Abstract
Objective(s):
The objectives of the current study were to evaluate the effects of hepatic ischemia/reperfusion (IR) injury on the activity of antioxidant enzymes, biochemical factors, and histopathological changes in rat kidney, and to investigate the effect of crocin on IR-related changes.
Materials and Methods:
Thirty-two male Wistar rats were randomly allocated into four groups (n=8). They were sham-operated, IR, crocin pre-treatment, and crocin pretreatment+IR groups. Sham-operated and Crocin pre-treatment groups received normal saline (N/S, 2 ml/day) and crocin (200 mg/kg) for seven consecutive days intraperitoneally (IP), respectively, then rats underwent laparotomy, only. IR and crocin pretreatment+IR groups received N/S and crocin with the same dose, time, and route, respectively, then rats underwent partial (70%) ischemia for 45 min that was followed by reperfusion for 60 min. At the end of the experiment, kidney specimens were taken for histopathological and antioxidant evaluations and also blood samples were obtained for biochemical analysis.
Results:
The results of the present study showed that crocin pre-treatment significantly increased the activity of antioxidants, decreased the serum levels of liver enzymes and blood urea nitrogen following IR-induced hepatic injury. Crocin also ameliorated kidney’s histopathological disturbance beyond IR-induced hepatic injury.
Conclusion:
Crocin as an antioxidant agent protected renal insult following liver IR injury by increasing the activity of antioxidant enzymes, reducing serum levels of liver enzymes, and improving histopathological changes.
Keywords: Antioxidant, Crocin, Ischemia/reperfusion Kidney, Liver, Rat
Introduction
Hepatic ischemia-reperfusion (IR) injury is a major complication which frequently occurs beyond liver graft, hepatectomy, liver trauma, and hepatic pedicle clamping during liver surgery (1). This phenomenon may affect other remote organs such as kidneys and the heart, due to hazardous agents release (2). The incidence of renal injury following liver IR is 40–85% which represents high mortality and morbidity (3).
The pathophysiology of insult to the renal tissue after liver IR is oxidative stress, particularly, reactive oxygen species (ROS) (4). It has been reported that free radicals can destroy endothelial, tubular epithelial, and mesangial cells, especially in proximal tubules of the kidney (5). In addition, beyond partial IR-induced liver injury, acute tubular damage may occur concomitantly with tubular necrosis and biochemical and inflammatory changes (6).
Furthermore, liver IR may lead to oxidative stress in kidneys via increasing the levels of malondialdehyde and decreasing the activity of superoxide dismutase (SOD), and catalase (CAT). These enzymes have protective effects against IR-induced cell insult through scavenging of ROS in intracellular space (6). In addition, CAT is an oxidoreductase enzyme, has ROS scavenger properties and protects cells against IR-induced damage with conversion of H2O2 into water and oxygen (7).
In recent years, phytomedicine has become an alternative remedy in developing countries for various aspects of health management (8). Various plant extracts and their ingredients such as flavonoids and phenolic compounds (9), due to their redox characteristics have antioxidant activity and are able to collect free radicals (10).
Crocin (Figure 1) a water soluble carotenoid (11), is the most abundant antioxidant compound of Crocus sativus linn stigma (saffron) and responsible for the red color of saffron (12). It has been shown that crocin has antiapoptotic, anti-inflammatory, and antioxidant properties (13). Moreover, reno- (14), gastro- (15), and retino-protective (16) effects of crocin against IR-induced injury are well established.
Figure 1.
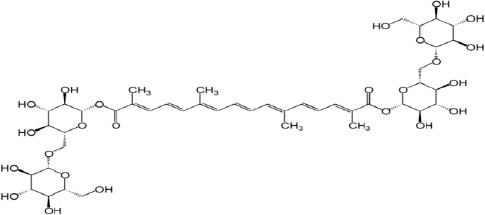
Chemical structure of crocin (C44H64O24)
Therefore, the protective effect of crocin as a potent antioxidant against renal IR-induced injury is well documented but to the best of our knowledge, its action on renal injury following liver IR-induced damage has not been determined. Therefore, the present study aimed to evaluate the effects of hepatic IR injury on the activity of antioxidant enzymes, biochemical factors, and histopathological changes in the rat kidney, and to investigate the effect of crocin on IR-related changes.
