Abstract
Penicillin is a bactericidal antibiotic that inhibits the synthesis of the peptidoglycan by targeting penicillin-binding proteins. This study aimed to assess through transcriptional profiling the stress response of S. pneumoniae strains after exposure to lethal penicillin concentrations to understand further the mode of action of penicillin. Two experimental designs (time-course and dose-response) were used for monitoring the effect of penicillin on the transcriptional profile. The expression of some genes previously shown to be modulated by penicillin was altered, including ciaRH, pstS and clpL. Genes of the glnRA and glnPQ operons were among the most downregulated genes in the three strains. These genes are involved in glutamine synthesis and uptake and LC-MS work confirmed that penicillin treatment increases the intracellular glutamine concentrations. Glutamine conferred a protective role against penicillin when added to the culture medium. Glutamine synthetase encoded by glnA catalyses the transformation of glutamate and ammonium into glutamine and its chemical inhibition by the inhibitor L-methionine sulfoximine is shown to sensitize S. pneumoniae to penicillin, including penicillin-resistant clinical isolates. In summary, a combination of RNA-seq and metabolomics revealed that penicillin interferes with glutamine metabolism suggesting strategies that could eventually be exploited for combination therapy or for reversal of resistance.
Introduction
Streptococcus pneumoniae is a Gram-positive bacteria responsible for several diseases such as otitis, sinusitis, pneumonia, sepsis and meningitis1. People at risk are the elderly and young children, pneumococcal infections being responsible for the death of about 393 000 children under the age of five every year2. Over the past decades, antibiotic resistance among S. pneumoniae has steadily increased, with 15 to 30% of the strains currently being classified as multi-resistant3. The introduction of two pneumococcal conjugate vaccines (PCV7 and PCV13) has slowed the spread of several epidemiologically significant serotypes, but antibiotic resistant rates continue to rise4,5.
Penicillin (PEN), a β-lactam antibiotic, remains a pillar against pneumococcal infections but resistant clones worldwide threaten its efficacy. This is especially true for pneumococcal meningitis against which third generation cephalosporins became the recommended treatment for non-susceptible PEN strains6. PEN is a bactericidal antibiotic inhibiting the synthesis of the peptidoglycan layer of the bacterial cell wall by targeting penicillin binding proteins (PBPs). PBPs are membrane-associated enzymes involved in the final steps of peptidoglycan assembly7. However, small molecules can have pleiotropic effects on cells and understanding the precise molecular events responsible for drug-induced events holds the promise of improving current therapies8. Nucleic acids sequencing, whether DNA or RNA has helped in detecting effector mutations linked to antibiotics mode of action and this has been applied to S. pneumoniae. Indeed, sequencing the genome of β-lactam resistant S. pneumoniae confirmed the role of PBPs in resistance9–12 but in addition also highlighted novel pathways and genes necessary for β-lactam resistance13,14.
Gene expression modulation, either at the transcriptional or translational levels, plays a central role in cellular adaptation to stresses15. Gene expression profiling is thus well suited for deciphering the mode of action of drugs given that expression alteration usually mirrors the cellular response to drug-induced damage16,17. For example, a microarray-based gene expression analysis in S. pneumoniae showed that the fluoroquinolone levofloxacin induces an upregulation of the fatDCEB operon coding for an iron transporter, leading to increased intracellular iron concentrations and ultimately to the accumulation of ROS18. Other microarray-based studies in S. pneumoniae revealed many genes whose expression is modulated in response to different antibiotics but did not assess their outcome on resistance or susceptibility19–21. Thus, a better understanding of the mode of action of PEN and of the cellular response induced by it would reveal the extent of metabolic alterations associated with cell death and identify new strategies to improve its effectiveness. Whole RNA sequencing is now surpassing DNA microarrays although not yet reported for studying β-lactam resistance or mode of action. However RNA-seq was successfully used in S. pneumoniae to look at the role of small RNAs on virulence22, on host-pathogen interactions23,24 or at tetracycline resistance in S. pneumoniae 25.
We performed here a detailed RNA-seq experiment of S. pneumoniae when subjected to penicillin. We discovered that glutamine metabolism is altered and that interfering with glutamine synthesis can alter susceptibility to penicillin.
Results
Transcriptional response of S. pneumoniae to penicillin
Two experimental designs were used to monitor the effect of PEN on the transcriptional profile of S. pneumoniae R6. The first design analysed gene expression over time in S. pneumoniae R6 exposed to PEN at its minimum inhibitory concentration (MIC) and in control cells grown in parallel in the absence of antibiotic. Because understanding metabolic alterations requires working with cells that are still metabolically active, gene expression was monitored at three time points corresponding to survival rates of ~85% (T1), ~70% (T2) and ~50% (T3) (Supplementary Fig. S1b), in addition to the T0 baseline. All time points were selected to fit into the S. pneumoniae R6 logarithmic phase of growth to minimize biases coming from growth-related regulation of gene expression. Only genes with a fold change (FC) higher than 2 (log2 FC ≤ −1 or log2 FC ≥ +1) and with a false discovery rate adjusted p value ≤ 0.05 (q-value) compared to the T0 baseline were considered as significantly modulated. Overall, 105 genes were differentially expressed upon exposure to PEN, of which 48 were overexpressed and 57 were downregulated (Table 1). Among the genes steadily upregulated over time were a number of molecular chaperones (spr0453–0456), metabolic enzymes (e.g. spr1074–1075) and uncharacterized proteins, as well as the two component regulator ciaRH (spr0707 and spr0708) already documented to be overexpressed upon PEN exposure19. The CiaRH two component system is known to repress the competence regulon26 and many competence-related genes had indeed their expression decreased from T0 to T3 (Table 1). These include the competence protein coding genes celB (spr0857), the operon cglA, cglB, cglC and cglD (spr1861–1864), the competence-specific transcription regulators comX1 and comX2 (spr0013, spr1819) and the single-stranded DNA-binding protein ssbB (spr1724) (Table 1). Genes coding for a phosphate ABC permease previously linked to PEN resistance was also overexpressed (spr1896–1899 in Table 1). Interestingly, the expression of several genes involved in glutamine metabolism steadily decreased upon PEN exposure (spr0443, spr0444 and spr1121 in Table 1). These are coding for the ABC transporter GlnQ, the transcriptional regulator GlnR and the glutamine synthetase GlnA, the latter two genes being the most downregulated from the dataset (Table 1).
Table 1.
Genes modulated by PEN in S. pneumoniae R6 in the time-course transcriptomic design.
| Entry no. in R6 genome database | Gene Symbol | Gene description | Log2FC (q-Value ≤ 0.05)a,b | |||||
|---|---|---|---|---|---|---|---|---|
| No PENc | PEN at 1 X MICd | |||||||
| T1/T0 | T2/T0 | T3/T0 | T1/T0 | T2/T0 | T3/T0 | |||
| Downregulated | ||||||||
| spr0013 | comX1 | competence- specific global transcription modulator | — | — | — | — | −1.22 | — |
| spr0020 | — | hypothetical protein | — | — | — | — | −1.68 | −2.42 |
| spr0024 | — | hypothetical protein | — | — | — | — | — | −1.04 |
| spr0041 | IS1167 | transposase | — | — | — | — | −1.17 | — |
| spr0042 | IS1167 | transposase | — | — | — | — | −1.22 | — |
| spr0117 | — | hypothetical protein | — | — | — | — | −1.22 | — |
| spr0120 | — | hypothetical protein | — | — | — | −1.32 | — | — |
| spr0123 | — | MutT/nudix family protein | — | — | — | — | — | −1.55 |
| spr0127 | orf51 | hypothetical protein | — | −1.23 | −1.23 | −1.00 | −1.74 | −3.00 |
| spr0128 | — | hypothetical protein | — | — | — | — | −1.58 | −2.26 |
| spr0210 | adk | adenylate kinase | — | — | — | — | — | −1.35 |
| spr0352a | — | DNA- binding protein | −2.00 | −1.00 | −1.00 | −2.00 | −2.00 | — |
| spr0379 | fabK | enoyl- acyl carrier protein(ACP) reductase | — | — | — | — | — | −1.14 |
| spr0387 | accA | acetyl- CoA carboxylase subunit alpha | — | — | — | −1.09 | — | — |
| spr0388 | — | hypothetical protein | −1.31 | — | — | −1.33 | −1.36 | −1.91 |
| spr0432 | cspR | rRNA methylase | — | — | — | — | −1.14 | −1.87 |
| spr0443 | glnR e | transcriptional repressor of the glutamine synthetase gene | — | — | — | — | −1.79 | −3.37 |
| spr0444 | glnA e | glutamine synthetase, type I | — | — | — | — | −1.62 | −3.02 |
| spr0445 | hsdS | type I restriction- modification system S subunit | — | — | — | — | −1.27 | −2.05 |
