Abstract
Survival rates for patients with medulloblastoma have improved in the last decades but for those who relapse outcome is dismal and new approaches are needed. Emerging drugs have been tested in the last two decades within the context of phase I/II trials. In parallel, advances in genetic profiling have permitted to identify key molecular alterations for which new strategies are being developed. We performed a systematic review focused on the design and outcome of early‐phase trials evaluating new agents in patients with relapsed medulloblastoma. PubMed, clinicaltrials.gov, and references from selected studies were screened to identify phase I/II studies with reported results between 2000 and 2015 including patients with medulloblastoma aged <18 years. A total of 718 studies were reviewed and 78 satisfied eligibility criteria. Of those, 69% were phase I; 31% phase II. Half evaluated conventional chemotherapeutics and 35% targeted agents. Overall, 662 patients with medulloblastoma/primitive neuroectodermal tumors were included. The study designs and the response assessments were heterogeneous, limiting the comparisons among trials and the correct identification of active drugs. Median (range) objective response rate (ORR) for patients with medulloblastoma in phase I/II studies was 0% (0–100) and 6.5% (0–50), respectively. Temozolomide containing regimens had a median ORR of 16.5% (0–100). Smoothened inhibitors trials had a median ORR of 8% (3–8). Novel drugs have shown limited activity against relapsed medulloblastoma. Temozolomide might serve as backbone for new combinations. Novel and more homogenous trial designs might facilitate the development of new drugs.
Keywords: Children, clinical trial, medulloblastoma, phase 1, phase 2, relapse or refractory tumor
Introduction
Medulloblastomas are aggressive embryonal tumors representing the most frequent primary malignant brain cancer in children 1. Maximal safe resection, chemotherapy, and craniospinal irradiation (CSI) remain the mainstays of first‐line treatment 2.
Long‐term survival rates have steadily improved over the last decades, from 22% by 1950 3 to up to 50% by late 1970 4 and even 85% with current approaches 5; this improvement is mostly due to the addition of systemic chemotherapy to the standard treatment with surgery and radiotherapy 6, 7, 8, superior surgical and radiotherapy techniques, intensification of therapy 9, 10, and improvement in supportive care measures. Unfortunately outcome is invariably poor for those who relapse 11, 12, with a long‐term survival of 6% 11 and new approaches are needed .
Clinical trials are the way forward to evaluate new therapies for high‐risk cancer patients 13. Patients with relapsed or refractory brain tumors represent between 36% 14 and 46% 15 of the population participating in pediatric oncology phase I studies; of those, medulloblastoma/primitive neuroectodermal tumors (PNET) patients represent up to a third. Moreover, patients with medulloblastoma and PNET have been traditionally treated together in trials although they are distinct molecular entities and PNETs are now called central nervous system (CNS) embryonal tumors 16.
The advent of the molecular classification 17 and the advances in genetic profiling of medulloblastomas open the horizon for more tailored therapeutic approaches. In this sense, classical criteria used to stratify patients based on residual tumor burden after surgery 18, age, and extent of disease may not accurately identify patients with better or worse outcome. The implementation of molecular variables into stratification schemes can help to refine risk definition and subsequent treatment 19. The identification of good‐prognosis patients may allow de‐escalating the intensity of frontline therapies and reducing long‐term sequelae. Conversely, high‐risk patients may benefit from adding new agents to conventional chemotherapeutics or even substituting those associated with more undesirable side effects by others with a better safety profile, while keeping their antitumor activity.
Hence, the number of potential patients with medulloblastoma for entering early‐phase trials or new therapies targeting a vast landscape of molecular alterations makes necessary an analysis of the activity that has already been carried out.
We performed a systematic review of the methodology and results of phase I/II clinical trials including pediatric patients with medulloblastoma at relapse/progression and we reviewed current molecularly driven trials in this population.
The objectives were as follows:
To stablish the level of activity and outcome of phase I/II studies for patients with medulloblastoma in the last 15 years;
To provide an update on the medulloblastoma clinical trials portfolio and to discuss current knowledge on biology and potential future targeted therapies;
To inform future trials and to discuss potential areas of improvement to optimize early clinical trials performance.
Material and Methods
Search strategy
PubMed (https://www.ncbi.nlm.nih.gov/pubmed) was searched with three different strategies to cover medulloblastoma‐specific trials, CNS tumor trials, and solid tumor trials (Data S1). Search was limited to articles published with patients aged <18 years old, between 2000 and 2015. No language restrictions were applied. The https://clinicaltrials.gov site was also searched, restricted to interventional phase I/II studies with results in children with medulloblastoma from 1st January 2000 to 31st December 2015, as well as the bibliographic references from the studies finally included in this review.
One reviewer (VF) evaluated the titles and abstracts of the identified publications and all potential relevant publications were retrieved for detailed evaluation. The final inclusion of studies was made by agreement of two reviewers (VF and FB). A third author (LM) reviewed ‘Potentially relevant publications retrieved for detailed evaluation’ independently and blindly to peer review the inclusion of papers. Two reviewers performed the data abstraction (VF and FB) by means of a standardized data collection form.
Inclusion and exclusion criteria
Inclusion and exclusion criteria were defined a priori. Phase I/II trials including patients with medulloblastoma aged <18 years at the time of enrolment were eligible. Stand‐alone radiotherapy trials were excluded.
Data extraction
Information was extracted regarding study design, inclusion/exclusion criteria, target population, type of intervention, outcome, and toxicity. Objective response rate (ORR) was calculated as the proportion of complete responses (CR) and partial responses (PR) among evaluable patients. Disease control rate (DCR) was calculated as the proportion of CR, PR, and stable diseases (SD) among evaluable patients.
Review of current molecularly driven trials in patients with medulloblastoma
The website https://clinicaltrials.gov was scrutinized to identify ongoing trials, using the advance search function. We used the term “medulloblastoma” and restricted our search to studies that were not yet recruiting or recruiting limited to the age group of child (birth–17 years); last accessed on 28th July 2017.
Results
Included studies
A total of 718 publications were identified (Data S1). Two hundred and thirteen articles were retrieved for detailed evaluation; 78 satisfied eligibility criteria. Adapted PRISMA flow diagram displays the process (Fig. 1) for including studies 20. Nine studies with results were identified in https://clinicaltrials.gov. Five had already been identified in Pubmed 21, 22, 23, 24, 25, 26 and one other (NCT01125800) had also been presented elsewhere 27. In three studies the data about patients with medulloblastoma were not available and they could not be analyzed (NCT01483820, NCT00867568, and NCT00024258).
Figure 1.
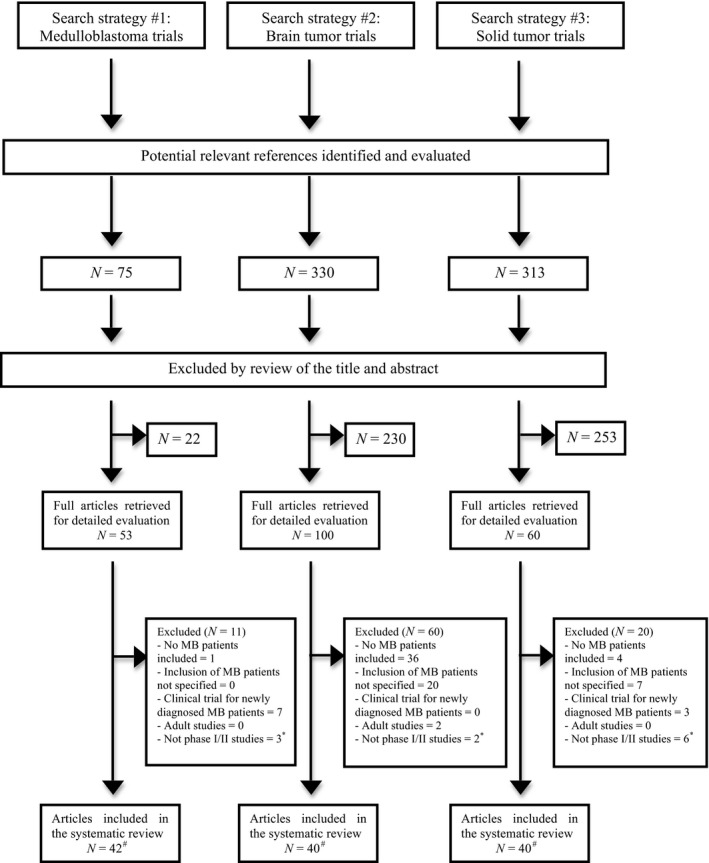
Flow diagram reporting results of the systematic review. MB, medulloblastoma. *In this category felt retrospective or observational studies. #Some studies finally included in the systematic review were identified by one or more search strategies. Therefore, there is an overlap of identified studies among research strategies yielding a final number of individual studies of 78.
Clinical trials description
There were 54 phase I (69%) 21, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78 and 24 phase II clinical trials (31%) 22, 23, 24, 25, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98. Half evaluated conventional chemotherapeutics (n = 40) and 35% (n = 27) targeted therapies (Table 1).
Table 1.
Clinical trials baseline characteristics and patient population description
| Description of clinical trials included in this study | Patient population description | |||||
|---|---|---|---|---|---|---|
| All patients | Medulloblastoma patientsa | |||||
| Variable | N | % | N | % | N | % |
| Total studies included in the analysis | 78 | 100 | 3531 | 100 | 662 | 100 |
| Participating centers | ||||||
| Unicenter | 9 | 12 | 148 | 4 | 30 | 5 |
| Multicenter | 69 | 88 | 3383 | 96 | 632 | 95 |
| Phase of development | ||||||
| Phase I | 54 | 69 | 1714 | 48 | 261 | 39 |
| Phase II | 24 | 31 | 1817 | 52 | 401 | 61 |
| Randomization | ||||||
| Yes | 1 | 1 | 44 | 1 | 12 | 2 |
| No | 77 | 99 | 3487 | 99 | 650 | 98 |
| Age at inclusion | ||||||
| Up to 18 years | 10 | 12 | 380 | 10 | 139 | 21 |
| Up to 21 years | 59 | 76 | 2906 | 83 | 464 | 70 |
| >22 years | 9 | 12 | 245 | 7 | 59 | 9 |
| Target population categories | ||||||
| Medulloblastoma | 4 | 5 | 125 | 4 | 125 | 19 |
| Central Nervous System tumors | 33 | 43 | 1452 | 41 | 325 | 49 |
| Solid tumors (CNS and extra‐CNS) | 41 | 52 | 1954 | 56 | 212 | 32 |
| Class of therapeutic(s) agent(s) | ||||||
| Conventional chemotherapeutic single agent | 24 | 31 | 1510 | 43 | 277 | 42 |
| Conventional chemotherapeutics combination | 15 | 19 | 631 | 18 | 134 | 20 |
| Targeted agent monotherapy | 25 | 32 | 880 | 25 | 164 | 25 |
| Targeted agents in combination | 2 | 3 | 29 | 1# | 2 | 0# |
| Chemotherapeutics + targeted agent in combination | 9 | 11 | 401 | 11 | 36 | 5 |
| Chemotherapeutics + HSCT | 3 | 4 | 80 | 2 | 49 | 7 |
96 out of the 662 patients included were presented in the original manuscript as medulloblastoma/PNET and figures could not be split.
Relative value expressed in percentage is 0.8%. # Relative value expressed in percentage is 0.3
Clinical trials design
The majority of phase I dose‐escalation trials followed a 3 + 3 design (n = 32, 60%), continual reassessment method (n = 9, 17%), or rolling six design (n = 8, 15%).
