“The good physician treats the disease; the great physician treats the patient who has the disease”
(Sir William Osler—1849 to 1919)
Medicine is about the science and art of healing and is guided by the pillars of correct diagnosis, proper treatment and more importantly in the prevention of diseases and disease mechanisms. The science behind the “art of medicine” encompasses an unlimited and ever growing variety of health care practices and new technologies evolved to maintain, restore, or improve general health and quality of life, guiding healthcare professionals to major results with minor interventions, thus avoiding excessive application of technology and unnecessary risks. With the advent of another type of ART (Assisted Reproductive Technologies), although it represents an outstanding advance in the management of the infertile male, making it possible to patients previously considered end-of-line for reproductive purposes, to father their own genetic offspring; a new concern arises in the traditional practice of medicine and that should be the health and wellbeing of not just the human being subject of treatment but of generations to come. Medical interventions in the field of ART have become so powerful, particularly with the introduction of intracytoplasmic sperm injection (ICSI) in 1992 (1), that they have the capacity to interfere and modify with the complex mechanism of evolution through natural selection that has been ruling life on Earth since 3.6 billion years ago. As the results with ICSI, are slightly better than traditional in vitro fertilization (IVF) since its introduction (2) even for non-male problems, from a practical point of view, the IVF industry has largely adopted this technique as the “silver bullet” for infertility-related issues, ignoring the value of andrological examinations and therapy and dehumanizing the male reducing him to the ability of obtaining a “single sperm”, from the ejaculate or the testis, regardless of clinical diagnosis and quality (3). There are substantial socioeconomic inequalities in both the abuse of ART in couples who do not need to go that further, but have the desire and the resources and have been persuaded to proceed; as well as in couples who suffer from the deprivation of not using the technology even though they desperately need it, but cannot afford. Involuntary childlessness is a multifactorial situation that is a result of a cascade of events in the reproductive organs and/or gametes and their interaction with the environment and lifestyle habits; therefore, not limited to the traditional explanations of male vs. female factors, genital infections, endocrine abnormalities, immunological, anatomical factors, obstructive and non-obstructive azoospermia or the concomitant presence of varicocele or other cofactors like cigarette, marijuana and alcohol abuse; but rather diving deep into genetic, epigenetic, and molecular causes as contributory explanations for the biological failure of reproduction, as the utmost important purpose of any living being’s life to perpetrate its species. Even though the results obtained after ICSI are reasonable, and it is very inviting to widen the scale of indications, the technique remains intrusive, bypasses the normal fertilization selection processes established in mammalian fertilization in the last 300 million years or so, ignoring the patient’s disease behind a low-quality sperm and from a cost-effectiveness perspective is way behind treating correctly the male problem (4-7). The cornerstone of the investigation of the infertile male is a detailed history combined with a careful physical examination. Next mandatory fundamental step in the initial investigation process, are two complete and technically correct semen analyses, although these preliminary parameters have not answered all the expectations deposited on them. Semen evaluation should be the first source for laboratory data. It should be carefully done as it provides important information on the male spermatogenesis and patency of the reproductive tract. Traditionally, the diagnosis of male infertility has depended upon a descriptive evaluation of the human ejaculate, with emphasis on the concentration, motility, and morphology of the spermatozoa. The fundamental purposeful misunderstanding underlying this approach by “not so few IVF labs” around the globe, and in special in countries with no regulatory mechanisms, nor oversight committees and neither a strong andrology, is that male fertility is conveniently solely defined and classified in terms of a threshold concentration of motile, morphologically normal spermatozoa that must be exceeded if a given patient is to be classified as fertile (…or infertile and directed straight to IVF/ICSI). It must be outlined that semen analysis is an initial step, not a proof of fertility neither a fertility test and that significant medical conditions are uncovered by a comprehensive evaluation (8). For example, it has been demonstrated that improved results with intra-uterine insemination and even IVF are achieved after varicocele repair, and that these better results were obtained without clear improvement in the basic semen parameters, so something must be going on in the inner sperm cell mechanism to improve its quality and biological function (6,9). As clinical experience has revealed, it is not so much the absolute number of spermatozoa that predicts fertility, but rather their functional competence. As a result, andrology has provided the medical community with in vitro tests that have been developed to monitor the functional competence of these cells in terms of