Materials and Methods
Animal
Male Wistar rats (200–250 g) were purchased from the animal house of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Animals were fed a conventional diet and tap water ad libitum. All protocols were in accordance with The Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (RDC-9416). Animals were housed in polyethylene cages in an air-conditioned room with controlled temperature 22 ±2 °C and under 12/12 hr light/dark cycle. Animals were deprived of food but not water overnight before experiments.
Animal grouping
In the current study, 32 rats were randomly assigned into four groups (8 in each). They were:
Sham-operated group: This group of rats received normal saline (N/S, 2 ml/day, seven consecutive days, IP) before surgery (17). Then animals underwent laparotomy without vascular occlusion.
IR group: Animals in this group received N/S (2 ml/day) with the same time and route as before surgery, then IR induction was performed.
Crocin pre-treatment group: This group of animals received crocin (200 mg/kg, seven consecutive days, IP) before surgery (18), then they underwent laparotomy without vascular occlusion.
Crocin pre-treatment+IR group: Animals received crocin (200 mg/kg, seven consecutive days, IP) before surgery, then they were subjected to IR induction.
Surgical procedure
Under anesthesia with a combination of ketamine (ketamine 10%, alfasan, Woerden-Holland, 80 mg/kg, IP) and xylazine (xylazine 2%, alfasan, Woerden-Holland, 10 mg/kg, IP) (19) rats underwent a surgical procedure. In summary, a small incision was made in the midline of the abdomen, then partial ischemia (70%) was induced via occlusion of portal triad (artery, vein, and bile duct) with an atraumatic microvascular clamp for 45 min, afterward, the clamp was removed and reperfusion established for 60 min. Liver ischemia was confirmed by prompt liver color change to a color pallor shade (4). At the end of the procedure, kidney tissue samples were obtained for evaluating the levels of antioxidant and histopathological changes. Also, blood sample was taken for biochemical factors measurement.
Biochemical assay
To determine the serum levels of liver enzymes (alanine aminotransaminase (ALT) and aspartate aminotransaminase (AST)), serum was separated by blood centrifugation at 3000 rpm for 5 min. Blood urea nitrogen (BUN) and creatinine were also measured as renal functional tests by commercial kits (Pars Azmoon) according to the manufacturer’s instructions and read by a serum autoanalyzer (BT-1500-A-A, Rome, Italy).
Measurement of antioxidants
To determine the activity of antioxidant enzymes (SOD, CAT, and glutathione peroxidase (GPX)) in kidney tissue, 1 ml of phosphate buffered saline (PBS, pH: 7.4) was added to 100 mg of each sample, homogenized for 3 min at 14,000 rpm by using a homogenizer (20). Then the homogenate was centrifuged for 20 min at 12000 rpm at +4 °C. The supernatant was administered for evaluating the activity of antioxidants by commercial kits (Zellbio GmbH, Germany) according to the manufacturer’s instructions and read by micro plate/ ELISA reader. Enzyme activity is expressed as unit per milliliter (U/ml).
Histopathological analysis
For histopathological evaluations, kidney tissue was fixed in 10% neutral formalin solution, after 72 hr, automatically dehydrated through a graded series of alcohols, embedded in paraffin. Afterward, it was cut into 5 μm sections using a microtome (Leica RM 2125, Leica Microsystems Nussloch GmbH, Germany) and stained with Hematoxylin and Eosin (12). Images were taken and assessed under a digital research microscope (BMZ-04-DZ, Behin Pajouhesh. ENG. CO. Iran). Renal specimens were evaluated for glomerular necrosis, dilation of Bowman’s capsule, necrosis in tubular epithelium, and congestion of blood vessels (21).