The majority of phase II studies followed a two‐stage Simon optimal design (n = 20, 83%). In four studies (6%) the design was not specified. The true response rate to declare the drug active ranged between 20% and 40% with probabilities ranging from 80% to 95%. Response was assessed by RECIST criteria (n = 5, 21%), World Health Organization (WHO) guidelines (n = 18, 75%), or other (n = 1, 4%) (Tables 2 and 3).
Table 2.
Intervention, population, design, and baseline characteristics of phase I studies including patients with medulloblastoma
| Drug(s) | Population & design | Baseline characteristics (All patients) | Patients with medulloblastoma | Reference (Year of publication) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease type | Statistical design | N | Median age (Y) | Range | Male/Female | Median prior Tx | N | % among all patients | ||
| Conventional chemotherapeutic single agent | ||||||||||
| Temozolomide | CNS | 3 + 3 | 27 | 10.8 | 4–19 | 13/14 | 1 | 6 | 22 | 28 (2006) |
| Fotemustine | CNS | 3 + 3 | 16 | 5 | 1.8–14.5 | 6/9 | NA | 6 | 38 | 29 (2009) |
| Cloretazine | CNS | CRM | 42 | 9.9 | 1.5–21.5 | 20/22 | NA | 7 | 16 | 30 (2008) |
| Irinotecan | All Tm | 3 + 3 | 81 | 7.9 | 0.9–18.5 | 50/31 | 2 | 19 | 23 | 31 (2003) |
| Liposomal Daunorubicine | All Tm | 3 + 3 | 48 | 9.6 | 1.3–18.5 | 28/20 | NA | 2 | 4 | 32 (2006) |
| Plitidepsin | All Tm | 3 + 3 | 41 | 10 | 2–17 | 21/20 | 3 | 3 | 7 | 33 (2012) |
| Depsipeptide | All Tm | 3 + 3 | 24 | 13 | 2–21 | 11/12 | NA | 1 | 4 | 34 (2006) |
| Fenretidine | All Tm | 3 + 3 | 54 | 9 | 2–20 | 35/19 | NA | 2 | 3 | 35 (2006) |
| Pemetrexed | All Tm | 3 + 3 | 33 | 12 | 1–21 | 21/12 | 2 | 1 | 3 | 36 (2007) |
| Oxaliplatin | All Tm | 3 + 3 | 26 | 11 | 5–21 | 17/9 | NA | 5 | 19 | 37 (2007) |
| Satraplatin | All Tm | 3 + 3 | 9 | 17 | 8–19 | 5/4 | 2 | 1 | 11 | 38 (2015) |
| Intrathecal lyposomal Ara‐C | All Tm | 3 + 3 | 18 | 10 | 4–19 | 12/6 | NA | 7 | 39 | 39 (2004) |
| Conventional chemotherapeutics combination | ||||||||||
| TMZ + VP‐16 | MB | 3 + 3 | 14 | 7.3 | 3–16.1 | 8/6 | NA | 14 | 100 | 40 (2010) |
| O6‐Benzylguanine + TMZ | CNS | CRM | 70 | 11.3 | 2.4–18.6 | 43/27 | NA | 10 | 14 | 41 (2007) |
| Cisplatin + Topotecan | All Tm | 3 + 3 | 36 | 12 | 2–21 | 20/16 | NA | 1 | 3 | 42 (2002) |
| Irinotecan + Cisplatin | All Tm | 3 + 3 | 24 | 15 | 4–21 | 10/14 | NA | 1 | 4 | 43 (2003) |
| CPM + Topotecan | All Tm | 3 + 3 | 16 | 11.9 | 2.8–18 | 10/6 | 2 | 3a | 2 | 44 (2004) |
| Cisplatin + TMZ | All Tm | CRM | 39 | 12.7 | 1.8–19.9 | 25/14 | NA | 2 | 5 | 45 (2005) |
| Carboplatin + Irinotecan | All Tm | 3 + 3 | 28 | 8.5 | 1–21 | 17/11 | NA | 2 | 7 | 46 (2009) |
| Oxaliplatin + VP16 | All Tm | 3 + 3 | 16 | 8 | 1–18 | 11/5 | 3 | 3 | 19 | 47 (2009) |
| Oxaliplatin + Irinotecan | All Tm | 3 + 3 | 13 | 16 | 5–21 | 4/9 | 1 | 1 | 8 | 48 (2009) |
| Irinotecan + TMZ + VCR | All Tm | 3 + 3 | 42 | 9.7 | 1–21 | 23/19 | 2 | 2 | 5 | 49 (2010) |
| Oxaliplatin + Ifosfamide + VP16 | All Tm | 3 + 3 | 17 | 7 | 2–21 | 12/5 | 3 | 2 | 12 | 50 (2015) |
| Targeted agent monotherapy | ||||||||||
| Vismodegib | MB | NA | 33 | 13 | 4.4–20.3 | 25/8 | NA | 33 | 100 | 26 (2013) |
| Lonarfarnib | CNS | CRM | 53 | 12.2 | 3.9–19.5 | 32/21 | NA | 2 | 4 | 51 (2007) |
| Cilengitide | CNS | CRM | 33 | 7.9 | 0.2–21.2 | 22/11 | NA | 3 | 9 | 52 (2008) |
| Lapatinib | CNS | CRM | 59 | 9.5 | 1.1–21.2 | 30/29 | NA | 15a | 25 | 21 (2010) |
| Valproic acid | CNS | R‐six | 26 | 13.5 | 3–21 | 10/16 | 3 | 2 | 8 | 53 (2011) |
| MK‐0752 | CNS | CRM | 23 | 8.1 | 2.6–17.7 | 10/13 | NA | 4a | 17 | 54 (2011) |
| MK‐0752 | CNS | R‐six | 10 | 8.8 | 3.1–19.2 | 6/4 | 2 | 1 | 10 | 78 (2015) |
| Erlotinib | CNS | 3 + 3 | 29 | 10 | 4–20 | 15/14 | 1 | 1 | 3 | 55 (2011) |
| Lenalidomide | CNS | CRM | 51 | 10.4 | 2.7–21.6 | 26/25 | 3 | 6a | 11 | 56 (2011) |
| Pazopanib | CNS | R‐six | 51 | 12.9 | 3.8–23.9 | 26/25 | 2 | 1 | 2 | 57 (2013) |
| Enzastaurin | CNS | CRM | 33 | 12 | 3–21 | 16/17 | NA | 1 | 3 | 58 (2015) |
| PTC299 | CNS | R‐six | 27 | 11.2 | 5.5–21.1 | 14/13 | 2 | 1 | 4 | 59 (2015) |
| Dendritic cells | CNS | NA | 9 | 15.5 | 9–22 | 1/8 | NA | 1 | 11 | 60 (2004) |
| 3F8 monoclonal antibody | CNS | NA | 15 | NA | 1–61 | NA | NA | 4 | 27 | 61 (2007) |
| RG1507 | All Tm | 3 + 3 | 31 | 11 | 3–17 | 17/14 | NA | 1 | 3 | 62 (2011) |
| AT9283 | All Tm | R‐six | 33 | 9 | 3–18 | 11/22 | 4 | 2 | 6 | 63 (2015) |
| Sonidegib | All Tm | Bayesian | 33 | 13 | 4–17 | NA | NA | 24 | 73 | 27 (2010) |
| SU101 | All Tm | 3 + 3 | 27 | 14 | 3–21 | 19/8 | 3 | 4 | 15 | 64 (2004) |
| Temsirolimus | All Tm | 3 + 3 | 19 | 11 | 4–21 | 11/8 | NA | 2 | 11 | 65 (2011) |
| MK‐2206 | All Tm | R‐six | 50 | 14.3 | 3.1–21.9 | 26/24 | NA | 3a | 6 | 66 (2014) |
| Vorinostat ± retinoic acid | All Tm | 3 + 3 | 63 | 11 | 2.6–22 | 40/23 | 2 | 9 | 14 | 67 (2010) |
| Targeted agent combination | ||||||||||
| Temsirolimus + Bevacizumab | CNS | NA | 6 | 6 | 3–14 | NA | NA | 2 | 33 | 68 (2014) |
| Vorinostat + Bortezomib | All Tm | R‐six | 23 | 12.6 | 1.1–20.1 | 17/6 | NA | 1 | 4 | 77 (2013) |
| Chemotherapeutics + targeted agent in combination | ||||||||||
| Vorinostat + TMZ | CNS | R‐six | 19 | 8.3 | 2.1–20.8 | 12/7 | 1 | 2 | 11 | 69 (2013) |
| Veliparib + TMZ | CNS | 3 + 3 | 31 | 8.5 | 1.8–21 | 16/15 | 1 | 2 | 6 | 70 (2014) |
| Carboplatin + Thalidomide | All Tm | 3 + 3 | 22 | 11 | 5–17 | 13/9 | 2 | 4 | 18 | 71 (2004) |
| Erlotinib + TMZ | All Tm | 3 + 3 | 46 | 11.5 | 3–20 | 30/16 | NA | 6 | 13 | 72 (2008) |
| VIT + Bevacizumab | All Tm | 3 + 3 | 12 | 11 | 3.9–19.4 | 8/4 | 2 | 1 | 8 | 73 (2013) |
| Bevacizumab + Irinotecan | All Tm | 3 + 3 | 11 | 9 | 3–22 | 5/6 | NA | 2 | 18 | 74 (2013) |
| Temsirolimus + Irinotecan + TMZ | All Tm | 3 + 3 | 71 | 11 | 1–21.5 | 45/26 | 2 | 2 | 3 | 75 (2014) |
| Chemotherapeutics + HSCT | ||||||||||
| Thiotepa + Carmustine + Carboplatin | CNS | 3 + 3 | 32 | 7 | 1.75–18 | 16/16 | NA | 18 | 56 | 76 (2011) |
All Tm, all tumors; CPM, cyclophosphamide; CRM, continual reassessment method; HSCT, hematopoietic stem cell transplantation; MB, medulloblastoma; NA, not available; R‐six, rolling six method; TMZ, temozolomide; Tx, therapies; VCR, vincristine; VIT, Vincristine + Temozolomide + Irinotecan; Y, years.
Medulloblastoma/PNET cohort that could not be split with the data obtained from the report.
Table 3.
