Abstract
Objective(s):
Low penetration depth of light is the main defect of photodynamic therapy (PDT), which could be improved by sonodynamic therapy (SDT). In this study, a combination of PDT and SDT known as sonophotodynamic therapy (SPDT) was investigated using two reverse arrangements in CT26 tumor model.
Materials and Methods:
The liposomal zinc phthalocyanine was synthesized and characterized. It was then administered to CT26 tumor models as a sensitizer. The animal models were subjected to PDT, SDT, and the combined treatment in different groups. The doubling time for the survival of tumors and animals was considered as a measure to evaluate treatments efficacy.
Results:
In all treatment groups there was a significant decline in tumor volume 15 days after treatment compared to the main control group, but the optimum response was observed in the group receiving a combined treatment with the priority of PDT. 120 days after treatment, in the groups treated by PDT and SDT, the tumor shrank by 20%, while in the group receiving SPDT with PDT priority, 80% of tumors was recovered. No case of complete tumor progression was observed in SPDT group with SDT priority. This could be due to the pores created in cell membranes during ultrasound irradiation of the tumor, which removed the sensitizer molecules from the cells and reduced PDT efficacy in SPDT group with SDT priority.
Conclusion:
It seems that SPDT with PDT priority offers a more efficient alternative than each of PDT, SDT individually or SPDT with the reverse arrangement.
Keywords: Sonophotodynamic –therapy, Photodynamic –herapy, Sonodynamic –therapy, Liposomal zinc, Phthalocyanine, Colon carcinoma
Introduction
Photodynamic and sonodynamic therapies are two minimally non-invasive methods to treat available malignancies (1). In photodynamic therapy, after inserting a proper dye (photosensitizer) in the body, the tumor is irradiated with visible light of appropriate wavelength. The due to excitation of the photosensitizing material and production of reactive oxygen species; cell death begins (2). One limitation of this technique is low penetration depth of visible light in tissues, which is well-suited for superficial and thin tumors (3). On the other hand, most photosensitizing agents are sensitive to ultrasound (US) waves. Stimulated by ultrasound, these agents can cause necrosis and cell death. This process is known as sonodynamic therapy (4). It is expected that a combination of PDT and SDT improves the efficacy of treatments by applying a proper dose of agents that are sensitive to light and ultrasound, so that the thickness or depth of treatment is increased (5). The approach uses a combination of two treatments called sonophotodynamic therapy (SPDT).
At the present, photodynamic therapy is conventiona-lly used in many countries including Russia, Britain and Italy (6). Also, some in vivo and in vitro studies on sonodynamic effects have been conducted (7-12).
Previous pre-clinical and clinical studies have shown potential antineoplastic effect of SPDT against a variety of malignancies (1, 13-15). According to some studies, utilizing this combined therapy induces more obvious anti-cancer effects in comparison with individual treatments. SPDT can reduce the necessary dosage of sensitizer and energy of ultrasound or light as activators (14, 15).
Today, sonophotodynamic therapy was used in many clinics. In England, this treatment has been applied to 115 patients over 4 years in Dove Clinic, with the results indicating the effectiveness of this treatment for primary and metastatic tumors (13).
In 2010, Sazgarnia et al. studied SPDT effect on CT26 tumor model mice BALB/c using the liposomal phthalocyanine. They reported the effect of individual treatments (sono or photodynamic therapy) and SPDT treatment in slowing tumor growth velocity. However, combined therapy with priority of sonodynamic did not yield improved results compared to individual treatments (1). Therefore, an arguable assumption is the possibility of removing sensitizer from the tumor tissue during ultrasound irradiation. It can in turn lessen the effectiveness of the photodynamic therapy (1).
The present study adopted a combined therapy of sonophotodynamic to examine the importance of irradiation sequence.
Materials and Methods
Chemicals and preparation of liposomal zinc phthalocyanine (ZnPc)
Zinc phthalocyanine (ZnPc) was obtained from Sigma-Aldrich (97% dye content). To prepare the liposomal zinc phthalocyanine, 300 mg of egg lecithin, 100 mg of cholesterol, 400 mg of glucose and zinc phthalocyanine powder were dissolved in 10 ml of Pyridine. The solution was frozen by dry ice and then processed by the freeze dryer (Labco Co-USA) (16). The estimated ZnPc encapsulation rate was more than 85%. The liposomes’ size distribution was recorded by a particle size analyzer (Zeta sizer, nano-ZS model, Malvern Ins., USA) and its UV-visible absorption spectrum was also obtained by a UV-visible spectrophotometer (UV 1700, Shimadzu Corp, Japan).
