Abstract
Objective(s):
This study was aimed to investigate the effect of Berberis integerrima (B. integerrima) extract on insulin sensitivity in high-fructose-fed insulin-resistant rats.
Materials and Methods:
Experimental rats were randomly divided into two groups: the control group was fed a regular chow diet while other group fed with a high-fructose diet for 8 weeks. After the first six weeks, the animals were treated with B. integerrima extract or pioglitazone for two weeks. Insulin and adiponectin levels were measured by ELISA. Additionally, Insulin resistance was calculated using a Homeostasis Model Assessment of Insulin resistance (HOMA-IR). The plasma free fatty acid (FFA) profile was obtained by gas chromatography. PPARγ and GLUT4 gene expression were assessed by real-time polymerase chain reaction (PCR) and western-blotting.
Results:
Comparing the B. integerrima treated group with the control group, weight gain (P=0.009) and levels of insulin (P=0.001), blood glucose (P<0.0001), and HOMA-IR (P<0.0001) were significantly reduced. Additionally, the adiponectin concentration was significantly increased (P<0.0001). Among the FFA fractions, the mean concentration of palmitoleic acid and stearic acid in the B. integerrima group were significantly higher than the control group (P<0.0001 and P=0.005, respectively). However, there was no significant difference at the mRNA and protein level of GLUT4 and PPAR-γ between B. integerrima treated group and control group.
Conclusion:
The study findings revealed that B. integerrima might be a protective candidate against Type 2 diabetes/insulin resistance through direct insulin-like effect and an increase in adiponectin levels. However, the mechanism of B. integerrima was independent of GLUT4 and PPARγ.
Keywords: Adiponectin, Diabetes, Free fatty acids, Insulin resistance, Metabolic syndrome
Introduction
Obesity is the primary disease of adipocytes and is strongly associated with serious comorbidities that impair an individual’s health (1, 2). Obesity is closely related to insulin resistance and other chronic diseases including all components of metabolic syndrome, cardiovascular diseases, type 2 diabetes mellitus (T2DM), and certain types of cancers (2–4). Chronic consumption of high-fat diets leading to the development of obesity can induce insulin resistance in both human and animals (5).
Insulin resistance is defined as impaired ability of insulin to promote glucose uptake and exert its metabolic effects in its target tissues (liver, skeletal muscle and adipose tissue) (6, 7). Moreover, intensive studies have demonstrated a strong association between obesity and insulin resistance with dysregulation of adipokines (8–10). Adiponectin, one of the most abundant adipokines secreted exclusively by adipocytes, plays an influential effect in insulin-sensitizing and anti-diabetic activity (8–12). Recent findings have revealed that adiponectin exerts its effect by binding to both AdipoR1 and AdipoR2 receptors, thereby leading to the activation of peroxisome proliferator-activated receptors (PPARs) and 5′AMP-activated protein kinase (AMPK) signaling pathways (11, 13). Subsequently, PPARs and AMPK cause an increase in fatty acid oxidation and glucose uptake (14, 15).
Several mechanisms have been proposed that increased free fatty acids (FFAs) interference with insulin function (16–19). High circulating levels of FFA not only inhibit lipolysis but also prevent insulin signaling pathway through activating protein kinases such as protein kinase C (PKC), and c-JUN NH2-terminal protein kinase (JNK) (2, 20). Since insulin acts as an effective inhibitor of lipolysis, increased circulating levels of FFAs contribute to insulin resistance (17, 21).
It is well established that insulin resistance is associated with increase in inflammatory mediators such as tumor necrosis factor α (TNF-α), interleukin 6 (IL-6), IL-10, plasminogen activator inhibitor-1 (PAI-1), macrophages and monocyte chemoattractant protein-1 (MCP-1), leptin and resistin (16, 21–23). These cytokines can interfere with the binding of IRS1/2 to the insulin receptor by activating the suppressor of cytokine signaling (SOCS) proteins and several serine/threonine kinases such as JNK, PKC, and IκB kinase β (IKK-β) (22, 24). Furthermore, an imbalance in energy metabolism of skeletal muscles plays an essential role in the development of metabolic diseases (25). The overproduction of lipids in skeletal muscles contributes to inhibition of glucose uptake through GLUT4 translocation from intracellular location to plasma membranes which can lead to insulin resistance (22, 23). In turn, regulation of GLUT4 is a major contributor of glucose metabolism in both skeletal muscle and adipose tissue (22, 23).
PPAR is a ligand-activated transcription factor which has three isotypes (α, γ, and ß/δ) (26). Activation of PPAR enhances insulin resistance and promotes lipid metabolism by increasing FFA oxide-tion and repressing the expression of leptin gene (2, 27). Rosiglitazone and pioglitazone which are subclasses of thiazolidinedione (TZD) are recognized as PPAR-γ agonists (28). These drugs have been used clinically as regulators of genes involved in glucose, lipid, and protein metabolism to control hyperglyc-emic status in patients with T2DM (29,30). However, drugs like fibrates (the PPARα agonists), and the mentioned PPAR-γ agonist cause an increase in the risk of cardiovascular disease and bladder cancer (31, 32). Consequently, much attention has been focused on functional food ingredients and herbal extracts to reduce the mortality rate and other serious side effects (1, 31).
Berberis integerrima, a plant belonging to Berberidaceae family, has exclusively been cultivated in Iran, especially in Northern and North-eastern regions where it is referred to as Zereshk in the Persian language (33–35). In traditional medicine, B. integerrima was mainly used as an impressive medicine for treating diabetes in the Alamut region, in the province of Qazvin, Iran (33). Furthermore, B. integerrima was used to treat various type of infectious fevers, diarrhea and it has also been reported to have other effects including hypolipidemic, hypotensive, and anti-inflammatory features (33, 34). Many components of B. integerrima have been identified. The main constitutes of the plant include berberine, palmatine chloride, berberine chloride, oxyacanthine, berbamine, and columbamine, among them, berberine is the most important active constituent found in the bark of the root, stem, and unripe fruit (33–37). Pharmaceutical and therapeutic effects of berberine have also been reported in treating diabetes. Previous studies have indicated that berberine improves insulin sensitivity and glucose uptake by activating protein kinase B and increasing glucose uptake through AMPK and AMPK- P38 pathways (36, 38).
