Abstract
Objective(s):
Metastasis is the main cause of death in patients with melanoma. Cannabis-based medicines are effective adjunctive drugs in cancer patients. Tau and Stathmin proteins are the key proteins in cancer metastasis. Here we have investigated the effect of a standardized Cannabis sativa extract on cell migration and Tau and Stathmin gene expression in the melanoma cell line.
Materials and Methods:
In the treatment group, melanoma (B1617) was treated 48 hr with various concentrations of standardized C. sativa extract. Cells with no treatment were considered as the control group, then study was followed by Quantitative RT-Real Time PCR assay. Relative gene expression was calculated by the ΔΔct method. Migration assay was used to evaluate cancer metastasis.
Results:
Tau and stathmin gene expression was significantly decreased compared to the control group. Cell migration was also significantly reduced compared to controls.
Conclusion:
C. sativa decreased tau and stathmin gene expression and cancer metastasis. The results may have some clinical relevance for the use of cannabis-based medicines in patients with metastatic melanoma.
Keywords: Cannabis plant extracts, Endothelial cells, Melanoma, Metastasis, Stathmin, Tau protein
Introduction
Melanoma is the most malignant skin cancer, incidence rate of which increases over time. At present no effective treatment exists for advanced melanoma. Metastasis accounts for the vast majority of death associated with malignant melanoma (1).
Metastasis is a complex phenomenon. Melanoma cells detach from the main tumor, reach blood circulation and migrate to distinct organs in order to form metastases. High motility of cancer cells is important during these stages (2, 3). Recent studies indicate that cellular cytoskeleton binding proteins mediate cell motility (4-6). Therefore, these proteins may have promising effects to attenuate the metastatic properties of melanoma cells (7).
Microtubules are components of the cytoskeleton; they have critical roles in cell invasion and motility (8, 9). Tau regulates the intracellular dynamic behavior of microtubules (10). Tau is part of the MAP2 family and increases microtubule assembly. Basically, tau is known as a neuronal MAP and is involved in neurite outgrowth. In cells attached to an extracellular matrix (ECM), tau enhances the extension of microtubule- based neurite-like protrusions (11-14).
Stathmin (STMN1) is an oncoprotein that has a critical role in the regulation of mitosis (15). Increased expression of STMN1 has been seen in different malignancies, especially melanoma. (16). It can precede tumor development and metastasis. High level of STMN1 expression is associated with poor prognosis in cancer patients (17, 18).
Cannabis sativa (marijuana) is a plant which is used both illegally and medicinally (19). The active compounds of this plant are cannabinoids. They exert their effects by binding to CB1 (central) and CB2 (peripheral) receptors (20, 21). Both types of cannabinoid receptors have been widely studied in cancer development, tumor progression, invasion, and metastasis models in vivo and in vitro. The preclinical data support the role of plant-derived CBs in inhibition of tumor progression. It has been proposed that cannabinoids can reduce the expression of tau protein in neuroblastoma (22, 23). So in this study, we aimed to evaluate the effect of standardized C. sativa extract on tau and STMN1 expression in melanoma cell line and its effect on cancer invasion.
Materials and Methods
Drugs
The extract has been prepared by Berij Esans (Kashan, Iran). Briefly, the seeds of C. sativa were collected from Iranian plant species and dried in the shade (25 °C) by the air drying method for 7 days and then, were ground using an electric grinder. The extract was obtained by the maceration method with 80% ethanol for 48 hr. It was standardized with 4% cannabidiol and cells were treated with different doses of 10, 50, and 100 µgr/ml.
Cell culture
B16F10 melanoma cells (cell bank of Pasteur Institute, Iran) were purchased from the Pasteur Institute (Tehran, Iran) and cultured in DMEM containing 10% FBS, 1 g/l glucose, 1% L-glutamine, and 1% penicillin-streptomycin at 37 °C in a CO2 atmosphere. They were incubated at 37 °C with 5% CO2 and 95% O2 concentration. The medium was changed every day and the third passage cells after reaching 80% confluency were used for experiments.
