 41, 44, 58, 60 and 15 did not refer to any pre-existing definition.25, 27, 31, 36, 42, 43, 45, 46, 47, 49, 50, 51, 56, 59, 65 Measures of sedentary behaviour (not technically a non-communicable disease behavioural risk factor) were reported in 19 studies; high or sufficient levels of activity were reported in ten studies. All data were derived from survey instruments rather than the use of accelerometers or other devices.
There was a paucity of studies reporting adjusted results that were statistically significant; however, most studies found that individuals with a high socioeconomic status were less active than groups with a lower socioeconomic status, irrespective of outcome and exposure measures. This trend was consistent across studies from southeast Asia, the western Pacific, Africa, and the eastern Mediterranean. The notable exceptions were found in populations from urban areas: residents of Aleppo,60 pre-diabetics in southern India,61 and residents of multiple Indian cities.43, 46 In these settings, low-income and less educated groups had the highest prevalence of inactivity. Eight of the ten studies that stratified findings by sex found men to be more active than women;25, 41, 45, 46, 50, 53, 54, 58 the remaining two found female residents of Jaipur to be more active than men in all educational groupings;42 and no clear association was found in two rural north Indian villages.44
Most participants were aged 15–65 years old. Studies that excluded people aged over 60 years, or those younger than 30 years, still found that higher socioeconomic groups were the least active.45, 47, 52 The eight high-quality physical activity studies corroborate these findings. Single studies from the capital cities of Vietnam and Burkina Faso found that wealthy and educated individuals were the least active.56, 57 The six papers from India and Bangladesh showed that higher socioeconomic status was associated with lower levels of physical activity in rural settings,41, 44, 52 and this association was reversed in urban settings.42, 43 None of these studies controlled for occupation; however, Reddy and colleagues46 examined physical activity in 20 000 industrial workers and found that those with primary or no education were eight times less active than the most educated workers in their leisure-time (p<0·001). This study did not account for other important sources of physical activity including commuting, employment, or housework.
24 studies from ten countries reported measures of alcohol use.25, 27, 28, 31, 32, 34, 35, 36, 38, 39, 40, 41, 43, 45, 47, 52, 66, 67, 68, 69, 70, 71, 72, 73 Three studies were graded as low quality (including the only randomised controlled trial), 13 were moderate, and eight were of high quality, including the only case-control study. Four studies reported prevalence of harmful alcohol use, defined in terms of the frequency and volume of alcohol consumed.35, 40, 43, 69 The remaining 18 reported measures of any alcohol use as the outcome variable. There was reasonable agreement between the various socioeconomic proxies; none of the studies that used multiple exposure measures found conflicting assessments.
One study found that alcohol users were more likely to experience impoverishment than non-users but this association was not statistically significant.66 Overall, low-income uneducated groups in rural areas were the most likely to engage in harmful drinking behaviour. The widest differences were observed between different educational groups; smaller gaps were observed when comparing income strata.
Analysing the findings by region, alcohol use—while not necessarily at harmful levels—was most prevalent in low-income and less-educated groups across India32, 34, 38, 41, 45, 47, 67, 68, 70, 71 and in the solitary study from the Americas.73 Prevalence of alcohol use tended to be higher in more affluent and well educated Africans;25, 27, 28, 31, 35 however, most of these studies were published in the 1990s25, 27, 28 and sample sizes were in the hundreds for all but one study—a randomised controlled trial graded as low quality.35 No studies from Europe, the eastern Mediterranean, or western Pacific regions were published. All five studies that reported results by sex found men to drink more than women;25, 27, 41, 45, 70 however, inequalities were often more pronounced between women. The smaller numbers of women in these studies rendered many of these findings not significant.
Six of the eight high-quality studies assessed alcohol use rather than harmful use of alcohol. With the exception of a 2012 survey of younger Indian adults,67 these studies all found that lower socioeconomic groups were the most likely to use alcohol.34, 41, 52, 70, 71 The largest differences were observed between members of different castes and educational groups. Gupta and colleagues43 assessed (undefined) alcohol abuse in middle-class urban Indians, finding minor differences between educational and self-assessed socioeconomic tertiles. Individuals in middle occupational classes had double the rate of alcohol abuse compared with the lowest occupational class (10·8% vs 5·1%); however, no measures of significance were presented.43
26 papers from 11 countries reported on eight different aspects of diet.24, 25, 29, 30, 34, 36, 38, 40, 41, 43, 45, 50, 51, 52, 56, 60, 65, 68, 72, 74, 75, 76, 77, 78, 79, 80 There was one case-control study34 and the remainder were cross-sectional. Four studies were low quality, 14 were moderate, and eight were of high quality. Six studies, from Pakistan,24 India,29, 30, 43, 65 and Nigeria25 found higher consumption of unhealthy fats in individuals of high socioeconomic status. Two studies examining salt intake found a higher prevalence in high-income households in Chennai77 and non-significant differences in a low quality multisite Indian survey.65 Two higher quality African studies found that the individuals of high socioeconomic status were more likely to consume diets high in processed foods.56, 78
Studies from Indonesia, Syria, Nepal, Benin, Eritrea, and Nigeria all found lower fruit and vegetable intake in less affluent and less educated groups.36, 50, 52, 60, 72, 79, 80, 81 These studies tended to present results that were either significant but unadjusted or adjusted but not significant; only two studies presented significant adjusted findings.80, 81 Six larger and higher quality studies from India predominantly found lower fruit and vegetable intake in groups of lower socioeconomic status.34, 38, 41, 45, 50, 65 One high-quality survey of low fibre intake found a low socioeconomic status preponderance76 and three large Indian studies found less affluent groups to consume the least fish and most meat.68, 74, 75 There was good agreement between different poverty markers; all but two studies that used more than one measure found that similar groups were identified as consuming the least healthy diet.43, 52 Women were found to consume less fruit and vegetables than were men in two Indian studies.41, 45 Four other studies reporting dietary findings by sex found non-significant differences.24, 25, 29, 50 In 320 elderly residents of Baroda city, India, men consumed twice as much fat as women.30
When we examined the effect of age on these dietary findings, we found that studies examining older populations came to the same conclusions as other studies examining the same dietary component.30, 34, 41, 76, 79, 80 One cross-sectional study examining cholesterol intake in Pakistani schoolchildren found the highest consumption in boys and girls from the highest socioeconomic group.24 After removing all medium and low-quality studies, five high-quality studies suggest that high socioeconomic groups consume more fat, fish, fibre, and fruit and vegetables than lower socioeconomic groups in southeast Asia.34, 41, 43, 52, 74, 75 The remaining small cross-sectional study in Burkina Faso56 found that those with the most education and assets were twice as likely to consume an unhealthy diet (high in fat and sugar, low in fibre, plant protein, and complex carbohydrates) as those with the lowest education and assets.