their potential for movement, cervical mucus penetration, capacitation, zona pellucida recognition, acrosome reaction, spermatozoa-oocyte fusion and pronucleus formation. In addition, a variety of biochemical markers have been developed in the last two decades and constantly under improvement in recent years providing information on such factors as the efficiency of spermiogenesis and the presence of oxidative stress. An example of such biochemical criteria is creatine-kinase (10-12), which can be used to assess the normality of spermiogenesis by providing information on the retention of excess residual cytoplasm by the spermatozoa, especially critical in the pathophysiology of varicocele-induced sperm abnormalities with ejaculation of immature spermatozoa. Another example of a biological marker for defective sperm function is the cellular generation of reactive oxygen species (ROS) (13,14). Produced at low physiological levels, these molecules are extremely important to spermatozoa in fueling the capacitation process (15). However, when produced in excessive amounts, oxidants such as hydrogen peroxide can disrupt sperm function by inducing peroxidative damage to the sperm plasma membrane and strand breakage in the sperm DNA. Spermatozoa are particularly susceptible to such damage, and assays of ROS generation in the washed ejaculate have shown to correlate with fertilization rates in vivo and in vitro. Furthermore, the presence of leukocytes highly influences levels of seminal ROS. Even in semen samples of a fertile population with leukocyte levels below 1×106/mL increased levels of ROS in washed and neat semen are found and have deleterious effects (16). There is an obvious need to objectively measure sperm quality both at initial evaluation as part of the diagnostic process, as well as after surgical or medical intervention to evaluate sperm quality improvement, but also to proceed with simple low-complexity forms of assisted reproduction, such as intrauterine insemination or high complex techniques such as IVF/ICSI, if that shall be the case. The difference between “the flash” or “lightning-bolt IVF/ICSI”, unfortunately the way it is being commercially performed nowadays in specific IVF centers, regions and countries, and a correct more “responsible IVF/ICSI” (17) relies in the correct diagnosis of the underlying cause, treating the patient accordingly and focusing in the proper timing of achieving optimal sperm function through selected intervention for best results; but also, to have in the andrology lab arsenal, reliable sperm functional tests to predict success rates or at least minimize chances of failure, diminishing the burden to the couple of repeated and often unexplained failure attempts. A growing body of evidence suggests that reproductive history influences post-reproductive mortality, factors like number of siblings, age at first delivery, multiple pregnancies, or the absence of pregnancy, are correlated with higher parental mortality, even in developed countries (18). This piece of evidence, must be another reason to raise a “red flag” towards not investigating the underlying cause of infertility and at the same time be a stimulus to improve methods to restore male general health, testicular function and consequently, sperm function. One of the measurements of sperm quality that can be assessed with validated protocols is sperm DNA fragmentation (SDF) (19). To understand SDF in the multifaceted and polymorphic scenario of male infertility, an initial navigation in the mechanisms underlying DNA strand breaks starts with the physiological concept of apoptosis or controlled cell death, an important part in multiplication of germ cells in the testis (20). The intratesticular cells primarily affected by apoptosis are high turnover cells, spermatogonia and spermatocytes; which depend on this physiological mechanism to adequate Sertoli’s cells capacity to support de degree of differentiation of the spermatogonia and its further development. Nondividing Sertoli cells can only maintain a limited number of germ cells at a given period, and that phenomenon depends on a series of events, like adequate Leydig cell number and function with its potent intratesticular testosterone production, genetic and epigenetic factors, guaranteeing that only a limited number of spermatogonia enter meiosis. When the number of spermatogonia is higher than the capacity to process then into normal spermatogenesis, and this capacity can be modified by an infinite of variables, including but not limited to environmental pollutants and endocrine-disrupting chemicals, the surplus will degenerate through apoptosis. Modern andrology can access through selective functional tests the status of the sperm cell and propose methods to enhance its function before proceeding to assisted reproduction. However, a warning should be launched as SDF tests have been used in a twisted manner as a poor and unfit substitute for the proper evaluation of the infertile male, in such a way that if results show elevated DNA fragmentation levels, it is said that there is little to do and as a consequence ICSI is solely “treatment”; on the other hand, if SDF levels are normal, the indication for ICSI is also there with the argument that “this is a good case to go straight for ICSI”, an array of excuses arise to justify for the misconduct, like: “the women is anxious” or “they do not want to lose time”! A powerful test that could be used as a prognostic factor or a marker of improvement is transformed into a “green card” to proceed with more of the same, regardless of being the best option at that time.