Real-time polymerase chain reaction (RT-PCR)
RT- PCR experiment using the rotor gene 6000 RT- PCR detection system (Corbett Research, Australia) was performed in order to determine tau and STMN1 gene expression. 1 µg of RNA was reverse transcribed using Quantitect Rev, transcription kit (Qiagen, Germany) and the complementary DNA (cDNA) was used for Real-time quantitative PCR. To make standard curves, 5-fold serial dilution of melanoma cDNA was used. For SYBR Green assay, primers were designed, using Beacon Designer (ver. 8.0), that span exon-junctions and were synthesized by TIB molbiol (TIB molbiol, Germany): tau forward primer 5-CAACAGCGGAAGATGTGACA, reverse primer: 5’-ACCATCTTGTACCGGAGAG-3’, STMN forward primer CTCGGACTGAGCAGGACTT, and reverse primer GGTGAATAGAAGACAAGCGACAG. The reaction mixture (25 µl) including 2 µl of cDNA template, 1.5 µl each of primers and Quantitect SYBR Green master mix (Qiagen, Germany) was amplified based on the SYBR Green method. Measuring the fluorescence produced due to SYBR Green dye binding to dsDNA after every cycle was used to determine direct detection of PCR products. Amplification efficiencies were tested for the gene of interest (GOI) and the housekeeping gene. In order to normalize data due to variations in RNA quality and quantity, all samples were assayed with the reference gene Beta Actin (ACTB). All samples were performed in triplicate.
Invasion assay
The in vitro invasion assay was performed in the uncoated membrane using transwell inserts with an 8-μm pore size (SPL, Germany). Melanoma was treated with different doses of standardized C. sativa (10, 50, 100 μg/ml) for 48 hr, whilst the control group did not receive treatment. Cells (1×104 in 200 μl serum-free medium) were seeded into the top chamber. After 48 hr incubation, non-migrated cells were removed from the top of the membrane. Invading cells were fixed and stained with a 0.05% (w/v) crystal violet solution. They were counted in five random microscopic fields (×200). Experiments were performed three times in triplicate.
Results
The effect of C. sativa on Tau protein expression
As it can be seen in Figure 1, C. sativa significantly reduces the expression of the tau gene. The most robust result was seen at the doses of 100 (µg/ml) after 48 hr of incubation.
Figure 1.
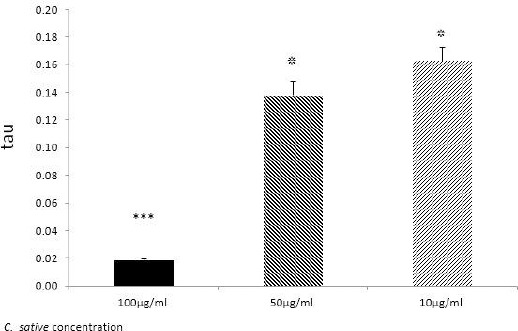
Tau expression level in melanoma cells, detected by Real-time PCR. After 48 hr incubation of treated melanoma cells with 100, 50, and 10 dose of Cannabis sativa extract (µgr/ml); *P≤0.05; ***P≤0.05
The effect of C. sativa on Stathmin protein expression
Figure 2 represents the effect of the extract on stathmin gene expression, however 10 µg/ml of extract had the most significant effect on gene expression.
Figure 2.
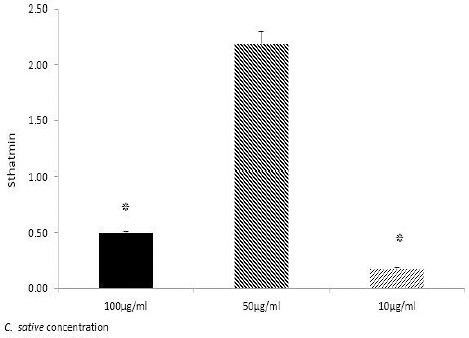
Stathmin expression level in melanoma cells, detected by Real-time PCR. After 48 hr incubation of treated melanoma cells with 100, 50, and 10 dose of Cannabis sativa extract (µg/ml); *P≤0.05; ***P≤0.05
The effect of C. sativa on melanoma cell invasion
C. sativa decreased melanoma cell line invasion significantly (Figure 3).
Figure 3.
