Abstract
Myopia is increasing worldwide. Although the exact etiology of myopia is unknown, outdoor activity is one of the most important environmental factors for myopia control. We previously reported that violet light (VL, 360–400 nm wavelength), which is abundant in the outdoor environment, suppressed myopia progression for individuals under 20 years of age. However, whether VL is also effective for adult high myopia, which can be sight-threatening, has remained unknown. To investigate the influence of VL for adult myopia, we retrospectively compared the myopic progression and the axial length elongation over five years in adult high myopic patients over 25 years of age after two types (non-VL transmitting and VL transmitting) of phakic intraocular lens (pIOL) implantation. We found that high myopic patients with the non-VL transmitting pIOLs implanted are almost two times more myopic in the change of refraction and four times longer in the change of axial length, compared to those implanted with the VL transmitting pIOLs. This result indicated that the VL transmitting pIOL suppressed myopia progression and axial length elongation compared with the non-VL transmitting one. In conclusion, our study showed the VL possibly has an anti-myopia effect for human adults with high myopia.
Introduction
The cause of the onset and progression of myopia (short-sightedness) is unknown, and the prevalence of myopia is increasing worldwide. If it continues to grow at its current rate, it is forecast that the world’s myopic population will be about 5 billion in 20501. Myopia has been increasing worldwide, especially over the past 50 years2, and it seems that environmental changes are bigger factors than genetic changes.
Some epidemiological studies have suggested that increased near vision tasks such as reading, using computers and smartphones are possible risk factors3. Recently, the time spent outdoors was proposed as a protective factor3–11, and the beneficial effect of high ambient light for the protection of myopia has been confirmed in chicks, mice, and monkeys12–16. Additionally, some clinical trials on students have indicated that increased outdoor activity had an anti-myopia effect11,17,18. Furthermore, we recently discovered that violet light (VL, 360–400 nm wavelength), which hardly exists indoors and can only be found in outdoor environments, suppressed myopia progression19. According to the international lighting vocabulary of the Commission Internationale de l’Eclairage (CIE)20, the lower limits of visible light’s wavelengths are defined to be between 360 and 400 nm, which overlaps with the upper end of the Ultraviolet A (UVA) spectrum21. This range, in fact, is visible as VL, but it is recognized as UV as well. We compared the degree of myopia progression in two groups of students aged 13 to 18 years with different transmittance of VL in contact lenses used for refractive correction. We found that the axial length elongation in the group wearing VL transmitting contact lenses was smaller than those in the group wearing partially VL-blocking contact lenses, finding that axial length elongation was significantly less in the group wearing VL transmitting contact lenses19. However, these samples were younger students under 20 years old and not adults, and we did not know whether VL is also effective for adult high myopia, which can be sight-threatening in the future.
McBrien and Adams22 reported that the incidence of adult myopia development for two years in an occupational group aged from 21 to 63 years was 45%, and they confirmed the elongation of the vitreous chamber depth. Furthermore, Saka et al.23 reported that the axial length elongation of adult high myopic patients (myopia ≤ −6 diopters (D) or axial length ≥ 26.5 mm) whether there was a staphyloma or not, was confirmed in adults aged from 22 to 84 years.
Phakic intraocular lens (pIOL) implantation has been accepted as a refractive surgery for correcting high myopia24,25. Several designs have been developed, including angle-supported anterior chamber pIOLs, posterior chamber pIOLs, and iris-fixated pIOLs. Among them, the ARTISAN® (Ophtec BV, Groningen, The Netherlands) and the ARTIFLEX® (Ophtec BV) are iris-fixated pIOLs for which long-term results have been previously published24,26–29.
Here we show the difference in transmittance of VL between the ARTISAN® and ARTIFLEX® pIOL and compare the change of the refraction and axial length elongation over a long-term period between the two lenses. Then, we evaluate whether VL had the anti-myopia effect even for human adults with high myopia.
Results
Comparing the myopic progression and axial length elongation over five-year period retrospectively between the patients implanted with ARTISAN® or ARTIFLEX® pIOL against adult high myopia
We compared the change of refraction and axial length elongation for five years in high myopic adult patients (average over −10.0 D) after refractive surgery, implanted with pIOLs to correct high myopia.
Optical coherence tomography (OCT) confirmed that there were no cases of posterior staphyloma in both groups 5 years postoperatively.
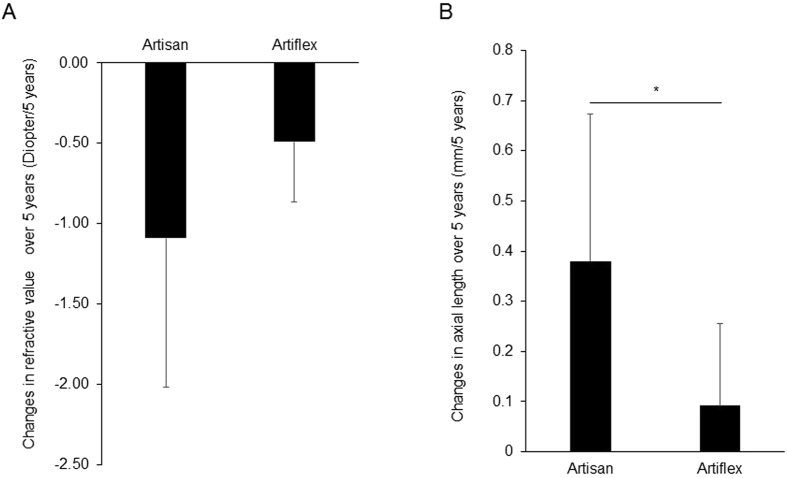
The mean change in the refraction after non-VL transmitting pIOL (11 cases of 11 eyes) (ARTISAN®, Fig. 1A,C) implantation was −1.09 D/5 years, whereas the change in the refraction after VL transmitting pIOL (15 cases of 15 eyes) (ARTIFLEX®, Fig. 1B,C) implantation was −0.49 D/5 years (P = 0.121) (Fig. 2A).