Nevertheless, available data regarding the effects of B. integerrima extract on the possible mechanisms of insulin resistance are scarce. As skeletal muscles and hepatic tissue are the chief parts involved in insulin function and energy homeostasis, we selected these two tissues. In the direction of unknown mechanisms and low evidence, the objective of the present study was to examine the effects of B. integerrima root extract on muscle and hepatic insulin resistance in rats to determine the possible mechanisms involved.
Materials and Methods
Preparation of extract
B. integerrima was collected from Fars province, Iran. Its genus and species were approved by the department of botany, at Bahonar University, Kerman, Iran (Voucher specimen no: KF1194). After collecting the plants, fruits were dried in the shade at 30 °C. Dried fruits were milled and converted into powder. The resulting powder was extracted by maceration method (300 g in 1000 ml distilled water) for 48 hrs at room temperature (39). After filtration water was evaporated at 40 °C in an oven and dried extract was stored at -20 °C.
Experimental animals and treatment
In this experimental study, male Wistar rats (weighing 250–300 g) were obtained from the animal house of Kerman University of Medical Sciences. The Ethics Committee of Kerman University of Medical Sciences approved the study procedure. Rats were maintained in the animal room at 22±3 °C under an automatic lighting schedule (08:00–20:00 hr). They had free access to standard chow and water for two weeks. They were then randomly divided into four groups (n=8). The first three groups were fed a 60% fructose diet, whereas the fourth group, as the healthy control group (HCG) was fed with normal chow for 8 weeks (40). The fructose-enriched diet contained 60% fructose, 21% protein, and 5 % fat, sodium 0.49%, and potassium 0.49% (40). After 6 weeks, insulin resistance was confirmed by oral glucose tolerance test (OGTT).
While rats were still on the fructose diet, they were further subdivided into three groups: Two groups received an intragastric injection of B. integerrima extract (1000 mg/kg) (41) and pioglitazone (Sigma, Saint Louis, MO, USA) (10 mg/kg) (42) as a B. integerrima extract group and pioglitazone group (Pio), respectively. The third group as a control group (Con) without any treatment. These treatment periods lasted for 2 weeks. Body weight was measured weekly throughout the study.
Blood and tissue collection
At the end of the treatment period, the food was removed for 12 hr before collecting blood samples from the heart under ether anesthesia. The blood samples were divided into two tubes with or without EDTA. Tubes were centrifuged at 6000 g for 10 min at 4 °C. Plasma (for FFA analysis) or serum (for other biochemical analyses) was immediately separated and stored at -20 °C. The hind limb skeletal muscle and liver tissue were quickly removed and then frozen in liquid nitrogen and kept at −75 °C until use.
Measurement of serum parameters
Serum concentrations of glucose, triglycerides, cholesterol, and high-density-lipoprotein-cholesterol (HDL-c) were measured by an RA-1000 autoanalyzer. Blood insulin and adiponectin levels were measured using ELISA commercial assay kits according to the manufacturer’s instructions (Mouse/Rat Adiponectin or insulin ELISA kit, USCN. China). Insulin resistance was calculated using Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) equation: [(insulin (μU/ml) × glucose (mmol/l))/22.5].
Measurement of plasma-free fatty acids
Plasma FFAs were extracted and analyzed by the method explained by Kangani et al. with slight modifications (43). Five hundred microliter of plasma was mixed with 20 μl of pentadecanoic acid (1 mg/ml) as an internal standard. Lipids were extracted from plasma by a reagent constituted from isopropanol–heptane–hydrochloric acid (1M) (40:10:1, v/v/v). After separating FFAs by TLC on silica gel plates using a heptane–ether–acetic acid [60:40:3] solvent system, FFAs were visualized by iodine vapor on TLC plates. FFA bands were scrapped, and free fatty acid methyl esters (FAME) were prepared by a reaction with BF3 containing methanol (Sigma). Then, FFA methyl esters were separated using an Agilent GC-7890A system equipped with a flame ionization detector. The injection volume was 1 μl in the split 30:1 injection mode. A capillary column (DB-225 20 m×0.1 mm I.D., 0.1 μm film thickness, J&W GC columns, USA) was employed.
RNA extraction and real-time quantitative PCR
RNA was isolated from the skeletal muscle (for GLUT4 assay) and liver (for PPARγ assay) tissues by RNeasy mini kit (Qiagen) according to manufacturer’s instruction. The concentration of RNA was quantified by the ultraviolet (UV) light spectrophotometry at 260 nm and 280 nm (ND-1000 nanodrop). The quality of the extracted RNA was confirmed by ethidium bromide staining of 18S and 28S ribosomal RNA bands after electrophoresis on a 2% agarose gel.
Reverse transcription of RNA to cDNA was performed using Quanti Tect Reverse Transcription Kit (Qiagen) according to the manufacture’s procedure. Relative Quantitative real-time PCR was conducted on a Qiagen Thermal Cycler (Rotor-Gene Q 5plex HRM System, Qiagen) using the corresponding QuantiFast SYBR Green PCR kit (Qiagen) according to manufacturer’s instructions. Primer sequences used for real-time PCR are shown in Table 1. The cycle of threshold (CT) value was determined for each sample. ΔCT was calculated using the equation: ΔCT= CT (target gene) –CT (endogenous reference gene (GAPDH)). Results were calculated by the equation: 2-∆∆CT. In the real-time PCR all suggestions that published by Qiagen in “Critical Factors for Success in Real Time PCR” were considered.