Statistical analysis
Data are shown as mean±standard errors of the mean (SEM). Statistical analysis was performed by one-way analysis of variance (ANOVA), followed by Dennett’s test. P<0.05 was considered statistically significant.
Results
Effects of crocin pretreatment on serum levels of ALT and AST following IR-induced hepatic injury
As shown in Figures 2A and 2B, pretreatment with crocin did not significantly affect the serum levels of AST and ALT in comparison with the sham group. These Figures also showed that 45 min liver ischemia followed by 60 min reperfusion increased the serum levels of AST and ALT in comparison with the sham group (P<0.001 and P<0.01, respectively). As presented in Figures 2A and 2B, serum levels of AST and ALT in crocin-pretreated IR rats were significantly lower than in the IR group (P<0.01 and P<0.001, respectively).
Figure 2.
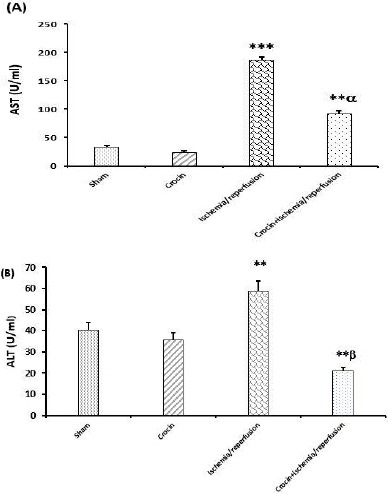
Effects of crocin pretreatment (200 mg/kg, IP, seven consecutive days) on serum levels of AST and ALT following ischemia/reperfusion-induced hepatic injury. Results expressed as mean±SEM, n=8. ∗P<0.05, ∗∗P<0.01 and ∗∗∗P<0.001 significant difference vs the sham group. αP<0.01, βP<0.001 significant difference vs the ischemia/reperfusion group. ALT: Alanine aminotransferase and AST: Aspartate aminotransferase
Effects of crocin pretreatment on serum levels of BUN and creatinine following IR-induced hepatic injury
As shown in Figures 3A and 3B, crocin pretreatment had no significant effects on serum levels of BUN and creatinine in comparison with the sham group. Figure 3A also shows that 45 min liver ischemia followed by 60 min reperfusion increased the serum level of BUN in comparison with the sham group (P<0.01). As presented in this Figure, serum levels of BUN in crocin-pretreated IR rats were significantly lower than in the IR group (P<0.01). As shown in Figure 3B, pretreatment with crocin did not affect the serum level of creatinine compared to other groups (P>0.05).
Figure 3.
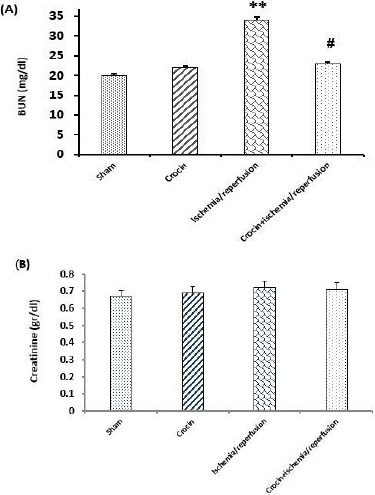
Effects of crocin pretreatment on serum levels of BUN (A) and creatinine (B) following hepatic ischemia/reperfusion injury. Data are expressed as mean±SEM, n=8, ∗∗P<0.01 significant difference vs the sham group. #P<0.05 significant difference vs the ischemia/reperfusion. Blood urea nitrogen; mg/dl: milligram/deciliter and g/dl: gram/deciliter
Effects of crocin pretreatment on SOD, CAT, and GPX activities in kidney tissue following IR-induced hepatic injury
Figure 4 (A-C), demonstrates that crocin pretreatment had significant effects on SOD and CAT activities in comparison with the sham group (P<0.05) but did not affect the activity of GPx. These Figures also show that 45 min liver ischemia followed by 60 min reperfusion significantly decreased the activity of SOD, CAT, and GPx in kidney homogenate in comparison with the sham group (P<0.05, P<0.05, and P<0.01, respectively). As presented in Figure 4 (A-C), the levels of SOD, CAT, and GPx activities in crocin pretreated IR rats were higher than in the IR group (P<0.01).