Cell line and culture conditions
CT26 cell line derived from a tumor colon carcinoma of a BALB/c mouse, provided by Iranian Pasteur Institute, were grown in RPMI-1640 culture medium supplemented with 10% (v/v) fetal bovine serum (FBS) at 37 °C in a 5% CO2 humidified incubator. After cells covering the bottom of the flask as a monolayer, they were trypsinized using 0.05% trypsin-EDTA. The cell number and survival rate were determined using a hemocytometer, trypan blue and a light microscope.
Tumor models
Male and female BALB/c mice, 6-8 weeks of age and weighting 20-22 g were obtained from Iranian Pasteur Institute. The mice were preserved in an animal house at 23 ± 2°C with 65% moisture and 12 hrs of alternative darkness and brightness. To create the colon carcinoma tumor model, 5×105 CT26 cells per mouse were injected subcutaneously in the right dorsal animals (1).
Animal anesthesia
The mice were anesthetized before the exposure to ultrasound and/or light using the intraperitoneal injection of ketamine hydrochloride (60 mg/kg), and Xylazine2% (6 mg/kg) (14).
PDT and SDT instruments
Tumor models were irradiated by a non-coherent light source, i.e. a Lumacare LC122-A (Ci-Tec, USA) equipped with a light probe with 8 mm spot diameter and 5% light homogeneity. It consisted of a band-pass filter at wavelengths of 670±20 nm. Light power density was 160 mW/cm2 and a total exposed light of 300 J/cm2 was considered (13). Radiation parameters were assessed by a radiometer (CON-TROL-CURE IL1400; UVPROCESS, USA).
Ultrasound irradiation was conducted by a 215A ultrasound generator in continuous mode with a frequency of 1.1 MHz and intensity (spatial average temporal average; SATA) of 1 W/cm2 for 10 mins (15). The ultrasound transducer with a surface area of 7.0 cm2 was horizontally submerged at the bottom of a chamber filled with degassed water to provide vertical exposure over the tumor. Acoustic calibration of the device power was performed by an ultrasound balance power meter (UPM 2000, Netech, USA).
Experimental protocols
With the tumors growing to a diameter of about 5 mm, animals were randomly divided into 9 groups and treatment protocols were applied. Each group consisted of 10 mice (5 males and 5 females). 1.46 µM/kg liposomal zinc phthalocyanine was intraperito-neally injected into five animal groups (17). After 24 hr (7), different tumor groups were irradiated by light, ultrasound or a combination of both. The sequence of light and ultrasound reception were inverted in groups of 8 and 9. Light and ultrasound were applied immediately burst to the combined group. In the control group, normal saline was injected instead of photo-sensitizer. The therapeutic protocols were followed using daily measurement of tumor dimensions by a digital caliper (small diameter: a; large diameter: b and tumor thickness: c). Tumor volume (V) was calculated as V= π/6 [a.b.c] (15). The measurements were sustained until the death of animals, which was recorded as an event.
Evaluation parameters for treatment efficacy and statistical analysis
For each tumor, the first day of treatment was considered as day zero and relative tumor volume in the following days were normalized accordingly. Based on the daily variations of the relative tumor volume, the doubling time of each tumor was determined and estimated in each group.
Following the normality test and selection of proper comparative tests, all data were analyzed using SPSS 11.5. According to Kolmogorov-Smirnov test, data distribution was abnormal. Thus, the Mann-Whitney test was used to compare relative tumor volume at a confidence level of 95%. Also, the doubling time of tumors was compared across different groups using t-test.
Results
Characterization of liposomal zinc phthalocyanine
Liposomal particles size distribution was estima-ted between 20 and 100 nm with an average size of 40 nm (z-average = 82.4 nm; PDI=0.261) as shown in Figure 1. The main absorption peak of the liposomal zinc phthalocyanine was recorded at a wavelength of 670 nm (Figure 2).
Figure 1.
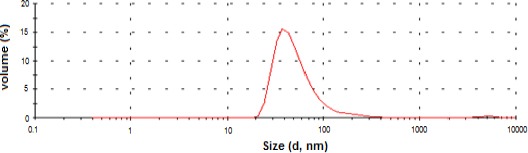
Size distribution of synthesized liposomal zinc phthalocyanine
Figure 2.