Table 1.
Primers used in this study
| Gene | Primers | PCR Product | Accession Number |
|---|---|---|---|
| GLUT4 | F: ACTGGCGCTTTCACTGAACT | 106 bp | NM_012751 |
| R: CGAGGCCAAGGCTAGATTTTG | |||
| PPAR.γ | F: CATGCTTGTGAAGGATGCAAG | 131 bp | NM_001145367 |
| R: TTCTGAAACCGACAGTACTGACAT | |||
| GAPDH | F: TGGAGTCTACTGGCGTCTT | 138 bp | NM_017008 |
| R: TGTCATATTTCTCGTGGTTCA |
Western immunoblotting analysis
Total protein was extracted from muscle or liver by homogenization in the radioimmune precipitation assay (RIPA) buffer (Sigma, cat number: R0278), followed by centrifugation at 14,000 rpm for 20 min and then supernatant containing proteins was collected. Total protein was measured by a Bradford protein assay using bovine serum albumin (BSA) as standard. Proteins were separated by using SDS- polyacrylamide gel electrophoresis and transferred on polyvinylidene difluoride (PVDF) membrane. Non-specific binding sites were blocked by overnight incubating with 5 % (w/v) non-fat skim milk in Tris-buffered saline containing 0.1% Tween 20 (TBS-T) buffer at 4 °C. Then, the membrane was washed with TBST three times (each time for 20 min) and incubated with appropriate polyclonal primary antibodies for PPARγ (ab27649, mouse polyclonal to PPAR gamma, Abcam, UK) or GLUT4 (ab33780, mouse polyclonal to GLUT4, Abcam, UK) antibody in TBS-T buffer for 1 hr. After washing, as mentioned above, membranes were incubated with the anti-rabbit secondary antibody (Goat Polyclonal HRP conjugated antibody to rabbit IgG, ab6112, Abcam, UK), for 1 hr at room temperature. Then PVDF was washed and incubated with substrate (Western lightening plus ECL, Perkin-Elmer) for 1 min. After exposing PVDF membrane to Hyblot film (Denvill) for 30 secs, in a dark room, band intensities were quantified by image j software.
Statistical analysis
All data are presented as mean±SEM (standard errors of the mean). The differences among groups were analyzed using one-way analysis of variance (ANOVA), and Post-hoc Tukey test was used to compare means between groups. The statistical analyses were performed using the SPSS software version 18.0 for Windows (SPSS Inc, Chicago, IL). P<0.05 were considered statistically significant.
Results
Weight gain, water and food intake
No significant difference was found among the groups in comparison of body weights at the beginning of treatment. A significant difference was observed in the body weight gain between B. integerrima and control groups (P=0.009). The difference between the initial body weight and body weight at the end of the 8th week were considered as body weight gain. B. integerrima reduced weight as compared to pioglitazone. However, no significant difference was observed in water or food intake between B. integerrima and controls groups (Table 2).
Table 2.
Effect of Berberis integerrima on body weight, water, and food intake and other insulin resistance related parameters
| Parameters | Groups | P-value* | |||
|---|---|---|---|---|---|
| HCG | Con | Pio | BIE | ||
| Initial weight (g) | 275±8 | 272±9 | 272±12 | 255±11 | 0.676 |
| Weight after 8 weeks (g) | 285±9 | 307±8 | 294±16 | 278±15 | 0.421 |
| Weight gain (g) | 10±2* | 35±2# | 22±5 | 14±6* | 0.009 |
| Water intake (ml) | 35±0.8* | 47±2# | 60±2*# | 45±1# | 0.749 |
| Food intake (g) | 21.6±0.7* | 13.4±0.4# | 13.5±0.3# | 13±0.2# | 0.84 |
| Insulin (pmol/l) | 50±4.8* | 137±34# | 40±2.7* | 25±6* | 0.001 |
| Adiponectin (μg/ml) | 2.9±0.16 | 3.9±0.15# | 5.6±0.4*# | 6±0.3*# | 0.000 |
| Glucose (mg/dl) | 132±4* | 187±15# | 129±5.8* | 115±3* | 0.000 |
| HOMA-IR | 2.7±0.37* | 9.7±2.1# | 2.1±0.12* | 1±0.3* | 0.000 |
| Cholesterol (mg/dl) | 71±12.1 | 59±3.2 | 63±4.2 | 63±2 | 0.967 |
| Triglyceride (mg/dl) | 85±13* | 217±18# | 200±51 | 121±9 | 0.179 |
| HDL-c (mg/dl) | 24.86±1.4 | 29.25±1.8 | 29.38±2.3 | 28±1 | 0.988 |
Biochemical parameters were measured by autoanalyzer and hormones by ELISA kits. HOMA-IR was calculated by the formula. All data were expressed as mean±SEM (n=8). HCG (was fed standard chow). Con (was fed 60% fructose), Pio (was fed 60% fructose with pioglitazone-treated (10 mg/Kg)) and BIE (was fed 60% fructose with Berberis integerrima extract-treated (1000mg/Kg))
HCG: healthy control group, Con: control, Pio: pioglitazone, BIE: Berberis integerrima extract
¥ P-value demonstrates the difference significance between Berberis integerrima and control group
Significant difference with control group (P<0.05);
Significant difference with HCG group (P<0.05)
Blood glucose, triglycerides, and cholesterol
Table 2 shows that the mean blood glucose level was significantly decreased in B. integerrima group (115±3 mg/dl) compared to the control group (187±15 mg/dl) (P<0.0001). However, no significant differences were observed in mean serum levels of triglycerides, cholesterol and HDL-c between B. integerrima and other groups. Pioglitazone did not show any effect on triglycerides in the current study (200±51 mg/dl).