Another key knowledge to better understand what andrology can do for the infertile male, comes from androgens and their action in the testis, for that the secretion of gonadotropin-releasing hormone (GnRH) from the hypothalamus is a fundamental pre-requisite for both initiation and maintenance of reproductive function. Two waves of Sertoli and germ cell proliferation occur in the lifetime: during the late fetal and early neonatal period and at early puberty (21). Disturbances of any of these interdependent events from fetal to adult life can result in hypogonadism with a huge variety of clinical scenarios according to time of onset, degree of hormone deficiency and presence of comorbidities (22). Additionally, the concomitant existence of cofactors for infertility, like varicocele, testicular dysgenesis syndrome, cryptorchidism, genetic and epigenetic alterations, lifestyle habits, use of drugs, tobacco, medications, antidepressants, anabolic steroids, exercises, diet, nutritional aspects, vitamin D levels, air pollution, toxicants, chemotherapy, radiation therapy and endocrine-disrupting chemicals can add stress to the underlying metabolic or clinical condition. Most of, if not all, these factors can be identified by an experienced andrologist and either, cured or mitigated. Therefore, the development of adult Leydig cells might be considered as a function in which stem cells are acted upon by endocrine and paracrine factors that induce cells to differentiate and start to produce hormones, this phenomenon can happen for the entire of adult lifetime and can be modified, stimulated and modulated by the use of gonadotropins, like human chorionic gonadotropins (hCG), recombinant follicular stimulating hormone (r-FSH), clomiphene citrate or a combination of them, before or after microsurgical interventions such as varicocele repair, orquidopexy, vasectomy reversal or even sperm extraction techniques, in such a way that there are effective tools to improve men’s fertility potential before choosing to go for complex laboratory assisted reproductive techniques that often carry hidden or unknown complications. By the way, the degree of complications implicit in the surrounding environment of IVF encompasses a growing list of events, most of times never fully explained and, many times even not mentioned, to infertile couples in the complex decision-making process of proceeding or not into the IVF rollercoaster. For instance, in a recent paper analyzing more than 300,000 births, 2% being from assisted conception, it was demonstrated that the overall percentage for any birth defect in pregnancies involving assisted conception was 8.3% as compared with pregnancies not involving assisted conception of 5.8%, (unadjusted odds ratio: 1.47; adjusted 1.28). The percentage of birth defects with IVF was 7.2% (unadjusted odds ratio: 1.26; adjusted 1.07). In IVF with ICSI is where higher percentage of birth defects of 9.9% were found (unadjusted odds ratio: 1.77; adjusted 1.57) (23). The real question is how many of these couples really had a real indication to undergo ICSI? How many of them had a clear diagnosis of an untreatable or irreversible andrological condition before proceeding to ART? How many of them could have been treated successfully without the most invasive technology of assisted conception? How will be the future of the children born after ICSI in twenty to thirty-years’ time? In another paper, transgenerational changes in somatic and germ line genetic integrity of first-generation offspring derived from DNA damaged sperm was evaluated in mice and demonstrated that genomic instability in the offspring is directly dependent on the amount of DNA damage present in the sire’s sperm at the time of fertilization (24). As in ICSI procedure the eyes of the embryologist cannot access DNA content of the randomly chosen sperm in the petri dish, even with the best of intentions, one can assume that if no treatment [antioxidants (25), hCG (26), microsurgery (6,7,9), etc.] was established before the ICSI cycle and neither, any test to evaluate functionally the quality of the sperm in the semen sample to be used, that considerable amount of DNA damaged sperm has been introduced that could largely been avoided.