Figure 4.
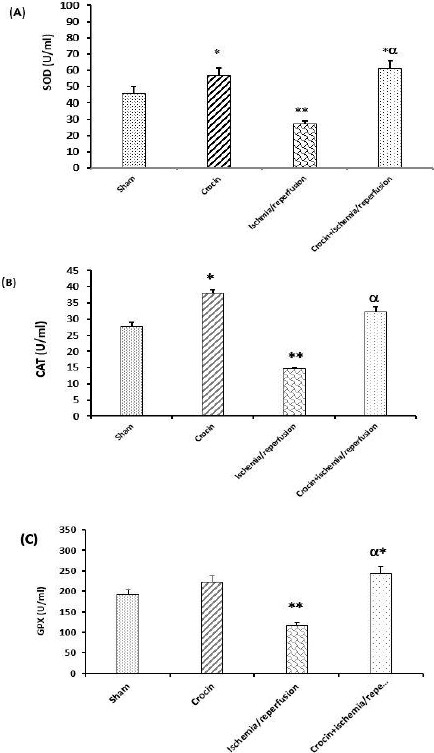
Effects of crocin pretreatment on the activity of antioxidant enzymes of SOD (A), CAT (B), and GPX (C) following hepatic ischemia/reperfusion-induced renal injury. Results expressed as mean±SEM, n=8. ∗P<0.05 and ∗∗P<0.01 significant difference vs sham group. αP<0.01 vs ischemia/reperfusion group. SOD: Superoxide dismutase; CAT: Catalase and GPX: Glutathione peroxidase
Crocin pretreatment ameliorated histopathological changes in kidney tissue following hepatic IR injury
As shown in Figure 5B histopathological alterations including hemorrhage and congestion of kidney blood vessels were seen following 45 min liver ischemia followed by 60 min reperfusion-induced hepatic injury. Crocin pretreatment (200 mg/kg, IP, seven consecutive days) effectively but not completely prevented these changes as illustrated in Figure 5D. As shown in Figures 5A and 5C the appearance of kidney tissue was normal in sham and crocin groups.
Figure 5.
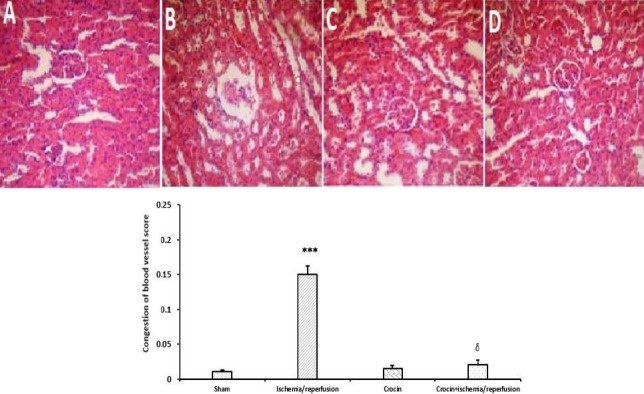
Representative microscopic images (magnification×300) of H&E stained kidney sections following hepatic IR injury. A. Sham-operated group: showed normal appearance; B: Ischemia/reperfusion group: showed moderate hemorrhage and congestion of kidney blood vessels; C. Crocin pretreatment group: showed approximately normal architecture. Crocin pretreatment+IR group: showed almost normal appearance. Data are expressed as mean±SEM, n=5, ∗∗∗P<0.001 significant difference vs the sham group and δP<0.001 significant difference vs the ischemia/reperfusion
Discussion
The present study demonstrated that crocin pretreatment prevented IR-induced hepato-renal injury in rats through 1. Reducing the serum levels of AST, ALT, and BUN; 2. Increasing antioxidant enzyme activities of SOD, CAT, and GPX in the kidney homogenate; and 3. Ameliorating the structural alterations of the renal tissue.