Serum levels of insulin and adiponectin
Comparison of B. integerrima group with controls, the mean level of insulin was significantly decreased (25±6 pmol/l and 137±34 pmol/l, respectively) (P=0.001). However, the mean level of adiponectin significantly increased in B. integerrima as compared to the control group (3.9±0.15 μg/ml) (P<0.0001). No significant difference was found in mean levels of insulin and adiponectin between B. integerrima and pioglitazone groups (Table 2).
Homeostasis model assessment of insulin resistance
As shown in Table 2, in B. integerrima treated animals (1±0.3), HOMA-IR was significantly lower compared to the control group (9.7±2.1) (P<0.0001). Moreover, no significant difference of HOMA-IR was observed in B. integerrima animals in comparison with pioglitazone treated animals.
Plasma free fatty acids
Among FFA fractions, the mean serum levels of palmitoleic acid and stearic acid were significantly higher in the B. integerrima group compared to the control group (P<0.0001 and P=0.005, respectively), while no significant difference was found in total FFAs between B. integerrima and control groups (Table 3).
Table 3.
Effect of Berberis integerrima on plasma free fatty acids profiles
| Parameters | Groups | P-value* | |||
|---|---|---|---|---|---|
| HCG | Con | Pio | BIE | ||
| Myristic acid (μmol/l) | 1.07±0.01 | 1.12±0.06 | 1.2±0.06 | 1.4±0.04 | 0.013 |
| Palmitic acid (μmol/l) | 5.9±0.57 | 11.1±2.6 | 5.1±0.18 | 6.7±0.32 | 0.252 |
| Palmitoleic acid (μmol/l) | 1.06±0.04 | 1.4±0.12 | 1.1±0.01 | 2.1±0.11 | 0.000* |
| Stearic acid (μmol/l) | 1.3±0.12 | 2.06±0.11 | 2.00±0.08 | 2.1±0.1 | 0.005* |
| Oleic acid (μmol/l) | 2.2±0.21 | 2.9±0.22 | 1.7±0.08 | 3±0.3 | 0.981 |
| Total free fatty acids (μmol/l) | 12.53±1.95 | 22±2.7 | 19.44±2.8 | 14.1±3.13 | 0.132 |
Plasma free fatty acids profiles were measured by GC. All data were expressed as mean±SEM (n=8)
HCG (was fed standard chow), Con (was fed 60% fructose), Pio (was fed 60% fructose with pioglitazone-treated (10mg/Kg)) and BIE (was fed 60% fructose with Berberis integerrima extract-treated (1000 mg/Kg))
HCG: healthy control group, Con: control, Pio: pioglitazone, BIE: Berberis integerrima extract
P-value demonstrates the difference between Berberis integerrima and control group
GLUT4 and PPAR-γ gene expression
Findings demonstrated that B. integerrima did not have any effect on PPAR-γ and GLUT4 gene expression at mRNA level (P=1 and P=0.88, respectively). Similarly, no significant difference was observed in the mRNA level of PPAR-γ and GLUT4 in pioglitazone group as compared to control group (Figure 1).
Figure 1.
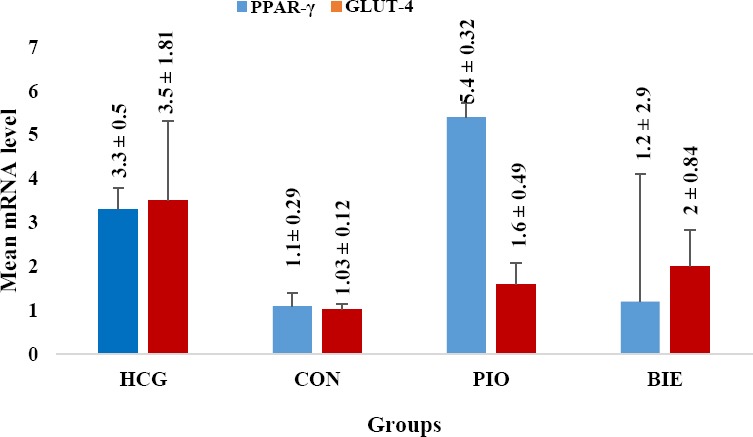
Effect of Berberis integerrima on mRNA level of liver PPARγ and muscle GLUT4 gene expression
The chart demonstrates that B. integerrima did not have any effect on PPARγ and GLUT4 gene expression at mRNA level (P=0.928 and P=0.995, respectively). However, pioglitazone significantly increased the mRNA level of PPARγ (Figure 1). All data are expressed as mean± SEM
HCG: healthy control group, Con: control, Pio: pioglitazone, BIE: Berberis integerrima extract
As shown in Figures 2 and 3, no significant difference was found in PPAR-γ protein level between B. integerrima and control group. However, the level of PPAR-γ protein in pioglitazone group was significantly higher compared to the control group (P<0.0001). Regarding the mean protein level of GLUT4, no significant difference was observed between the B. integerrima and control groups (Figures 2 and 3).
Figure 2.