Intervention, population, design, and baseline characteristics of phase II studies including patients with medulloblastoma
| Drug(s) | Population & design | Baseline characteristics (All patients) | Patients with medulloblastoma | Reference (Year of publication) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Disease type | True response rate to declare the drug active (%) (Probability,%) | N | Median age (Y) | Range | Male/Female | Median prior Tx | N | % among all patients | ||
| Conventional chemotherapeutic single agent | ||||||||||
| Oral methotrexate | CNS | 30 (90) | 82 | NA | NA | NA | NA | 18 | 22 | 79 (2000) |
| Placitaxel | CNS | 30 (95) | 73 | 7.7 | 0.3–19 | 41/32 | NA | 16 | 22 | 80 (2001) |
| Idarubicin | CNS | 30 (87) | 91 | NA | 3–19 | 50/41 | NA | 21 | 23 | 81 (2003) |
| Oxaliplatin | CNS | 35 (95) | 43 | 8.5 | 0.6–18.9 | 30/13 | NA | 30 | 70 | 82 (2006) |
| Temozolomide | CNS | 30 (95) | 121 | 11 | 1–23 | 63/85 | NA | 29a | 24 | 83 (2007) |
| Temozolomide | CNS | 25 (80) | 40 | 10 | 2–21 | 31/9 | NA | 37 | 93 | 84 (2014) |
| Topotecan | All Tm | 30 (95) | 53 | 12.9 | 2–23 | 23/30 | NA | 2 | 4 | 85 (2006) |
| Docetaxel | All Tm | 30 (95) | 173 | 13 | 1–27 | 107/66 | NA | 20 | 12 | 86 (2006) |
| Irinotecan | All Tm | 30 (80) | 161 | 9 | 1–23 | 104/67 | NA | 25a | 16 | 87 (2007) |
| Rebeccamycin analog | All Tm | 25 (88) | 133 | 9 | 0–21 | 72/61 | NA | 7 | 5 | 88 (2008) |
| Vinorelbine | All Tm | 30 (88) | 50 | 8.5 | 0–20 | 24/26 | NA | 2 | 4 | 89 (2009) |
| Pemetrexed | All Tm | 30 (95) | 72 | 11 | 3–23 | 39/33 | NA | 10 | 14 | 23 (2013) |
| Conventional chemotherapeutics combination | ||||||||||
| Temozolomide + Irinotecan | MB | 30 (80) | 66 | 10.5 | 2–17 | 45/21 | NA | 66 | 100 | 24 (2013) |
| Lobradimil + Carboplatin | CNS | 20 (90) | 40 | 9 | 2–21 | 20/20 | NA | 6a | 15 | 90 (2006) |
| Gemcitabine + Oxaliplatin | All Tm | 40 (95) | 93 | 11.7 | 1.3–20.8 | 52/41 | NA | 14 | 15 | 91 (2011) |
| Vinorelbine + CPM | All Tm | NA | 117 | 12 | 1–24 | 61/56 | NA | 7 | 6 | 92 (2012) |
| Targeted agent monotherapy | ||||||||||
| Tipifarnib | CNS | 25 (95) | 97 | 11.2 | 3.2–21.9 | 45/52 | NA | 12 | 12 | 93 (2007) |
| Imatinib | CNS | NA | 19 | 9 | 2–18 | 12/7 | 2 | 8a | 42 | 94 (2009) |
| Lapatinib | CNS | 25 (90) | 44 | 9.4 | 1.2–21.3 | 20/24 | NA | 12 | 27 | 22 (2013) |
| Vismodegib | MB | 25 (90) | 12 | 10.4 | 3.9–20 | 6/6 | NA | 12 | 100 | 98 (2015) |
| Targeted agent combination (n = 0) | ||||||||||
| Chemotherapeutics + targeted agent in combination | ||||||||||
| Bevacizumab + Irinotecan | CNS | NA | 92 | NA | 0.6–20.1 | NA | NA | 10 | 11 | 25 (2013) |
| Multiagent metronomic | All Tm | 30 (95) | 97 | 10 | 0–21 | 50/47 | NA | 6 | 6 | 95 (2014) |
| Chemotherapeutics + HSCT | ||||||||||
| Multiagent conditioning | CNS | NA | 19 | NA | 0.2–17 | 13/6 | NA | 9 | 47 | 96 (2010) |
| CPM + Melphalan | CNS | NA | 29 | 9.8 | 4.3–17.1 | 17/12 | NA | 22 | 76 | 97 (2008) |
All Tm, all tumors; CPM, cyclophosphamide; HSCT, hematopoietic stem cell transplantation; MB, medulloblastoma; NA, not available; OR, objective response; Tx, therapies; Y, years.
Medulloblastoma/PNET cohort that could not be split with the data obtained from the report.
Study population
A total of 3531 patients were included in the 78 studies that satisfied the eligibility criteria. Of those, 566 patients (16%) had medulloblastoma. In nine studies (12%), medulloblastoma and CNS‐PNET patients (n = 96) were presented together and figures could not be split; all were included in the analysis (Total = 662 patients). The proportion of patients with medulloblastoma was 11% in trials for patients with solid tumors (n = 212/1954 patients) and 22% in CNS tumors trials (n = 325/1452 patients). Median number of patients with medulloblastoma per trial was 4 (range, 1–66).
Response and outcome in patients with medulloblastoma
Data about response could be extracted from 48 of 54 phase I studies (89%) and 21 of 24 phase II (88%) (Tables 4 and 5). Median ORR (range) for all patients with medulloblastoma (n = 662) was 0% (0–100). Median ORR (range) in phase I studies was 0% (0–100) and 6.5% (0–50) in phase II. Median DCR in phase I studies was 16% (0–100) and 25% (0–75) in phase II.
Table 4.
Response rates of phase I studies including patients with medulloblastoma
| N (MB patients) | CR | PR | SD | PD | Objective response rate (%) | Disease control rate (%) | Reference (year of publication) | |
|---|---|---|---|---|---|---|---|---|
| Conventional chemotherapeutic single agent | ||||||||
| Temozolomide | 6 | 2 | 0 | NA | NA | 33 | 33 | 28 (2006) |
| Fotemustine | 6 | 0 | 0 | 1 | 5 | 0 | 16 | 29 (2009) |
| Cloretazine | 7 | 0 | 0 | 1 | 6 | 0 | 14 | 30 (2008) |
| Irinotecan | 19 | 0 | 1 | 1 | 17 | 5 | 11 | 31 (2003) |
| Liposomal Daunorubicine | 2 | NA | NA | NA | NA | NA | NA | 32 (2006) |
| Plitidepsin | 3 | 0 | 0 | 1 | 2 | 0 | 33 | 33 (2012) |
| Depsipeptide | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 34 (2006) |
| Fenretidine | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 35 (2006) |
| Pemetrexed | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 36 (2007) |
| Oxaliplatin | 5 | 0 | 0 | 1 | 4 | 0 | 20 | 37 (2007) |
| Satraplatin | 1 | 0 | 0 | 1 | 0 | 0 | 100 | 38 (2015) |
| Intrathecal lyposomal Ara‐C | 7 | 0 | 0 | 2 | 5 | 0 | 29 | 39 (2004) |
| Total | 60 | 2 | 1 | 8 | 43 | – | – | |
| ORR/DCRb | – | ORR(3/58 = 5% | DCR 11/58 = 19% | – | – | – | ||
| Median objective response/disease control rate (Range)c | 0 (0–33) | 16 (0–100) | ||||||
| Conventional chemotherapeutics combination | ||||||||
| TMZ + VP16 | 14 | 1 | 1 | 7 | 3 | 17a | 75 | 40 (2010) |
| O6‐Benzylguanine + TMZ | 10 | 0 | 0 | 2 | 8 | 0 | 20 | 41 (2007) |
| Cisplatin + Topotecan | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 42 (2002) |
| Irinotecan + Cisplatin | 1 | 0 | 0 | 1 | 0 | 0 | 100 | 43 (2003) |
| CPM + Topotecan | 3d | 0 | 0 | 1 | 2 | 0 | 33 | 44 (2004) |
| Cisplatin + TMZ | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 45 (2005) |
| Carboplatin + Irinotecan | 2 | 1 | 1 | 0 | 0 | 100 | 100 | 46 (2009) |
| Oxaliplatin + VP16 | 3 | 1 | 0 | 0 | 2 | 33 | 33 | 47 (2009) |
| Oxaliplatin + Irinotecan | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 48 (2009) |
| Irinotecan + TMZ + VCR | 2 | 0 | 0 | 2 | 0 | 0 | 100 | 49 (2010) |
| Oxaliplatin + Ifosfamide + VP16 | 2 | 0 | 1 | 0 | 1 | 50 | 50 | 50 (2015) |
| Total | 41 | 3 | 3 | 13 | 20 | – | – | |
| ORR/DCR | – | ORR 6/39 = 15% | DCR 19/39 = 48% | – | – | – | ||
| Median objective response/disease control rate (Range)c | 0 (0–100) | 33 (0–100) | ||||||
| Targeted agent monotherapy | ||||||||
| Vismodegib | 33 | 1 | 0 | 0 | 32 | 3 | 3 | 26 (2013) |
| Lonarfarnib | 2 | 0 | 0 | 1 | 1 | 0 | 50 | 51 (2007) |
| Cilengitide | 3 | 0 | 0 | 1 | 2 | 0 | 33 | 52 (2008) |
| Lapatinib | 15d | 0 | 0 | 1 | 14 | 0 | 7 | 21 (2010) |
| Valproic acid | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 53 (2011) |
| MK‐0752 | 4d | 0 | 0 | 0 | 4 | 0 | 0 | 54 (2011) |
| Erlotinib | 1 | NA | NA | NA | NA | NA | NA | 78 (2015) |
| Lenalidomide | 6d | NA | NA | NA | NA | NA | NA | 55 (2011) |
| Pazopanib | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 56 (2011) |
| Enzastaurin | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 57 (2013) |
| PTC299 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 58 (2015) |
| Dendritic cells | 1 | NA | NA | NA | NA | NA | NA | 59 (2015) |
| 3F8 monoclonal antibody | 4 | 0 | 0 | 0 | 4 | 0 | 0 | 60 (2004) |
| MK‐0752 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 61 (2007) |
| RG1507 | 1 | NA | NA | NA | NA | NA | NA | 62 (2011) |
| AT9283 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 63 (2015) |
| Sonidegib | 24 | 2 | 0 | 0 | 22 | 8 | 8 | 27 (2010) |
| SU101 | 4 | 0 | 0 | 1 | 3 | 0 | 25 | 64 (2004) |
| Temsirolimus | 2 | 0 | 0 | NA | NA | 0 | NA | 65 (2011) |
| MK‐2206 | 3d | 0 | 0 | 0 | 3 | 0 | 0 | 66 (2014) |
| Vorinostat ± retinoic acid | 9 | 0 | 0 | 1 | 8 | 0 | 11 | 67 (2010) |
| Total | 120 | 3 | 0 | 5 | 101 | – | – | |
| ORR/DCR | – | ORR 3/110 = 2.8% | DCR)8/110 = 7% | – | – | – | ||
| Median objective response/disease control rate (Range)c | 0 (0–8) | 0 (0–50) | ||||||
| Targeted agent combination | ||||||||
| Temsirolimus + Bevacizumab | 2 | 0 | 0 | 1 | 1 | 0 | 50 | 68 (2014) |
| Vorinostat + Bortezomib | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 77 (2013) |
| Total | 2 | 0 | 0 | 1 | 1 | – | – | |
| ORR/DCR | – |
ORR 0/3 = 0% |
DCR 1/3 = 33% |
– | – | – | ||
| Median objective response/disease control rate (Range)c | 0 (0–0) | 25 (0–50) | ||||||
| Chemotherapeutics + targeted agent in combination | ||||||||
| Vorinostat + TMZ | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 69 (2013) |
| Veliparib + TMZ | 2 | NA | NA | NA | NA | NA | NA | 70 (2014) |
| Carboplatin + Thalidomide | 4 | 0 | 0 | 1 | 3 | 0 | 25 | 71 (2004) |
| Erlotinib + TMZ | 6 | 0 | 1 | 0 | 5 | 17 | 17 | 72 (2008) |
| VIT + Bevacizumab | 1 | 0 | 1 | 0 | 0 | 100 | 100 | 73 (2013) |
| Bevacizumab + Irinotecan | 2 | 0 | 0 | 1 | 1 | 0 | 50 | 74 (2013) |
| Temsirolimus + Irinotecan + TMZ | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 75 (2014) |
| Total | 19 | 0 | 2 | 2 | 13 | – | – | |
| ORR/DCR | – |
ORR 2/17 = 12% |
DCR 4/17 = 24% |
– | – | – | ||
| Median objective response/disease control rate (Range)c | 0 (0–100) | 21 (0–100) | ||||||
| Chemotherapeutics + HSCT | ||||||||
| Thiotepa + Carmustine + Carboplatin | 18 | 4 | 0 | 0 | 14 | 22 | 22 | 76 (2011) |
| Total | 18 | 4 | 0 | 0 | 14 | – | – | |
| ORR/DCR | – |
ORR 4/18 = 22% |
DCR 4/18 = 22% |
|||||
| Median objective response/disease control rate (Range)c | 22 | 22 | ||||||
CPM, cyclophosphamide; CR, complete response; DCR, disease control rate; HSCT, hematopoietic stem cell transplantation; MB, medulloblastoma; NA, not available; ORR, overall response rate; PD, progressive disease; PNET, primitive neuroectodermal tumor; PR, partial response; SD, stable disease; TMZ, temozolomide; VCR, vincristine; VIT, Vincristine + Temozolomide + Irinotecan.