From a market and financial view, with increasing globalization, a new wave of fusion of IVF labs is taking place very fast in all continents. IVF labs are being partially or completely acquired and/or controlled by private investment banks, equity funds and investment banks, therefore IVF babies are becoming part of investment portfolios around the globe. This could be a threat or an opportunity for improvement of healthcare in assisted reproduction, provided that these rapid-growing businesses understand the importance of a high-complexity andrology laboratory workout in the context of correct andrological care using guidelines for male infertility and applying cost-effectiveness solutions with less risk for the woman and future generations. Another key point to be addressed is that private insurance companies are being legally requested to pay for IVF cycles, the real question is: do they have the knowledge that there are more effective, less expensive, and less-risky treatments available for their insured couples that, could benefit a large amount if not, the majority of couples with male infertility as a primary or adjuvant cause of infertility? Health insurance companies and healthcare professionals should be informed that it is much more cost-effective to expend money in the evaluation and treatment of the infertile male or female partner before proceeding to IVF/ICSI (27). More importantly, IVF costs do not end-up in the IVF lab neither, in the delivery room with higher miscarriage rates; nor in the intensive care unit with ovarian hyperstimulation syndrome (24), premature, low-birth weight births (28) and multiple gestations with consequently higher maternal death (29). They go beyond and include epigenetic concerns (30), imprinting disorders (31), birth defects (32), increased risk of hypospadias (33), genetic considerations (34), fostering international reproductive tourism bypassing local and regional strict regulations that could impose limitations based on current approved and established guidelines and protocols (35). Of even greater concern, in a recent published article comparing the incidence of neoplasms in 247,187 children (median follow-up of 10.55 years), 98.3% conceived spontaneously, 1.1% born from IVF and 0.7% conceived after simple ovulation induction treatments, a total of 1,498 neoplasms (0.6%) were diagnosed. Incidence density rate for neoplasms was close to three times higher among children conceived after IVF (1.5/1,000 person years) and around two times higher after ovulation induction treatments (1.0/1,000 person years), as compared with naturally conceived children (0.59/1,000 person years, P<0.001) in a demonstration of strong positive association between IVF or any fertility treatments and higher incidence of pediatric malignancies (36).
In conclusion, the incredible positive advances made possible with the introduction of IVF with intracytoplasmic sperm injection are among the great achievements of medicine in the 20th century and should be encouraged, the real message is to use this powerful tool strictly when necessary avoiding abuse, banalization and mitigating risks for generations to come. At stake is the protection of the children born after ICSI, the still unknown fate of their future offspring and ultimately, the survival of the IVF industry itself.