50 studies reported data for tobacco use in 39 countries (appendix), almost twice the number of studies examining other risk factors.22, 23, 26, 27, 31, 32, 33, 34, 37, 38, 39, 41, 42, 43, 44, 45, 46, 47, 49, 50, 51, 52, 58, 59, 60, 65, 66, 67, 68, 71, 73, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100 Eight studies were low quality, 18 were moderate, and 23 were of high quality—a much higher proportion than for other risk factors. 33 studies reported smoking as an outcome variable using a range of definitions,23, 26, 27, 31, 32, 37, 38, 39, 41, 42, 45, 50, 51, 52, 60, 68, 73, 83, 91, 93, 94, 95, 96, 97, 99, 100 two reported hardcore smoking (very low chances of ever quitting),22, 88, 101 two reported quitting,87, 98 and one study each was included on use of manipuri,33 betel quid,90 and the likelihood of smokers experiencing financial distress.66
The popularity of chewing versus smoking tobacco varied by setting, but women were more likely to chew tobacco than smoke.84, 89 Although levels of smoking and chewing were broadly commensurate within populations, socioeconomic inequalities were more pronounced with smoking. Jena and colleagues88 found an adjusted but non-significant higher prevalence of hardcore smoking in well educated individuals in a large nationally representative Indian sample.88 Illiterate individuals and those who were poorly educated were more likely to smoke manipuri33 and betel quid,90 and less likely to quit all forms of tobacco.87, 98
One moderate quality survey of 233 young Keralan men found a significantly higher prevalence of tobacco use in middle-class students, adjusting for age and other confounders.86 The remaining 49 studies found tobacco use, in all forms, to be more prevalent in low socioeconomic groups than in high socioeconomic groups, including 17 studies presenting statistically significant adjusted results from 18 different countries.23, 26, 41, 42, 43, 46, 47, 58, 67, 71, 82, 83, 89, 94, 97, 98, 99
The finding that low socioeconomic groups were more likely to use tobacco than high socioeconomic groups was the same in every geographical region. Studies that examined older populations came to the same conclusions.34, 47, 92 Two studies examined tobacco use in young adults, both from southern India; Lal and Nair's subanalysis86 of Keralan data from the Global Adult Tobacco Survey, which has been previously mentioned, uniquely found higher tobacco usage in 233 highly educated and middle socioeconomic status men aged 15–24 years. Samuel and colleagues found the highest usage in the poorest and least educated 26–32 year olds in a cross sectional survey of 2218 26–32 year olds living in south India.67
Differences between educational groups were larger than differences between castes and income or wealth strata.26, 43, 45, 71 Neufeld and colleagues found that measures of caste and state-defined poverty were associated with wider inequalities for chewing tobacco than cigarette use.71 All 15 studies that stratified prevalence by sex found men to smoke more than women, often by a large margin.23, 26, 27, 41, 42, 44, 45, 46, 50, 58, 83, 84, 85, 89, 96 Removing all low and moderate quality studies did not change the findings. Among the 24 high-quality studies, education remained the strongest predictor of betel quid and tobacco use. Those with no formal education were between 1·75 and 6·50 times more likely to smoke than those with at least a secondary education.26, 34, 42, 46, 67, 83 Low income, caste, and socioeconomic status were associated with a tobacco use prevalence roughly twice that of high-status groups.
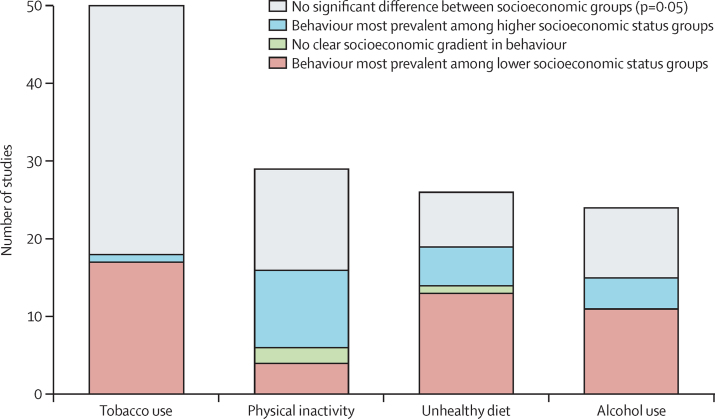
Overall, low socioeconomic groups in most of the LLMICs in which evidence was available were more likely to use tobacco and alcohol, and to consume less fruit, vegetables, fish, and fibre, and more meat than high socioeconomic groups. High socioeconomic status groups tended to have higher levels of physical inactivity and consume more fats, salt, and processed foods than low socioeconomic groups (figure 3). While the included studies presented clear patterns for tobacco use and physical activity, heterogeneity between dietary outcome measures and a paucity of evidence around harmful alcohol use limit the certainty of these findings.
Figure 3.
Number of studies for each risk factor showing the socioeconomic group with the highest risk
Discussion
This systematic review identifies broad trends in global behavioural risk factors for non-communicable diseases, finding that low socioeconomic groups in many countries are more likely to drink alcohol, use tobacco, and consume insufficient fruit and vegetables than are high socioeconomic groups. High socioeconomic groups tend to be more inactive and consume more fats, salt, and processed food.
This systematic review is the first to examine the socioeconomic distribution of all four major behavioural risk factors within LLMICs. Our findings substantially augment the scant evidence from previous LLMIC-based reviews on individual risk factors; Ciapponi and colleagues12 showed a significant income gradient for tobacco use but their focus on income only excluded many of the studies included in our systematic review. With the use of a broad range of socioeconomic indicators, we found significant differences between castes, classes, sexes, and occupational groups with the widest differences observed across educational strata.