| spr0446 | hsdS | type I restriction- modification system S subunit | — | — | — | — | −1.49 | −2.37 |
| spr0480 | — | hypothetical protein | — | — | — | — | — | −1.00 |
| spr0499a | — | hypothetical protein | — | −1.00 | −1.00 | −1.00 | — | — |
| spr0504 | licT | BglG family transcriptional antiterminator | — | −1.58 | −2.43 | — | — | −1.05 |
| spr0560a | — | hypothetical protein | — | — | — | −1.17 | −2.17 | — |
| spr0629 | thiM | hydroxyethylthiazole kinase | — | — | — | — | — | −1.00 |
| spr0683 | — | hypothetical protein | — | — | — | — | — | −1.25 |
| spr0857 | celB | competence protein CelB | — | — | — | — | −1.17 | — |
| spr0936 | ABC-MSP | iron- compound ABC transporter permease | — | — | — | — | — | −1.37 |
| spr0940 | — | hypothetical protein | — | — | — | — | −1.05 | −1.76 |
| spr0941 | — | hypothetical protein | — | — | — | — | −1.32 | −2.06 |
| spr0942 | ccrB | hypothetical protein | — | — | — | — | −1.26 | |
| spr0943 | — | hypothetical protein | — | — | — | — | −1.03 | −1.53 |
| spr0988 | IS1167 | transposase | — | — | — | — | −1.16 | −1.36 |
| spr1064 | nrdH | glutaredoxin- like protein | −1.28 | — | — | — | −1.18 | — |
| spr1121 | glnQ e | amino acid ABC transporter ATP- binding protein | — | — | — | — | −1.14 | −1.83 |
| spr1144 | smf | DNA processing protein DprA | −1.00 | — | — | −1.42 | −1.42 | — |
| spr1155 | pyrB | aspartate carbamoyltransferase | — | — | — | — | — | −1.30 |
| spr1156 | pyrR | bifunctional pyrimidine regulatory protein PyrR uracil phosphoribosyltransferase | — | — | — | — | — | −1.41 |
| spr1208 | — | hypothetical protein | — | — | — | — | — | −1.58 |
| spr1210 | — | hypothetical protein | — | — | — | −1.32 | −1.32 | −2.32 |
| spr1316 | — | hypothetical protein | — | — | — | — | −1.17 | — |
| spr1409 | — | glutathione S- transferase YghU | — | — | — | — | — | −1.11 |
| spr1440 | — | ATP- dependent RNA helicase | — | — | — | — | — | −1.11 |
| spr1724 | ssbB | single- stranded DNA- binding protein | — | — | — | — | −1.32 | −1.74 |
| spr1806 | — | cell wall surface anchor family protein | — | — | — | — | −1.04 | −1.49 |
| spr1817 | ABC-NBD | ABC transporter ATP- binding protein | — | — | — | — | −1.03 | −1.67 |
| spr1818 | — | hypothetical protein | — | — | — | −1.03 | −1.37 | −2.46 |
| spr1819 | comX2 | competence- specific global transcription modulator | — | — | — | — | −1.22 | — |
| spr1858 | — | hypothetical protein | — | — | −1.28 | — | −1.50 | — |
| spr1859 | — | hypothetical protein | — | — | — | — | −1.00 | — |
| spr1861 | cglD | competence protein CglD | — | — | — | −1.16 | −1.38 | −2.16 |
| spr1862 | cglC | competence protein CglC | — | −1.14 | −1.14 | −1.46 | −1.87 | −2.14 |
| spr1863 | cglB | competence protein CglB | — | — | −1.00 | −1.00 | −1.49 | −1.81 |
| spr1864 | cglA | competence protein CglA | — | — | — | −1.00 | −1.26 | — |
| spr1993 | hslO | Hsp33- like chaperonin | — | — | — | — | — | −1.19 |
| spr2023 | mreC | rod shape- determining protein MreC | — | — | — | — | — | −1.19 |
| spr2024 | ABC-MSP | ABC transporter permease | — | — | — | — | — | −1.07 |
| Upregulated | ||||||||
| spr0096 | — | LysM domain-containing protein | — | — | — | — | 1.46 | 1.84 |
| spr0262 | adhP | alcohol dehydrogenase | — | — | — | — | — | 1.17 |
| spr0287 | kdgA | keto-hydroxyglutarate-aldolase/keto-deoxy-phosphogluconate aldolase | 1.00 | — | 1.00 | — | 1.58 | — |
| spr0289 | — | hypothetical protein | 1.00 | — | — | 1.00 | 1.58 | — |
| spr0307 | clpL | ATP-dependent protease ATP-binding subunit | — | — | — | — | — | 2.16 |
| spr0311 | — | hypothetical protein | — | — | — | 1.32 | — | 2.04 |
| spr0342 | — | hypothetical protein | — | — | — | — | — | 1.25 |
| spr0343 | hk03 | sensor histidine kinase | — | — | — | — | — | 1.29 |
| spr0441 | pgk | phosphoglycerate kinase | — | 1.11 | 1.26 | 1.11 | 1.18 | — |
| spr0453 | hrcA | heat-inducible transcription repressor | 1.31 | — | — | 1.36 | 1.87 | — |
| spr0454 | grpE | heat shock protein GrpE | — | — | — | 1.23 | 1.64 | 1.36 |
| spr0455 | dnaK | molecular chaperone DnaK | 1.34 | — | 1.08 | 1.80 | 2.24 | 2.07 |
| spr0456 | dnaJ | molecular chaperone DnaJ | 1.40 | 1.00 | 1.03 | 1.57 | 1.91 | 1.73 |
| spr0471 | — | hypothetical protein | — | 1.28 | 1.10 | — | 1.00 | — |
| spr0562 | PTS-EII | PTS system transporter subunit IIA | — | — | — | — | — | 1.54 |
| spr0563 | — | hypothetical protein | — | — | — | — | — | 1.22 |
| spr0633 | — | hypothetical protein | — | — | — | — | 1.00 | — |
| spr0635 | — | hypothetical protein | — | — | — | — | 2.00 | — |
| spr0707 | ciaR | DNA-binding response regulator CiaR | — | — | — | — | 1.24 | 1.81 |
| spr0708 | ciaH | sensor histidine kinase CiaH | — | — | — | — | 1.52 | 2.09 |
| spr0782 | — | hypothetical protein | — | — | — | 1.67 | 2.66 | 3.63 |
| spr0810 | — | hypothetical protein | — | — | — | — | 1.59 | 2.27 |
| spr0884 | prsA | foldase PrsA | — | — | — | — | 1.30 | 1.63 |
| spr0931 | - | hypothetical protein | — | — | — | — | — | 1.94 |
| spr0959 | — | hypothetical protein | — | - | — | — | 2.00 | 3.00 |
| spr0997 | — | hypothetical protein | — | — | — | — | — | 1.15 |
| spr1028 | gapN | glyceraldehyde-3-phosphate dehydrogenase | 1.34 | 1.04 | 1.00 | — | — | 1.20 |
| spr1074 | lacC | tagatose-6-phosphate kinase | — | — | — | — | — | 1.17 |
| spr1075 | lacB | galactose-6-phosphate isomerase subunit LacB | — | — | — | 1.08 | 1.35 | 1.30 |
| spr1097 | nirC | formate/nitrate transporter | — | 1.32 | 1.22 | 1.50 | 1.94 | 3.17 |
| spr1293 | ABC-NBD | ABC transporter ATP-binding protein | — | — | — | — | — | 2.81 |
| spr1536 | nanA | neuraminidase A | — | — | — | — | — | 3.00 |
| spr1538 | axe1 | acetyl xylan esterase | — | — | — | — | 1.70 | 2.65 |
| spr1683 | — | NAD-dependent epimerase/dehydratase | — | — | — | — | 1.32 | 2.46 |
| spr1700 | treR | trehalose operon transcriptional repressor | —— | — | — | 1.28 | — | — |
| spr1722 | groEL | molecular chaperone GroEL | — | — | — | — | 1.49 | — |
| spr1800 | — | hypothetical protein | 1.00 | — | — | 1.00 | — | — |
| spr1837 | adhE | bifunctional acetaldehyde-CoA/alcohol dehydrogenase | 2.56 | 2.43 | 2.57 | 2.28 | 3.05 | 3.45 |
| spr1866 | adh | zinc-containing alcohol dehydrogenase | — | — | 1.03 | 1.06 | — | — |
| spr1875 | — | hypothetical protein | — | — | — | — | 1.71 | 2.45 |
| spr1895 | pstS | phosphate ABC transporter substrate + B766-binding protein | — | — | — | 1.24 | — | — |
| spr1896 | pstC | phosphate ABC transporter permease | — | — | — | 1.42 | — | — |
| spr1898 | pstB | phosphate transporter ATP-binding protein | — | — | — | 1.21 | — | — |
| spr1899 | phoU | phosphate transporter PhoU | — | — | — | 1.58 | — | — |
| spr1916 | malP | maltodextrin phosphorylase | — | — | — | — | 1.33 | 2.12 |
| spr2011 | — | ribosomal subunit interface protein | — | — | — | — | 1.40 | 1.94 |
| spr2045 | sphtra | serine protease | — | — | — | — | 1.49 | 1.97 |
| spr2046 | spo0J | chromosome segregation protein | — | — | — | — | 1.64 | 2.09 |
aPEN was added at 1 X MIC at T0 and the differential gene expression was tested for three time points (T1, T2 and T3). Genes included showed significant variations of log2 FC ≤ −1 or log2 FC ≥ +1 with a q-value ≤ 0.05.
b ‘-’ means no significant change in expression.
c‘No PEN’ corresponds to the genes modulated in the untreated R6.
d‘PEN’ corresponds to the genes modulated in the treated R6.
eGenes involved in glutamine metabolism are shown in bold.
The second experimental design analysed the transcriptional response of S. pneumoniae R6 exposed to increasing concentrations of PEN (0.5X, 1X, 5X and 10X PEN MIC) for 15 minutes. These conditions were selected so the survival rate at the highest concentration would not be less than 30% to ensure the presence of metabolically active cells (Supplementary Fig. S1d). Of the 129 genes modulated, 42 were common to the time course design (underlined in Table 2). Among these, the expression of the hcrA-grpE-dnaK-dnaJ operon (spr0453–0456) coding for heat shock proteins and molecular chaperones, as well as the expression of the chaperone groEL (spr1722) and of a PTS system (spr0562–0563), was similarly increased in both designs. Specific to this incremental dosing design was the upregulation of operons involved in gluconate metabolism (spr0287-spr0290) and iron transport (spr1684-spr1687) (Table 2), the latter being of interest given the reported role of iron in PEN lethality13,17. Remarkably, the glutamine metabolic and transporter genes glnR, glnA, glnP and glnQ were again among the most downregulated genes (Table 2). To further validate this finding, and because S. pneumoniae isolates are highly polymorphic27, the time-course RNA-seq based transcriptomics were replicated with two PEN-susceptible clinical isolates (CCRI-21487 and CCRI-8970). Strikingly, while several genes had their expression changed in the clinical strains (Supplementary Tables S1 and S2), glnR, glnA and glnQ were among the few genes modulated in a common fashion in the presence of PEN in every condition tested (Table 3). Quantitative RT-PCR was used to monitor the expression of these genes at T3 compared to T0 and confirmed that PEN enhances the downregulation of glnA, glnR, glnP and glnQ in the three S. pneumoniae strains (Fig. 1).
Table 2.