Clinical case
To illustrate the discussion above, a clinical case is presented: 36-year-old male, 2.03 m high, 126 kg weight, BMI =31.2; married to a 31-year-old wife with normal investigation, with primary infertility. At physical examination, he was discovered with a left GII varicocele and a right sub inguinal varicocele with total reflux time >3 s, left testis volume of 14.5 mL; right testis volume of 16 mL. Initial semen analysis (×2) revealed total progressive motile sperm count (TPMSP) of 0.2 to 0.66 million, concentration (C) of 1.09 to 3.70 M/mL sperm, motility grade B =10% to 15%, WHO morphology of 2% to 4%, strict criteria =0 to 1%, creatine kinase activity =21.25 U/108 spermatozoa (normal <0.36 U/108 spermatozoa), normal hormonal levels, submitted to a bilateral microsurgical varicocele repair with artery and lymphatic preservation, achieved natural pregnancy after 5 months. Semen analysis revealed total progressive motile sperm count =11.50 million, sperm concentration =10.6 M/mL, no improvement in morphology, creatine kinase dropped to 3.6 U/108 spermatozoa, still high than normal but he achieved natural pregnancy. At that time, we did not have SDF tests available in the lab. The same patient comes with secondary infertility after three years, and went for the same evaluation, except that this time he had a TPMSP =0.47 million sperm, C =2.2 M/mL, grade B =15%, WHO =7% and Strict criteria =2%. Even though this semen analysis looks worse than the one after achieving pregnancy, it is noticeable that quality did show an improvement. ROS levels were = 6.02×104 counted photons per minute (cpm) (normal without leukocytes = 0.55×104 cpm). This time SDF tests revealed an index of 76% abnormal DNA fragmentation. I recommended that he tried medical treatment before proceeding to IVF/ICSI, but reproductive gynecologist considered that the wife’s age was advancing (34 years old) and that there was no time to waste and they should hurry towards ART. Patient disappeared for four months, after failing two cycles of ICSI and returned for additional investigation. As an urologist, I was intrigued with the fact that his semen analysis got better and then worst again. A complete physical examination was performed and blood test were ordered. A thyroid cancer was suspected and then confirmed by Doppler-color ultrasound and he was submitted to total removal of the thyroid gland and then submitted to iodotherapy. Now his semen analysis showed the presence of rare spermatozoa in the ejaculate. He cryopreserved sperm before treatment, but also with no good quality. After failing one more attempt of ICSI with cryopreserved semen, we decided to stimulate spermatogenesis using hCG, aromatase inhibitors and antioxidants. His semen analysis improved and he achieved a TPMSP =8 million, C =29 M/mL, ROS =3.0×104 cpm and SDF =37% by SCSA. Couple did not escape from having to go through ICSI, but at this time he had a proper diagnosis and is doing the procedure more confident. He was submitted to another two cycles of ART to achieve pregnancy (Figures 1,2).
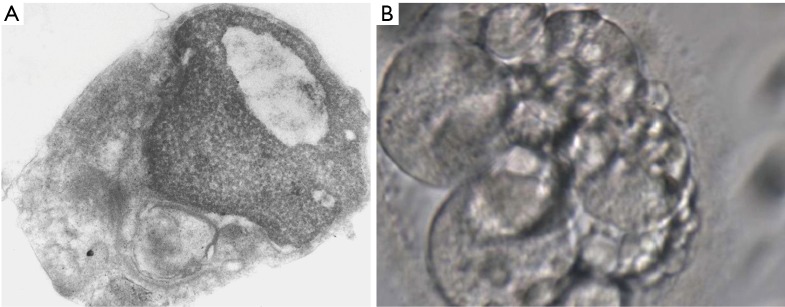
Figure 1.
Electron microscopy (EM) of sperm before diagnosis and treatment. SCSA =76%, and embryo obtained through ICSI with that sperm. (A) EM ultrastructural diagnosis revealed: sperm head showing granular immature chromatin and a big nuclear rarefaction with moderate increase (50–59%) of anomalies in sperm chromatin condensation and compaction, abundant non-specific flagellar abnormalities. Magnification 23,000×. (B) Embryo transferred: D+3; 8 cell CII BIII, with a lot of debris. SCSA, sperm chromatin structure assay; ICSI, intracytoplasmic sperm injection.
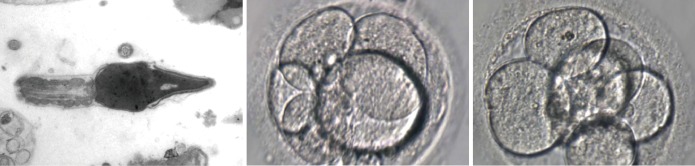
Figure 2.