Our tobacco findings mirror the well established inequalities from high-income countries, where low-income groups are the most likely to smoke, start smoking earlier, consume more tobacco, quit less successfully, experience more adverse health effects, and die at a younger age than affluent groups.23, 102, 103, 104, 105 The last study to examine socioeconomic status and tobacco in LMICs was performed by Blakely and colleagues in 2005.11 They found that low-income groups from 11 LMIC WHO subregions had a marginally higher prevalence of tobacco use and lower use of alcohol than did higher-income groups.11 Most studies included in our systematic review used direct surveys, whereas Blakely and colleagues relied on estimates of consumption derived from household economic data.11 Evidence summarised in the Global Alcohol Report also suggests that abstinence is more common in low-income groups and that alcohol-related harm is more prevalent in low socioeconomic groups than in high-income groups; however, these data are mainly drawn from high-income countries.106, 107 Our findings suggest that alcohol use and harmful alcohol use tend to be most prevalent in low socioeconomic groups. We note that data for harmful alcohol use were lacking from 79 of 84 LLMICs and the few existing African studies are of low quality. There is an urgent need to quantify the burden of risky alcohol use in LLMICS.
Our dietary findings complement studies from high-income countries that have consistently found a positive association between socioeconomic status and consumption of fruit, vegetables, fibre, and fish.108, 109, 110, 111, 112, 113, 114 Whereas low socioeconomic status groups in high-income settings tend to consume higher levels of salt and processed food,111, 115 we found the opposite in LLMICs, but there was a conspicuous absence of studies on salt intake given the impact of this dietary risk factor.1 Our findings corroborate those of Mayén and colleagues116 who found higher consumption of all foods except fibre in high socioeconomic status groups in their systematic review of dietary patterns in LMICs; however, three quarters of their included studies were from upper-middle-income countries.
Our finding that rural high socioeconomic status groups tend to be the most physically inactive departs from the experience of high-income countries.117, 118, 119, 120 A possible explanation is that rural low socioeconomic status groups tend to work in physically demanding occupations in LLMICs.121 In cities, this association was reversed and evidence from China suggests that low socioeconomic status migrants take up less physically demanding jobs when they move to cities.122 If cities truly attenuate the socioeconomic gradient in occupational activity, then leisure-time physical activity might be proportionally more important as an explanatory variable. Reddy and colleagues found that higher socioeconomic status Indian groups engaged in more leisure-time physical activity than low socioeconomic status groups in urban areas.46 A large systematic review from mainly high-income countries has shown that leisure activity is associated with larger health gains than occupational activity.123 Our findings highlight the need for more research in LLMICs to explore the health effects of various domains of physical activity on different socioeconomic groups in rural and urban settings.
This systematic review was done in line with PRISMA and Cochrane guidance, following a registered protocol and assessing risk of bias using well established criteria. The bidirectional association between socioeconomic status and health is widely averred but infrequently assessed within LLMICs.11, 124, 125, 126, 127, 128 To our knowledge, this is the first systematic review to explore intranational socioeconomic patterning of behavioural risk factors in these countries and the first study to report that increasing wealth and education are associated with physical inactivity and increasing consumption of fats, salt, and processed food in a number of LLMICs. Our work demonstrates important associations and emphasises the importance of context; trends vary by region, sex, urbanicity, and exposure. Most included studies were moderate to high quality and almost invariably used a cross-sectional, survey-based approach.
Our method was designed to capture all studies on socioeconomic status and non-communicable disease behavioural risk factors. As a result, our findings are extremely heterogeneous and require careful interpretation. We treated the highest and lowest groupings of each exposure (eg, education, income, or social class) as if they were interchangeable even though each study tended to use a unique definition, cutoff, and study population. This allowed us to identify broad trends for future research to examine in detail; however, it meant that our findings should not be seen as definitive. The large amount of data also prevented us from presenting deep analysis of each risk factor in this systematic review; however, our comprehensive data extraction and presentation of all original data and subgroup descriptors in the appendix allows further study to build upon this initial global assessment. The heterogeneity in outcome measures for each risk factor limits the ability of any systematic review to synthesise findings cleanly, and the surfeit of smoking and alcohol definitions is especially noteworthy given the relative homogeneity of the products. A further source of bias was a dependence on survey instruments rather than objective measurements to establish tobacco use, alcohol use, diet, and physical activity between the studies. Survey responses are not very reliable and socioeconomic differences in recall bias might affect observed gradients in behaviour.
Use of the 2013 World Bank classification of income excluded countries that have only recently been reclassified as upper-middle-income; however, our focus on countries that are currently low-income enhances the usefulness of this systematic review as development agencies are moving away from upper-middle-income settings.129 Because of resource constraints, we were unable to perform a duplicate screen and data extraction for every record. Our high levels of agreement at each stage, including perfect agreement in triple-checked data extraction samples, provide reassurance that this systematic review includes all relevant data.
In view of the broad scope of this systematic review, the fact that over half of the countries classed as low-income or lower-middle-income were not represented in our search results is striking. Almost half of the included studies relate to India, and the evidence from the Americas, the eastern Mediterranean, and Europe is relatively scant. The fact that so many LLMICs were not represented is a major finding, but also a weakness in itself; the excellent evidence from India is not generalisable to all low socioeconomic groups in LLMICs and research is needed to explore whether the patterns we identify hold true in countries where surveillance is non-existent.
Of the 47 publicly available LLMIC-based WHO STEPS surveys,130 only five present behavioural risk factors stratified by any marker of socioeconomic status.35, 36, 37, 38, 39 All STEPS reports should make these routinely collected data publicly available.
Our findings provide an overview of the current evidence, underlining intranational trends and data gaps. Policy makers and national development agencies working in the countries where 82% of premature deaths occur should review the evidence relevant to their setting and consider whether their current non-communicable disease prevention strategies are appropriate. Where low socioeconomic status correlates with non-communicable disease risk factors, governments can use development funds to simultaneously improve literacy, living standards, and income alongside health. The definitions used to identify behavioural risk factors are inconsistent, and data are not available for most LLMICs. Rectification of these issues, with surveillance reporting risk factors stratified by socioeconomic status, is an obvious research priority. Nevertheless, this should not delay action in the countries where data exist.