Genes modulated by PEN in S. pneumoniae R6 in the dose-response transcriptomic design.
| Entry no. in R6 genome databasea | Gene Symbol | Gene description | Log2FC (q-Value ≤ 0.05)b,c | ||||
|---|---|---|---|---|---|---|---|
| NO PENd | 0.5X MIC | 1X MIC | 5X MIC | 10X MIC | |||
| Downregulated | |||||||
| spr0020 | — | hypothetical protein | — | — | — | −1.26 | −2.58 |
| spr0078 | rpsD | 30S ribosomal protein S4 | — | — | — | — | −1.30 |
| spr0123 | — | MutT/nudix family protein | — | — | — | — | −1.33 |
| spr0127 | orf51 | hypothetical protein | −1.00 | — | — | −1.62 | — |
| spr0128 | — | hypothetical protein | — | — | — | −1.27 | — |
| spr0187 | rpsJ | 30S ribosomal protein S10 | — | — | — | — | −1.26 |
| spr0210 | adk | adenylate kinase | — | — | — | −1.03 | −1.43 |
| spr0211 | infA | translation initiation factor IF−1 | — | — | — | — | −1.71 |
| spr0216 | rplQ | 50S ribosomal protein L17 | — | — | — | — | −1.34 |
| spr0327 | aliA | oligopeptide ABC transporter substrate-binding protein | — | — | — | — | −1.30 |
| spr0381 | fabG | 3-ketoacyl-ACP reductase | — | −1.19 | — | — | — |
| spr0382 | fabF | 3-oxoacyl-ACP synthase | — | −1.01 | — | — | — |
| spr0383 | accB | acetyl-CoA carboxylase biotin carboxyl carrier protein subunit | — | −1.16 | — | — | — |
| spr0384 | fabZ | (3R)-hydroxymyristoyl-ACP dehydratase | — | −1.05 | −1.26 | — | — |
| spr0386 | accD | acetyl-CoA carboxylase subunit beta | — | −1.05 | — | — | — |
| spr0388 | — | hypothetical protein | — | −1.27 | — | −1.30 | −1.45 |
| spr0398 | rpmB | 50S ribosomal protein L28 | — | — | — | — | −1.87 |
| spr0443 | glnR e | transcriptional repressor of the glutamine synthetase gene | — | — | — | −1.61 | −2.38 |
| spr0444 | glnA e | glutamine synthetase, type I | — | — | — | −1.45 | −2.06 |
| spr0682 | rpsP | 30S ribosomal protein S16 | — | — | — | — | −1.26 |
| spr0691 | bioY | biotin synthase | — | — | — | — | −1.00 |
| spr0714 | gph | phosphoglycolate phosphatase | — | — | — | — | −1.02 |
| spr0767 | IS1167 | transposase | — | — | — | — | −1.13 |
| spr0861 | infC | translation initiation factor IF-3 | — | — | — | — | −1.20 |
| spr0864 | lguL | lactoylglutathione lyase | — | — | — | — | −1.20 |
| spr1012 | rplU | 50S ribosomal protein L21 | — | — | −1.41 | — | — |
| spr1120 | glnP e | amino acid ABC transporter substrate-binding protein | — | — | — | — | −1.36 |
| spr1121 | glnQ e | amino acid ABC transporter ATP-binding protein | — | — | — | −1.05 | — |
| spr1144 | smf | DNA processing protein DprA | — | — | — | −1.58 | — |
| spr1208 | — | hypothetical protein | — | — | −1.00 | — | — |
| spr1210 | — | hypothetical protein | — | — | — | −1.58 | — |
| spr1409 | — | glutathione S-transferase YghU | — | — | — | −1.17 | −1.44 |
| spr1410 | pacL | calcium transporter P-type ATPase | — | — | — | −1.08 | −1.26 |
| spr1440 | — | ATP-dependent RNA helicase | — | — | — | −1.24 | −1.52 |
| spr1604 | aqpZ | aquaporin | — | — | — | −1.11 | −1.60 |
| spr1623 | — | hypothetical protein | — | — | — | −1.11 | — |
| spr1624 | — | hypothetical protein | — | — | — | — | −1.26 |
| spr1626 | — | hypothetical protein | — | — | — | −1.31 | — |
| spr1859 | — | hypothetical protein | −1.00 | — | — | −1.00 | — |
| spr1883 | — | hypothetical protein | — | — | −1.00 | — | — |
| spr1912 | — | hypothetical protein | — | — | −1.06 | — | — |
| Upregulated | |||||||
| spr0079 | — | degenerative transposase | — | — | 1.29 | — | — |
| spr0096 | — | LysM domain-containing protein | — | — | — | 1.04 | — |
| spr0102 | argG | argininosuccinate synthase | — | 1.00 | — | — | — |
| spr0104 | — | hypothetical protein | — | 1.00 | — | 1.32 | 1.81 |
| spr0151 | — | hypothetical protein | — | — | — | 1.05 | — |
| spr0225 | — | hypothetical protein | — | 1.00 | — | 2.00 | — |
| spr0247 | pulA | alkaline amylopullulanase | — | — | — | — | 1.84 |
| spr0280 | celC | PTS system cellobiose transporter subunit IIA | 1.00 | 1.00 | — | 2.00 | 2.32 |
| spr0282 | celD | PTS system cellobiose transporter subunit IIC | — | 1.00 | — | — | — |
| spr0287 | kdgA | keto-deoxy-phosphogluconate aldolase | — | 1.00 | 1.32 | — | — |
| spr0288 | kdgK | 2-keto-3-deoxygluconate kinase | 1.00 | 1.00 | — | — | 1.58 |
| spr0289 | — | hypothetical protein | 1.00 | 1.58 | — | 1.58 | 2.00 |
| spr0290 | gno | gluconate 5-dehydrogenase | 1.00 | 1.00 | — | — | — |
| spr0295 | PTS-EII | PTS system transporter subunit IID | — | — | — | 1.00 | 1.00 |
| spr0311 | — | hypothetical protein | — | — | — | 1.38 | — |
| spr0344 | rr03 | DNA-binding response regulator | — | — | — | — | 1.36 |
| spr0373 | — | hypothetical protein | — | — | 1.33 | — | — |
| spr0453 | hrcA | heat-inducible transcription repressor | — | 1.32 | — | 1.72 | 1.83 |
| spr0454 | grpE | heat shock protein GrpE | — | 1.03 | 1.11 | 1.70 | 1.79 |
| spr0455 | dnaK | molecular chaperone DnaK | — | 1.30 | 1.23 | 1.88 | 2.18 |
| spr0456 | dnaJ | molecular chaperone DnaJ | — | 1.23 | 1.74 | — | 1.85 |
| spr0506 | bglH | 6-phospho-beta-glucosidase | — | — | — | — | 1.24 |
| spr0534 | glnH | amino acid ABC transporter amino acid-binding protein | — | — | — | 1.44 | 1.65 |
| spr0562 | PTS-EII | PTS system transporter subunit IIA | 1.13 | 1.23 | — | — | — |
| spr0563 | — | hypothetical protein | — | 1.03 | — | — | — |
| spr0565 | bgaA | beta-galactosidase | — | — | — | — | 1.11 |
| spr0613 | pyrF | orotidine 5′-phosphate decarboxylase | 1.09 | 1.14 | — | 1.27 | — |
| spr0614 | pyrE | orotate phosphoribosyltransferase | 1,00 | 1.11 | 1.21 | 1.28 | — |
| spr0615 | — | hypothetical protein | 1.25 | 1.17 | — | — | — |
| spr0634 | tenA | extracellular enzyme gene transcriptional regulator | — | 1.00 | — | — | — |
| spr0644 | Transposase_C | transposase | 1.00 | 1.00 | — | — | — |
| spr0645 | — | hypothetical protein | — | 1.00 | — | — | — |
| spr0664 | — | acetoin utilization protein AcuB | — | — | 1.12 | 1.12 | — |
| spr0782 | — | hypothetical protein | — | — | — | 1.92 | 2.07 |
| spr0791 | hsdS | type I restriction-modification system S subunit | — | — | — | 1.15 | 1.32 |
| spr0793 | argR | arginine repressor ArgR | — | 1.14 | — | 1.38 | — |
| spr0810 | — | hypothetical protein | — | — | — | 1.51 | — |
| spr0811 | — | hypothetical protein | — | — | — | 1.22 | 1.42 |
| spr0812 | ABC-NBD | ABC transporter ATP-binding protein | — | — | — | 1.00 | — |
| spr0840 | — | hypothetical protein | — | 1.00 | — | — | — |
| spr0887 | gpmB | phosphoglycerate mutase | — | — | 1.21 | — | — |
| spr0946 | — | hydrolase | — | — | — | 1.00 | — |
| spr0959 | — | hypothetical protein | — | — | — | 1.58 | 2.00 |
| spr0997 | — | hypothetical protein | — | 1.04 | — | — | — |
| spr1028 | gapN | glyceraldehyde-3-phosphate dehydrogenase | 1.28 | 1.32 | 1.24 | 1.51 | 1.65 |
| spr1069 | lacG | 6-phospho-beta-galactosidase | — | — | — | 1.00 | 1.50 |
| spr1073 | lacD | tagatose 1,6-diphosphate aldolase | — | 1.00 | 1.05 | 1.41 | 1.96 |
| spr1075 | lacB | galactose-6-phosphate isomerase subunit LacB | — | 1.20 | — | — | 1.93 |
| spr1079 | — | hypothetical protein | — | — | — | — | 1.58 |
| spr1080 | — | hypothetical protein | — | — | — | — | 1.58 |
| spr1081 | — | hypothetical protein | — | — | — | 1.00 | 1.22 |
| spr1097 | nirC | formate/nitrate transporter | 1.42 | 1.66 | 2.17 | 2.12 | 2.58 |
| spr1112 | — | hypothetical protein | — | — | 1.21 | — | — |
| spr1291 | — | hypothetical protein | — | 1.00 | — | — | — |
| spr1293 | ABC-NBD | ABC transporter ATP-binding protein | — | — | — | 1.26 | — |
| spr1382 | aliB | peptide ABC transporter substrate-binding protein | 1.06 | 1.06 | 1.13 | 1.67 | 1.86 |
| spr1467 | rpsO | 30S ribosomal protein S15 | — | — | 1.11 | — | — |
| spr1475 | — | hypothetical protein | — | — | — | 1.00 | — |
| spr1527 | ABC-SBP | sugar ABC transporter substrate-binding protein | — | — | — | — | 1.32 |
| spr1528 | PTS-EII | PTS system transporter subunit IIBC | — | — | — | — | 1.42 |
| spr1536 | nanA | neuraminidase A | — | 1.58 | — | 2.00 | 2.58 |
| spr1630 | — | hypothetical protein | — | — | — | 1.08 | — |
| spr1646 | — | hypothetical protein | 1.58 | 1.00 | — | 1.58 | — |
| spr1649 | — | phosphate transporter PhoU | 1.00 | 1.00 | — | 1.58 | — |
| spr1667 | galT | galactose-1-phosphate uridylyltransferase | — | — | 1.32 | 1.00 | 1.50 |
| spr1668 | galK | galactokinase | — | — | — | 1.12 | 1.42 |
| spr1684 | fatD | iron-compound ABC transporter permease | — | — | 1.78 | — | — |
| spr1685 | fatC | iron-compound ABC transporter permease | — | 1.00 | — | — | — |
| spr1686 | fecE | iron-compound ABC transporter ATP-binding protein | — | 1.22 | — | — | — |
| spr1687 | fatB | iron-compound ABC transporter substrate-binding protein | — | 1.12 | — | — | — |
| spr1700 | treR | trehalose operon transcriptional repressor | — | — | — | 1.43 | 1.38 |
| spr1715 | birA | biotin–protein ligase | — | — | — | 1.00 | — |
| spr1721 | — | transposase | — | 1.00 | — | — | — |
| spr1722 | groEL | molecular chaperone GroEL | — | — | 1.30 | 1.43 | 1.58 |
| spr1800 | — | hypothetical protein | 1.00 | — | — | 1.22 | 1.22 |
| spr1801 | ABC-NBD | ABC transporter ATP-binding protein | — | — | 1.22 | — | — |
| spr1810 | — | hypothetical protein | — | — | — | 1.22 | — |
| spr1837 | adhE | bifunctional acetaldehyde-CoA/alcohol dehydrogenase | 2.20 | 2.27 | 2.15 | 3.07 | 3.09 |
| spr1866 | adh | zinc-containing alcohol dehydrogenase | — | — | — | 1.23 | 1.45 |
| spr1895 | pstS | phosphate ABC transporter substrate + B766-binding protein | — | — | — | 1.25 | 1.43 |
| spr1896 | pstC | phosphate ABC transporter permease | — | — | 1.38 | — | — |
| spr1915 | — | hypothetical protein | — | — | — | 1.05 | — |
| spr1953 | — | hypothetical protein | — | 1.00 | — | — | — |
| spr1962 | — | hypothetical protein | — | — | — | 1.35 | 1.80 |
| spr1983 | — | hypothetical protein | — | 1.00 | — | 1.93 | 1.93 |
| spr2011 | — | ribosomal subunit interface protein | — | — | — | 1.41 | 1.57 |
| spr2016 | transposase_G | hypothetical protein | — | 1.00 | — | — | — |
aGenes underlined are common between Table 1 and Table 2.