In these series there were patients with medulloblastoma who were not evaluable for response. Therefore, the number of responses is not equal to the number of patients with medulloblastoma included in the study.
ORR/DCR was calculated as the proportion of evaluable patients for which response was available in each category (CR, PR, SD, and PD).
Median ORR/DCR was calculated only based on the studies for which data on response (CR, PR, and SD) were available. It is expressed in percentage.
Medulloblastoma/PNET cohort that could not be split with the data obtained from the report.
Table 5.
Response rates of phase II studies including patients with medulloblastoma
| N (MB patients) | CR | PR | SD | PD | Objective Response Rate (%) | Disease control rate (%) | Reference (Year of publication) | |
|---|---|---|---|---|---|---|---|---|
| Conventional chemotherapeutic single agent | ||||||||
| Oral methotrexate | 18 | 0 | 0 | 6 | 11a | 0 | 35 | 79 (2000) |
| Placitaxel | 16 | 1 | 0 | 5 | 8a | 7 | 43 | 80 (2001) |
| Idarubicin | 21 | 0 | 1 | 6 | 11a | 6 | 39 | 81 (2003) |
| Oxaliplatin | 30 | 0 | 2 | 5 | 23 | 7 | 23 | 82 (2006) |
| Temozolomide | 29f | 1 | 3 | 7 | 14a | 16 | 56 | 83 (2007) |
| Temozolomide | 37 | 6 | 9 | 10 | 12 | 41 | 67 | 84 (2014) |
| Topotecan | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 85 (2006) |
| Docetaxel | 20 | 0 | 1 | 18 | 18b | 5 | NA | 86 (2006) |
| Irinotecan | 25f | 0 | 4 | NA | NA | 16 | NA | 87 (2007) |
| Rebeccamycin analog | 7 | 0 | 0 | 0 | 7 | 0 | 0 | 88 (2008) |
| Vinorelbine | 2 | 0 | 1 | 0 | 1 | 50 | 50 | 89 (2009) |
| Pemetrexed | 10 | 0 | 0 | 1 | 9 | 0 | 11 | 23 (2013) |
| Total | 217 | 8 | 21 | 58 | 116 | |||
| ORR/DCRd | – | ORR 29/207 = 14% | NAc | – | – | – | ||
| Median objective response/disease control rate (Range)e | 7 (0–50) | 37 (0–67) | ||||||
| Conventional chemotherapeutics combination | ||||||||
| Temozolomide + Irinotecan | 66 | 1 | 20 | 26 | 15a | 34 | 75 | 24 (2013) |
| Lobradimil + Carboplatin | 6f | 0 | 0 | 0 | 6 | 0 | 0 | 90 (2006) |
| Gemcitabine + Oxaliplatin | 14 | 0 | 1 | 6 | 7 | 7 | 50 | 91 (2011) |
| Vinorelbine + CPM | 7 | 0 | 0 | 1 | 6 | 0 | 14 | 92 (2012) |
| Total | 93 | 1 | 21 | 33 | 34 | |||
| ORR/DCR | – | ORR 21/89 = 23% | DCR 53/89 = 59% | – | – | – | ||
| Median objective response/disease control rate (Range)e | 3.5 (0–34) | 32 (0–75) | ||||||
| Targeted agent monotherapy | ||||||||
| Tipifarnib | 12 | 0 | 0 | 0 | 12 | 0 | 0 | 93 (2007) |
| Imatinib | 8f | 0 | 0 | 1 | 7 | 0 | 13 | 94 (2009) |
| Lapatinib | 12 | 0 | 0 | 3 | 9 | 0 | 25 | 22 (2013) |
| Vismodegib | 12 | 0 | 1 | 0 | 11 | 8 | 8 | 98 (2015) |
| Total | 44 | 0 | 1 | 4 | 39 | |||
| ORR/DCR | – | ORR 1/44 = 2% | DCR 5/44 = 11% | – | – | – | ||
| Median objective response/disease control rate (Range)e | 0 (0–8) | 11 (0–25) | ||||||
| Targeted agent combination (n = 0) | ||||||||
| Chemotherapeutics + targeted agent in combination | ||||||||
| Bevacizumab + Irinotecan | 10 | NA | NA | NA | NA | NA | NA | 25 (2013) |
| Multiagent metronomic | 6 | 1 | 0 | 2 | 3 | 17 | 50 | 95 (2014) |
| Total | 16 | 1 | 0 | 2 | – | – | – | |
| ORR/DCR | – | ORR 1/6 = 17% | DCR 3/6 = 50% | – | – | – | ||
| Median objective response/disease control rate (Range)e | 17 | 50 | ||||||
| Chemotherapeutics + HSCT | ||||||||
| Multiagent conditioning | 9 | NA | NA | NA | NA | NA | NA | 96 (2010) |
| CPM + Melphalan | 22 | NA | NA | NA | NA | NA | NA | 97 (2008) |
| Total | 31 | – | – | – | – | – | – | |
| ORR/DCR | – | – | – | – | – | – | – | |
| Median objective response/disease control rate (Range)e | NA | NA | ||||||
CPM, cyclophosphamide; CR, complete response; DCR, disease control rate; HSCT, hematopoietic stem cell transplantation; MB, medulloblastoma; NA, not available; ORR, overall response rate; PD, progressive disease; PNET, primitive neuroectodermal tumor; PR, partial response; SD, stable disease.
In these series there were patients with medulloblastoma who experienced early death or for whom disease evaluation was unknown. Therefore, the number of responses is not equal to the number of patients with medulloblastoma included in the study.
In these series, 18 patients experienced either SD or PD but figures were presented together in the original manuscript and therefore could not be split in this table. One of the 20 patients was not evaluable.
Calculation of DCR cannot be made because there were two studies for which data about SD and PD could not be obtained.
ORR/DCR was calculated as the proportion of evaluable patients for whom response was available.
Median ORR/DCR was calculated only based on the studies for which data on response (CR, PR, and SD) were available. It is expressed in percentage.
Medulloblastoma/PNET cohort that could not be split with the data obtained from the report.
Conventional single‐agent chemotherapeutics yielded the highest response rates in phase I (median DCR 16%, 0–100) and II studies (median DCR 37%, 0–67). Within phase II trials there were three studies in which patients died of documented progressive disease before their first scheduled evaluation (n = 4 patients, 0.6% of 662 patients) 79, 80, 81.
Response and outcome in medulloblastoma‐/PNET‐specific trials
Four studies were addressed exclusively to patients with medulloblastoma evaluating the smoothened (SMO) inhibitor vismodegib (n = 2) 26, 98, temozolomide, and etoposide 40, and the combination of temozolomide with irinotecan 24. In the phase II study evaluating temozolomide and irinotecan, ORR and DCR were 33% and 73%, respectively; 46.2% of the patients were progression free at 6 months and 79.7% were still alive, which is the best response obtained among these four studies, although with a short follow‐up for progression free 24. One study including patients with medulloblastoma and PNET, investigated temozolomide as a single agent 84. Within 37 patients with medulloblastoma, ORR was 46%, including six CR and a progression‐free survival rate among those with objective response at 6 and 12 months of 70.6% and 17.5%, respectively.
Description of response and outcome by therapeutic class of agents
In this section we describe the results for specific therapeutic class of agents that have been tested more frequently.
Platinum salts
Platinum salts were the most frequent class of agent tested (n = 15, 19%). Median ORR varied from 0 to 7% 37, 82 when used as a single agent, and up to 33% 47 when combined with etoposide and 100% 46 with irinotecan.
Temozolomide
Temozolomide was the second most common agent tested (n = 13, 17%). Temozolomide containing studies have shown a median ORR of 16.5% (range, 0–100%) and a median DCR of 36.5% (range, 0–100%). Phase II studies containing temozolomide had a median ORR of 33% (range, 16–46) and a median DCR of 57% (range, 40–73). Toxicity is mainly represented by hematological and gastrointestinal events.
Targeted therapies
Three different categories of targeted agents (n = 36) have been evaluated: small molecules (n = 30, 83%), antibodies (n = 5, 14%), and immunotherapeutic agents (n = 1, 3%).
The smoothened (SMO) inhibitors
Three studies have evaluated two different SMO inhibitors. Sonidegib was evaluated in a phase I–II study where the cohort included patients with relapsed tumors potentially dependent on sonic hedgehog (Shh) signaling 27; 33 patients were included, 24 of whom had a medulloblastoma. ORR for the whole population was 6% (two CR in Hh‐activated medulloblastoma; of note, only 14 patients with medulloblastoma were evaluated with the 5‐gene Hh signature assay, and only the two patients who responded had an Hh‐activated medulloblastoma). In the phase I study of Vismodegib, seven of 33 patients were found to have Hh‐activated disease, of which only one responded (unsustained 8‐week CR, ORR 3%) 26. In the phase II part of the study, 12 other patients were included and only one experienced sustained response 98.
Antiangiogenic therapies
A total of nine studies evaluated antiangioenic therapies. A phase II trial with multiagent oral antiangiogenic regimen in patients with medulloblastoma (n = 6) reported one CR (ORR 17%) and two disease stabilizations (DCR 50%) with a tolerable toxicity profile 95. The combination of bevacizumab with vincristine, irinotecan, and temozolomide resulted in one partial response after four cycles (3 months) allowing the patient to be consolidated with radiotherapy (ORR 100%) 73. The combination of bevacizumab and temsirolimus resulted in a 5‐month sustained disease stabilization in one of two patients included (DCR 50%) 68 and one patient receiving bevacizumab and irinotecan achieved a 14‐month disease stabilization (DCR 50%) 74. Other evaluated antiangiogenic agents such as cilengitide 52 or thalidomide and its analogs, either in monotherapy 56 or in combination with platinum agents 71, have yielded only short‐lasting disease stabilizations.
Current and forthcoming molecularly stratified studies and targeted and immunotherapeutic agents in clinical trials for medulloblastoma patients
Fifty‐one studies were identified in the https://clinicaltrials.gov website, of which 20 were molecularly stratified studies and targeted/immunotherapeutic trials addressed to patients with medulloblastoma: five (25%) in first line and fifteen (75%) in second or subsequent lines (Table 6).
Table 6.