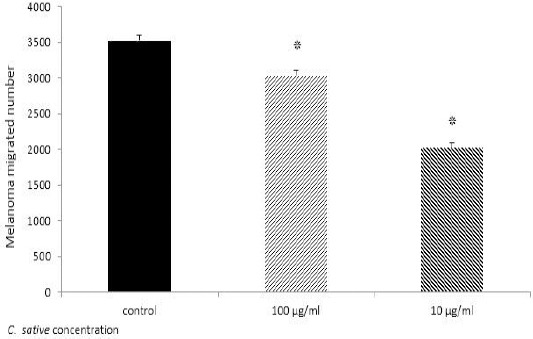
Melanoma migration assay. After 48 hr incubation of treated Melanoma with 10 and 100 μg/ml standardized Cannabis sativa and no treated cells, the number of migrated cells across membrane counted by flowcytometry. Results indicate 100 and 10 μg/ml standardized C. sativa significantly decreased migration of Melanoma; The result compared with control *P≤0.05
Discussion
To the best of our knowledge, this is the first study to investigate the effects of C. sativa extract on tau and stathmin protein expression in cancer cell line.
Microtubule-associated proteins (MAPs) are part of the cytoskeleton and have different functional roles. Tau protein is a part of MAPs, which regulates microtubules dynamic behavior (10). It has been studied in different types of cancer. It has been proposed that tau expression decreases chemotherapy response in cancer patients. Tau is the most expressed gene during paclitaxel chemotherapy (24). Growing evidence indicates that tau contributes to the metastatic efficiency of breast tumor cells. Tau-overexpressing cell lines are more trapped compared to tau-deficient cells in in vitro metastasis assays, however, tau can provide a selective advantage during metastasis in cancer patients (25). So, anti-tau drugs may hold potential to inhibit tumor metastasis. As it can be seen, C. sativa extract effectively reduced tau expression in melanoma.
STMN1 interferes with microtubule dynamics and promotes depolymerization and/or inhibits microtubule formation (26). STMN1 also promotes cancer cell proliferation, differentiation, migration, and invasion (27). In addition, a high stathmin level in tumor tissues predicts a poor overall survival (28), a previous study has shown that complete down-regulation of stathmin expression by using an siRNA treatment strategy in osteosarcoma cell lines, led to significant inhibition of in vitro proliferation and colony formation (29). In our study, we showed that C. sativa significantly decreased stathmin expression. We also observed that extract reduced expression in the melanoma cell line.
There are some results from preclinical studies which show the beneficial effects of cannabinoids, however, use of them is still restricted to palliative therapies (30). Cannabinoid and cannabinoid agonists exert anticancer actions in preclinical models of cancer through a well-established mechanism of action. It has been shown that cannabinoids enhance the anticancer activity of TMZ and ALK inhibitors in animal models of cancer (31).
Anti-tumorigenic effects of cannabinoids are shown to be mediated via CB1 and CB2 depending on cancer cell types (32). However, many antitumorigenic effects of cannabinoids have been associated with molecular events independent of cannabinoid receptors (33).
The role of cannabinoids in cancer metastasis and invasion is new. It has been demonstrated that increased ICAM-1 levels, which are enhanced by CBD, can decrease tumor cell invasion and metastasis (34).
Conclusion
In this study, we demonstrated that C. sativa can significantly reduce the migration of melanoma cell line and part of this effect is due to reduction in tau and stathmin gene expression.
More preclinical and clinical studies are needed to evaluate the advantage of these substances for cancer patients.
Acknowledgment
This study was supported by Isfahan University of Medical Sciences, Isfahan, Iran. The authors thank the staffs of the Applied Physiology Research Centre.