bPenicillin (PEN) was added at 0.5, 1, 5 and 10X MIC at T0 and the differential gene expression was tested between 15 min and T0. Genes included showed significant variations of log2 FC ≤ −1 or log2 FC ≥ +1 with a q-value ≤0.05.
c‘−’ means no significant change in expression.
d‘No PEN’ corresponds to the genes modulated in the untreated R6.
eGenes involved in glutamine metabolism are shown in bold.
Table 3.
Genes modulated in a common fashion by PEN in S. pneumoniae R6, CCRI-8970 and CCRI-21487.
| Entry no. in R6 genome database | Gene Symbol | Gene description | Condition | Log2FC (q-Value ≤ 0.05)a,b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R6 | CCRI-8970 | CCRI-21487 | ||||||||||
| T1/T0 | T2/T0 | T3/T0 | T1/T0 | T2/T0 | T3/T0 | T1/T0 | T2/T0 | T3/T0 | ||||
| Downregulated | ||||||||||||
| spr0443 | glnR e | transcriptional repressor of the glutamine synthetase gene | NO PEN c | — | — | — | — | −2.53 | −2.35 | — | — | — |
| PEN 1X MIC d | — | −1.79 | −3.37 | −2.08 | −3.02 | −2.93 | — | −1.08 | −2.32 | |||
| spr0444 | glnA e | glutamine synthetase, type I | NO PEN | — | — | — | −1.42 | — | — | — | — | −1.26 |
| PEN 1X MIC | — | −1.62 | −3.02 | −1.75 | −1.45 | −1.65 | — | −1.45 | −2.82 | |||
| spr1121 | glnQ e | amino acid ABC transporter ATP-binding protein | NO PEN | — | — | — | −1.02 | −1.55 | −1.80 | — | — | — |
| PEN 1X MIC | — | −1.14 | −1.83 | −2.05 | −2.63 | −2.96 | — | −1.10 | −2.23 | |||
| Upregulated | ||||||||||||
| spr0307 | clpL | ATP-dependent protease ATP-binding subunit | NO PEN | — | — | — | — | 1.33 | 1.81 | — | — | — |
| PEN 1X MIC | — | — | 2.16 | 1.30 | 2.03 | 2.22 | — | — | 1.13 | |||
| spr0455 | dnaK | molecular chaperone DnaK | NO PEN | 1.34 | — | 1.08 | — | 1.50 | 1.49 | — | — | — |
| PEN 1X MIC | 1.80 | 2.24 | 2.07 | 1.33 | 1.71 | 1.75 | — | — | 1.01 | |||
| spr0456 | dnaJ | molecular chaperone DnaJ | NO PEN | 1.40 | 1.00 | 1.03 | — | 2.01 | 1.86 | — | — | — |
| PEN 1X MIC | 1.57 | 1.91 | 1.73 | 1.80 | 1.97 | 2.10 | — | — | 1.15 | |||
| spr1866 | adh | zinc-containing alcohol dehydrogenase | NO PEN | — | — | 1.03 | — | — | — | — | 1.11 | — |
| PEN 1X MIC | 1.06 | — | — | — | — | 1.04 | — | 1.13 | 1.25 | |||
| spr2011 | — | ribosomal subunit interface protein | NO PEN | — | — | — | — | — | — | — | 1.33 | 1.27 |
| PEN 1X MIC | — | 1.40 | 1.94 | — | — | 1.06 | 1.31 | 1.55 | 2.15 | |||
aPenicillin (PEN) at 1 X MIC was added at T0 and the differential gene expression was tested for three time points (T1, T2 and T3). Genes included showed significant variations of log2 FC ≤ −1 or log2 FC ≥ +1 with a q-value ≤ 0.05.
b‘−’ means no significant change in expression.
c‘No PEN’ corresponds to the genes modulated in the untreated S. pneumoniae.
d‘PEN 1 X MIC’ corresponds to the genes modulated in the treated bacteria.
eGenes involved in glutamine metabolism are shown in bold.
Figure 1.
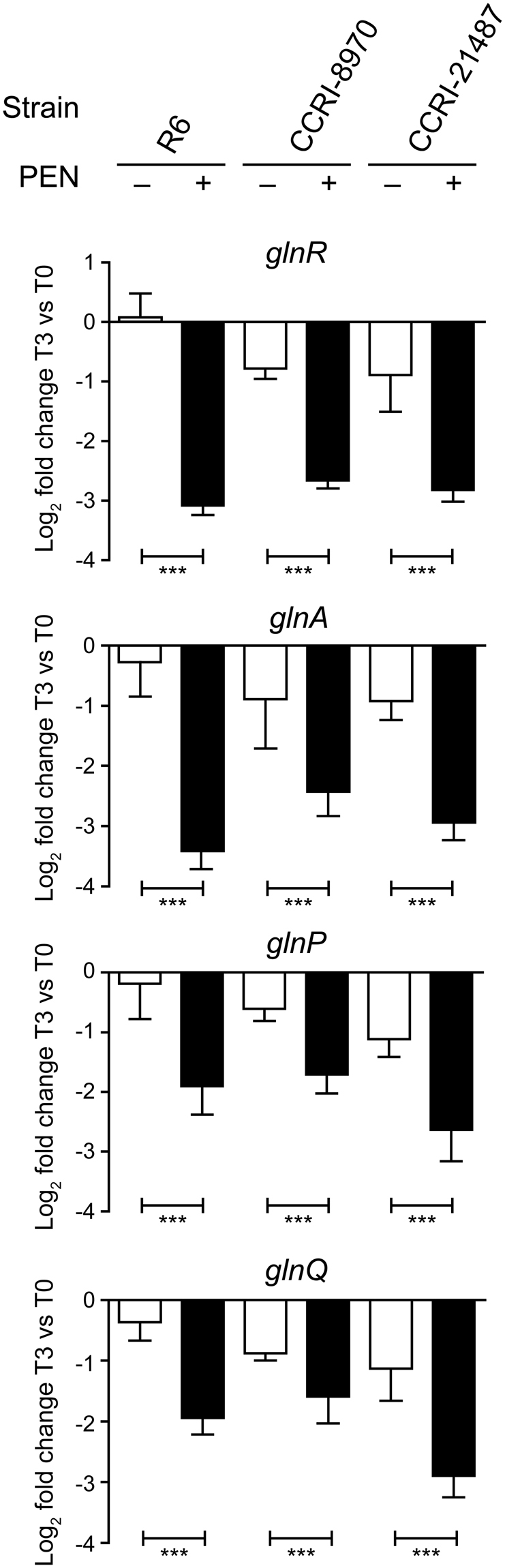
Validation of penicillin-induced alterations to glutamine metabolism gene expression in S. pneumoniae by qRT-PCR. Genes involved in glutamine metabolism (glnR, glnA, glnP and glnQ) found to be down-regulated after exposure to PEN by RNA-seq were validated by qRT-PCR. RNA levels were normalized based on the amplification signals of 16S ribosomal RNA. Graphs show the log2 fold change of expression at time T3 (corresponding to 40 min for R6, 28 min for CCRI-21487 and 85 min for CCRI-8970) over T0 in untreated (white bars) and PEN-treated (black bars) S. pneumoniae isolates. Results are displayed as mean ± SD of three biological replicates and significant differences are identified as determined by split plot design and Fisher’s F-test (***p ≤ 0.001).