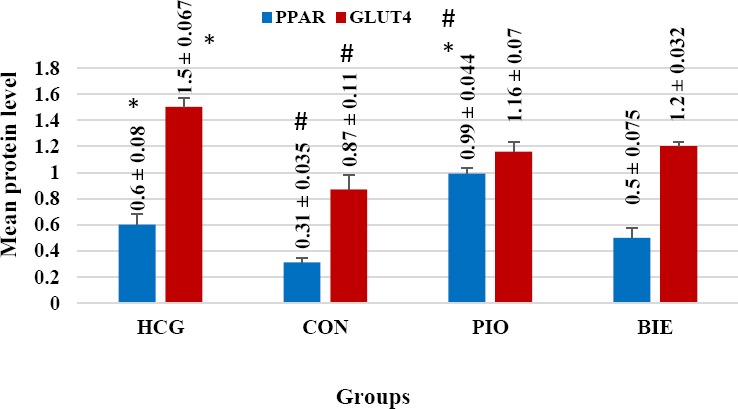
Effect of Berberis integerrima on protein level of liver PPARγ and muscle GLUT4
The chart illustrates that the level of PPAR-γ protein in pioglitazone group was significantly higher than the control group (P<0.0001). Regarding the mean protein level of GLUT4 and PPARγ, no significant difference was found between B. integerrima group and the control group. All data are expressed as mean ± SEM.
HCG: healthy control group, Con: control, Pio: pioglitazone, BIE: Berberis integerrima extract
* Significant difference with control group (P<0.0001)
# Significant difference with HCG group (P<0.05)
Figure 3.
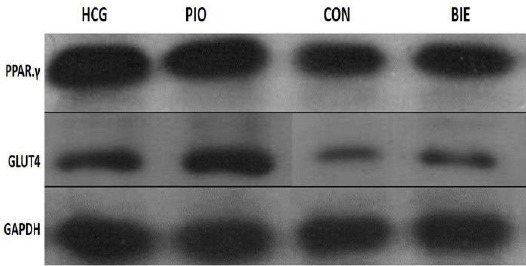
Western blot assay of protein level of liver PPARγ and muscle GLUT4 from HCG (was fed standard chow). Con (was fed 60% fructose), Pio (was fed 60% fructose with pioglitazone-treated (10mg/Kg)) and BIE (was fed 60% fructose with Berberis integerrima extract-treated (1000mg/Kg)). Immunodetection was performed using polyclonal anti- PPARγ or anti- GLUT4 antibodies HCG: healthy control group, Con: control, Pio: pioglitazone, BIE: Berberis integerrima extract
Discussion
In the present study, findings demonstrated that B. integerrima clearly improved insulin resistance in high-fructose-fed insulin-resistant rats. We showed that B. integerrima extract alters body weight gain, insulin, adiponectin, blood glucose levels, and HOMA-IR. In agreement with several studies, the present findings indicated that hyperlipidemia, insulin resis-tance, and hyperinsulinemia states were induced after treating the animals with a high-fructose diet (3, 39, 40). Significant decreases in body weight, insulin, blood glucose, and HOMA-IR were observed after administration of B. integerrima in insulin-resistant rats compared to the control group. To the best of author’s knowledge, there was no study on the effects of B. integerrima on insulin resistance until now; however, the current findings clearly indicate that B. integerrima improves insulin resistance.
In agreement with our previous study, B. integerrima like Zataria multiflora extract showed protective effects on glucose homeostasis and HOMA-IR (21). Decreases in insulin level along with the reduction in glucose and HOMA-IR suggest that B. integerrima has a direct insulin-like activity and acts as a hypoglycemic factor through improving insulin action rather than insulin secretion (44). Adipocyte accumulation plays a pivotal role in glucose homeostasis imbalance and adipose tissue inflammation (9, 10). Particularly, body weight is defined by the balance between food intake and energy expenditure (45). The present results have demonstrated that B. integerrima reduced body weight gain. Hence, decreases in visceral adiposity and adipocyte size could lead to improving insulin resistance.
Several studies have been put forward to explain the relationship between insulin resistance and dyslipidemia (46–48). Controversially, Ashraf et al have reported that administration of B. integerrima increased the serum levels of HDL-cholesterol and decreased triglycerides and total cholesterol (36). Further studies on the B. integerrima in different doses are needed to clarify the discrepancy.
Recently, it has been reported that lower production of adiponectin is directly linked to incidence of insulin resistance and metabolic syndrome (8–10). A reverse correlation has been recorded between adiponectin with adipocyte size and circulating adiponectin levels increased with weight loss (8, 49, 50). Consistent with other studies, Matsubara et al. findings indicated that adiponectin has a negative correlation with enlarged visceral fat, body mass index (BMI), and insulin resistance (51). Furthermore, adiponectin has been shown to come into play as major insulin-sensitizer adipokines by binding to their receptors in the liver and skeletal muscle (8, 9). TZDs increase the gene expression and plasma concentration of adiponectin and other genes involved in glucose uptake (52, 53). The serum level of adiponectin in the B. integerrima group was significantly higher as compared to the control group. Taken together, it could be deduced that B. integerrima exerts its antihyperglycemic effect, at least in part, by improving adiponectin production.
Most of the recent studies revealed that the improvement of insulin sensitivity has already been related to increased GLUT4 and PPAR-γ expression (40, 54). To clarify the molecular mechanisms involved in insulin resistance, we measured GLUT4 and PPAR-γ genes expression at the protein and mRNA levels. According to the recent studies on insulin sensitivity improvement, the activation of PPAR-γ can establish an important part of the molecular mechanism behind the adipogenesis. PPAR-γ is the most important member of the PPARs family that is involved in the adipogenesis adjustment. PPAR-γ, as a regulator of lipid and glucose metabolism, improves the insulin and glucose parameters and increases whole-body insulin sensitivity. PPAR-γ is predominantly expressed in the adipose tissue and it is the target of some anti-diabetic drugs such as pioglitazone. PPAR-γ agonists contribute to enhancing the insulin sensitivity and glucose metabolism, and promoting the differentiation of pre-adipocytes into adipocytes. Comparing the B. integerrima treated group with the control group, no significant difference was found at the mRNA level between GLUT4 and PPAR-γ. Moreover, B. integerrima administrated group did not has any effect on protein level of GLUT4 and PPAR-γ. It can be assumed that the mechanism of decreased expression might be related to the composition of B. integerrima. However, PPAR-γ protein level was significantly higher in pioglitazone treated group in comparison with control group. Results derived from Sakamoto et al. demonstrated pioglitazone as a high-affinity selective PPAR-γ activator (55). PPAR-γ agonists ameliorate insulin resistance, reduce infla-mmation and enhance the differentiation of adipocytes and macrophages (56, 57). TZDs, particularly pioglitazone, have been extensively used in insulin resistance and T2DM.