Acknowledgments
Acknowledgments
This study was funded and commissioned by WHO. NT (grant number 006/P&C/CORE/2013/OXFSTATS), KW (grant number 006/P&C/CORE/2013/OXFSTATS), and CF (006/PSS/CORE/2016/OXFORD) received funding from the British Heart Foundation. JW is a DPhil student receiving funding from the Nuffield Department of Population Health, University of Oxford. NR is employed by the Bodleian Health Care Libraries.
Contributors
BM, KW, and NT conceived the research project, coordinated the contributors, and revised drafts of the manuscript. LA selected the studies, designed and executed the analyses, interpreted the findings, wrote the first draft, revised subsequent drafts, and prepared the manuscript. JW, NT, and KW contributed to analysis design, study selection, data extraction, and data analysis. CF revised drafts of the report and contributed to the data analysis. NR developed the search strategy, searched the databases, and contributed to the revision of subsequent drafts.
Declaration of interests
We declare no competing interests. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Supplementary Material
References
- 1.Institute for Health Metrics and Evaluation Global Burden of Disease data visualizations. 2015. http://vizhub.healthdata.org/gbd-compare/ (accessed July 30, 2015).
- 2.Horton R. Offline: Chronic diseases—the social justice issue of our time. Lancet. 2015;386:2378. [Google Scholar]
- 3.WHO Noncommunicable disease fact sheet. http://www.who.int/mediacentre/factsheets/fs355/en/ (accessed July 30, 2015).
- 4.UN General Assembley Resolution . Resolution 70/1. Transforming our world: the 2030 Agenda for Sustainable Development. A/Res/70/1. United Nations General Assembley; New York: 2015. [Google Scholar]
- 5.United Nations General Assembley . RES/66/2. Political declaration of the high-level meeting of the General Assembly on the prevention and control of non-communicable diseases. Resolutions adopted by the General Assembly at its 66th session. United Nations General Assembley; New York: 2011. [Google Scholar]
- 6.WHO . 2008–2013 action plan for the global strategy for the prevention and control of noncommunicable diseases. WHO; Geneva: 2009. [Google Scholar]
- 7.WHO . Global action plan for the prevention and control of noncommunicable diseases 2013–2020. WHO; Geneva: 2013. [Google Scholar]
- 8.World Health Organization Global Coordination Mechanism on non-communicable diseases . Report of the first dialogue convened by the World Health Organization Global Coordination mechanism on noncommunicable diseases. WHO; Geneva: 2015. [Google Scholar]
- 9.WHO . Global status report on noncommunicable diseases. WHO; Geneva: 2014. [Google Scholar]
- 10.United Nations General Assembley . Political declaration of the high-level meeting of the general assembly on the prevention and control of non-communicable diseases. United Nations; New York: 2011. [Google Scholar]
- 11.Blakely T, Hales S, Kieft C, Wilson N, Woodward A. The global distribution of risk factors by poverty level. Bull World Health Organ. 2005;83:118–126. [PMC free article] [PubMed] [Google Scholar]
- 12.Ciapponi A. Systematic review of the link between tobacco and poverty. WHO; Geneva: 2011. [Google Scholar]
- 13.Hosseinpoor AR, Bergen N, Kunst A. Socioeconomic inequalities in risk factors for non communicable diseases in low-income and middle-income countries: results from the World Health Survey. BMC Public Health. 2012;12:1. doi: 10.1186/1471-2458-12-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen L, Cobiac L, Townsend N. Quantifying the global distribution of premature mortality from non-communicable diseases. J Public Health (in press). [DOI] [PubMed]
- 15.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 16.World Bank Country and lending groups: analytic classifications. 2016. http://data.worldbank.org/about/country-and-lending-groups (accessed July 30, 2015).
- 17.WHO . Noncommunicable diseases fact sheet. World Health Organization; Geneva: 2013. [Google Scholar]
- 18.Fleiss JL, Levin B, Paik MC. The measurement of interrater agreement, in statistical methods for rates and proportions. 3rd edn. John Wiley & Sons; Hoboken, NJ: 2003. [Google Scholar]
- 19.Cochrane Effective Practice and Organisation of Care Group Data collection checklist. 2015. http://epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/datacollectionchecklist.pdf (accessed July 30, 2015).
- 20.Wells G, Shea B, O'connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. McGill University; Montreal: 2000. [Google Scholar]
- 21.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Wiley Online Library; New Jersey: 2008. [Google Scholar]
- 22.Kishore J, Jena PK, Bandyopadhyay C, Swain M, Das S, Banerjee I. Hardcore smoking in three south-east Asian countries: results from the Global Adult Tobacco Survey. Asian Pac J Cancer Prev. 2013;14:625–630. doi: 10.7314/apjcp.2013.14.2.625. [DOI] [PubMed] [Google Scholar]
- 23.Hosseinpoor AR, Parker LA, Tursan d'Espaignet E, Chatterji S. Socioeconomic inequality in smoking in low-income and middle-income countries: results from the World Health Survey. PLoS One. 2012;7:e42843. doi: 10.1371/journal.pone.0042843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badruddin S, Molla A, Khurshid M, Vaz S, Hassanali S. Cardiovascular risk factors in school children from low middle income families in Karachi, Pakistan. J Pak Med Assoc. 1994;44:106–112. [PubMed] [Google Scholar]
- 25.Bunker CH, Ukoli FA, Okoro FI. Correlates of serum lipids in a lean black population. Atherosclerosis. 1996;123:215–225. doi: 10.1016/0021-9150(96)05810-8. [DOI] [PubMed] [Google Scholar]
- 26.Narayan KV, Chadha S, Hanson R. Prevalence and patterns of smoking in Delhi: cross sectional study. BMJ. 1996;312:1576–1579. doi: 10.1136/bmj.312.7046.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor O, Oyediran O, Bamgboye A, Afolabi B, Osuntokun B. Profile of some risk factors for coronary heart disease in a developing country: Nigeria. Afr J Med Med Sci. 1996;25:341–346. [PubMed] [Google Scholar]