References
- 1.Damsky WE, Rosenbaum LE, Bosenberg M. Decoding melanoma metastasis. Cancers (Basel) 2011;3:126–163. doi: 10.3390/cancers3010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 3.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights andevolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang ZL, Miao X, Xiong L, Zou Q, Yuan Y, Li J, et al. CFL1 and Arp3 are biomarkers for metastasis and poor prognosis of squamous cell/adenosquamous carcinomas and adenocarcinomas of gallbladder. Cancer Invest. 2013;31:132–139. doi: 10.3109/07357907.2012.756113. [DOI] [PubMed] [Google Scholar]
- 5.Byrne FL, Yang L, Phillips PA, Hansford LM, Fletcher JI, Ormandy CJ, et al. Kavallaris M: RNAi-mediated stathmin suppression reduceslung metastasis in an orthotopic neuroblastoma mouse model. Oncogene. 2013;10:1038. doi: 10.1038/onc.2013.11. [DOI] [PubMed] [Google Scholar]
- 6.Stevenson RP, Veltman D, Machesky LM. Actin-bundling proteins in cancer progression at a glance. J Cell Sci. 2012;125:1073–1079. doi: 10.1242/jcs.093799. [DOI] [PubMed] [Google Scholar]
- 7.Riplinger SM, Wabnitz GH, Kirchgessner H, Jahraus B, Lasitschka F, Schulte B, et al. Metastasis of prostate cancer and melanoma cells in a preclinical in vivo mouse model is enhanced by L-plastin expression and phosphorylation. Mol Cancer. 2014;13:10. doi: 10.1186/1476-4598-13-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden JH, Bowser SS, Rieder CL. Kinetochores capture astral microtubules during chromosome attachment to the mitotic spindle: direct visualization in live newt lung cells. J Cell Biol. 1990;111:1039–1045. doi: 10.1083/jcb.111.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai Y, Kronebusch PJ, Simon PM, Borisy GG. Microtubule dynamics at the G2/Mtransition: abrupt breakdown of cytoplasmic microtubules at nuclear envelope breakdown and implications for spindle morphogenesis. J Cell Biol. 1996;135:201–214. doi: 10.1083/jcb.135.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andersen SSL. Spindle assembly and the art of regulating microtubule dynamics byMAPs and stathmin/Op18. Trends Cell Biol. 2000;10:261–267. doi: 10.1016/s0962-8924(00)01786-4. [DOI] [PubMed] [Google Scholar]
- 11.Cassimeris L, Spittle C. Regulation of microtubule-associated proteins. Int Rev Cytol. 2001;210:163–226. doi: 10.1016/s0074-7696(01)10006-9. [DOI] [PubMed] [Google Scholar]
- 12.Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975;72:1858–1868. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goedert M. Tau protein and neurodegeneration. Semin Cell Dev Biol. 2004;15:45–49. doi: 10.1016/j.semcdb.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Knops J, Kosik KS, Lee G, Pardee JD, Cohen-Gould L, McConlogue L. Overexpression of tau in a nonneuronal cell induces long cellular processes. J Cell Biol. 1991;114:725–733. doi: 10.1083/jcb.114.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mistry SJ, Atweh GF. Stathmin inhibition enhances okadaic acid-induced mitotic arrest. A potential role for stathmin in mitotic exit. J Biol Chem. 2001;276:1209–1215. doi: 10.1074/jbc.M011654200. [DOI] [PubMed] [Google Scholar]
- 16.Mistry S, Atweh G. Role of stathmin in the regulation of the mitotic spindle: potential applications in cancer therapy. Mt Sinai J Med. 2002;69:299–304. [PubMed] [Google Scholar]
- 17.Brattsand G, Roos G, Marklund U, Ueda H, Landberg G, Nånberg E, et al. Quantitative analysis of the expression and regulation of an activation-regulated phosphoprotein (oncoprotein in normal and neoplastic cells. Leukemia. 1993;7:569–579. [PubMed] [Google Scholar]
- 18.Brattsand G. Correlation of oncoprotein 18/stathmin-expression in human breast cancer with establishedprognostic factors. Br J Cancer. 2000;83:311–318. doi: 10.1054/bjoc.2000.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams R. Marihuana: Harvey lecture. Bull N Y Acad Med. 1942;18:705–730. [PMC free article] [PubMed] [Google Scholar]