Active and forthcoming molecularly stratified and tumor‐specific studies and targeted agents tested in clinical trials for medulloblastoma patients
| First line treatments | |||||
|---|---|---|---|---|---|
| Population | Intervention | Phase | Sponsor | Responsible party | Reference |
| Classical MB WNT positive tumors and absence of other high‐risk clinical and molecular featuresa |
Surgery + combination chemotherapy No radiotherapy |
II | Academia | Sidney Kimmel Cancer Center | NCT02212574 |
| Classical MB WNT positive tumors and absence of other high‐risk clinical and molecular featuresa | Surgery + Combination chemotherapy and reduced local and craniospinal irradiation | II | Academia | Children′s Oncology Group | NCT02724579 |
| Low‐risk (LR)b and standard‐risk (SR) MB patients |
LR: Surgery + Radiotherapy and reduced radiotherapy and maintenance chemotherapy SR: Surgery + Radiotherapy (± carboplatin) and radiotherapy and maintenance chemotherapy |
II–III | Academia | Universitätsklinikum Hamburg‐Eppendorf |
NCT02066220 (PNET‐5) |
| WNT, SHH, and Non‐WNT or Non‐SHH MB patients |
LR WNT tumors: Lower dose of radiotherapy and chemotherapy SHH patients: Value of adding vismodegib IR and HR Non‐WNT/Non‐SHH: Value of adding pemetrexed and gemcitabine |
II | Academia | St. Jude Children′s Research Hospital | NCT01878617 |
| Standard‐Risk MB patients |
Postoperative radioimmunotherapy (intrathecal 131‐I‐3F8) Reduced doses of CSI, primary site boost, and standard adjuvant chemotherapy |
II | Academia | Memorial Sloan Kettering Cancer Center | NCT00058370 |
| Second and subsequent lines of treatment | |||||
|---|---|---|---|---|---|
| Population | Intervention | Phase | Sponsor | Reference | |
| Studies with a specific cohort for medulloblastoma patients | |||||
| MB and PNET | Vaccine immunotherapy (TTRNA‐xALT) | I | Academia | University of Florida | NCT01326104 |
| MB and ATRT | Modified measles virus (MV‐NIS) | I | Academia | University of California, San Francisco | NCT02962167 |
| MB and CNS and other solid tumors | AZD1175 (Wee1 inhibitor) + Irinotecan | I | Academia | NCI | NCT02095132 |
| MB and CNS tumors | Indoximod (IDO checkpoint inhibitor) + TMZ | I | Industry | NewLink Genetics Corporation | NCT02502708 |
| MB | Metronomic and targeted antiangiogenesis therapy | II | Academia | Medical University of Vienna | NCT01356290 |
| MB and other solid tumors (carcinoid, neuroblastoma and neuroendocrine tumors) | Dosimetry‐Guided 90Y‐DOTA‐tyr3‐Octreotide Peptide Receptor Radiotherapy | II | Academia | University of Iowa | NCT02441088 |
| MB | TB‐403 (monoclonal antibody against placental growth factor [PlGF]) | I–II | Industry | Oncurious NV | NCT02748135 |
| Studies addressed to patients with relapsed malignancies including also medulloblastoma patients | |||||
| CNS tumors | Wild‐Type Reovirus in Combination With Sargramostim | I | Academia | Mayo Clinic | NCT02444546 |
| CNS tumors | Palbociclib (CDK 4–6 inhibitor) | I | Academia | Pediatric Brain Tumor Consortium | NCT02255461 |
| Solid tumors that are 8H9 reactive | Iodine I 131 monoclonal antibody 8H9 | I | Academia | Memorial Sloan Kettering Cancer Center | NCT00089245 |
| Solid tumors undergoing autologous hematopoietic stem cell transplantation | Antiangiogenic therapy: Cyclophosphamide or thalidomide beginning Day +30 (30 days posttransplant) and continued until at least Day +86 | I | Academia | Washington University School of Medicine | NCT01661400 |
| Solid tumors | Talazoparib (PARP inhibitor) + Irinotecan ± temozolomide | I | Academia | St. Jude Children′s Research Hospital | NCT02392793 |
| Solid tumors and hematologic malignancies | Multiarm targeted therapies ± conventional chemotherapeutics (ESMART) | I–II | Academia | Gustave Roussy | NCT02813135 |
| Solid tumors and hematologic malignancies | Multiarm targeted therapies (Pediatric MATCH) | II | Academia | National Cancer Institute |
NCT03233204 NCT03213665 NCT03213678 NCT03213704 NCT03210714 NCT03155620 |
| Solid tumors | Erlotinib (EGFR inhibitor) and TMZ | II | Academia | Washington University School of Medicine | NCT02689336 |
ATRT, atypical teratoid rhaboid tumor; CNS, central nervous system; CSI, craniospinal irradiation; HR, high risk; IR, intermediate risk; LR, low risk; MB, medulloblastoma; NCI, National Cancer Institute; PNET, primitive neuroectodermal tumors; PlGF, placental growth factor; SHH, sonic hedgehog; SR, standard risk; TMZ, temozolomide.
High‐risk features are defined as metastatic disease, >1.5 cm2 postoperative residual tumor, presence of MYC or MYCN amplification, absence of nuclear beta‐catenin reactivity, and unfavorable histology (large‐cell or anaplastic subtypes).
In the PNET V study the Low‐Risk group is defined as the WNT subgroup positivity.
Discussion
The outcome of patients with medulloblastoma has improved over the last decades. This has been largely achieved as a result of international collaborative efforts through clinical trials 99. Still, outcome for those with metastatic disease, adverse molecular or cytogenetic features, infants 99, and relapsed or refractory patients 11 remains challenging.
In addition, for those who survive long‐term side effects are of major importance. Hearing and cognitive impairment can hamper independent living and these patients are endured an increased risk of stroke and secondary neoplasms 100, 101, 102, among other late effects.
Therefore, clinical trials are clearly needed to find new strategies to improve their outcome and reduce long‐term sequelae.
This study covers an expanded period of time in which new agents and strategies have been tested giving a precise landscape of the attempts to improve the outcome of patients with relapsed medulloblastoma.
Some limitations must be pointed out. Firstly, the search strategy was limited to articles indexed in Pubmed, those with results in https://clinicaltrials.gov, and references from selected studies. We did not search meetings’ abstracts books, where preliminary results from ongoing trials are presented before definitive publication. Secondly, results disclosing response need to be interpreted cautiously due to heterogeneity between studies as regards to eligibility criteria, patient population (e.g., first or subsequent relapse), and, more importantly, the limited number of patients with medulloblastoma in each trial. In addition, the radiological response criteria used across phase II studies were heterogeneous, with 75% using WHO and 21% using RECIST. Finally, we identified in phase II studies that true response rates to declare a drug active were heterogeneous, even when evaluating the same drug in similar scenarios. This means that a trial might be deemed successful or not based on how we predefine the true response rates. Activity data from historical controls are used to calculate true response rates for interventional clinical trials, although it still has major limitations 103. Yet randomized trials remain the best method to discern true effects in interventional studies.
Of note, only a small number of patients died of rapid disease progression before the first scheduled trial evaluation (4/662; 0.6) 79, 80, 81 and it has been shown that poor performance status at enrolment correlates with worse survival in children with brain tumors participating in phase I trials 104.
Objective response rates remain modest. Median ORR rate for patients with medulloblastoma was 0% (range, 0–100) in phase I studies and 6.5% (range, 0–50) in phase II. Median DCR for patients with medulloblastoma was 16% (0–100) in phase I studies and 25% (0–75) in phase II.
Among conventional chemotherapeutics, temozolomide‐containing regimens have shown most promising activity. Two studies, one in monotherapy 84 and another in combination with irinotecan 24, have shown the best results in a relatively large population, although follow up for disease‐free survival is short. Its tolerable toxicity profile and synergies with other chemotherapeutics and targeted agents make it an attractive compound to serve as backbone for new strategies. Indeed, temozolomide has been brought to frontline trials as maintenance therapy after intensive chemotherapy and hematopoietic stem cell transplantation in metastatic CNS‐PNET patients (NCT00936156).
The advent of the molecular classification of medulloblastoma in 2012 17 and the progressive implementation of molecular techniques able to clarify key biology aspects have permitted to improve our understanding of this disease and develop more specific strategies.
More recently, the identification of novel molecular subgroups has permitted to further stratify patients into four prognostic categories (favorable, standard, high, and very high risk) 105; this implies that our current frontline therapeutic approach needs to be revised.
In this sense, serial characterization of medulloblastomas at diagnosis and at the time of relapse has shown that medulloblastoma does not change subgroup at recurrence but have drastically different genomes than the primary disease, and that the pattern of recurrence is driven by subgroup affiliation rather than treatment 106 (e.g., SHH tumors recur mostly locally and groups 3 and 4 recur almost exclusively with metastases with prolonged long‐term postrecurrence survival). Future strategies addressed to patients with groups 3 and 4 medulloblastoma should consider intensification of treatments aimed at the metastatic compartment (e.g., intrathecal consolidation) 106.
Based on the fact that pediatric tumors evolve under therapy with emerging new molecular alterations 107 and behave differently at the time of relapse 106 or develop secondary events that require a complete distinct approach 106, several platforms in Europe (iTHER, INFORM) look to identify changes in the tumor molecular profile by comparing tissue from diagnosis with that at relapse in order to identify new therapeutic opportunities.
The sonic hedgehog pathway plays a critical role in normal cerebellar development; desmoplastic, nodular, and extensive nodularity subtypes are universally associated with Shh pathway activation. Alterations in this pathway are characteristics of one of the four molecular subgroups in medulloblastoma, the so‐called Shh group 2. The application of the first smoothened inhibitor showed extraordinary (although short‐lasting) response in first‐in‐human studies 108. But subsequent studies in selected Shh‐activated patients have yielded only limited and short‐lasting responses 26, 98. Nonetheless, prolonged complete responses have also been reported 27. For this reason, vismodegib is currently being evaluated as maintenance treatment postradiotherapy and chemotherapy for skeletally mature children with newly diagnosed standard‐risk Shh medulloblastoma (NCT01878617). Whether SMO inhibitors are called to play a major role in this subset of patients remains unclear. The genomic aberration relative to SMO is predictive of SMO inhibitor activity 98 and current efforts are focusing on identifying which subset of Hh‐activated tumors are more likely to respond by means of a complete molecular profiling. The Shh pathway can also be targeted at different levels to disrupt tumorigenesis and to overcome the limitations of single‐agent therapies; for instance, blocking GLI1 with arsenic trioxide 2, or combining SMO inhibitors with PI3K inhibitors 98, whose aberrations are frequent in this subset of patients.
Non‐WNT/Non‐SHH medulloblastomas comprise groups 3 and 4 of the molecular classification. Altogether they represent up to 60% of all medulloblastoma, but the underlying molecular drivers yet remain to be fully characterized and therefore no specific targeted treatments are available at present 2. A phase II clinical trial (NCT01878617) is currently evaluating the addition of pemetrexed and gemcitabine in consolidation. Both pemetrexed 23, 36 and gemcitabine 91 have been previously tested per separate in medulloblastoma patients. In our analysis, only the combination of gemcitabine with oxaliplatin was found to have promising results (one PR and six disease stabilizations of 14 treated medulloblastoma patients; ORR 7% and DCR 50%) 91. Interestingly, a recent preclinical study identified the combination of these two drugs as active, both in cellular assays and in mouse models of group 3 medulloblastoma 109, further supporting the interest of combination in prospective studies (NCT01878617). For patients with group 4 medulloblastomas, there may be a role for epigenetic‐based therapies, such as demethylating agents and histone deacetylase inhibitors 2, 99. The combination of vorinostat and retinoic acid resulted in a 5‐month disease stabilization 67, while no responses were seen when combining vorinostat with temozolomide 69 or with bortezomib 77.
Ongoing and forthcoming phase I‐II trials in medulloblastoma are addressed to specific cancer vulnerabilities (Table 6). New strategies look to identify genetic aberrations through exhaustive molecular screening, which permits patients with individual alterations to receive a coupled treatment (ESMART trial; NCT02813135).