- 28.Rahlenbeck S, Gebre-Yohannes A. Cardiovascular risk factors in ethiopian medical students. Ann Biol Clin (Paris) 1997;56:705–709. (in French). [PubMed] [Google Scholar]
- 29.Singh RB, Niaz MA, Thakur AS, Janus ED, Moshiri M. Social class and coronary artery disease in a urban population of North India in the Indian Lifestyle and Heart Study. Int J Cardiol. 1998;64:195–203. doi: 10.1016/s0167-5273(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 30.Mehta P, Shringarpure B. Diet nutrition and health profile of elderly population of urban Baroda. Indian J Public Health. 1999;44:124–128. [PubMed] [Google Scholar]
- 31.Sossa C, Delisle H, Agueh V, Sodjinou R, Ntandou G, Makoutode M. Lifestyle and dietary factors associated with the evolution of cardiometabolic risk over four years in west-African adults: the Benin study. J Obes. 2013;2013:298024. doi: 10.1155/2013/298024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deepa M, Anjana RM, Manjula D, Narayan KV, Mohan V. Convergence of prevalence rates of diabetes and cardiometabolic risk factors in middle and low income groups in urban India: 10-year follow-up of the Chennai Urban Population Study. J Diabetes Sci Technol. 2011;5:918–927. doi: 10.1177/193229681100500415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Channa NA, Khan A. Hazards of Mainpuri chewing in Hyderabad and adjoining areas, Pakistan. RMJ. 2014;39:216–219. [Google Scholar]
- 34.Hashibe M, Jacob BJ, Thomas G. Socioeconomic status, lifestyle factors and oral premalignant lesions. Oral Oncol. 2003;39:664–671. doi: 10.1016/s1368-8375(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 35.Cubbins LA, Kasprzyk D, Montano D, Jordan LP, Woelk G. Alcohol use and abuse among rural Zimbabwean adults: a test of a community-level intervention. Drug Alcohol Depend. 2012;124:333–339. doi: 10.1016/j.drugalcdep.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.WHO . WHO STEPS Survey: Eritrea. World Health Organization; Geneva: 2004. [Google Scholar]
- 37.WHO . WHO STEPS Survey: Cote d'Iviore. World Health Organization; Geneva: 2005. [Google Scholar]
- 38.WHO . WHO STEPS Survey: India. World Health Organization; Geneva: 2007. [Google Scholar]
- 39.WHO . WHO STEPS Survey: Zambia. World Health Organization; Geneva: 2008. [Google Scholar]
- 40.WHO . WHO STEPS Survey: Togo. World Health Organization; Geneva: 2010. [Google Scholar]
- 41.Kinra S, Bowen LJ, Lyngdoh T. Sociodemographic patterning of non-communicable disease risk factors in rural India: a cross sectional study. BMJ. 2010;341:c4974. doi: 10.1136/bmj.c4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta R, Gupta V, Sarna M, Prakash H, Rastogi S, Gupta K. Serial epidemiological surveys in an urban Indian population demonstrate increasing coronary risk factors among the lower socioeconomic strata. J Assoc Physicians India. 2003;51:470–478. [PubMed] [Google Scholar]
- 43.Gupta R, Deedwania PC, Sharma K. Association of educational, occupational and socioeconomic status with cardiovascular risk factors in Asian Indians: a cross-sectional study. PLoS One. 2012;7:e44098. doi: 10.1371/journal.pone.0044098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh R, Sharma J, Rastogi V. Social class and coronary disease in a rural population of north India. Eur Heart J. 1997;18:588–595. doi: 10.1093/oxfordjournals.eurheartj.a015301. [DOI] [PubMed] [Google Scholar]
- 45.Zaman MJ, Patel A, Jan S. Socio-economic distribution of cardiovascular risk factors and knowledge in rural India. Int J Epidemiol. 2012;41:1302–1314. doi: 10.1093/ije/dyr226. [DOI] [PubMed] [Google Scholar]
- 46.Reddy KS, Prabhakaran D, Jeemon P. Educational status and cardiovascular risk profile in Indians. Proc Natl Acad Sci USA. 2007;104:16263–16268. doi: 10.1073/pnas.0700933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kar S, Thakur J, Virdi N, Jain S, Kumar R. Risk factors for cardiovascular diseases: is the social gradient reversing in northern India? Natl Med J India. 2010;23:206–209. [PubMed] [Google Scholar]
- 48.Anjana RM, Pradeepa R, Das AK. Physical activity and inactivity patterns in India—results from the ICMR-INDIAB study (phase-1)[ICMR-INDIAB-5] Int J Behav Nutr Phys Act. 2014;11:26. doi: 10.1186/1479-5868-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Safraj S, Anish T, Vijayakumar K, Kutty VR, Soman CR. Socioeconomic position and prevalence of self-reported diabetes in rural Kerala, India results from the PROLIFE study. Asia Pac J Public Health. 2012;24:480–486. doi: 10.1177/1010539510387822. [DOI] [PubMed] [Google Scholar]
- 50.Dewi FS, Stenlund H, Ohman A, Hakimi M, Weinehall L. Mobilising a disadvantaged community for a cardiovascular intervention: designing PRORIVA in Yogyakarta, Indonesia. Glob Health Action. 2010 doi: 10.3402/gha.v3i0.4661. published online July 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mumu S, Saleh F, Ara F, Afnan F, Ali L. Non-adherence to life-style modification and its factors among type 2 diabetic patients. Indian J Public Health. 2014;58:40. doi: 10.4103/0019-557X.128165. [DOI] [PubMed] [Google Scholar]
- 52.Dhungana RR, Devkota S, Khanal MK. Prevalence of cardiovascular health risk behaviors in a remote rural community of Sindhuli district, Nepal. BMC Cardiovasc Disord. 2014;14:92. doi: 10.1186/1471-2261-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vaidya A, Krettek A. Physical activity level and its sociodemographic correlates in a peri-urban Nepalese population: a cross-sectional study from the Jhaukhel-Duwakot health demographic surveillance site. Int J Behav Nutr Phys Act. 2014;11:39. doi: 10.1186/1479-5868-11-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katulanda P, Jayawardana R, Ranasinghe P, Rezvi Sheriff M, Matthews DR. Physical activity patterns and correlates among adults from a developing country: the Sri Lanka diabetes and cardiovascular study. Public Health Nutr. 2013;16:1684–1692. doi: 10.1017/S1368980012003990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oyewole OO, Odusan O, Oritogun KS, Idowu AO. Physical activity among type-2 diabetic adult Nigerians. Ann Afr Med. 2014;13:189. doi: 10.4103/1596-3519.142290. [DOI] [PubMed] [Google Scholar]