It has been shown that the serum levels of AST and ALT elevate beyond IR-induced liver insult (4, 22). A rise in plasma levels of AST and ALT often represents liver cell injury, but these parameters are not specific to the liver (23). The present study showed a significant increase in the serum levels of these enzymes beyond IR-induced hepatic injury in rats, and crocin pretreatment significantly prevented these increases. In agreement, it has been reported that crocin reduced the level of these enzymes after nicotine-induced hepatic injury (12).
Consistent with a previous study (24), the findings of the current study show an increase in serum level of BUN after IR-induced hepatic injury. The present results show that the level of BUN in crocin-pretreated hepatic IR rats was significantly lower than in IR group. Therefore, our results showed that crocin pretreatment improved the kidney function by improving the serum concentration of BUN.
Creatinine is an important biomarker for indicating glomerular filtration rate but it is reported that following hepatic IR injury the serum level of this marker decreased owing to disability of the liver to convert creatine to creatinine; therefore, renal secretion of this parameter was enhanced (3). In line, in this study, we showed that following IR-induced hepatic injury, the serum level of creatinine did not change.
Several studies reported that beyond IR-induced liver injury, mild to moderate changes can occur in the architecture of the renal tissue. These studies reported the structural changes occurred concomitantly with biochemical alterations (3, 25). In this regard, one hypothesis implies that following portal hypertension, splanchnic vasodilation may occur (26), which leads to systemic hypotension and activation of reninangiotensin system secondary to intrarenal ischemia. Finally, hypoxia leads to renal tubular necrosis and disturbances in this organ (27). The present histopathological results indicate that pretreatment with crocin effectively but not entirely inhibits destructive effects of renal tissue following IR-induced hepatic injury.
In the present study, we showed that the activity of SOD, CAT, and GPx decreased in the renal tissue after IR-induced hepatic injury. In agreement, one study reported that mRNA expression of SOD and CAT decreased following oxidative stress (28). It has been suggested that crocin protected the renal tissue against IR injury partly by increasing the antioxidant enzyme activities of SOD and CAT (5).
Recently, the authors have shown that crocin pretreatment protected the liver against IR-induced injury through increasing the activity of antioxidant enzymes (SOD, CAT, and GPx) in liver homogenate in rats (29). Moreover, in another study, authors demonstrated that crocin pretreatment protected the gastric mucosa against IR-induced insult via upregulating the mRNA expression and activity of antioxidant enzymes in rats (30). The present results also showed that the activity of all studied antioxidant enzymes (SOD, CAT, and GPx) increased after crocin pretreatment.
Conclusion
This study demonstrated that crocin pretreatment has renoprotective action following IR-induced hepatic injury through increasing the activity of antioxidant enzymes, improving renal function tests, and also improving histopathological changes in the kidney tissue. Therefore, this natural substance may be effective for perioperative hepatorenal problems.