Modulation in glutamine levels in penicillin treated S. pneumoniae
The transcriptomics data revealed a possible alteration to the metabolism of glutamine in S. pneumoniae upon exposure to PEN and this was further analysed using liquid chromatography coupled to mass spectrometry (LC-MS). The levels of glutamine and glutamate were quantified in the three S. pneumoniae R6, CCRI-8970 and CCRI-21487 strains in the presence or absence of PEN at time point equivalent to T3 above, in addition to the T0 baseline (Table 3). In S. pneumoniae R6 the level of glutamine increased close to 40-fold after exposure to PEN, whereas it increased just by 2 fold in untreated bacteria (Fig. 2a). The levels of glutamate changed more modestly, an increase of 6-fold, in bacteria treated with PEN (Fig. 2a). PEN also increased glutamine concentration in S. pneumoniae CCRI-21487 close to 8 fold (Fig. 2b), while glutamate level were only modestly increased (Fig. 2b). Glutamine and glutamate levels remained unchanged in untreated CCRI-21487 (Fig. 2b). As for S. pneumoniae CCRI-8970, glutamine level increased close to 2 fold after exposure to PEN, whereas it decreased by 0.5 fold in the untreated bacteria (Fig. 2c). Glutamate level remained unchanged after exposure to PEN and similarly to the level of glutamine, it decreased by around 0.5 fold in untreated CCRI-8970 (Fig. 2c).
Figure 2.
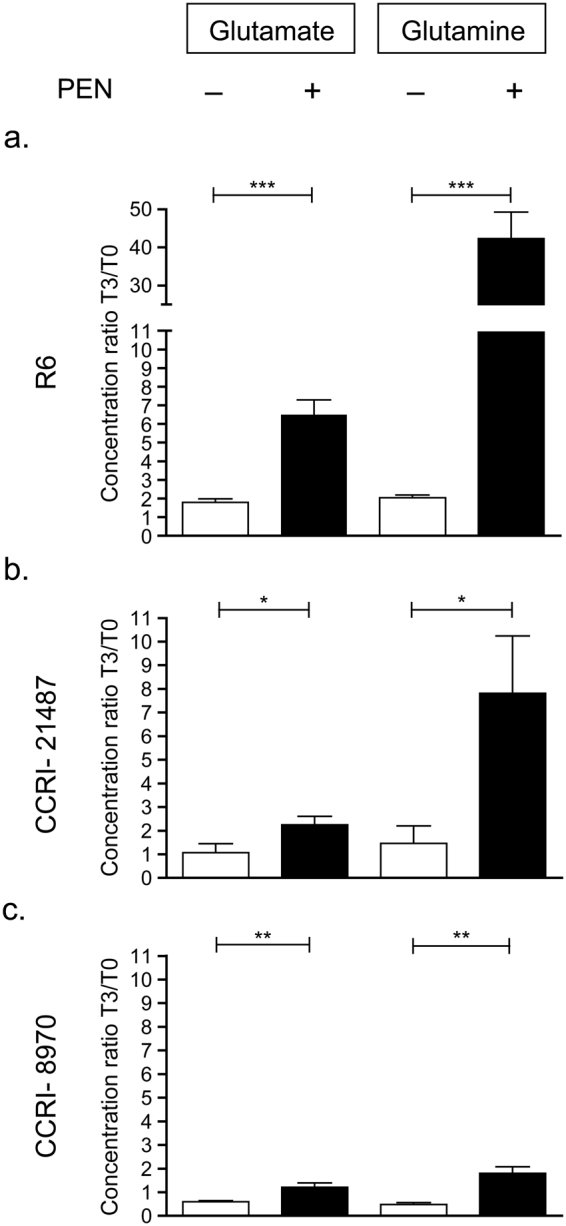
Quantification of intracellular levels of glutamine and glutamate by LC-MS. Glutamine and glutamate relative concentrations at time point T3 (corresponding to 40 min for R6, 28 min for CCRI-21487 and 85 min for CCRI-8970) over T0 in untreated (white bars) and PEN-treated (black bars) S. pneumoniae R6 (a), CCRI-21487 (b) and CCRI-8970 (c). The data was normalized according to the bacterial counts. Results are displayed as mean ± SD of three biological replicates and significant differences were determined by unpaired student t-test (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Glutamine confers low-level protection against penicillin in S. pneumoniae R6
Penicillin increases glutamine levels (Fig. 2). We tested whether glutamine could protect against penicillin action. S. pneumoniae R6 was grown in BHI medium supplemented with glutamine at 0, 6 and 12 mM at early log-phase, half an hour before the addition of PEN at 1X MIC (Supplementary Fig. S2a). The survival rates at 30 min and 60 min were derived from the ratio of bacterial counts in the presence of PEN compared to untreated bacteria at each time point. Interestingly, glutamine at 6 mM and 12 mM conferred considerable protection against PEN by increasing survival rates at 30 min from 40% to 60% and from 40% to 75%, respectively (Fig. 3a). Survival rates also increased from 5% to 11% at 60 min in the presence of glutamine (Fig. 3a). The protection conferred by glutamine supplementation was specific to PEN and was not observed with ciprofloxacin (CIP), a fluoroquinolone antibiotic inhibiting DNA replication (Fig. 3b & Supplementary Fig. S2b). In fact in the case of CIP, glutamine at 6 mM or 12 mM was detrimental and apparently enhanced its lethality by decreasing survival rates from 60% to 38% and from 20% to 5% at 30 and 60 min, respectively (Fig. 3b).
Figure 3.
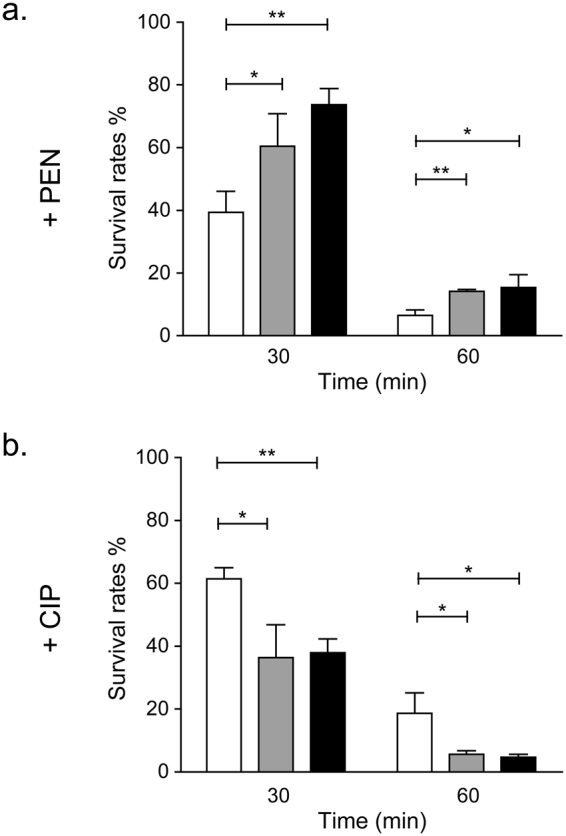
Protective role of glutamine against penicillin in S. pneumoniae R6. Survival rates at 30 min and 60 min of S. pneumoniae R6 in BHI media supplemented with penicillin (a) or ciprofloxacin (b) with the addition of 0 mM (white bars), 6 mM (gray bars) or 12 mM (black bars) glutamine. Untreated S. pneumoniae R6 was used as control. Results display the mean ± SD of at least three biological repeats and significant differences were determined by unpaired Student’s t test (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
Inhibiting the glutamine synthetase GlnA renders S. pneumoniae more susceptible to penicillin
Because glutamine supplementation decreased susceptibility to PEN, we next sought whether inhibiting the glutamine synthetase GlnA would enhance PEN lethality. The MIC of PEN against S. pneumoniae R6 was determined in the presence of L-methionine sulfoximine (MSO), a specific inhibitor of GlnA28–30. Attempts to determine the toxicity of MSO in S. pneumoniae were not possible by microdilution and bacteria were growing albeit at a lesser density up to 32 mM by macrodilution. The larger culture volume of macrodilution (see Methods) facilitated measurements. Several concentrations of MSO were tested in combination with PEN, and 0.5 mM MSO was found to have maximal effect on PEN susceptibility while alone having minimal activity against S. pneumoniae. Indeed, 0.5 mM MSO increased the susceptibility of S. pneumoniae R6 to PEN by four-fold, from a MIC of 0.03 µg/mL in the absence of MSO to a MIC of 0.008 µg/mL (Table 4). Addition of glutamine at 12 mM completely reverted this PEN hyper-susceptibility phenotype (Table 4). A two- to four-fold sensitization to PEN induced by MSO was also observed in the PEN-susceptible S. pneumoniae clinical isolates CCRI-21487 and CCRI-8970, but also in PEN-resistant clinical isolates (CCRI-1397, CCRI-1414 and CCRI-1983) (Table 4). Again, glutamine fully (or partially in the case of CCRI-1983) rescued the hyper-susceptibility phenotype (Table 4).
Table 4.
Inhibition of GlnA by L-Methionine Sulfoximine sensitizes S. pneumoniae to PEN.
| Condition | MIC of PEN (µg/mL)a,b | |||||
|---|---|---|---|---|---|---|
| R6 | CCRI-8970 | CCRI-21487 | CCRI-1397 | CCRI-1414 | CCRI-1983 | |
| CAMHBc | 0.03 | 0.015 | 0.03 | 1 | 2 | 8 |
| CAMHB + MSOd | 0.008 | 0.008 | 0.015 | 0.5 | 1 | 2 |
| CAMHB + MSO + Glne | 0.03 | 0.015 | 0.03 | 1 | 2 | 4 |
aMIC of PEN: Minimum Inhibitory Concentration of penicillin.
bAverage of three biological replicates.
cCAMHB: Cation Adjusted Muller Hinton Broth.
dMSO: L-Methionine Sulfoximine added at 0.5 mM.
eGln: glutamine added at 12 mM.