Down-regulation of GLUT4 expression may enhance whole body insulin resistance and glucose imbalance (7). The GLUT4 content was lower in skeletal muscle fibers and fat tissue of type-2 diabetic patients. Besides, insulin begins insulin signaling cascade, which results in GLUT4 translocation and increased glucose uptake at the cell membrane in muscle cells. In this pathway, the impairment of any of these steps leads to the decrease of GLUT4 translocation and causes insulin resistance. Insulin decreases circulating glucose levels by suppressing hepatic glucose production and also activating GLUT4. GLUT4 increases insulin-mediated glucose uptake in muscle and adipose tissue (7, 58). In the present study, GLUT4 mRNA and protein levels were not significantly different among groups. Therefore, we speculate that the extracted B. integerrima contents do not increase the GLUT4 genes expression at protein and mRNA levels. However, it could improve GLUT4 translocation and glucose uptake. Moreover, no significant differences were found in GLUT4 protein and mRNA levels between the two groups. Expression and translocation of GLUT4 is increased in adipose tissue by PPARγ (21, 57). The reason for this issue can be administration of a single dose to these groups. In striated muscle, no significant difference was found at the total GLUT4 protein between two groups, therefore, it could be supposed that translocation of GLUT4 to the cell membrane is increased after treating animals with B. integerrima and pioglitazone.
Many studies in animals and humans have highlighted the association of obesity with T2DM/insulin resistance (2, 9). Although the exact mechanisms have not been clearly elucidated, it could be deduced from previous studies that in most obese individuals, FFA levels are obviously too high, also acute and chronic increases in FFA levels lead to insulin resistance (18, 19). Meanwhile, this FFA elevation causes interference in insulin signaling and function (19, 39). On the other hand, Insulin has a profound influence on the metabolic homeostasis and potently affects the metabolism of fuel molecules in adipose tissue, skeletal muscle, and the liver (58, 59). Accordingly, we analyzed the FFAs profile in high-fructose-fed insulin-resistant rats. Total plasma FFA level was not significantly different; however, palmitoleic acid and stearic acid were significantly increased in B. integerrima treated group in comparison with the control group. PPARγ agonists enhance the insulin sensitivity in adipose tissue and decrease serum FFA levels (57). In the present study, no significant difference was found between pioglitazone and control groups regarding the PPARγ gene expression, since we evaluated PPARγ gene expression in the liver to investigate hepatic insulin resistance. This, in turn, could be suggested that further studies on PPARγ gene expression in adipocytes are required to clarify the direct effect of PPARγ on FFA levels. In the present study, using single dose of B. integerrima is one of the limitations. We could not confirm the optimal dose of B. integerrima because of the limited relevant investigations. Moreover, the lack of enough time and budget, and inadequate literature were some limitations of the present study.
Conclusion
The B. integerrima water extract, significantly improved insulin resistance in insulin-resistant rats. We demonstrated that B. integerrima not only increased adiponectin but also significantly reduced plasma glucose level and HOMA-IR. However, the mechanism of B. integerrima was independent of GLUT4 and PPARγ. We suggest that B. integerrima might be a protective candidate against T2DM/insulin resistance. Therefore, further studies are needed to identify precisely which components of B. integerrima have the main role in its therapeutic effect.
Acknowledgment
This study was granted by Kerman University of Medical Sciences, Kerman, Iran. Authors would like to thank all the participants for help us to perform our study.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Soares FL, de Oliveira Matoso R, Teixeira LG, Menezes Z, Pereira SS, Alves AC, et al. Gluten-free diet reduces adiposity, inflammation and insulin resistance associated with the induction of PPAR-alpha and PPAR-gamma expression. J Nutr Biochem. 2013;24:1105–1111. doi: 10.1016/j.jnutbio.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 3.Araújo TG, de Oliveira AG, Vecina JF, Marin RM, Franco ES, Abdalla Saad MJ, et al. Parkinsonia aculeata (Caesalpineaceae) improves high-fat diet-induced insulin resistance in mice through the enhancement of insulin signaling and mitochondrial biogenesis. J Ethnopharmacol. 2016;183:95–102. doi: 10.1016/j.jep.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 4.Joo H, Kim CT, Kim IH, Kim Y. Anti-obesity effects of hot water extract and high hydrostatic pressure extract of garlic in rats fed a high-fat diet. Food Chem Toxicol. 2013;55:100–105. doi: 10.1016/j.fct.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 5.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koo SH, Satoh H, Herzig S, Lee CH, Hedrick S, Kulkarni R, et al. PGC-1 promotes insulin resistance in liver through PPAR-alpha-dependent induction of TRB-3. Nat Med. 2004;10:530–534. doi: 10.1038/nm1044. [DOI] [PubMed] [Google Scholar]