In conclusion, this systematic review shows that there have been a large number of studies evaluating new therapies in children with medulloblastoma but with limited impact in their survival outcomes. The heterogeneity between trials in terms of their design and study population limits the generalization of those results and no randomized studies have been conducted. Temozolomide‐containing regimens are tolerable and have demonstrated antitumor activity against relapsed/refractory medulloblastoma. Future studies may consider using this drug as a backbone for new combinations. Targeted therapies have shown modest antitumor activity; SMO inhibitors are promising agents in Hh‐activated tumors, although still we need to identify which subset of patients can benefit more from this approach. New high‐throughput molecular platforms permitting to dissect and compare tumor biology at diagnosis and at relapse will allow identifying patients harboring specific genetic aberrations who are suitable candidates for new targeted therapies and therefore more likely to derive benefit from these novel agents.
Conflict of Interest
The authors declare that they have no conflict of interest.
Supporting information
Data S1. Search strategy (PUBMED).
Acknowledgments
We are grateful to all patients (children and adults) participating in the clinical trials here reported and their families. We are also grateful to the research teams that have conducted these studies.
Cancer Medicine 2017; 6(11):2606–2624
References
- 1. Gilbertson, R. J. 2004. Medulloblastoma: signalling a change in treatment. Lancet Oncol. 5:209–218. [DOI] [PubMed] [Google Scholar]
- 2. DeSouza, R.‐M. , Jones B. R. T., Lowis S. P., and Kurian K. M.. 2014. Pediatric medulloblastoma ‐ update on molecular classification driving targeted therapies. Front. Oncol. 4:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farwell, J. R. , Dohrmann G. J., and Flannery J. T.. 1984. Medulloblastoma in childhood: an epidemiological study. J. Neurosurg. 61:657–664. [DOI] [PubMed] [Google Scholar]
- 4. Packer, R. J. , Sutton L. N., Goldwein J. W., Perilongo G., Bunin G., Ryan J., et al. 1991. Improved survival with the use of adjuvant chemotherapy in the treatment of medulloblastoma. J. Neurosurg. 74:433–440. [DOI] [PubMed] [Google Scholar]
- 5. Ramaswamy, V. , Remke M., Adamski J., Bartels U., Tabori U., Wang X., et al. 2016. Medulloblastoma subgroup‐specific outcomes in irradiated children: who are the true high‐risk patients? Neuro. Oncol. 18:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taylor, R. E. , Bailey C. C., Robinson K., Weston C. L., Ellison D., Ironside J., et al. 2003. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: the International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET‐3 study. J. Clin. Oncol. 21:1581–1591. [DOI] [PubMed] [Google Scholar]
- 7. Lannering, B. , Rutkowski S., Doz F., Pizer B., Gustafsson G., Navajas A., et al. 2012. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard‐risk medulloblastoma: results from the randomized multicenter HIT‐SIOP PNET 4 trial. J. Clin. Oncol. 30:3187–3193. [DOI] [PubMed] [Google Scholar]
- 8. von Hoff, K. , Hinkes B., Gerber N. U., Deinlein F., Mittler U., Urban C., et al. 2009. Long‐term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT'91. Eur. J. Cancer 45:1209–1217. [DOI] [PubMed] [Google Scholar]
- 9. Jakacki, R. I. , Burger P. C., Zhou T., Holmes E. J., Kocak M., Onar A., et al. 2012. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children‘s Oncology Group Phase I/II study. J. Clin. Oncol. 30:2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Allen, J. , Donahue B., Mehta M., Miller D. C., Rorke L. B., Jakacki R., et al. 2009. A phase II study of preradiotherapy chemotherapy followed by hyperfractionated radiotherapy for newly diagnosed high‐risk medulloblastoma/primitive neuroectodermal tumor: a report from the Children's Oncology Group (CCG 9931). Int. J. Radiat. Oncol. Biol. Phys. 74:1006–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sabel, M. , Fleischhack G., Tippelt S., Gustafsson G., Doz F., Kortmann R., et al. 2016. Relapse patterns and outcome after relapse in standard risk medulloblastoma: a report from the HIT‐SIOP‐PNET4 study. J. Neurooncol. 129:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Perreault, S. , Lober R. M., Carret A.‐S., Zhang G., Hershon L., Décarie J.‐C., et al. 2013. Relapse patterns in pediatric embryonal central nervous system tumors. J. Neurooncol. 115:209–215. [DOI] [PubMed] [Google Scholar]
- 13. Bautista, F. , Gallego S., Cañete A., Mora J., Diaz de Heredia C., Cruz O., et al. 2016. Landscape of early clinical trials for childhood and adolescence cancer in Spain. Clin. Transl. Oncol. 18:708–713. [DOI] [PubMed] [Google Scholar]
- 14. Kim, A. , Fox E., Warren K., Blaney S. M., Berg S. L., Adamson P. C., et al. 2008. Characteristics and outcome of pediatric patients enrolled in phase I oncology trials. Oncologist 13:679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carceller, F. , Bautista F. J., Jiménez I., Hladun‐Álvaro R., Giraud C., Bergamaschi L., et al. 2016a. Prognostic factors of overall survival in children and adolescents enrolled in dose‐finding trials in Europe: an Innovative Therapies for Children with Cancer study. Eur. J. Cancer 67:130–140. [DOI] [PubMed] [Google Scholar]
- 16. Louis, D. N. , Perry A., Reifenberger G., von Deimling A., Figarella‐Branger D., Cavenee W. K., et al. 2016. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131:803–820. [DOI] [PubMed] [Google Scholar]
- 17. Taylor, M. D. , Northcott P. A., Korshunov A., Remke M., Cho Y.‐J., Clifford S. C., et al. 2012. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 123:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Thompson, E. M. , Hielscher T., Bouffet E., Remke M., Luu B., Gururangan S., et al. 2016. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 17:484–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ellison, D. W. , Kocak M., Dalton J., Megahed H., Lusher M. E., Ryan S. L., et al. 2011. Definition of disease‐risk stratification groups in childhood medulloblastoma using combined clinical, pathologic, and molecular variables. J. Clin. Oncol. 29:1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liberati, A. , Altman D. G., Tetzlaff J., Mulrow C., Gotzsche P. C., Ioannidis J. P. A., et al. 2009. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700–b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fouladi, M. , Stewart C. F., Blaney S. M., Onar‐Thomas A., Schaiquevich P., Packer R. J., et al. 2010a. Phase I trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J. Clin. Oncol. 28:4221–4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fouladi, M. , Stewart C. F., Blaney S. M., Onar‐Thomas A., Packer R. J., Goldman S., et al. 2013. A molecular biology and phase II trial of lapatinib in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium Study. J. Neurooncol. 114:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Warwick, A. B. , Malempati S., Krailo M., Melemed A., Gorlick R., Ames M. M., et al. 2013. Phase 2 trial of pemetrexed in children and adolescents with refractory solid tumors: a Children's Oncology Group study. Pediatr. Blood Cancer 60:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grill, J. , Geoerger B., Gesner L., Perek D., Leblond P., Cañete A., et al. 2013. Phase II study of irinotecan in combination with temozolomide (TEMIRI) in children with recurrent or refractory medulloblastoma: a joint ITCC and SIOPE brain tumor study. Neuro. Oncol. 15:1236–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fangusaro, J. , Gururangan S., Poussaint T. Y., McLendon R. E., Onar‐Thomas A., Warren K. E., et al. 2013. Bevacizumab (BVZ)‐associated toxicities in children with recurrent central nervous system tumors treated with BVZ and irinotecan (CPT‐11): a Pediatric Brain Tumor Consortium study (PBTC‐022). Cancer 119:4180–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gajjar, A. , Stewart C. F., Ellison D. W., Kaste S., Kun L. E., Packer R. J., et al. 2013. Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: a Pediatric Brain Tumor Consortium study. Clin. Cancer Res. 19:6305–6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Geoerger, B. , Aerts I., Casanova M., Chisholm J., Hargrave D., Leary S., et al. 2012a. Phase I/II study of LDE225, a Smoothened (Smo) antagonist, in pediatric patients with recurrent medulloblastoma or other solid tumors. J. Clin. Oncol. 30:abstr e9519. [Google Scholar]
- 28. Baruchel, S. , Diezi M., Hargrave D., Stempak D., Gammon J., Moghrabi A., et al. 2006. Safety and pharmacokinetics of temozolomide using a dose‐escalation, metronomic schedule in recurrent paediatric brain tumours. Eur. J. Cancer 42:2335–2342. [DOI] [PubMed] [Google Scholar]
- 29. Hargrave, D. R. , Bouffet E., Gammon J., Tariq N., Grant R. M., Baruchel S., et al. 2002. Phase I study of fotemustine in pediatric patients with refractory brain tumors. Cancer 95:1294–1301. [DOI] [PubMed] [Google Scholar]
- 30. Gururangan, S. , Turner C. D., Stewart C. F., O'Shaughnessy M., Kocak M., Poussaint T. Y., et al. 2008. Phase I trial of VNP40101M (Cloretazine) in children with recurrent brain tumors: a Pediatric Brain Tumor Consortium study. Clin. Cancer Res. 14:1124–1130. [DOI] [PubMed] [Google Scholar]
- 31. Vassal, G. , Doz F., Frappaz D., Imadalou K., Sicard E., Santos A., et al. 2003. A phase I study of irinotecan as a 3‐week schedule in children with refractory or recurrent solid tumors. J. Clin. Oncol. 21:3844–3852. [DOI] [PubMed] [Google Scholar]
- 32. Lowis, S. , Lewis I., Elsworth A., Weston C., Doz F., Vassal G., et al. 2006. A phase I study of intravenous liposomal daunorubicin (DaunoXome) in paediatric patients with relapsed or resistant solid tumours. Br. J. Cancer 95:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geoerger, B. , Estlin E. J., Aerts I., Kearns P., Gibson B., Corradini N., et al. 2012b. A phase I and pharmacokinetic study of plitidepsin in children with advanced solid tumours: an Innovative Therapies for Children with Cancer (ITCC) study. Eur. J. Cancer 48:289–296. [DOI] [PubMed] [Google Scholar]
- 34. Children's Oncology Group , M., Fouladi , W. L., Furman , Chin T., Freeman B. B., Dudkin L., et al. 2006. Phase I study of depsipeptide in pediatric patients with refractory solid tumors: a Children's Oncology Group report. J. Clin. Oncol. 24:3678–3685. [DOI] [PubMed] [Google Scholar]
- 35. Children's Oncology Group (CCG 09709) , J. G., Villablanca , M. D., Krailo , Ames M. M., Reid J. N., Reaman G. H., et al. 2006. Phase I trial of oral fenretinide in children with high‐risk solid tumors: a report from the Children's Oncology Group (CCG 09709). J. Clin. Oncol. 24:3423–3430. [DOI] [PubMed] [Google Scholar]
- 36. Malempati, S. , Nicholson H. S., Reid J. M., Blaney S. M., Ingle A. M., Krailo M., et al. 2007. Phase I trial and pharmacokinetic study of pemetrexed in children with refractory solid tumors: the Children's Oncology Group. J. Clin. Oncol. 25:1505–1511. [DOI] [PubMed] [Google Scholar]