EM of the sperm cell after microsurgical treatment of varicocele, followed by complementary treatment with hCG, ARIs and antioxidants. Ultrastructural changes now reveal: normal head-neck attachment, normal mitochondria, symmetrical axis, small rarefaction in the sperm head, normal compaction of the chromatin. SCSA =37%. Embryos transferred after two attempts. EM, electron microscopy; hCG, human chorionic gonadotropins; ARIs, aromatase inhibitors; SCSA, sperm chromatin structure assay.
SDF evaluation is for infertility evaluation, not fertility, based on sperm DNA integrity, there are many variables that will end-up in a viable pregnancy, this is one of them as it is too much to predict the outcome of a complex, complicated, multifactorial process based on a single variable. And that is exactly why SDF tests should not be used to define if patients go or not straight to IVF/ICSI in borderline levels, or even higher fragmentation results. Patients should always have a chance to improve sperm quality with andrological treatment, which is completely feasible in competent hands, for at least two to six months before moving to IVF. The proposal is a dramatic change in the way assisted reproduction is being conducted, without short-termism and primitive driven medicine based in attempt-error. Time for change has arrived and it should be emphasized that considerable harm is being produced by some reproductive specialists who do not understand that the male, the testis, the sperm, are not in a “static state”, like in a photograph, but on the contrary they are in a dynamic state, like in a movie, in constant change, for better or for worst, depending on genetic, metabolic, hormonal, nutritional, medical, physical and anatomical conditions as well external factors like environmental and lifestyle. Andrology needs to impose itself, moving forward and use the resources already available to measure outcomes of andrological interventions and treatments, ultimately guiding patients, general gynecologists, and IVF specialists into the “easy to get lost maze of ARTs”, with the goal of more than just suboptimal results, but mainly with fewer complications. Future will tell how far it will take us, but one thing is for sure: it will take us forward in the search for what we chose when we decided to take care of human beings. Paraphrasing Sir William Osler: “do not bypass the disease behind the patient, identify and treat both accordingly”.
Acknowledgements
Dr. Hector Chemes, CEDIE Centro de Investigaciones Endocrinologicas, Buenos Aires, Argentina, for performing electron microscopy of spermatozoa.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Palermo G, Joris H, Devroey P, et al. Pregnancies after intracytoplasmic injection of single spermatozoa into an oocyte. Lancet 1992;340:17-8. 10.1016/0140-6736(92)92425-F [DOI] [PubMed] [Google Scholar]
- 2.Van Steirteghem AC, Liu J, Joris H, et al. Higher success rate by intracytoplasmic sperm injection than by subzonal insemination. Report of a second series of 300 consecutive treatment cycles. Hum Reprod 1993;8:1055-60. 10.1093/oxfordjournals.humrep.a138191 [DOI] [PubMed] [Google Scholar]
- 3.Payne D, Flaherty SP, Jeffrey R, et al. Successful treatment of severe male factor infertility in 100 consecutive cycles using intracytoplasmic sperm injection. Hum. Reprod 1994;9:2051-7. 10.1093/oxfordjournals.humrep.a138392 [DOI] [PubMed] [Google Scholar]
- 4.Penson DF, Paltiel D, Krumholz HM, et al. The Cost-effectiveness of treatment for varicocele related infertility. J Urol 2002;168:2490-4. 10.1016/S0022-5347(05)64175-4 [DOI] [PubMed] [Google Scholar]