Discussion
Gene expression modulation is central to bacterial adaptation and can mirror the cellular response to stress-induced damages. In this study, we used RNA-seq to assess the metabolic consequences of exposure to PEN in S. pneumoniae. A number of genes previously shown to be implicated in PEN resistance were detected, including ciaRH 19, pstS 19,31 and clpL 32,33. The transcriptional regulator CiaR was previously shown to influence on natural competence and susceptibility to β-lactams in S. pneumoniae 34. It is known to activate fourteen promoters35 and many of the genes under its control had indeed increased expression in the presence of PEN, such as the foldase prsA, the acetyl xylan esterase axe1, the maltodextrin phosphorylase malP, the serine protease htrA, the chromosome segregation protein spoOJ and the hypothetical protein coding genes spr0782 and spr0931 (Table 1). CiaR also drives the expression of small regulatory non-coding RNAs36 and one target of such RNA (the formate/nitrate transporter nirC) was found to be overexpressed (Table 1). Apart from the CiaRH regulon, adenylate kinase, glutathione S-transferase, the fatty acid and phospholipid-related genes fabK, fabG, fabF, fabZ and accD and a gene from the MutT/nudix family whose expression was decreased in our RNA-seq data had previously been shown to be downregulated by PEN using microarrays19, so is the case for the overexpression of LysM domain-containing proteins and molecular chaperones (Tables 1 & 2).
Common to all S. pneumoniae strains and transcriptomics designs was the downregulation of the glnRA and glnPQ operons (Table 3). The transcriptional regulator glnR mediates the repression of its own glnRA operon, the glnPQ-zwf operon and the gdhA gene by binding to a conserved operator sequence37. The gene glnA encodes glutamine synthetase which is responsible for the conversion of glutamate and ammonium into glutamine37. GlnA also indirectly influences the transcription of its own operon by stimulating the binding of GlnR. The glnPQ genes are coding for a transporter involved in glutamine scavenging37. Targeted metabolomics using LC-MS revealed an increase in glutamine concentrations following exposure to PEN in all three S. pneumoniae strains studied (Fig. 2). The increase is considerable with 4-to-20 fold increase compared to the untreated cells control. Since high concentration of glutamine down-regulates the glnRA and glnPQ operons37,38, the decreased expression of those two operons (Table 3) are most likely due to the penicillin-induced increase in glutamine levels (Fig. 2). Glutamate levels increased moderately (2–4 fold) in the S. pneumoniae R6, CCRI-21487 and CCRI-8970 when compared to untreated cells at the same time point (Fig. 2). Intriguingly, glutamine levels were increased two-fold in R6 during growth (Fig. 2a) but not in the two other strains. One possible candidate is glnH (spr0534) coding for an ABC transporter that binds glutamine and glutamate39. Indeed, glnH remained constant in R6 untreated cells but downregulated in untreated CCRI-8970 and CCRI-21487 (Tables S1 and S2). These RNA seq data were confirmed by qRT-PCR (Supplementary Fig. S3). Upon penicillin treatment, glnH is downregulated in the two clinical isolates but upregulated in R6 (Supplementary Fig. S3). This differential expression of glnH in R6 may contribute to the higher uptake and accumulation of glutamate and glutamine observed in R6 (Fig. 2).
The mechanism by which PEN increases the levels of glutamine is still unclear. However, glutamine is a major nitrogen donor for the synthesis of cell building blocks and is used by the aminotransferase GlmS to convert fructose-6-phosphate into glucosamine-6-phosphate (Glc-6P)40. GlmS occupies a central position between glycolysis and peptidoglycan synthesis in catalysing the first of a series of reactions leading to the cell wall precursor UDP-N-acetylglucosamine40. Inactivation or chemical inhibition of GlmS was shown to synergize with a broad set of cell wall synthesis inhibitors in Staphylococcus aureus 41,42. Similarly, disruption of the downstream phosphoglucosamine mutase GlmM increased the susceptibility of Streptococcus gordonii to PEN43 in addition to decrease methicillin resistance in S. aureus without affecting the production of endogenous PBPs44–46. Similarly, inhibiting the wall teichoic acid transporter protein TarG was shown to potentiate the activity of imipenem against methicillin-resistant Staphylococcus aureus 47. Consistent with these findings, here we observed that the inhibition of GlnA leads to penicillin sensitization in S. pneumoniae clinical isolates (Table 4). Chemical inhibition of GlmS in methicillin-resistant S. aureus 42 and of GlnA in PEN-resistant S. pneumoniae clinical isolates (Table 4) decreased their levels of resistance. Conversely, the mere addition of glutamine was found to be partly protective against the action of penicillin (Fig. 3). Glutamine is also a co-factor for the amidation of the second amino acid residue of lipid II by MurT/GatD, which is required for efficient cross-linking of the peptidoglycan48. It remains to be established whether glutamine accumulation is a secondary response from bacteria to PEN attack against the peptidoglycan assembly machinery or if the role of PEN is more direct, for example by hampering the use of glutamine by GlmS or by MurT/GatD. Additional work may shed further light on this.
In an era of ever increasing antibiotic resistance and shortage of new molecules, new strategies are required for increasing the activity of current antibiotics against sensitive and resistant bacteria. Our study showed a new link between glutamine metabolism and PEN susceptibility. The search for antibiotic adjuvants is now an intensive field of investigations8. Metabolites are now emerging as possible adjuvants and one possible strategy is the inhibition of metabolic pathways. For example exogenous alanine was shown to revert kanamycin resistance in a number of bacterial species49 and tetracycline resistance and thiamine biosynthesis were linked in S. pneumoniae 25 while bacterial resistance to tetracycline can be reversed using tryptophan analogues50. Further investigations are warranted in the attempt of restoring PEN activity using strategies to lower cellular glutamine levels. For example, untargeted metabolomics experiments may help elucidating the mechanism by which PEN leads to cell death by revealing the extent of metabolic alterations associated with it. This may have added benefits as glutamine was also shown to be involved in virulence, and deletion of glnA and glnP led to attenuated colonization38 and to decreased bloodstream invasiveness and survival in the lungs38, respectively. While still speculative, interfering with the metabolism of glutamine such as inhibiting GlnA would increase PEN susceptibility but also reduces virulence and drug-like leads against this target may thus serve dual purposes when paired with existing β-lactam antibiotics.
Methods
Bacterial strains and growth conditions
S. pneumoniae strains were grown in brain heart infusion broth (BHI, Becton Dickinson), or in blood agar containing 5% defibrinated sheep’s blood (Becton Dickinson). Cultures were incubated for 16 to 24 hours in a 5% CO2 atmosphere at 35 °C as previously described51. All strains were maintained frozen at −80 °C in BHI containing 15% glycerol. For the RNA-seq experiments, S. pneumoniae was grown in BHI broth at 35 °C until early log-phase (OD600 = 0.11). At this time (T0), cultures were divided into 18 tubes for the time-course design, half of which contained 1 X MIC PEN and incubated until survival rates of 95–75% (T1), 75–60% (T2) and 60–40% (T3) (6 tubes each). These time points correspond to 15, 30 and 40 min for R6, 8, 18 and 28 min for CCRI-21487 and 45, 65 and 85 min for CCRI-8970. For the dose-response design, at T0 S. pneumoniae R6 culture was divided into 30 tubes, half of which contained either 0.5X, 1X, 5X or 10X PEN MIC, and incubated for 15 min. At each time point, a serial dilution in PBS 1x was carried out to determine survival rates.
Antibacterial susceptibility testing
MIC of PEN or CIP were determined with E-test strips (AB BioMérieux) on Müller-Hinton agar plates supplemented with 5% sheep blood (Becton Dickinson) using manufacturer’s instructions. The MICs were further confirmed by the microdilution method in a 96 wells plate according to the Clinical Laboratory Standards Institute (CLSI) guidelines in 0.1 ml Cation Adjusted Müller-Hinton Broth (CAMHB) supplemented with 5% lysed sheep blood (Difco). All MIC were determined from three independent biological replicates, each replicate being further assessed in technical duplicates. PEN and CIP were purchased from Sigma-Aldrich.
Macrodilution was performed in triplicate in a volume of 3 ml in CAMHB or CAMHB supplemented with 12 mM glutamine. S. pneumoniae inoculum were prepared by suspending colonies grown overnight on TSA agar (Becton Dickinson) using 1× PBS to achieve a turbidity of 0.5 McFarland (1 × 108 CFU/ml). Fifteen microliters of this suspension was inoculated to the CAMBH broth to reach a concentration of 5 × 105 CFU/mL. MICs of PEN were determined in the presence or absence of varying concentrations (0.008 to 32 mM) of L-methionine sulfoximine (MSO; Sigma-Aldrich) but 0.5 mM was the lowest MSO concentration to allow maximal effects on the MIC of PEN. For sensitive strains, the range of PEN concentrations tested varied between 0.004 and 0.12 µg/mL in doubling dilutions. For resistant isolates this varied from 0.06 to 8 µg/mL. Tubes were incubated at 35 °C and turbidity was checked after 20–24 h.
RNA sequencing
Total RNA was isolated at different time points from S. pneumoniae R6, CCRI-8970 and CCRI-21487 grown in BHI using the RNeasy Mini Kit (Qiagen) according to manufacturer’s instructions. The RNAs were treated with DNase I (Ambion) to avoid any DNA contamination. RNAs were quantified using 2100 BioAnalyzer RNA6000 Nano chips (Agilent) and 1 µg of total RNA was treated with Ribo-ZeroTM Magnetic Kit for Gram-Positive Bacteria (Epicentre). The rRNA-depleted samples were purified using RNeasy MinElute Cleanup kit (Qiagen). RNA-seq libraries were produced from 50 ng of rRNA-depleted samples using the ScriptSeqTM v2 RNA-Seq Library Preparation Kit (Epicentre). The libraries were analysed using 2100 BioAnalyser High Sensitivity DNA Chips (Agilent) and quantified by QuantiFluor. The libraries were pooled, diluted to 8 pM and sequenced on an Illumina HiSeq system using a 101 bp paired-ends reads protocol.
RNA-seq data analysis
The S. pneumoniae R6 genome52 (NCBI accession number AE007317) was used as reference. Sequencing reads were aligned to the S. pneumoniae R6 genome and analysed using the software Rockhopper53 with default settings. The RNA-seq data are available under the accessions number from SAMN07298959 to SAMN07298986 under the BioProject PRJNA392406.