- 7.Kahn BB, Flier JS. On diabetes : insulin resistance Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol. 2010;314:1–16. doi: 10.1016/j.mce.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 9.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15:6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206–218. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Kalupahana NS, Moustaid-Moussa N, Claycombe KJ. Immunity as a link between obesity and insulin resistance. Mol Aspects Med. 2012;33:26–34. doi: 10.1016/j.mam.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 12.Esfahani M, Movahedian A, Baranchi M, Goodarzi MT. Adiponectin: an adipokine with protective features against metabolic syndrome. Iran J Basic Med Sci. 2015;18:430–442. [PMC free article] [PubMed] [Google Scholar]
- 13.Caselli C. Role of adiponectin system in insulin resistance. Mol Genet Metab. 2014;113:155–160. doi: 10.1016/j.ymgme.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Wu L, Wang C, Liu L, Zhao Y. Adiponectin modulates carnitine palmitoyltransferase-1 through AMPK signaling cascade in rat cardiomyocytes. Regul Pept. 2007;139:72–79. doi: 10.1016/j.regpep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Dupont J, Chabrolle C, Ramé C, Tosca L, Coyral-Castel S. Role of the peroxisome proliferator-activated receptors, adenosine monophosphate-activated kinase, and adiponectin in the ovary. PPAR Res. 2008;2008:176275. doi: 10.1155/2008/176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maebuchi M, Machidori M, Urade R, Ogawa T, Moriyama T. Low resistin levels in adipose tissues and serum in high-fat fed mice and genetically obese mice: Development of an ELISA system for quantification of resistin. Arch Biochem Biophys. 2003;416:164–170. doi: 10.1016/s0003-9861(03)00279-0. [DOI] [PubMed] [Google Scholar]
- 17.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capurso C, Capurso A. From excess adiposity to insulin resistance: The role of free fatty acids. Vasc Pharmacol. 2012;57:91–97. doi: 10.1016/j.vph.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E. Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab. 2007;292:E1775–1781. doi: 10.1152/ajpendo.00624.2006. [DOI] [PubMed] [Google Scholar]
- 20.Hotamisligil GS. Inflammation and metabolic disorders 1. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 21.Mohammadi A, Gholamhoseinian A, Fallah H. Zataria multiflora increases insulin sensitivity and PPARγgene expression in high fructose fed insulin resistant rats. Iran J Basic Med Sci. 2014;17:263–270. [PMC free article] [PubMed] [Google Scholar]
- 22.Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42:1331–1346. doi: 10.1016/j.clinbiochem.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 23.Leguisamo NM, Lehnen AM, Machado UF, Okamoto MM, Markoski MM, Pinto GH, et al. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc Diabetol. 2012;11:100. doi: 10.1186/1475-2840-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butler M, McKay RA, Popoff IJ, Gaarde WA, Witchell D, Murray SF, et al. Specific inhibition of PTEN expression reverses hyperglycemia in diabetic mice. Diabetes. 2002;51:1028–1034. doi: 10.2337/diabetes.51.4.1028. [DOI] [PubMed] [Google Scholar]
- 25.Westerblad H, Bruton JD, Katz A. Skeletal muscle: Energy metabolism, fiber types, fatigue and adaptability. Exp Cell Res. 2010;316:3093–3099. doi: 10.1016/j.yexcr.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 26.Kajiya T, Ho C, Wang J, Vilardi R, Kurtz TW. Molecular and cellular effects of azilsartan: a new generation angiotensin II receptor blocker. J Hypertens. 2011;29:2476–2483. doi: 10.1097/HJH.0b013e32834c46fd. [DOI] [PubMed] [Google Scholar]
- 27.Lee JY, Hashizaki H, Goto T, Sakamoto T, Takahashi N, Kawada T. Activation of peroxisome proliferator-activated receptor-alpha enhances fatty acid oxidation in human adipocytes 3332. Biochem Biophys Res Commun. 2011;407:818–822. doi: 10.1016/j.bbrc.2011.03.106. [DOI] [PubMed] [Google Scholar]
- 28.Papaetis GS, Orphanidou D, Panagiotou TN. Thiazolidinediones and type 2 diabetes: from cellular targets to cardiovascular benefit. Curr Drug Targets. 2011;12:1498–512. doi: 10.2174/138945011796818243. [DOI] [PubMed] [Google Scholar]
- 29.Taylor C, Hobbs FDR. Type 2 diabetes, thiazolidinediones, and cardiovascular risk. Br J Gen Pract. 2009;59:520–524. doi: 10.3399/bjgp09X453440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papaetis GS, Orphanidou D, Panagiotou TN. Thiazolidinediones and type 2 diabetes: from cellular targets to cardiovascular benefit 2553. Curr Drug Targets. 2011;12:1498–1512. doi: 10.2174/138945011796818243. [DOI] [PubMed] [Google Scholar]
- 31.Seymour EM, Tanone II, Urcuyo-Llanes DE, Lewis SK, Kirakosyan A, Kondoleon MG, et al. Blueberry ikntake alters keletal muscle and adipose tissue peroxisome proliferator-activated receptor activity and reduces insulin resistance in obese rats. J Med Food. 2011;14:1511–1518. doi: 10.1089/jmf.2010.0292. [DOI] [PubMed] [Google Scholar]
- 32.Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: Interim report of a longitudinal cohort study. Diabetes Care. 2011;34:916–922. doi: 10.2337/dc10-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahvazi M, Akbarzadeh M, Khalighi Sigaroodi F, Kohandel A. Introduce some of the medicinal plants species with the most traditional usage in East Mazandaran. J Med Plants. 2012;11:164–175. [PMC free article] [PubMed] [Google Scholar]
- 34.Alemardan A, Asadi W, Rezaei M, Tabrizi L, Mohammadi S. Cultivation of iranian seedless barberry (Berberis Integerrima“bidaneh”): A medicinal shrub. Ind Crops Prod. 2013;50:276–287. [Google Scholar]
- 35.Potdar D, Hirwani RR, Dhulap S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia. 2012;83:817–830. doi: 10.1016/j.fitote.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Ashraf H, Heidari R, Nejati VIM. Preventive effect of Berberis Integerrima on the serum levels of glucose and lipids in streptozotocin (STZ)-induced diabetes in rats. J Fasa Univ Med Sci. 2012;2:148–155. [Google Scholar]