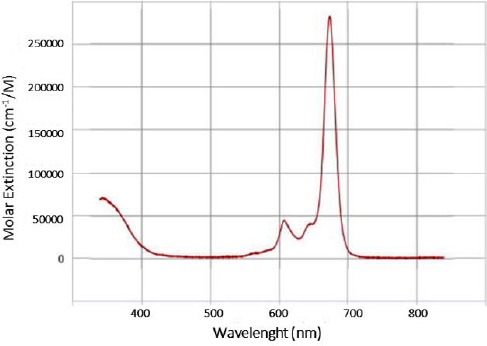
Absorbance spectrum of liposomal zinc phthalocyanine
Experimental treatments
Treatment results of different groups were evalua-ted on the basis of the relative tumor volume at the first day after treatment, mathematical function of relative tumor volume versus day, and the doubling time of tumor volume at the different groups. The relative tumor volume variations are shown in Figure 3. In all groups, the growth of CT26 tumor was significantly inhibited 15 days after treatment compared to the control group (P≤0.05). The optimum treatment response was observed in the SPDT group with the priority of photodynamic therapy. This group was significantly different from all treatment groups (P<0.01).
Figure 3.
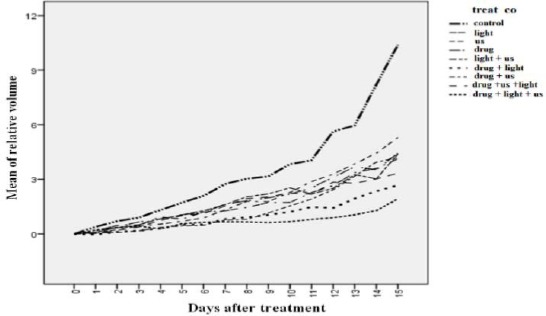
Relative variations of tumor volume in different groups for the first 15 days post treatment.
drug: liposomal zinc phthalocyanine (1.46 µM/kg)
Us: Ultrasound irradiation was performed in continuous mode with a frequency of 1.1 MHz and intensity (spatial average temporal average; SATA) of 1 W/cm2 for 10 min
After this group, the best response belonged to groups treated with SDT or PDT. The two groups were no significantly difference in this regard.
During the study (120 days), groups treated by SDT or PDT revealed only 20% tumor shrinkage, while in the group receiving SPDT with light priority, 80% of tumors was recovered. Also, no case of complete tumor progression was observed in other groups.
The doubling time of tumors for different groups is shown in Figure 4. The longest doubling times was observed in SPDT group with priority of light. The shortest doubling time of the tumors was found in the control group.
Figure 4.
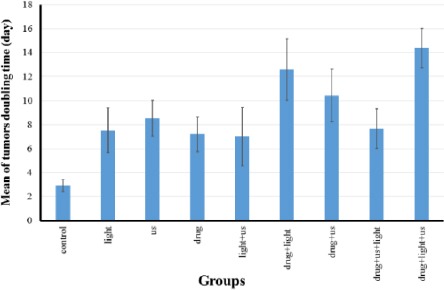
Mean of tumors’ doubling time ± standard deviation in the various groups.
drug: liposomal zinc phthalocyanine (1.46 µM/kg)
Us: Ultrasound irradiation was performed in continuous mode with a frequency of 1.1 MHz and intensity (spatial average temporal average; SATA) of 1 W/cm2 for 10 min
The survival fraction in various groups is presented in Figure 5.
Figure 5.
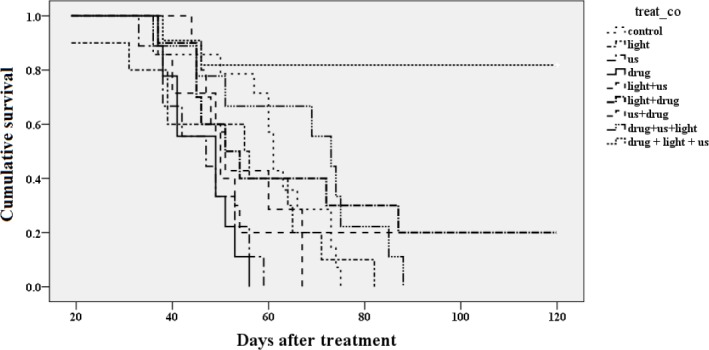
Variations of cumulative survival fraction in different treatment groups versus day based on Kaplan-Meier calculations
drug: liposomal zinc phthalocyanine (1.46 µM/kg)
Us: Ultrasound irradiation was performed in continuous mode with a frequency of 1.1 MHz and intensity (spatial average temporal average; SATA) of 1 W/cm2 for 10 min
The highest survival rate was achieved in the SPDT group irradiated with light and ultrasound, respectively. In this group, survival rate increase was more significant than other groups (P<0.01), as shown in Table 1. The next highest survival rates belonged to groups receiving PDT, SDT and the combined treatment with ultrasound priority respectively. As expected, these groups were not significantly different than the control group (P>0.05).