Quantitative reverse transcription (qRT-PCR)
The qRT-PCR experiment was done as previously described25. Briefly, total RNAs were extracted as described above and treated with DNase I (Ambion) to avoid any DNA contamination. RNA quality and integrity was assessed by agarose gel electrophoresis. cDNAs were generated from 50 ng of total RNA using the Superscript II reverse transcriptase (Invitrogen) and random hexamers according to the manufacturer’s instructions. qRT-PCR assays were carried out on a Bio-Rad Cycler using SYBR Green I. A final volume of 10 µl was used for each reaction containing specific primers (Table S4) and iQ SYBR Green Supermix (Bio-Rad). The relative quantitation of gene expression was performed using the relative standard curve method. All qRT-PCR data were normalized according to the amplification signals of 16S rRNA.
Metabolites extraction
S. pneumoniae strains were grown in BHI broth at 35 °C until they reached early log-phase (OD600 = 0.11). At this time (T0), cultures were divided into 6 tubes of 10 mL, half of which contained 1X MIC PEN, and incubated for a duration equivalent to time point T3 of the time-course RNA-seq experiments. Samples were rapidly quenched by submersion into dry ice/ethanol for 20 sec. The quenched samples were centrifuged at 1500 RCF for 10 min at 4 °C then washed twice with ice-cold PBS 1× and centrifuged for 5 min at 4 °C. Pellets were flash frozen in liquid nitrogen before being stored at −80 °C until use. Cell lysis and protein denaturation was achieved by addition of 200 µL of cooled methanol: water (4:1) supplemented with a mixture of deuterated glutamine and glutamate standards (at 300 and 500 ng/mL respectively) along with 100 mg acid washed glass beads (≤106 µm, Sigma) to the pellets followed by agitation in a bead beater at 4 °C for 6 cycles of 1 min agitation and 1 min cooling (Mini-BeadBeater-24, BioSpec Products, Inc.). Samples were then centrifuged at 13000 rpm for 5 min at 4 °C. Supernatant (150 µL) were speed-vac dried for 65 min at medium heat. Lyophilised samples were kept at −80 °C until further analysis. The LC-MS data were normalized according to the bacterial count obtained by plating on Trypticase soy agar supplemented with 5% sheep blood (BD).
LC-MS
Lyophilised samples were resuspended in 150 µL of 50:50 of mobile phase A: mobile phase B (MPA: MPB), sonicated 5 min and filtrated on a 0.45 µm filter before injection. Liquid chromatography was performed on an Acquity UPLC I-Class binary pump system. The MPA consisted of 0.1% formic acid in water and the MPB consisted of 0.1% formic acid in acetonitrile. The column used was a BEH Amide 1.7 µM × 2.1 mm × 150 mm (Waters Corporation, part no. 186004802) at 45 °C. A VanGuard pre-column with 2.1 × 5 mm filter unit (Waters corporation, part no 205000343) was used to protect the analytical column from impurities. Gradient conditions were: 0.01 to 0.1 min = 99% B; 0.1 to 7.00 min = 99 to 50% B; 7.00 to 7.10 min = 50% to 99% B; 7.10 to 10.00 min = 99% B. Injection volume was 2.0 µL, and flow rate was 0.4 mL/min. Single reaction monitoring (SRM) analysis were performed on a Q-TOF Synapt G2-Si system with an electrospray ionization source (ESI) in positive ionization mode. Source conditions and MS parameters were optimized by direct infusion of standards in sensitivity mode. MS parameters included: capillary voltage 2.75 kV, source temperature 120 °C, desolvation temperature 325 °C and desolvation gas 900 L*min−1. The specific SRM transitions for quantification were: glutamate 147.0764 > 130.0499, glutamine 148.0604 > 130.0499, deuterated glutamate 153.0979 > 135.0838 and deuterated glutamine 152.1155 > 135.0838. All data were collected by MassLynx software version 4.1. Deuterated glutamate (DLM-357-0.25) and deuterated glutamine (DLM-1826-0.1) were purchased from Cambridge Isotope Laboratories. Calibration curves of both metabolites using seven concentration points (23-46-94-187-375-750 and 1500 ng/mL) were obtained by comparison of the area ratios. Deuterated standards and QC samples at low (80 ng/mL), medium (320 ng/mL) and high (640 ng/mL) concentration were directly diluted in a pool of matrix from S. pneumoniae untreated samples. The relative concentrations of intracellular glutamine and glutamate in metabolite extracts were determined against the calibration curves. For a detailed description of the LC-MS analysis, see the supplementary data (Supplementary Tables S5 & S6).
Statistical analysis
Statistical analysis for the qRT-PCR experiment was done using a split plot design and Fisher’s F-test using the SAS software. Significant differences in the LC-MS quantification of glutamine and glutamate and in survival rates in the presence of glutamine were determined using unpaired (two-tailed) Student’s t test in GraphPad Prism. Comparative data with a p value ≤ 0.05 were considered statistically different.
Electronic supplementary material
Acknowledgements
This work was supported by Canadian Institutes of Health Research grant number 81266 to M.O. M.O. is the holder of a Tier 1 Canada Research Chair in Antimicrobial Resistance. We would also like to thank Pier-Luc Plante for his helpful advice and Francis Brière for his technical assistance in the LC-MS experiment. We thank Awa Diop from « Service de consultation statistique, Université Laval » for performing the statistical analysis for the qRT-PCR experiment.
Author Contributions
J.Y.E.K., P.L. and M.O. designed the study. J.Y.E.K. performed the experiments, analysed the data and drafted the manuscript; N.B. performed the L.C.-M.S. analysis. M.G.B. provided materials. P.L., M.G.B. and M.O. revised the manuscript and provided critical comments. All authors approved the final version of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-15035-y.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pneumococcal Vaccines WHO Position Paper--2012. Wkly Epidemiol Rec. 87, 129–144 (2012). [PubMed]
- 2.Global, Regional, and National Life Expectancy, All-Cause Mortality, and Cause-Specific Mortality for 249 Causes of Death, 1980-2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet. 388, 1459–1544, 10.1016/S0140-6736(16)31012-1 (2016). [DOI] [PMC free article] [PubMed]
- 3.Lynch JP, 3rd, Zhanel GG. Streptococcus Pneumoniae: Does Antimicrobial Resistance Matter? Semin Respir Crit Care Med. 2009;30:210–238. doi: 10.1055/s-0029-1202939. [DOI] [PubMed] [Google Scholar]
- 4.Picazo JJ. Management of Antibiotic-Resistant Streptococcus Pneumoniae Infections and the Use of Pneumococcal Conjugate Vaccines. Clin Microbiol Infect. 2009;15(Suppl 3):4–6. doi: 10.1111/j.1469-0691.2009.02723.x. [DOI] [PubMed] [Google Scholar]
- 5.Jones RN, Sader HS, Mendes RE, Flamm RK. Update on Antimicrobial Susceptibility Trends among Streptococcus Pneumoniae in the United States: Report of Ceftaroline Activity from the Sentry Antimicrobial Surveillance Program (1998–2011) Diagn Microbiol Infect Dis. 2013;75:107–109. doi: 10.1016/j.diagmicrobio.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Therapy for Children with Invasive Pneumococcal Infections. American Academy of Pediatrics Committee on Infectious Diseases. Pediatrics. 99, 289–299 (1997). [PubMed]
- 7.Tomasz A. Penicillin-Binding Proteins and the Antibacterial Effectiveness of Beta-Lactam Antibiotics. Rev Infect Dis. 1986;8(Suppl 3):S260–278. doi: 10.1093/clinids/8.Supplement_3.S260. [DOI] [PubMed] [Google Scholar]
- 8.Wright GD. Antibiotic Adjuvants: Rescuing Antibiotics from Resistance. Trends Microbiol. 2016;24:862–871. doi: 10.1016/j.tim.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Tait-Kamradt AG, Cronan M, Dougherty TJ. Comparative Genome Analysis of High-Level Penicillin Resistance in Streptococcus Pneumoniae. Microb Drug Resist. 2009;15:69–75. doi: 10.1089/mdr.2009.0891. [DOI] [PubMed] [Google Scholar]
- 10.Pillai DR, et al. Genome-Wide Dissection of Globally Emergent Multi-Drug Resistant Serotype 19a. Streptococcus Pneumoniae. BMC Genomics. 2009;10:642. doi: 10.1186/1471-2164-10-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fani F, Leprohon P, Zhanel GG, Bergeron MG, Ouellette M. Genomic Analyses of DNA Transformation and Penicillin Resistance in Streptococcus Pneumoniae Clinical Isolates. Antimicrob Agents Chemother. 2014;58:1397–1403. doi: 10.1128/AAC.01311-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, Y. et al. Penicillin-Binding Protein Transpeptidase Signatures for Tracking and Predicting β-Lactam Resistance Levels in Streptococcus pneumoniae. MBio. 7, 10.1128/mBio.00756-16 (2016). [DOI] [PMC free article] [PubMed]
- 13.Fani F, Leprohon P, Legare D, Ouellette M. Whole Genome Sequencing of Penicillin-Resistant Streptococcus Pneumoniae Reveals Mutations in Penicillin-Binding Proteins and in a Putative Iron Permease. Genome Biol. 2011;12:R115. doi: 10.1186/gb-2011-12-11-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fani F, Brotherton MC, Leprohon P, Ouellette M. Genomic Analysis and Reconstruction of Cefotaxime Resistance in Streptococcus Pneumoniae. J Antimicrob Chemother. 2013;68:1718–1727. doi: 10.1093/jac/dkt113. [DOI] [PubMed] [Google Scholar]