- 20.Sarfaraz S, Adhami VM, Syed DN, Afaq F, Mukhtar H. Cannabinoids for cancer treatment: progress and promise. Cancer Res. 2008;68:339–342. doi: 10.1158/0008-5472.CAN-07-2785. [DOI] [PubMed] [Google Scholar]
- 21.Bosier B, Muccioli GG, Hermans E, Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem Pharmacol. 2010;80:1–12. doi: 10.1016/j.bcp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Hermanson D, Marnett L. Cannabinoids, endocanna-binoids, and cancer. Cancer Metastasis Rev. 2011;30:599–612. doi: 10.1007/s10555-011-9318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joseph J, Niggemann B, Zaenker K, Entschladen F. Anandamide is an endogenous inhibitor for the migration of tumor cells and T lymphocytes. Cancer Immunol Immunother. 2004;53:723–728. doi: 10.1007/s00262-004-0509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rouzier R, Rajan R, Wagner P, Hess KR, Gold DL, Stec J, et al. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc Natl Acad Sci U S A. 2005;102:8315–8320. doi: 10.1073/pnas.0408974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matrone MA, Whipple RA, Thompson K, Cho EH, Vitolo MI, Balzer EM, et al. Metastatic breast tumors express increased tau, which promotes microtentacle formation and the reattachment of detached breast tumor cells. Oncogene. 2010;29:3217–3227. doi: 10.1038/onc.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matrone MA, Whipple RA, Thompson K, Cho EH, Vitolo MI, Balzer EM, et al. Metastatic breast tumors express increased tau, which promotes microtentacle formation and the reattachment of detached breast tumor cells. Oncogene. 2010;29:3217–3227. doi: 10.1038/onc.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt S, Safferling K, Westphal K. Stathmin regulates keratinocyte proliferation and migration during cutaneous regeneration. PLoS One. 2013;8:750–775. doi: 10.1371/journal.pone.0075075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu HP, Li CF, Lee SW, Wu WR, Chen TJ, Chang KY, et al. Overexpression of stathmin 1 confers an independent prognostic indicator in nasopharyngeal carcinoma. Tumour Biol. 2013:2619–29. doi: 10.1007/s13277-013-1345-3. [DOI] [PubMed] [Google Scholar]
- 29.Wang R, Dong K, Lin F, Wang X, Gao P, Wei SH, et al. Inhibiting proliferation and enhancing chemosensitivity to taxanes in osteosarcoma cells by RNA interference-mediated downregulation of stathmin expression. Mol Med. 2007;13:567–575. doi: 10.2119/2007-00046.Wang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Velasco G, Hernández-Tiedra S, Dávila D. Lorente ar: The use of cannabinoids as anticancer agents. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2015:259–266. doi: 10.1016/j.pnpbp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Cudaback E, Marrs W, Moeller T, Stella N. The expression level of CB1 and CB2 receptors determines their efficacy at inducing apoptosis in astrocytomas. PLoS One. 2010;5:e8702. doi: 10.1371/journal.pone.0008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freimuth N, Ramer N, Hinz B. Antitumorigenic effects of cannabinoids beyond apoptosis J. Pharmacol Exp Ther. 2010;332:336–344. doi: 10.1124/jpet.109.157735. [DOI] [PubMed] [Google Scholar]
- 33.Fogli S, Nieri P, Chicca A, Adinolfi B, Mariotti V, Iacopetti P, et al. Cannabinoid derivatives induce cell death in pancreatic MIA PaCa-2 cells via a receptor-independent mechanism FEBS Lett. 2006;589:1733–1739. doi: 10.1016/j.febslet.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 34.Ramer R, Bublitz K, Freimuth N, Merkord J, Rohde H, Haustein H, et al. Cannabidiol inhibits lung cancer cell invasion and metastasis via intercellular adhesion molecule-1. FASEB J. 2012;26:1535–1548. doi: 10.1096/fj.11-198184. [DOI] [PubMed] [Google Scholar]