- 5.Meng MV, Greene KL, Turek P. Surgery or assisted reproduction? A decision analysis of treatment costs in male infertility. J Urol 2005;174:1926-31. 10.1097/01.ju.0000176736.74328.1a [DOI] [PubMed] [Google Scholar]
- 6.Kirby EW, Wiener LE, Rajanahally S, et al. Undergoing varicocele repair prior to assisted reproduction improves pregnancy rate and live birth rate in azoospermic and oligozoospermic men with varicocele: A systematic review and meta-analysis. Fertil Steril 2016;106:1338-43. 10.1016/j.fertnstert.2016.07.1093 [DOI] [PubMed] [Google Scholar]
- 7.Kolettis PN, Thomas AJ. Vasoepididymostomy for vasectomy reversal: A critical assessment in the era of intracytoplasmic sperm injection. J Urol 1997;158:467-70. 10.1016/S0022-5347(01)64504-X [DOI] [PubMed] [Google Scholar]
- 8.Honig SC, Lipshults LI, Jarow J. Significant medical pathology uncovered by a comprehensive male infertility evaluation. Fertil Steril 1994;62:1028-34. 10.1016/S0015-0282(16)57069-1 [DOI] [PubMed] [Google Scholar]
- 9.Daitch JA, Bedaiwy MA, Pasqualotto EB, et al. Varicocelectomy improves intrauterine insemination success rates in men with varicocele. J Urol 2001;165:1510-3. 10.1016/S0022-5347(05)66338-0 [DOI] [PubMed] [Google Scholar]
- 10.Huszar G, Vigue L, Corrales M. Sperm creatine kinase activity in fertile and infertile men. J Androl 1990;11:40-6. [PubMed] [Google Scholar]
- 11.Sidhu RS, Hallak J, Sharma RK, et al. Relationship between creatine kinase levels and clinical diagnosis of infertility. J Assist Reprod Genet 1998;15:188-92. 10.1023/A:1023096201880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallak J, Sharma RK, Pasqualotto FF, et al. Creatine kinase as an indicator of sperm quality and maturity in men with oligospermia. Urology 2001;58:446-51. 10.1016/S0090-4295(01)01224-9 [DOI] [PubMed] [Google Scholar]
- 13.Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil 1987;81:459-69. 10.1530/jrf.0.0810459 [DOI] [PubMed] [Google Scholar]
- 14.Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology 1996;48:835-50. 10.1016/S0090-4295(96)00313-5 [DOI] [PubMed] [Google Scholar]
- 15.de Lamirande E, Leduc BE, Iwasaki A, et al. Increased reactive oxygen species formation in semen of patients with spinal cord injury. Fertil Steril 1995;63:637-42. 10.1016/S0015-0282(16)57438-X [DOI] [PubMed] [Google Scholar]
- 16.Athayde KS, Cocuzza MS, Agarwal A, et al. Development of normal reference values for seminal reactive oxygen species and their correlation with leukocytes and semen parameters in a fertile population. J Androl 2007;28:613-20. 10.2164/jandrol.106.001966 [DOI] [PubMed] [Google Scholar]
- 17.Hallak J. Asymptomatic male currently not desiring fertility with bilateral subclinical varicocele found on ultrasound evaluation and borderline semen analysis results. Asian J Androl 2016;18:315-6. 10.4103/1008-682X.172645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barclay K, Keenan K, Grundy E, et al. Reproductive history and post-reproductive mortality: A sibling comparison analysis using Swedish register data. Social Science & Medicine 2016;155:82-92. 10.1016/j.socscimed.2016.02.043 [DOI] [PubMed] [Google Scholar]
- 19.Agarwal A, Majzoub A, Esteves SC, et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2016;5:935-50. 10.21037/tau.2016.10.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Billig H, Furuta I, Rivier C, et al. Apoptosis in the testis germ cells: Developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology 1995;136:5-12. 10.1210/endo.136.1.7828558 [DOI] [PubMed] [Google Scholar]