- 15.Janga SC, Contreras-Moreira B. Dissecting the Expression Patterns of Transcription Factors across Conditions Using an Integrated Network-Based Approach. Nucleic Acids Res. 2010;38:6841–6856. doi: 10.1093/nar/gkq612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dwyer DJ, Kohanski MA, Hayete B, Collins JJ. Gyrase Inhibitors Induce an Oxidative Damage Cellular Death Pathway in Escherichia Coli. Mol Syst Biol. 2007;3:91. doi: 10.1038/msb4100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A Common Mechanism of Cellular Death Induced by Bactericidal Antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 18.Ferrandiz MJ, de la Campa AG. The Fluoroquinolone Levofloxacin Triggers the Transcriptional Activation of Iron Transport Genes That Contribute to Cell Death in Streptococcus Pneumoniae. Antimicrob Agents Chemother. 2014;58:247–257. doi: 10.1128/AAC.01706-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers PD, et al. Gene Expression Profiling of the Response of Streptococcus Pneumoniae to Penicillin. J Antimicrob Chemother. 2007;59:616–626. doi: 10.1093/jac/dkl560. [DOI] [PubMed] [Google Scholar]
- 20.Haas W, Kaushal D, Sublett J, Obert C, Tuomanen EI. Vancomycin Stress Response in a Sensitive and a Tolerant Strain of Streptococcus Pneumoniae. J Bacteriol. 2005;187:8205–8210. doi: 10.1128/JB.187.23.8205-8210.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ng WL, Kazmierczak KM, Robertson GT, Gilmour R, Winkler ME. Transcriptional Regulation and Signature Patterns Revealed by Microarray Analyses of Streptococcus Pneumoniae R6 Challenged with Sublethal Concentrations of Translation Inhibitors. J Bacteriol. 2003;185:359–370. doi: 10.1128/JB.185.1.359-370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mann B, et al. Control of Virulence by Small RNAs in Streptococcus Pneumoniae. PLoS Pathog. 2012;8:e1002788. doi: 10.1371/journal.ppat.1002788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pettigrew MM, et al. Dynamic Changes in the Streptococcus Pneumoniae Transcriptome During Transition from Biofilm Formation to Invasive Disease Upon Influenza A Virus Infection. Infect Immun. 2014;82:4607–4619. doi: 10.1128/IAI.02225-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aprianto R, Slager J, Holsappel S, Veening JW. Time-Resolved Dual RNA-Seq Reveals Extensive Rewiring of Lung Epithelial and Pneumococcal Transcriptomes During Early Infection. Genome Biol. 2016;17:198. doi: 10.1186/s13059-016-1054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lupien A, Gingras H, Bergeron MG, Leprohon P, Ouellette M. Multiple Mutations and Increased RNA Expression in Tetracycline-Resistant Streptococcus Pneumoniae as Determined by Genome-Wide DNA and mRNA Sequencing. J Antimicrob Chemother. 2015;70:1946–1959. doi: 10.1093/jac/dkv211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mascher T, et al. The Streptococcus Pneumoniae Cia Regulon: CiaR Target Sites and Transcription Profile Analysis. J Bacteriol. 2003;185:60–70. doi: 10.1128/JB.185.1.60-70.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakenbeck R, et al. Mosaic Genes and Mosaic Chromosomes: Intra- and Interspecies Genomic Variation of Streptococcus Pneumoniae. Infect Immun. 2001;69:2477–2486. doi: 10.1128/IAI.69.4.2477-2486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronzio RA, Rowe WB, Meister A. Studies on the Mechanism of Inhibition of Glutamine Synthetase by Methionine Sulfoximine. Biochemistry. 1969;8:1066–1075. doi: 10.1021/bi00831a038. [DOI] [PubMed] [Google Scholar]
- 29.Rowe WB, Ronzio RA, Meister A. Inhibition of Glutamine Synthetase by Methionine Sulfoximine. Studies on Methionine Sulfoximine Phosphate. Biochemistry. 1969;8:2674–2680. doi: 10.1021/bi00834a065. [DOI] [PubMed] [Google Scholar]
- 30.Brusilow, W. S. & Peters, T. J. Therapeutic Effects of Methionine Sulfoximine in Multiple Diseases Include and Extend Beyond Inhibition of Glutamine Synthetase. Expert Opin Ther Targets. 1–9, 10.1080/14728222.2017.1303484 (2017). [DOI] [PubMed]
- 31.Soualhine H, et al. A Proteomic Analysis of Penicillin Resistance in Streptococcus Pneumoniae Reveals a Novel Role for PstS, a Subunit of the Phosphate ABC Transporter. Mol Microbiol. 2005;58:1430–1440. doi: 10.1111/j.1365-2958.2005.04914.x. [DOI] [PubMed] [Google Scholar]
- 32.Tran TD, et al. Decrease in Penicillin Susceptibility Due to Heat Shock Protein ClpL in Streptococcus Pneumoniae. Antimicrob Agents Chemother. 2011;55:2714–2728. doi: 10.1128/AAC.01383-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran TD, et al. Heat-Shock Protein ClpL/HSP100 Increases Penicillin Tolerance in. Streptococcus Pneumoniae. Adv Otorhinolaryngol. 2011;72:126–128. doi: 10.1159/000324658. [DOI] [PubMed] [Google Scholar]
- 34.Guenzi E, Gasc AM, Sicard MA, Hakenbeck R. A Two-Component Signal-Transducing System Is Involved in Competence and Penicillin Susceptibility in Laboratory Mutants of Streptococcus Pneumoniae. Mol Microbiol. 1994;12:505–515. doi: 10.1111/j.1365-2958.1994.tb01038.x. [DOI] [PubMed] [Google Scholar]
- 35.Halfmann A, Kovacs M, Hakenbeck R, Bruckner R. Identification of the Genes Directly Controlled by the Response Regulator CiaR in Streptococcus Pneumoniae: Five out of 15 Promoters Drive Expression of Small Non-Coding RNAs. Mol Microbiol. 2007;66:110–126. doi: 10.1111/j.1365-2958.2007.05900.x. [DOI] [PubMed] [Google Scholar]
- 36.Schnorpfeil A, et al. Target Evaluation of the Non-Coding csRNAs Reveals a Link of the Two-Component Regulatory System CiaRH to Competence Control in Streptococcus Pneumoniae R6. Mol Microbiol. 2013;89:334–349. doi: 10.1111/mmi.12277. [DOI] [PubMed] [Google Scholar]
- 37.Kloosterman TG, et al. Regulation of Glutamine and Glutamate Metabolism by GlnR and GlnA in. Streptococcus Pneumoniae. J Biol Chem. 2006;281:25097–25109. doi: 10.1074/jbc.M601661200. [DOI] [PubMed] [Google Scholar]
- 38.Hendriksen WT, et al. Site-Specific Contributions of Glutamine-Dependent Regulator GlnR and GlnRRegulated Genes to Virulence of Streptococcus Pneumoniae. Infect Immun. 2008;76:1230–1238. doi: 10.1128/IAI.01004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartel T, et al. Impact of Glutamine Transporters on Pneumococcal Fitness under Infection-Related Conditions. Infect Immun. 2011;79:44–58. doi: 10.1128/IAI.00855-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanehisa M, et al. Data, Information, Knowledge and Principle: Back to Metabolism in Kegg. Nucleic Acids Res. 2014;42:D199–205. doi: 10.1093/nar/gkt1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komatsuzawa H, et al. The Gate Controlling Cell Wall Synthesis in. Staphylococcus Aureus. Mol Microbiol. 2004;53:1221–1231. doi: 10.1111/j.1365-2958.2004.04200.x. [DOI] [PubMed] [Google Scholar]
- 42.Lee SH, et al. Antagonism of Chemical Genetic Interaction Networks Resensitize MRSA to β-Lactam Antibiotics. Chem Biol. 2011;18:1379–1389. doi: 10.1016/j.chembiol.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Shimazu K, et al. Identification of the Streptococcus Gordonii glmM Gene Encoding Phosphoglucosamine Mutase and Its Role in Bacterial Cell Morphology, Biofilm Formation, and Sensitivity to Antibiotics. FEMS Immunol Med Microbiol. 2008;53:166–177. doi: 10.1111/j.1574-695X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 44.Wu S, de Lencastre H, Sali A, Tomasz A. A Phosphoglucomutase-Like Gene Essential for the Optimal Expression of Methicillin Resistance in Staphylococcus Aureus: Molecular Cloning and DNA Sequencing. Microb Drug Resist. 1996;2:277–286. doi: 10.1089/mdr.1996.2.277. [DOI] [PubMed] [Google Scholar]
- 45.Jolly L, et al. The Femr315 Gene from Staphylococcus Aureus, the Interruption of Which Results in Reduced Methicillin Resistance, Encodes a Phosphoglucosamine Mutase. J Bacteriol. 1997;179:5321–5325. doi: 10.1128/jb.179.17.5321-5325.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glanzmann P, Gustafson J, Komatsuzawa H, Ohta K, Berger-Bachi B. glmM Operon and Methicillin-Resistant glmM Suppressor Mutants in Staphylococcus Aureus. Antimicrob Agents Chemother. 1999;43:240–245. doi: 10.1128/aac.43.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, et al. Discovery of Wall Teichoic Acid Inhibitors as Potential Anti-MRSA β-Lactam Combination Agents. Chem Biol. 2013;20:272–284. doi: 10.1016/j.chembiol.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zapun A, et al. In Vitro Reconstitution of Peptidoglycan Assembly from the Gram-Positive Pathogen Streptococcus Pneumoniae. ACS Chem Biol. 2013;8:2688–2696. doi: 10.1021/cb400575t. [DOI] [PubMed] [Google Scholar]
- 49.Peng B, et al. Exogenous Alanine and/or Glucose Plus Kanamycin Kills Antibiotic-Resistant Bacteria. Cell Metab. 2015;21:249–261. doi: 10.1016/j.cmet.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 50.Meisel JW, Patel MB, Garrad E, Stanton RA, Gokel GW. Reversal of Tetracycline Resistance in Escherichia Coli by Noncytotoxic Bis(Tryptophan)S. J Am Chem Soc. 2016;138:10571–10577. doi: 10.1021/jacs.6b05578. [DOI] [PubMed] [Google Scholar]
- 51.Munoz R, et al. Genetics of Resistance to Third-Generation Cephalosporins in Clinical Isolates of Streptococcus Pneumoniae. Mol Microbiol. 1992;6:2461–2465. doi: 10.1111/j.1365-2958.1992.tb01422.x. [DOI] [PubMed] [Google Scholar]
- 52.Hoskins J, et al. Genome of the Bacterium Streptococcus Pneumoniae Strain R6. J Bacteriol. 2001;183:5709–5717. doi: 10.1128/JB.183.19.5709-5717.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McClure R, et al. Computational Analysis of Bacterial RNA-Seq Data. Nucleic Acids Res. 2013;41:e140. doi: 10.1093/nar/gkt444. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