- 56.Zeba AN, Delisle HF, Renier G. Dietary patterns and physical inactivity, two contributing factors to the double burden of malnutrition among adults in Burkina Faso, west Africa. J Nutr Sci. 2014;3:e50. doi: 10.1017/jns.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trinh OTH, Nguyen ND, Dibley MJ, Phongsavan P, Bauman AE. The prevalence and correlates of physical inactivity among adults in Ho Chi Minh City. BMC Public Health. 2008;8:204. doi: 10.1186/1471-2458-8-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hosey GM, Samo M, Gregg EW, Padden D, Bibb SG. Socioeconomic and demographic predictors of selected cardiovascular risk factors among adults living in Pohnpei, Federated States of Micronesia. BMC Public Health. 2014;14:895. doi: 10.1186/1471-2458-14-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Abd-Elhady AS, El-Sadek A. degree of compliance towards therapeutic tasks among diabetic patients attending a health insurance setting in Cairo. Egypt J Hosp Med. 2007;27:234–244. [Google Scholar]
- 60.Al Ali R, Rastam S, Fouad FM, Mzayek F, Maziak W. Modifiable cardiovascular risk factors among adults in Aleppo, Syria. Int J Public Health. 2011;56:653–662. doi: 10.1007/s00038-011-0278-0. [DOI] [PubMed] [Google Scholar]
- 61.Anjana RM, Ranjani H, Unnikrishnan R, Weber MB, Mohan V, Narayan KM. Exercise patterns and behaviour in Asian Indians: data from the baseline survey of the Diabetes Community Lifestyle Improvement Program (D-CLIP) Diabetes Res Clin Pract. 2015;107:77–84. doi: 10.1016/j.diabres.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 62.Booth ML, Ainsworth BE, Pratt M. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1396. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 63.WHO . Global physical activity questionnaire (GPAQ) analysis guide. World Health Organization; Geneva: 2012. [Google Scholar]
- 64.WHO . Global Recommendations on physical activity for health: 18–64 years old. WHO; Geneva: 2011. [Google Scholar]
- 65.Singh RB, Beegom R, Verma SP. Association of dietary factors and other coronary risk factors with social class in women in five Indian cities. Asia Pac J Clin Nutr. 2000;9:298–302. doi: 10.1046/j.1440-6047.2000.00177.x. [DOI] [PubMed] [Google Scholar]
- 66.Bonu S, Rani M, Peters DH, Jha P, Nguyen SN. Does use of tobacco or alcohol contribute to impoverishment from hospitalization costs in India? Health Policy Plan. 2005;20:41–49. doi: 10.1093/heapol/czi005. [DOI] [PubMed] [Google Scholar]
- 67.Samuel P, Antonisamy B, Raghupathy P, Richard J, Fall CH. Socio-economic status and cardiovascular risk factors in rural and urban areas of Vellore, Tamilnadu, south India. Int J Epidemiol. 2012;41:1315–1327. doi: 10.1093/ije/dys001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menon J, Vijayakumar N, Joseph JK. Below the poverty line and non-communicable diseases in Kerala: the Epidemiology of Non-communicable Diseases in Rural Areas (ENDIRA) study. Int J Cardiol. 2015;187:519–524. doi: 10.1016/j.ijcard.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 69.Pillai A, Nayak MB, Greenfield TK, Bond JC, Nadkarni A, Patel V. Patterns of alcohol use, their correlates, and impact in male drinkers: a population-based survey from Goa, India. Soc Psychiatry Psychiatr Epidemiol. 2013;48:275–282. doi: 10.1007/s00127-012-0538-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Subramanian S, Nandy S, Irving M, Gordon D, Smith GD. Role of socioeconomic markers and state prohibition policy in predicting alcohol consumption among men and women in India: a multilevel statistical analysis. Bull World Health Organ. 2005;83:829–836. [PMC free article] [PubMed] [Google Scholar]
- 71.Neufeld KJ, Peters DH, Rani M, Bonu S, Brooner RK. Regular use of alcohol and tobacco in India and its association with age, gender, and poverty. Drug Alcohol Depend. 2005;77:283–291. doi: 10.1016/j.drugalcdep.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 72.Houehanou YC, Lacroix P, Mizehoun GC, Preux PM, Marin B, Houinato DS. Magnitude of cardiovascular risk factors in rural and urban areas in Benin: findings from a nationwide steps survey. PLoS One. 2015;10:e0126441. doi: 10.1371/journal.pone.0126441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laux TS, Bert PJ, González M, Unruh M, Aragon A, Lacourt CT. Prevalence of obesity, tobacco use, and alcohol consumption by socioeconomic status among six communities in Nicaragua. Rev Panam Salud Publica. 2012;32:217–225. doi: 10.1590/s1020-49892012000900007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agrawal S, Millett C, Subramanian S, Ebrahim S. Frequency of Fish Intake and Diabetes among Adult Indians. J Am Coll Nutr. 2014;33:215–230. doi: 10.1080/07315724.2013.867420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Agrawal S, Millett CJ, Dhillon PK, Subramanian S, Ebrahim S. Type of vegetarian diet, obesity and diabetes in adult Indian population. Nutr J. 2014;13:89. doi: 10.1186/1475-2891-13-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ganesan S, Raman R, Kulothungan V, Sharma T. Influence of dietary–fibre intake on diabetes and diabetic retinopathy: Sankara Nethralaya—diabetic retinopathy epidemiology and molecular genetic study (report 26) Clin Exp Ophthalmol. 2012;40:288–294. doi: 10.1111/j.1442-9071.2011.02594.x. [DOI] [PubMed] [Google Scholar]
- 77.Radhika G, Sathya R, Sudha V, Ganesan A, Mohan V. Dietary salt intake and hypertension in an urban south Indian population—[CURES-53] J Assoc Physicians India. 2007;55:405–411. [PubMed] [Google Scholar]