- 37. Spunt, S. L. , Freeman B. B., Billups C. A., Mcpherson V., Khan R. B., Pratt C. B., et al. 2007. Phase I clinical trial of oxaliplatin in children and adolescents with refractory solid tumors. J. Clin. Oncol. 25:2274–2280. [DOI] [PubMed] [Google Scholar]
- 38. Akshintala, S. , Marcus L., Warren K. E., Murphy R. F., Sissung T. M., Srivastava A., et al. 2015. Phase 1 trial and pharmacokinetic study of the oral platinum analog satraplatin in children and young adults with refractory solid tumors including brain tumors. Pediatr. Blood Cancer 62:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bomgaars, L. , Geyer J. R., Franklin J., Dahl G., Park J., Winick N. J., et al. 2004. Phase I trial of intrathecal liposomal cytarabine in children with neoplastic meningitis. J. Clin. Oncol. 22:3916–3921. [DOI] [PubMed] [Google Scholar]
- 40. Ruggiero, A. , Rizzo D., Attin G., Lazzareschi I., Mastrangelo S., Maurizi P., et al. 2010. Phase I study of temozolomide combined with oral etoposide in children with recurrent or progressive medulloblastoma. Eur. J. Cancer 46:2943–2949. [DOI] [PubMed] [Google Scholar]
- 41. Broniscer, A. , Gururangan S., MacDonald T. J., Goldman S., Packer R. J., Stewart C. F., et al. 2007. Phase I trial of single‐dose temozolomide and continuous administration of o6‐benzylguanine in children with brain tumors: a pediatric brain tumor consortium report. Clin. Cancer Res. 13:6712–6718. [DOI] [PubMed] [Google Scholar]
- 42. Wells, R. J. , J. M., Reid , Ames M. M., Mares W. L., Krailo M. D., Seibel N. L., et al. 2002. Phase I trial of cisplatin and topotecan in children with recurrent solid tumors: Children's Cancer Group study 0942. J. Pediatr. Hematol. Oncol. 24:89–93. [DOI] [PubMed] [Google Scholar]
- 43. Tumors, S. , Souid A., Dubowy R. L., Blaney S. M., Hershon L., Sullivan J., et al. 2003. Phase I clinical and pharmacologic study of weekly cisplatin and irinotecan combined with amifostine for refractory solid tumors. Clin. Cancer Res. 9:703–710. [PubMed] [Google Scholar]
- 44. Bowers, D. C. , Aquino V. M., Leavey P. J., Bash R. O., Journeycake J. M., Tomlinson G., et al. 2004. Phase I study of oral cyclophosphamide and oral topotecan for children with recurrent or refractory solid tumors. Pediatr. Blood Cancer 42:93–98. [DOI] [PubMed] [Google Scholar]
- 45. Geoerger, B. , Vassal G., Doz F., O'Quigley J., Wartelle M., Watson A. J., et al. 2005. Dose finding and O6‐alkylguanine‐DNA alkyltransferase study of cisplatin combined with temozolomide in paediatric solid malignancies. Br. J. Cancer 93:529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Levy, A. S. , Meyers P. A., Wexler L. H., Jakacki R., Angiolillo A., Ringuette S. N., et al. 2009. Phase 1 and pharmacokinetic study of concurrent carboplatin and irinotecan in subjects aged 1 to 21 years with refractory solid tumors. Cancer 115:207–216. [DOI] [PubMed] [Google Scholar]
- 47. McGregor, L. M. , Spunt S. L., Santana V. M., Stewart C. F., Ward D. A., Watkins A., et al. 2009a. Phase 1 study of an oxaliplatin and etoposide regimen in pediatric patients with recurrent solid tumors. Cancer 115:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McGregor, L. M. , Spunt S. L., Furman W. L., Stewart C. F., Schaiquevich P., Krailo M. D., et al. 2009b. Phase 1 study of oxaliplatin and irinotecan in pediatric patients with refractory solid tumors: a Children's Oncology Group Study. Cancer 115:1765–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wagner, L. M. , Perentesis J. P., Reid J. M., Ames M. M., Safgren S. L., Nelson M. D., et al. 2010. Phase I trial of two schedules of vincristine, oral irinotecan, and temozolomide (VOIT) for children with relapsed or refractory solid tumors: a Children's Oncology Group Phase I Consortium study. Pediatr. Blood Cancer 54:538–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lam, C. G. , Furman W. L., Wang C., Spunt S. L., Wu J., Ivy P., et al. 2015. Phase I clinical trial of ifosfamide, oxaliplatin, and etoposide (IOE) in pediatric patients with refractory solid tumors. J. Pediatr. Hematol. Oncol. 37:e13–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kieran, M. W. , Packer R. J., Onar A., Blaney S. M., Phillips P., Pollack I. F., et al. 2007. Phase I and pharmacokinetic study of the oral farnesyltransferase inhibitor lonafarnib administered twice daily to pediatric patients with advanced central nervous system tumors using a modified continuous reassessment method: a Pediatric Brain Tumor Consortium study. J. Clin. Oncol. 25:3137–3143. [DOI] [PubMed] [Google Scholar]
- 52. Macdonald, T. J. , Stewart C. F., Kocak M., Goldman S., Ellenbogen R. G., Phillips P., et al. 2008. Phase I clinical trial of cilengitide in children with refractory brain tumors: Pediatric Brain Tumor Consortium study PBTC‐012. J. Clin. Oncol. 26:919–924. [DOI] [PubMed] [Google Scholar]
- 53. Su, J. M. , Li X.‐N., Thompson P., Ou C. ‐N., Ingle A. M., Russell H., et al. 2011. Phase 1 study of valproic acid in pediatric patients with refractory solid or CNS tumors: a Children's Oncology Group Report. Clin. Cancer Res. 17:589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fouladi, M. , Stewart C. F., Olson J., Wagner L. M., Onar‐Thomas A., Kocak M., et al. 2011. Phase I trial of MK‐0752 in children with refractory CNS malignancies: a Pediatric Brain Tumor Consortium study. J. Clin. Oncol. 29:3529–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Geoerger, B. , Hargrave D., Thomas F., Ndiaye A., Frappaz D., Andreiuolo F., et al. 2011a. Innovative Therapies for Children with Cancer pediatric phase I study of erlotinib in brainstem glioma and relapsing/refractory brain tumors. Neuro. Oncol. 13:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Warren, K. E. , Goldman S., Pollack I. F., Fangusaro J., Schaiquevich P., Stewart C. F., et al. 2011. Phase I trial of lenalidomide in pediatric patients with recurrent, refractory, or progressive primary CNS tumors: Pediatric Brain Tumor Consortium study PBTC‐018. J. Clin. Oncol. 29:324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bender, J. L. G. , Lee A., Reid J. M., Baruchel S., Roberts T., Voss S. D., et al. 2013. Phase I pharmacokinetic and pharmacodynamic study of pazopanib in children with soft tissue sarcoma and other refractory solid tumors: a Children's Oncology Group Phase I Consortium Report. J. Clin. Oncol. 31:3034–3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kilburn, L. B. , Kocak M., Decker R. L., Wetmore C., Chintagumpala M., Su J., et al. 2015. A phase 1 and pharmacokinetic study of enzastaurin in pediatric patients with refractory primary central nervous system tumors: a Pediatric Brain Tumor Consortium study. Neuro. Oncol. 17:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Packer, R. J. , Rood B. R., Turner D. C., Stewart C. F., Fisher M., Smith C., et al. 2015. Phase I and pharmacokinetic trial of PTC299 in pediatric patients with refractory or recurrent central nervous system tumors: a PBTC study. J. Neurooncol. 121:217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Caruso, D. A. , Orme L. M., Neale A. M., Radcliff F. J., Amor G. M., Maixner W., et al. 2004. Results of a phase 1 study utilizing monocyte‐derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro. Oncol. 6:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kramer, K. , Humm J. L., Souweidane M. M., Zanzonico P. B., Dunkel I. J., Gerald W. L., et al. 2007. Phase I study of targeted radioimmunotherapy for leptomeningeal cancers using intra‐Ommaya 131‐I‐3F8. J. Clin. Oncol. 25:5465–5470. [DOI] [PubMed] [Google Scholar]
- 62. Bagatell, R. , Herzog C. E., Trippett T. M., Grippo J. F., Fox E., and Macy M.. 2011. Pharmacokinetically guided phase 1 trial of the IGF‐1 receptor antagonist RG1507 in children with recurrent or refractory solid tumors. Clin. Cancer Res. 1:611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moreno, L. , Marshall L. V., Pearson A. D. J., et al. 2015. A phase I trial of AT9283 (a selective inhibitor of aurora kinases) in children and adolescents with solid tumors: a Cancer Research UK study. Clin. Cancer Res. 21:267–273. [DOI] [PubMed] [Google Scholar]
- 64. Adamson, P. C. , Blaney S. M., Widemann B. C., Kitchen B., Murphy R. F., Hannah A. L., et al. 2004. Pediatric phase I trial and pharmacokinetic study of the platelet‐derived growth factor (PDGF) receptor pathway inhibitor SU101. Cancer Chemother. Pharmacol. 53:482–488. [DOI] [PubMed] [Google Scholar]
- 65. Spunt, S. L. , Grupp S. A., Vik T. A., Santana V. M., Greenblatt D. J., Clancy J., et al. 2011. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J. Clin. Oncol. 29:2933–2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fouladi, M. , Perentesis J. P., Phillips C. L., Leary S., Reid J. M., McGovern R. M., et al. 2014. A phase I trial of MK‐2206 in children with refractory malignancies: a Children's Oncology Group study. Pediatr. Blood Cancer 61:1246–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fouladi, M. , Park J. R., Stewart C. F., Gilbertson R. J., Schaiquevich P., Sun J., et al. 2010b. Pediatric phase I trial and pharmacokinetic study of vorinostat: a Children's Oncology Group phase I consortium report. J. Clin. Oncol. 28:3623–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Piha‐Paul, S. A. , Shin S. J., Vats T., Guha‐Thakurta N., Aaron J., Rytting M., et al. 2014. Pediatric patients with refractory central nervous system tumors: experiences of a clinical trial combining bevacizumab and temsirolimus. Anticancer Res. 34:1939–1945. [PMC free article] [PubMed] [Google Scholar]
- 69. Hummel, T. R. , Wagner L., Ahern C., Fouladi M., Reid J. M., McGovern R. M., et al. 2013. A pediatric phase 1 trial of vorinostat and temozolomide in relapsed or refractory primary brain or spinal cord tumors: a Children's Oncology Group Phase 1 Consortium study. Pediatr. Blood Cancer 60:1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Su, J. M. , Thompson P., Adesina A., Li X.‐N., Kilburn L., Onar‐Thomas A., et al. 2014. A phase I trial of veliparib (ABT‐888) and temozolomide in children with recurrent CNS tumors: a Pediatric Brain Tumor Consortium report. Neuro. Oncol. 16:1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chintagumpala, M. , Blaney S. M., Bomgaars L. R., Aleksic A., Kuttesch J. F., Klenke R. A., et al. 2004. Phase I and pharmacokinetic study of thalidomide with carboplatin in children with cancer. J. Clin. Oncol. 22:4394–4400. [DOI] [PubMed] [Google Scholar]