Table 1.
P-values obtained from the equality statistical test of survival distributions for the different treatment group (based on Kaplan-Meier calculations); Drug: liposomal zinc phthalocyanine (1.46 µM/kg).
| Control | Light | US | Drug | Light+US | Light+Drug | US+Drug | Drug + US + light | |
|---|---|---|---|---|---|---|---|---|
| Light | 0.62 | |||||||
| US | 0.00 | 0.10 | ||||||
| Drug | 0.00 | 0.09 | 0.67 | |||||
| Light+US | 0.19 | 0.74 | 0.12 | 0.12 | ||||
| Light+Drug | 0.47 | 0.22 | 0.08 | 0.06 | 0.26 | |||
| US+Drug | 0.7 | 0.75 | 0.24 | 0.19 | 0.81 | 0.83 | ||
| Drug+US+Light | 0.10 | 0.08 | 0.08 | 0.08 | 0.03 | 0.72 | 0.87 | |
| Drug+Light+US | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 |
Us: Ultrasound irradiation was performed in continuous mode with a frequency of 1.1 MHz and intensity (spatial average temporal average; SATA) of 1 W/cm2 for 10 mins.
Discussion
SPDT combines ultrasonic and light irradiations with toxicity induced by a photo and sono- sensitizing drug. The activated sensitizer generates this lethality via apoptosis and necrosis processes which can damage malignant cells selectively. Besides enjoying the features of PDT, SPDT overcome one main limitation of PDT, namely low penetration depth. Several key factors should be optimized to obtain successful SPDT, including the sensitizer structure and dosage, interval between drug administrative and exposure, specifications of ultrasound and light, and the sequence of ultrasound and light exposure, among other things. In the previous study, we evaluated the therapeutic effect of SPDT with SDT priority, but the combined treatment was not significantly better than each treatment individually (1). Therefore, we proposed the withdrawal of drug from tumor tissue during ultrasound irradiation. For this reason, the present study explored variation in the sequence of light and sound irradiation. The optimum response to treatment was observed in SPDT group with PDT priority. In this context, the doubling time and percentage of tumor as well as relative tumor volume were greater than groups receiving each treatments individually. In the group receiving SPDT with PDT priority, 80% of tumors were recovered, whereas in groups treated by PDT or SDT, only 20% tumor shrinkage was observed.
This is consistent with the report of Jin et al. in 2000 (17). They utilized PH-1126 and ATX-70 as photo and sonosensitizing agents respectively. After applying SPDT, the observed inhibitory rate of tumor growth was nearly 92-98%. The reported inhibitory effect of tumor growth using one of these methods was 27-77%. Also, Liu et al. in 2016 reported similar results. They showed that sinoporphyrin sodium (DVDMS) mediated with SPDT yielded stronger therapeutic effects compared to SDT or PDT individual. As for the sequence of application, they found that if PDT was applied before SDT, the combinational effect was relatively stronger than the case SDT was employed prior to PDT (18). Based on the results, it can be posited that a combination of PDT with SDT can be the first choice of PDT dependent on oxygen concentration, followed by SDT, which is not entirely dependent on oxygen. This can be due to the pores created in cell membranes during ultrasound irradiation of the tumor, which with- draws sensitizer molecules from cells and reduces PDT efficacy. The destruction and/or bleaching of photosensitizer molecules by ultrasound is another predictable hypothesis that eliminates the photosensi-tivity of the tumor.
Conclusion
SPDT has more confirmed anti-cancer effects than does any other individual treatments. It can reduce the necessary dosage of sensitizer, ultrasound and light, which in turn further reduces side effects of treatment. In SPDT, if PDT is performed before SDT, treatment results would be more efficient compared to case when the reverse arrangement of SDT and PDT is employed. In any case, these findings suggest that the irradiation arrangement of light and ultrasound serves as an important factor in SPDT, thereby playing a major role in the treatment efficiency. However, anticipating the exact mecha-nism of this dependency requires further studies.
Acknowledgment
The authors would like to thank Ms Soudmand for her help in the preparation of animal models. This study was financially supported by Research Deputy of Mashhad University of Medical Sciences, Mashhad, Iran.