Acknowledgment
The authors would like to gratefully acknowledge the financial support of the Vice Chancellor of Ahvaz Jundishapur University of Medical Sciences and Physiology Research Center (grant no. RDC-9416), Ahvaz, Iran.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Salomão LS, Young SB, Galhardo MA, Pereira LA, Pires ARC, Boaventura GT, et al. Evaluation of liver regeneration by modulation with ischemic preconditioning after ischemia and reperfusion and partial hepatectomy. Rev Col Bras Cir. 2012;39:211–215. [PubMed] [Google Scholar]
- 2.Tanaka Y, Maher JM, Chen C, Klaassen CD. Hepatic ischemia-reperfusion induces renal heme oxygenase-1 via NF-E2-related factor 2 in rats and mice. Mol Pharmacol. 2007;71:817–825. doi: 10.1124/mol.106.029033. [DOI] [PubMed] [Google Scholar]
- 3.Lee HT, Park SW, Kim M, D'Agati V. Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab Invest. 2009;89:196–208. doi: 10.1038/labinvest.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sözen S, Kisakürek M, Yildiz F, Gönültas M, Dinçel A. The effects of glutamine on hepatic ischemia reperfusion injury in rats. Hippokratia. 2011;15:161–166. [PMC free article] [PubMed] [Google Scholar]
- 5.Hosseinzadeh H, Sadeghnia HR, Ziaee T, Danaee A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J Pharm Pharm Sci. 2005;8:387–393. [PubMed] [Google Scholar]
- 6.Kadkhodaee M, Mikaeili S, Zahmatkesh M, Golab F, Seifi B, Arab H-A, et al. Alteration of renal functional, oxidative stress and inflammatory indices following hepatic ischemia-reperfusion. Gen Physiol Biophys. 2012;31:195–202. doi: 10.4149/gpb_2012_024. [DOI] [PubMed] [Google Scholar]
- 7.Yabe Y, Kobayashi N, Nishihashi T, Takahashi R, Nishikawa M, Takakura Y, et al. Prevention of neutrophil-mediated hepatic ischemia/reperfusion injury by superoxide dismutase and catalase derivatives. J Pharmacol Exp Ther. 2001;298:894–899. [PubMed] [Google Scholar]
- 8.Jin G, Prabhakaran MP, Kai D, Annamalai SK, Arunachalam KD, Ramakrishna S. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials. 2013;34:724–734. doi: 10.1016/j.biomaterials.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Ebrahimzadeh MA, Nabavi SM, Nabavi SF, Bahramian F, Bekhradnia AR. Antioxidant and free radical scavenging activity of H. officinalis L. var. angustifolius, V. odorata, B. hyrcana and C. speciosum. Pak J Pharm Sci. 2010;23:29–34. [PubMed] [Google Scholar]
- 10.Padma VV, Sowmya P, Felix TA, Baskaran R, Poornima P. Protective effect of gallic acid against lindane induced toxicity in experimental rats. Food Chem Toxicol. 2011;49:991–998. doi: 10.1016/j.fct.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Abdullaev FI. Biological effects of saffron. Biofactors. 1993;4:83–86. [PubMed] [Google Scholar]
- 12.Jalili C, Tabatabaei H, Kakaberiei S, Roshankhah S, Salahshoor MR. Protective role of Crocin against nicotine-induced damages on male mice liver. Int J Prev Med. 2015;6:92. doi: 10.4103/2008-7802.165203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mard SA, Pipelzadeh MH, Teimoori A, Neisi N, Mojahedin S, Khani MZS, et al. Protective activity of crocin against indomethacin-induced gastric lesions in rats. J Nat Med. 2016;70:62–74. doi: 10.1007/s11418-015-0938-0. [DOI] [PubMed] [Google Scholar]
- 14.Najafi H, Yarijani ZM, Pourmotabed A, Madani SH. The effect of Crocin on the renal tissue damages and leukocyte infiltration induced by ischemia-reperfusion among rats. J Kermanshah Univ Med Sci. 2013;17:394–403. [Google Scholar]
- 15.Mard SA, Nikraftar Z, Farbood Y, Mansouri E. A preliminary study of the anti-inflammatory and anti-apoptotic effects of crocin against gastric ischemia-reperfusion injury in rats. Braz J Pharm Sci. 2015;51:637–642. [Google Scholar]