- 78.Babalola D, Makinde Y, Afodu J. Nutrition transition and indicators of hypertension among farming households in nigeria: evidence from ikenne local government area of ogun state. Can J Pure App Sci. 2009;5:1349–1353. [Google Scholar]
- 79.Delisle H, Ntandou-Bouzitou G, Agueh V, Sodjinou R, Fayomi B. Urbanisation, nutrition transition and cardiometabolic risk: the Benin study. Br J Nutr. 2012;107:1534–1544. doi: 10.1017/S0007114511004661. [DOI] [PubMed] [Google Scholar]
- 80.Nwamarah JU, Otitoju GTO. Fruit and vegetable consumption pattern and health challenges of elderly (≥ 60 years) staff in the University of Nigeria, Nsukka and Enugu campuses: a case study. Pak J Nutr. 2014;13:626–630. [Google Scholar]
- 81.Delisle H, Agueh V, Fayomi B. Partnership research on nutrition transition and chronic diseases in West Africa—trends, outcomes and impacts. BMC Int Health Hum Rights. 2011;11(suppl 2):S10. doi: 10.1186/1472-698X-11-S2-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jindal S, Aggarwal A, Chaudhry K. Tobacco smoking in India: prevalence, quit-rates and respiratory morbidity. Indian J Chest Dis Allied Sci. 2006;48:37. [PubMed] [Google Scholar]
- 83.Corsi DJ, Subramanian SV, Lear SA. Tobacco use, smoking quit rates, and socioeconomic patterning among men and women: a cross-sectional survey in rural Andhra Pradesh, India. Eur J Prev Cardiol. 2014;21:1308–1318. doi: 10.1177/2047487313491356. [DOI] [PubMed] [Google Scholar]
- 84.Dixit AM, Jain PK, Agarwal R, Gupta S, Shukla SK, Rani V. Prevalence and pattern of tobacco use in rural community of Jaipur, Rajasthan (India): a cross sectional study. Natl J Commun Med. 2015;6:16–20. [Google Scholar]
- 85.Singh RB, Singh S, Chattopadhya P. Tobacco consumption in relation to causes of death in an urban population of north India. Int J Chron Obstruct Pulmon Dis. 2007;2:177. [PMC free article] [PubMed] [Google Scholar]
- 86.Lal P, Nair S. Socio-economic influence on tobacco use among male youth in Kerala. Health Popul Perspect Issues. 2012;35:47–60. [Google Scholar]
- 87.Gupta R, Sharma KK, Gupta BK, Gupta A, Gupta RR, Deedwania PC. Educational status-related disparities in awareness, treatment and control of cardiovascular risk factors in India. Heart Asia. 2015;7:1–6. doi: 10.1136/heartasia-2014-010551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jena PK, Kishore J. Prevalence and correlates of hardcore smoking in India. Res Rev J Med. 2012;2:16–24. [Google Scholar]
- 89.Rani M, Bonu S, Jha P, Nguyen S, Jamjoum L. Tobacco use in India: prevalence and predictors of smoking and chewing in a national cross sectional household survey. Tob Control. 2003;12:e4. doi: 10.1136/tc.12.4.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heck JE, Marcotte EL, Argos M. Betel quid chewing in rural Bangladesh: prevalence, predictors and relationship to blood pressure. Int J Epidemiol. 2012;41:462–471. doi: 10.1093/ije/dyr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goon S, Bipasha MS. Prevalence and pattern of smoking among bus drivers of Dhaka, Bangladesh. Tob Use Insights. 2014;7:21–25. doi: 10.4137/TUI.S13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zaman MM, Bhuiyan MR, Huq SM, Rahman MM, Sinha DN, Fernando T. Dual use of tobacco among Bangladeshi men. Indian J Cancer. 2014;51(suppl 1):S46–S49. doi: 10.4103/0019-509X.147481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chawla R, Sathian B, Mehra A, Kiyawat V, Garg A, Sharma K. Awareness and assessment of risk factors for lung cancer in residents of Pokhara valley, Nepal. Asian Pac J Cancer Prev. 2010;11:1789–1793. [PubMed] [Google Scholar]
- 94.Bovet P, Ross AG, Gervasoni J-P. Distribution of blood pressure, body mass index and smoking habits in the urban population of Dar es Salaam, Tanzania, and associations with socioeconomic status. Int J Epidemiol. 2002;31:240–247. doi: 10.1093/ije/31.1.240. [DOI] [PubMed] [Google Scholar]
- 95.Kebede Y. Cigarette smoking and khat chewing among university instructors in Ethiopia. East Afr Med J. 2002;79:274–278. doi: 10.4314/eamj.v79i5.8869. [DOI] [PubMed] [Google Scholar]
- 96.Owusu-Dabo E, Lewis S, McNeill A, Gilmore A, Britton J. Smoking uptake and prevalence in Ghana. Tob Control. 2009;18:365–370. doi: 10.1136/tc.2009.030635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Minh H, Byass P, Dao LH, Nguyen T, Wall S. Risk factors for chronic disease among rural Vietnamese adults and the association of these factors with sociodemographic variables: findings from the WHO STEPS survey in rural Vietnam, 2005. Prev Chronic Dis. 2007;4:A22. [PMC free article] [PubMed] [Google Scholar]
- 98.Tonstad S, Job JS, Batech M, Yel D, Kheam T, Singh PN. Adult tobacco cessation in Cambodia: I. Determinants of quitting tobacco use. Asia Pac J Public Health. 2013;25(5 suppl):10S–19S. doi: 10.1177/1010539512451853. [DOI] [PubMed] [Google Scholar]
- 99.Ahmad K, Jafary F, Jehan I. Prevalence and predictors of smoking in Pakistan: results of the National Health Survey of Pakistan. Eur J Cardiovasc Prev Rehabil. 2005;12:203–208. doi: 10.1097/S1741-82670312303-1. [DOI] [PubMed] [Google Scholar]
- 100.Ali S, Sathiakumar N, Delzell E. Prevalence and socio-demographic factors associated with tobacco smoking among adult males in rural Sindh, Pakistan. Southeast Asian J Trop Med Public Health. 2006;37:1054–1060. [PubMed] [Google Scholar]
- 101.Emery S, Gilpin EA, Ake C, Farkas AJ, Pierce JP. Characterizing and identifying” hard-core” smokers: implications for further reducing smoking prevalence. Am J Public Health. 2000;90:387. doi: 10.2105/ajph.90.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Soc Sci Med. 1997;44:809–819. doi: 10.1016/s0277-9536(96)00191-8. [DOI] [PubMed] [Google Scholar]
- 103.Mathur C, Stigler MH, Perry CL, Arora M, Reddy KS. Differences in prevalence of tobacco use among Indian urban youth: the role of socioeconomic status. Nicotine Tob Res. 2008;10:109–116. doi: 10.1080/14622200701767779. [DOI] [PubMed] [Google Scholar]