References
- 1.Bakhshizadeh M, Sazgarnia A, Rajabi O, Khooei AR, Esmaily H. Effects of combined sonodynamic and photodynamic therapies on a colon carcinoma tumor model. Iran J Basic Med Sci. 2011;14:205–212. [Google Scholar]
- 2.Huang Z. A review of progress in clinical photodynamic therapy. Technol Cancer Res Treat. 2005;4:283–293. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brendan GB, Quirka J, Donlonb S, Verab JC, Mangc S, Andrew B, et al. Photodynamic therapy (PDT) for malignant brain tumors--where do we stand?Photodiagnosis Photodyn Ther. 2015;12:530–544. doi: 10.1016/j.pdpdt.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Miyoshi N, Mišík V, Fukuda M, Riesz P. Effect of gallium-porphyrin analogue ATX-70 on nitroxide formation from a cyclic secondary amine by ultrasound: on the mechanism of sonodynamic activation. Rad Res. 1995;143:194–202. [PubMed] [Google Scholar]
- 5.Xiaohuai Wang WZ, Zhiyong X, Luo Y, Mitchell M. Sonodynamic and photodynamic therapy in advanced breast carcinoma: a report of 3 cases. Integr Cancer Ther. 2009;8:283–287. doi: 10.1177/1534735409343693. [DOI] [PubMed] [Google Scholar]
- 6.Jori G, Fabris C. Relative contribution of apoptosis and random necrosis in tumor response to photodynamic therapy: Effects of the chemical structure of Zn(II) Phtalocyanines. J Photochem Photobiol B. 1998;43:181–185. doi: 10.1016/s1011-1344(98)00106-7. [DOI] [PubMed] [Google Scholar]
- 7.Milowska K, Gabryelak T. Synergistic effect of ultrasound and phthalocyanines on nucleated erythrocytes in vitro . Ultrasound Med Biol. 2005;31:1707–1712. doi: 10.1016/j.ultrasmedbio.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Yumita N, Okuyama N, Sasaki K, Umemura SI. Umemura;Sonodynamic therapy on chemically induced mammary tumor: Pharmacokinetics, tissue distribution and sonodynamically induced antitumor effect of porfimer sodium. Cancer Sci. 2004;95:765–769. doi: 10.1111/j.1349-7006.2004.tb03259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy: a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11:349–363. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Yasui A, Haga Y, Chen J, Wada H. Focused ultrasonic device for sonodynamic therapy in the human body, in Microtechnology in Medicine and Biology, 2005. 3rd IEEE/EMBS Special Topic Conference on 2005. IEEE [Google Scholar]
- 11.Yumita Y, Umemura S. Sonodynamic therapy with photofrin II on AH130 solid tumor. Cancer Chemother Pharmacol. 2003;51:174–178. doi: 10.1007/s00280-002-0523-6. [DOI] [PubMed] [Google Scholar]
- 12.Sazgarnia A, Shanei A, Meibodi NT, Eshghi H, Nassirli H. A novel nanosonosensitizer for sonodynamic therapy. J Ultrasound Med. 2011;30:1321–1329. doi: 10.7863/jum.2011.30.10.1321. [DOI] [PubMed] [Google Scholar]
- 13.Kenyon JN, Fulle RJ, Lewis TJ. Activated cancer therapy using light and ultrasound-a case series of sonodynamic photodynamic therapy in 115 patients Over a 4 Year Period. Curr Drug Ther. 2009;4:179–193. [Google Scholar]
- 14.Liu Q, Wang X, Wang P, Xiao L. Sonodynamic antitumor effect of protoporphyrin IX disodium salt on S180 solid tumor. Chemotherapy. 2007;53:429–436. doi: 10.1159/000110008. [DOI] [PubMed] [Google Scholar]
- 15.Payne TP, Ambrose CV, Ward MD, Ridgway F. Proliposomes: A novel solution to an old problem. Pharm Sci. 1986:75. doi: 10.1002/jps.2600750402. [DOI] [PubMed] [Google Scholar]
- 16.Li C, Deng Y. A novel method for the preparation of liposomes: Freeze drying of monophase solutions, J Pharm Sci. 2004;93:1403–1414. doi: 10.1002/jps.20055. [DOI] [PubMed] [Google Scholar]
- 17.Jin ZH, Ishiguro K, Umemura S, Kawabata K, Yumita N, Sakata I, et al. Combination effect of photodynamic and sonodynamic therapy on experimental skin squamous cell carcinoma in C3H/HeN mice. Dermatal. 2000;27:294–306. doi: 10.1111/j.1346-8138.2000.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Wang P, Liu Q, Wang X. Sinoporphyrin sodium triggered sono-photodynamic effects on breast cancer both in vitro and in vivo. Ultrason Sonochem. 2016;31:437–448. doi: 10.1016/j.ultsonch.2016.01.038. [DOI] [PubMed] [Google Scholar]