- 72. Jakacki, R. I. , Hamilton M., Gilbertson R. J., Blaney S. M., Tersak J., Krailo M. D., et al. 2008. Pediatric phase I and pharmacokinetic study of erlotinib followed by the combination of erlotinib and temozolomide: a Children's Oncology Group Phase I Consortium study. J. Clin. Oncol. 26:4921–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Venkatramani, R. , Malogolowkin M., Davidson T. B., May W., Sposto R., and Mascarenhas L.. 2013. A phase I study of vincristine, irinotecan, temozolomide and bevacizumab (vitb) in pediatric patients with relapsed solid tumors. Stemmer SM, ed. PLoS ONE 8:e68416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Okada, K. , Yamasaki K., Tanaka C., Fujisaki H., Osugi Y., and Hara J.. 2013. Phase I study of bevacizumab plus irinotecan in pediatric patients with recurrent/refractory solid tumors. Jpn. J. Clin. Oncol. 43:1073–1079. [DOI] [PubMed] [Google Scholar]
- 75. Bagatell, R. , Norris R., Ingle A. M., Ahern C., Voss S., Fox E., et al. 2014. Phase 1 trial of temsirolimus in combination with irinotecan and temozolomide in children, adolescents and young adults with relapsed or refractory solid tumors: a Children's Oncology Group study. Pediatr. Blood Cancer 61:833–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gilman, A. L. , Jacobsen C., Bunin N., Levine J., Goldman F., Bendel A., et al. 2011. Phase I study of tandem high‐dose chemotherapy with autologous peripheral blood stem cell rescue for children with recurrent brain tumors: a Pediatric Blood and MarrowTransplant Consortium study. Pediatr. Blood Cancer 57:506–513. [DOI] [PubMed] [Google Scholar]
- 77. Muscal, J. A. , Thompson P. A., Horton T. M., Ingle A. M., Ahern C. H., McGovern R. M., et al. 2013. A phase I trial of vorinostat and bortezomib in children with refractory or recurrent solid tumors: a Children's Oncology Group phase I Consortium study (ADVL0916). Pediatr. Blood Cancer 60:390–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hoffman, L. M. , Fouladi M., Olson J., Daryani V. M., Stewart C. F., Wetmore C., et al. 2015. Phase I trial of weekly MK‐0752 in children with refractory central nervous system malignancies: a Pediatric Brain Tumor Consortium study. Childs Nerv. Syst. 31:1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mulne, A. F. , Ducore J. M., Elterman R. D., Friedman H. S., Krischer J. P., Kun L. E., et al. 2000. Oral methotrexate for recurrent brain tumors in children: a Pediatric Oncology Group study. J. Pediatr. Hematol. Oncol. 22:41–44. [DOI] [PubMed] [Google Scholar]
- 80. Hurwitz, C. A. , Strauss L. C., Kepner J., Kretschmar C., Harris M. B., Friedman H., et al. 2001. Paclitaxel for the treatment of progressive or recurrent childhood brain tumors: a Pediatric Oncology Phase II study. J. Pediatr. Hematol. Oncol. 23:277–281. [DOI] [PubMed] [Google Scholar]
- 81. Dreyer, Z. E. , Kadota R. P., Stewart C. F., Friedman H. S., Mahoney D. H., Kun L. E., et al. 2003. Phase 2 study of idarubicin in pediatric brain tumors: Pediatric Oncology Group study POG 9237. Neuro. Oncol. 5:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fouladi, M. , Blaney S. M., Poussaint T. Y., Freeman B. B., McLendon R., Fuller C., et al. 2006. Phase II study of oxaliplatin in children with recurrent or refractory medulloblastoma, supratentorial primitive neuroectodermal tumors, and atypical teratoid rhabdoid tumors: a Pediatric Brain Tumor Consortium study. Cancer 107:2291–2297. [DOI] [PubMed] [Google Scholar]
- 83. Nicholson, H. S. , Kretschmar C. S., Krailo M., Bernstein M., Kadota R., Fort D., et al. 2007. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors. Cancer 110:1542–1550. [DOI] [PubMed] [Google Scholar]
- 84. Cefalo, G. , Massimino M., Ruggiero A., Barone G., Ridola V., Spreafico F., et al. 2014. Temozolomide is an active agent in children with recurrent medulloblastoma/primitive neuroectodermal tumor: an Italian multi‐institutional phase II trial. Neuro. Oncol. 16:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hawkins, D. S. , Bradfield S., Whitlock J. A., Krailo M., Franklin J., Blaney S. M., et al. 2006. Topotecan by 21‐day continuous infusion in children with relapsed or refractory solid tumors: a Children's Oncology Group study. Pediatr. Blood Cancer 47:790–794. [DOI] [PubMed] [Google Scholar]
- 86. Zwerdling, T. , Krailo M., Monteleone P., Byrd R., Sato J., Dunaway R., et al. 2006. Phase II investigation of docetaxel in pediatric patients with recurrent solid tumors: a report from the Children's Oncology group. Cancer 106:1821–1828. [DOI] [PubMed] [Google Scholar]
- 87. Bomgaars, L. R. , Bernstein M., Krailo M., Kadota R., Das S., Chen Z., et al. 2007. Phase II trial of irinotecan in children with refractory solid tumors: a Children's Oncology Group study. J. Clin. Oncol. 25:4622–4627. [DOI] [PubMed] [Google Scholar]
- 88. Langevin, A.‐M. , Bernstein M., Kuhn J. G., Blaney S. M., Ivy P., Sun J., et al. 2008. A phase II trial of rebeccamycin analogue (NSC #655649) in children with solid tumors: a Children's Oncology Group study. Pediatr. Blood Cancer 50:577–580. [DOI] [PubMed] [Google Scholar]
- 89. Kuttesch, J. F. , Krailo M. D., Madden T., Johansen M., and Bleyer A.. 2009. Phase II evaluation of intravenous vinorelbine (Navelbine) in recurrent or refractory pediatric malignancies: a Children's Oncology Group study. Pediatr. Blood Cancer 53:590–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Warren, K. , Jakacki R., Widemman B., Aikin A., Libucha M., Packer R., et al. 2006. Phase II trial of intravenous lobradimil and carboplatin in childhood brain tumors: a report from the Children's Oncology group. Cancer Chemother. Pharmacol. 58:343–347. [DOI] [PubMed] [Google Scholar]
- 91. Geoerger, B. , Chisholm J., Le Deley M., Gentet J.‐C. C., Michel C., Dias N., et al. 2011b. Phase II study of gemcitabine combined with oxaliplatin in relapsed or refractory paediatric solid malignancies: an innovative therapy for children with Cancer European Consortium study. Eur. J. Cancer 47:230–238. [DOI] [PubMed] [Google Scholar]
- 92. Minard‐Colin, V. , Ichante J.‐L., Nguyen L., Paci A., Orbach D., Bergeron C., et al. 2012. Phase II study of vinorelbine and continuous low doses cyclophosphamide in children and young adults with a relapsed or refractory malignant solid tumour: good tolerance profile and efficacy in rhabdomyosarcoma–a report from the Société Française des Can. Eur. J. Cancer 48:2409–2416. [DOI] [PubMed] [Google Scholar]
- 93. Fouladi, M. , Nicholson H. S., Zhou T., Laningham F., Helton K. J., Holmes E., et al. 2007. A phase II study of the farnesyl transferase inhibitor, tipifarnib, in children with recurrent or progressive high‐grade glioma, medulloblastoma/primitive neuroectodermal tumor, or brainstem glioma: a Children's Oncology Group study. Cancer 110:2535–2541. [DOI] [PubMed] [Google Scholar]
- 94. Baruchel, S. , Sharp J. R., Bartels U., Hukin J., Odame I., Portwine C., et al. 2009. A Canadian paediatric brain tumour consortium (CPBTC) phase II molecularly targeted study of imatinib in recurrent and refractory paediatric central nervous system tumours. Eur. J. Cancer 45:2352–2359. [DOI] [PubMed] [Google Scholar]
- 95. Robison, N. J. , Campigotto F., Chi S. N., Manley P. E., Turner C. D., Zimmerman M. A., et al. 2014. A phase II trial of a multi‐agent oral antiangiogenic (metronomic) regimen in children with recurrent or progressive cancer. Pediatr. Blood Cancer 61:636–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rosenfeld, A. , Kletzel M., Duerst R., Jacobsohn D., Haut P., Weinstein J., et al. 2010. A phase II prospective study of sequential myeloablative chemotherapy with hematopoietic stem cell rescue for the treatment of selected high risk and recurrent central nervous system tumors. J. Neurooncol. 97:247–255. [DOI] [PubMed] [Google Scholar]
- 97. Kadota, R. P. , Mahoney D. H., Doyle J., Duerst R., Friedman H., Holmes E., et al. 2008. Dose intensive melphalan and cyclophosphamide with autologous hematopoietic stem cells for recurrent medulloblastoma or germinoma. Pediatr. Blood Cancer 51:675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Robinson, G. W. , Orr B. A., Wu G., Gururangan S., Lin T., Qaddoumi I., et al. 2015. Vismodegib exerts targeted efficacy against recurrent sonic hedgehog‐subgroup medulloblastoma: results from phase II Pediatric Brain Tumor Consortium Studies PBTC‐025B and PBTC‐032. J. Clin. Oncol. 33:2646–2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gajjar, A. , Bowers D. C., Karajannis M. A., Leary S., Witt H., and Gottardo N. G.. 2015. Pediatric brain tumors: innovative genomic information is transforming the diagnostic and clinical landscape. J. Clin. Oncol. 33:2986–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Uday, S. , Murray R. D., Picton S., Chumas P., Raju M., Chandwani M., et al. 2015. Endocrine sequelae beyond 10 years in survivors of medulloblastoma. Clin. Endocrinol. (Oxf) 83:663–670. [DOI] [PubMed] [Google Scholar]
- 101. Christopherson, K. M. , Rotondo R. L., Bradley J. A., Pincus D. W., Wynn T. T., Fort J. A., et al. 2014. Late toxicity following craniospinal radiation for early‐stage medulloblastoma. Acta Oncol. (Madr). 53:471–480. https://doi.org/10.3109/0284186X.2013.862596. [DOI] [PubMed] [Google Scholar]
- 102. Lassaletta, A. , Bouffet E., Mabbott D., and Kulkarni A. V.. 2015. Functional and neuropsychological late outcomes in posterior fossa tumors in children. Childs Nerv. Syst. 31:1877–1890. [DOI] [PubMed] [Google Scholar]
- 103. Moroz, V. , Wilson J. S., Kearns P., and Wheatley K.. 2014. Comparison of anticipated and actual control group outcomes in randomised trials in paediatric oncology provides evidence that historically controlled studies are biased in favour of the novel treatment. Trials 15:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Carceller, F. , Bautista F., Jimenez I., Hladun‐Alvaro R., Giraud C., Bergamaschi L., et al. 2016b. Participation of children and adolescents with central nervous system tumours in phase I trials within the ITCC European consortium. Neuro. Oncol. 18:iii25. [DOI] [PubMed] [Google Scholar]
- 105. Schwalbe, E. C. , Lindsey J. C., Nakjang S., Crosier S., Smith A. J., Hicks D., et al. 2017. Novel molecular subgroups for clinical classification and outcome prediction in childhood medulloblastoma: a Cohort study. Lancet Oncol. 18:958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ramaswamy, V. , Remke M., Bouffet E., Faria C. C., Perreault S., Cho Y.‐J., et al. 2013. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 14:1200–1207.24140199 [Google Scholar]
- 107. Schleiermacher, G. , Javanmardi N., Bernard V., Leroy Q., Cappo J., Rio Frio T., et al. 2014. Emergence of new ALK mutations at relapse of neuroblastoma. J. Clin. Oncol. 32:2727–2734. [DOI] [PubMed] [Google Scholar]
- 108. Rudin, C. M. , Hann C. L., Laterra J., Yauch R. L., Callahan C. A., Fu L., et al. 2009. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC‐0449. N. Engl. J. Med. 361:1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Morfouace, M. , Shelat A., Jacus M., Freeman B. B., Turner D., Robinson S., et al. 2014. Pemetrexed and gemcitabine as combination therapy for the treatment of Group3 medulloblastoma. Cancer Cell 25:516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Search strategy (PUBMED).