- 104.Bauld L, Judge K, Platt S. Assessing the impact of smoking cessation services on reducing health inequalities in England: observational study. Tob Control. 2007;16:400–404. doi: 10.1136/tc.2007.021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jha P, Peto R, Zatonski W, Boreham J, Jarvis MJ, Lopez AD. Social inequalities in male mortality, and in male mortality from smoking: indirect estimation from national death rates in England and Wales, Poland, and North America. Lancet. 2006;368:367–370. doi: 10.1016/S0140-6736(06)68975-7. [DOI] [PubMed] [Google Scholar]
- 106.WHO . Global status report on alcohol and health—2014. World Health Organization; Geneva: 2014. [Google Scholar]
- 107.Grittner U, Kuntsche S, Graham K, Bloomfield K. Social inequalities and gender differences in the experience of alcohol-related problems. Alcohol Alcohol. 2012;47:597–605. doi: 10.1093/alcalc/ags040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Irala-Estevez J, Groth M, Johansson L, Oltersdorf U, Prattala R, Martínez-González MA. A systematic review of socio-economic differences in food habits in Europe: consumption of fruit and vegetables. Eur J Clin Nutr. 2000;54:706–714. doi: 10.1038/sj.ejcn.1601080. [DOI] [PubMed] [Google Scholar]
- 109.Lallukka T, Laaksonen M, Rahkonen O, Roos E, Lahelma E. Multiple socio-economic circumstances and healthy food habits. Eur J Clin Nutr. 2007;61:701–710. doi: 10.1038/sj.ejcn.1602583. [DOI] [PubMed] [Google Scholar]
- 110.Darmon N, Drewnowski A. Does social class predict diet quality? Am J Clin Nutr. 2008;87:1107–1117. doi: 10.1093/ajcn/87.5.1107. [DOI] [PubMed] [Google Scholar]
- 111.Giskes K, Avendaňo M, Brug J, Kunst A. A systematic review of studies on socioeconomic inequalities in dietary intakes associated with weight gain and overweight/obesity conducted among European adults. Obes Rev. 2010;11:413–429. doi: 10.1111/j.1467-789X.2009.00658.x. [DOI] [PubMed] [Google Scholar]
- 112.Thornton LE, Bentley RJ, Kavanagh AM. Individual and area-level socioeconomic associations with fast food purchasing. J Epidemiol Community Health. 2011;65:873–880. doi: 10.1136/jech.2009.099614. [DOI] [PubMed] [Google Scholar]
- 113.Pechey R, Monsivais P, Ng Y-L, Marteau TM. Why don't poor men eat fruit? Socioeconomic differences in motivations for fruit consumption. Appetite. 2015;84:271–279. doi: 10.1016/j.appet.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maguire ER, Monsivais P. Socio-economic dietary inequalities in UK adults: an updated picture of key food groups and nutrients from national surveillance data. Br J Nutr. 2015;113:181–189. doi: 10.1017/S0007114514002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Turrell G, Vandevijvere S. Socio-economic inequalities in diet and body weight: evidence, causes and intervention options. Public Health Nutr. 2015;18:759–763. doi: 10.1017/S1368980015000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mayén A-L, Marques-Vidal P, Paccaud F, Bovet P, Stringhini S. Socioeconomic determinants of dietary patterns in low- and middle-income countries: a systematic review. Am J Clin Nutr. 2014:1520–1531. doi: 10.3945/ajcn.114.089029. [DOI] [PubMed] [Google Scholar]
- 117.Drenowatz C, Eisenmann JC, Pfeiffer KA. Influence of socio-economic status on habitual physical activity and sedentary behavior in 8- to 11-year old children. BMC Public Health. 2010;10:214. doi: 10.1186/1471-2458-10-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Elhakeem A, Cooper R, Bann D, Hardy R. Childhood socioeconomic position and adult leisure-time physical activity: a systematic review. Int J Behav Nutr Phys Act. 2015;12:1–27. doi: 10.1186/s12966-015-0250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Stalsberg R, Pedersen AV. Effects of socioeconomic status on the physical activity in adolescents: a systematic review of the evidence. Scand J Med Sci Sports. 2010;20:368–383. doi: 10.1111/j.1600-0838.2009.01047.x. [DOI] [PubMed] [Google Scholar]
- 120.Gidlow C, Johnston LH, Crone D, Ellis N, James D. A systematic review of the relationship between socio-economic position and physical activity. Health Educ J. 2006;65:338–367. [Google Scholar]
- 121.Sallis JF, Bull F, Guthold R, Heath GW. Progress in physical activity over the Olympic quadrennium. Lancet. 2016;388:1325–1336. doi: 10.1016/S0140-6736(16)30581-5. [DOI] [PubMed] [Google Scholar]
- 122.Monda KL, Gordon-Larsen P, Stevens J, Popkin BM. China's transition: the effect of rapid urbanization on adult occupational physical activity. Soc Sci Med. 2007;64:858–870. doi: 10.1016/j.socscimed.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Samitz G, Egger M, Zwahlen M. Domains of physical activity and all-cause mortality: systematic review and dose–response meta-analysis of cohort studies. Int J Epidemiol. 2011;40:1382–1400. doi: 10.1093/ije/dyr112. [DOI] [PubMed] [Google Scholar]
- 124.Sachs JD. Macroeconomics and health: investing in health for economic development. Rev Panam Salud Publica. 2002;12:143–144. [Google Scholar]
- 125.Sen A. Development as freedom. Oxford University Press; Oxford: 2001. [Google Scholar]
- 126.Wagstaff A. Poverty and health sector inequalities. Bull World Health Organ. 2002;80:97–105. [PMC free article] [PubMed] [Google Scholar]
- 127.Pampel FC, Krueger PM, Denney JT. Socioeconomic disparities in health behaviors. Annu Rev Sociol. 2010;36:349. doi: 10.1146/annurev.soc.012809.102529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.World Health Organization NCD Summary Report. 2011. http://www.who.int/nmh/publications/ncd_report_summary_en.pdf (accessed July 30, 2015).
- 129.UK International Development Committee . Minutes of Evidence—HC 334. The Stationary Office; London: 2013. [Google Scholar]
- 130.World Health Organization STEPS Country Reports. 2015. http://www.who.int/chp/steps/reports/en/ (accessed July 30, 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.