- 21.Russell LD, Bartke A, Goh JC. Postnatal development of the Sertoli cell barrier, tubular lumen, and cytoskeleton of Sertoli and myoid cells in the rat, and their relationship to tubular fluid secretion and flow. Am J Anat 1989;184:179. 10.1002/aja.1001840302 [DOI] [PubMed] [Google Scholar]
- 22.Rey RA, Campo SM, Bedecarras P, et al. Is infancy a quiescent period of testicular development? Histological, morphometric, and functional study of the seminiferous tubules of the cebus monkey from birth to the end of puberty. J Clin Endocrinol Metab 1993;76:1325. [DOI] [PubMed] [Google Scholar]
- 23.Davies MJ, Moore V, Willson KJ, et al. Reproductive Technologies and the Risk of Birth Defects. N Engl J Med 2012;366:1803-13. 10.1056/NEJMoa1008095 [DOI] [PubMed] [Google Scholar]
- 24.Feinberg EC. Ovarian hyperstimulation: past, present, and future. Fertil Steril 2016;106:1330. 10.1016/j.fertnstert.2016.08.032 [DOI] [PubMed] [Google Scholar]
- 25.Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod 2011;26:1628-40. 10.1093/humrep/der132 [DOI] [PubMed] [Google Scholar]
- 26.Riccetti L, Yvinec R, Klett D, et al. Human Luteinizing Hormone and Chorionic Gonadotropin Display Biased Agonism at the LH and LH/CG Receptors. Sci Rep 2017;7:940. 10.1038/s41598-017-01078-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connolly MP, Hoorens S, Chambers GM, et al. The costs and consequences of assisted reproductive technology: an economic perspective. Hum Reprod Update 2010;16:603-13. 10.1093/humupd/dmq013 [DOI] [PubMed] [Google Scholar]
- 28.Shih W, Rushford DD, Bourne H, et al. Factors affecting low birthweight after assisted reproduction technology: difference between transfer of fresh and cryopreserved embryos suggests an adverse effect of oocyte collection. Hum Reprod 2008;23:1644-53. 10.1093/humrep/den150 [DOI] [PubMed] [Google Scholar]
- 29.Braat DD, Schutte JM, Bernardus RE, et al. Maternal death related to IVF in the Netherlands 1984-2008. Hum Reprod 2010;25:1782-6. 10.1093/humrep/deq080 [DOI] [PubMed] [Google Scholar]
- 30.Shufaro Y, Laufer N. Epigenetic concerns in assisted reproduction: update and critical review of the current literature. Fertil Steril 2013;99:605-6. 10.1016/j.fertnstert.2013.01.126 [DOI] [PubMed] [Google Scholar]
- 31.Vermeiden JP, Bernardus RE. Are imprinting disorders more prevalent after human in vitro fertilization or intracytoplasmic sperm injection? Fertil Steril 2013;99:642-51. 10.1016/j.fertnstert.2013.01.125 [DOI] [PubMed] [Google Scholar]
- 32.El-Chaar D, Yang Q, Gao J, et al. Risk of birth defects increased in pregnancies conceived by assisted human reproduction. Fertil Steril 2009;92:1557-61. 10.1016/j.fertnstert.2008.08.080 [DOI] [PubMed] [Google Scholar]
- 33.Silver RI, Rodriguez R, Chang TS, et al. In vitro fertilization is associated with an increased risk of hypospadias. J Urol 1999;161:1954-7. 10.1016/S0022-5347(05)68863-5 [DOI] [PubMed] [Google Scholar]
- 34.Practice Committee of American Society for Reproductive Medicine. Practice Committee of Society for Assisted Reproductive Technology Genetic considerations related to intracytoplasmic sperm injection (ICSI). Fertil Steril 2008;90:S182-4. 10.1016/j.fertnstert.2008.08.048 [DOI] [PubMed] [Google Scholar]
- 35.Smith E, Behrmann J, Martin C, et al. Reproductive tourism in Argentina: clinic accreditation and its implications for consumers, health professionals and policy makers. Dev World Bioeth 2010;10:59-69. 10.1111/j.1471-8847.2009.00256.x [DOI] [PubMed] [Google Scholar]
- 36.Tamar W, Walfisch A, Shoham-Vardi I, et al. Fertility treatments and pediatric neoplasms of the offspring: results of a population-based cohort with a median follow-up of 10 years. Am J Obstet Gynecol 2017;216:314.e1-314.e14. 10.1016/j.ajog.2017.01.015 [DOI] [PubMed] [Google Scholar]