- 37.Ashraf H, Zare S. Preventive effects of aqueous extract of Berberis integerrima Bge. Root on liver injury induced by diabetes Mellitus (Type 1) in rats. Iran J Pharm Res. 2015;14:335–343. [PMC free article] [PubMed] [Google Scholar]
- 38.Chang W, Chen L, Hatch GM. Berberine as a therapy for type 2 diabetes and its complications: From mechanism of action to clinical studies. Biochem Cell Biol Biol Cell. 2015;93:479–486. doi: 10.1139/bcb-2014-0107. [DOI] [PubMed] [Google Scholar]
- 39.Mohammadi A, Gholamhosseinian A, Fallah H. Trigonella foenum-graecum water extract improves insulin sensitivity and stimulates PPAR and γgene expression in high fructose-fed insulin-resistant rats. Adv Biomed Res. 2016;5:54. doi: 10.4103/2277-9175.178799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shih CC, Lin CH, Lin WL, Wu J, Bin Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J Ethnopharmacol. 2009;123:82–90. doi: 10.1016/j.jep.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 41.Elrashidy R, Asker M. Pioglitazone attenuates cardiac fibrosis and hypertrophy in a rat model of diabetic nephropathy. J Cardiovasc. 2012;17:324–333. doi: 10.1177/1074248411431581. [DOI] [PubMed] [Google Scholar]
- 42.Gholamhoseinian A, Falah H, SHarififar F. Anti-hyperglycemic activity of four plants extracts effective against alpha glucosidase in normal and diabetic rats. J Kerman. 2009;15:35–44. [Google Scholar]
- 43.Kangani C, Kelley D, DeLany J. New method for GC/FID and GC-C-IRMS analysis of plasma free fatty acid concentration and isotopic enrichment. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873:95–101. doi: 10.1016/j.jchromb.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abdin AA, Baalash AA, Hamooda HE. Effects of rosiglitazone and aspirin on experimental model of induced type 2 diabetes in rats: focus on insulin resistance and inflammatory markers. J Diabetes Complications. 2010;24:168–178. doi: 10.1016/j.jdiacomp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Paradis S, Cabanac M, Marceau P, Frankham P. Body weight and satiation after duodenal switch:2 years later. Obes Surg. 2007;17:631–6. doi: 10.1007/s11695-007-9115-1. [DOI] [PubMed] [Google Scholar]
- 46.Lee YS, Cha BY, Choi SS, Choi BK, Yonezawa T, Teruya T, et al. Nobiletin improves obesity and insulin resistance in high-fat diet-induced obese mice. J Nutr Biochem. 2013;24:156–162. doi: 10.1016/j.jnutbio.2012.03.014. [DOI] [PubMed] [Google Scholar]
- 47.Liu X, Li G, Zhu H, Huang L, Liu Y, Ma C, et al. Beneficial effect of berberine on hepatic insulin resistance in diabetic hamsters possibly involves in SREBPs, LXRαand PPARαtranscriptional programs. Endocr J. 2010;57:881–893. doi: 10.1507/endocrj.k10e-043. [DOI] [PubMed] [Google Scholar]
- 48.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456–480. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bambace C, Telesca M, Zoico E, Sepe A, Olioso D, Rossi A, et al. Adiponectin gene expression and adipocyte diameter: A comparison between epicardial and subcutaneous adipose tissue in men. Cardiovasc Pathol. 2011;20:e153–156. doi: 10.1016/j.carpath.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- 51.Matsubara M, Maruoka S, Katayose S. Inverse relationship between plasma adiponectin and leptin concentrations in normal-weight and obese women. Eur J Endocrinol. 2002;147:173–180. doi: 10.1530/eje.0.1470173. [DOI] [PubMed] [Google Scholar]
- 52.Natali A, Natali A, Ferrannini E, Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: a systematic review. Diabetologia. 2006;49:434–441. doi: 10.1007/s00125-006-0141-7. [DOI] [PubMed] [Google Scholar]
- 53.Papaetis GS, Orphanidou D, Panagiotou TN. Thiazolidinediones and type 2 diabetes: from cellular targets to cardiovascular benefit. Curr Drug Targets. 2011;12:1498–1512. doi: 10.2174/138945011796818243. [DOI] [PubMed] [Google Scholar]
- 54.Elmazar MM, El-Abhar HS, Schaalan MF, Farag NA. Phytol/Phytanic acid and insulin resistance: potential role of phytanic acid proven by docking simulation and modulation of biochemical alterations. PLoS One. 2013;8:e45638. doi: 10.1371/journal.pone.0045638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sakamoto J, Kimura H, Moriyama S, Odaka H, Momose Y, Sugiyama Y, et al. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem Biophys Res Commun. 2000;278:704–711. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- 56.Ye JM, Tid-Ang J, Turner N, Zeng XY, Li HY, Cooney GJ, et al. PPARδagonists have opposing effects on insulin resistance in high fat-fed rats and mice due to different metabolic responses in muscle. Br J Pharmacol. 2011;163:556–566. doi: 10.1111/j.1476-5381.2011.01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Derosa G, Maffioli P. Peroxisome proliferator-activated receptor- γ(PPAR- γ) agonists on glycemic control, lipid profile and cardiovascular risk. Curr Mol Pharmacol. 2012:272–281. doi: 10.2174/1874467211205020272. [DOI] [PubMed] [Google Scholar]
- 58.Olson AL. Insulin resistance: cross-talk between adipose tissue and skeletal muscle, through free fatty acids, liver X receptor, and peroxisome proliferator-activated receptor-αsignaling. Horm Mol Biol Clin Investig. 2013;15:115–121. doi: 10.1515/hmbci-2013-0019. [DOI] [PubMed] [Google Scholar]
- 59.Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms. J Endocrinol. 2014;220:T1–23. doi: 10.1530/JOE-13-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]