- 16.Qi Y, Chen L, Zhang L, Liu W-B, Chen X-Y, Yang X-G. Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp Eye Res. 2013;107:44–51. doi: 10.1016/j.exer.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Naghizadeh B, Boroushaki MT, Vahdati Mashhadian N, Mansouri MT. Protective effects of crocin against cisplatin-induced acute renal failure and oxidative stress in rats. Iran Biomed J. 2008;12:93–100. [PubMed] [Google Scholar]
- 18.Naghizadeh B, Mansouri SMT, Mashhadian NV. Crocin attenuates cisplatin-induced renal oxidative stress in rats. Food Chem Toxicol. 2010;48:2650–2655. doi: 10.1016/j.fct.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 19.Askaripour M, Fatemi-Tabatabaei SR, Hosseini F, Rashno M, Ghaderi S. Effects of aqueous extract of purslane (Portulaca oleracea) on hepatic enzymes in Two models of renal ischemia-reperfusion injury in rats. Zahedan J Res Med Sci. 2015:29–31. [Google Scholar]
- 20.Tüfek A, Tokgöz O, Aliosmanoglu İ, Alabalik U, Evliyaoglu O, Çiftçi T, et al. The protective effects of dexmedetomidine on the liver and remote organs against hepatic ischemia reperfusion injury in rats. Int J Surg. 2013;11:96–100. doi: 10.1016/j.ijsu.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Kiris I, Kapan S, Kılbas A, Yılmaz N, Altuntaş I, Karahan N, et al. The protective effect of erythropoietin on renal injury induced by abdominal aortic-ischemia-reperfusion in rats. J Surg Res. 2008;149:206–213. doi: 10.1016/j.jss.2007.12.752. [DOI] [PubMed] [Google Scholar]
- 22.Basbug M, Yildar M, Yaman I, Ozkan OF, Aksit H, Cavdar F, et al. Effects of boric acid in an experimental rat model of hepatic ischemia-reperfusion injury. Acta Medica Mediterr. 2015;31:1067–1073. [Google Scholar]
- 23.Unsal C, Celik J, Toy H, Esen H, Otelcioglu S. Protective role of zinc pretreatment in hepatotoxicity induced by halothane. Eur J Anaesthesiol. 2008;25:810–815. doi: 10.1017/S0265021508004523. [DOI] [PubMed] [Google Scholar]
- 24.Seifi B, Kadkhodaee M, Delavari F, Mikaeili S, Shams S, Ostad SN. Pretreatment with pentoxifylline and N-acetylcysteine in liver ischemia reperfusion-induced renal injury. Ren Fail. 2012;34:610–615. doi: 10.3109/0886022X.2012.660827. [DOI] [PubMed] [Google Scholar]
- 25.Polat C, Tokyol Ç, Kahraman A, Sabuncuoğlu B, Yìlmaz S. The effects of desferrioxamine and quercetin on hepatic ischemia–reperfusion induced renal disturbance. Prostaglandins Leukot Essent Fatty Acids. 2006;74:379–383. doi: 10.1016/j.plefa.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Schepke M. Hepatorenal syndrome: current diagnostic and therapeutic concepts. Nephrol Dial Transplant. 2007;22:viii2–viii4. doi: 10.1093/ndt/gfm656. [DOI] [PubMed] [Google Scholar]
- 27.Wadei HM, Mai ML, Ahsan N, Gonwa TA. Hepatorenal syndrome: pathophysiology and management. Clin J Am Soc Nephrol. 2006;1:1066–1079. doi: 10.2215/CJN.01340406. [DOI] [PubMed] [Google Scholar]
- 28.Rajaei Z, Hadjzadeh M-A-R, Nemati H, Hosseini M, Ahmadi M, Shafiee S. Antihyperglycemic and antioxidant activity of crocin in streptozotocin-induced diabetic rats. J Med Food. 2013;16:206–210. doi: 10.1089/jmf.2012.2407. [DOI] [PubMed] [Google Scholar]
- 29.Akbari G, Mard SA, Dianat M, Mansouri E. The Hepatoprotective and MicroRNAs downregulatory effects of crocin following hepatic ischemia-reperfusion injury in rats. Oxid Med Cell Longev. 2017;2017:1702967. doi: 10.1155/2017/1702967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mard SA, Azad M, Ahangarpour A. Protective effect of crocin on gastric mucosal lesions induced by ischemia-reperfusion. Iran J Pharm Res. 2016;15:93–99. [PMC free article] [PubMed] [Google Scholar]