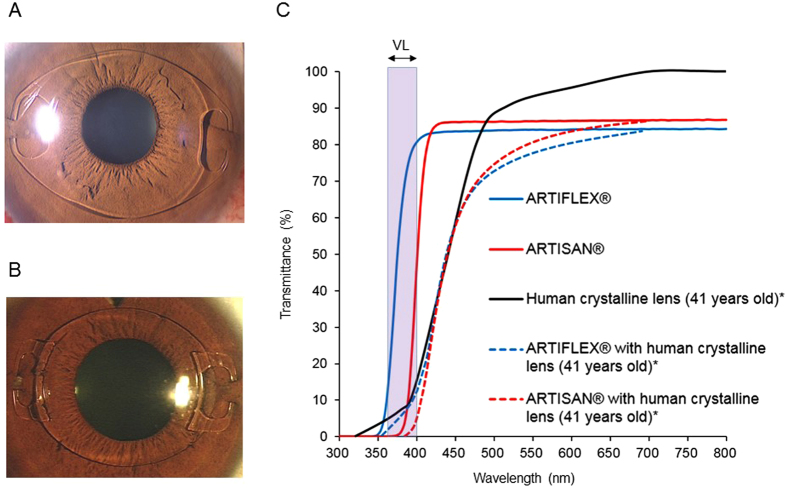
Figure 1.
The two types of iris-fixated phakic intraocular lens (pIOL). (A) ARTISAN® (Ophtec BV, Groningen, Netherlands) pIOL. (B) ARTIFLEX® (Ophtec BV) pIOL. (C) Spectral transmission of ARTISAN® and ARTIFLEX® pIOLs. ARTISAN® pIOL transmits minimal violet light (VL). ARTIFLEX® is a VL transmitting pIOL. The calculated transmittance of ARTIFLEX® or ARTISAN® combined with human crystalline lens (41 years old)* was also shown. *Data from the spectral transmission of a 41-year-old human crystalline lens was taken from the figure of a published paper31, and the Association for Research in Vision and Ophthalmology is the copyright holder of this paper31.
Figure 2.
Violet light (VL) through eye suppressed myopia progression and axial length elongation in adult humans who were implanted with non-VL transmitting phakic intraocular lens (pIOL) and VL transmitting pIOL. (A) Changes in the refraction for about 5 years (5 years postoperatively minus 3 months postoperatively). The refractive values are more myopic in the Artisan group (11 cases of 11 eyes) than the Artiflex group (15 cases of 15 eyes). (B) Changes in axial length over 5 years (5 years postoperatively minus preoperative data). Changes in axial length in the Artiflex group (13 cases of 13 eyes) was significantly lower than the Artisan group (10 cases of 10 eyes). *P < 0.05, Mann-Whitney U Test. Data are shown as mean ± standard deviation (SD).
The mean axial length elongation after non-VL transmitting pIOL (10 cases of 10 eyes) (ARTISAN®) implantation was 0.38 mm/5 years, whereas the axial length elongation after VL transmitting pIOL (13 cases of 13 eyes) (ARTIFLEX®) implantation was only 0.09 mm/5 years (P = 0.030) (Fig. 2B).
There were no significant differences in preoperative age, cycloplegic refractive value, axial length, uncorrected visual acuity, or best corrected visual acuity between the two groups (Table 1). Table 2 shows the difference in some parameters including spherical aberration30 and the transmission of VL, 360–400 nm wavelength between the two lenses. These findings indicated that VL transmittance through pIOLs was possibly related to myopic progression in adult high myopia after pIOL implantation. Table 3 shows the results of all the individual subjects.
Table 1.
Violet light (VL) exposure to the eye suppressed axial length elongation in adult humans: Comparing patients who were implanted with non-VL transmitting phakic intraocular lens (pIOL) or VL transmitting pIOL.
| Parameter | VL (−) pIOLs | VL (+) pIOLs | P value |
|---|---|---|---|
| Artisan group mean ± SD (range) | Artiflex group mean ± SD (range) | ||
| Number | 11 cases 11 eyes | 15 cases 15 eyes | − |
| Race | All Japanese | ||
| Age (years) | 39.9 ± 8.9 (28 ~ 58) | 36.3 ± 7.2 (26 ~ 53) | 0.259 |
| Cycloplegic refractive value (diopter) (Spherical equivalent) | −12.96 ± 4.19 (−18.13 ~ −6.88) | −11.14 ± 1.65 (−13.88 ~ −7.63) | 0.318 |
| Axial length (mm) | 28.54 ± 1.85 (26.22~32.37) | 28.13 ± 1.41 (25.30~30.53) | 0.799 |
| UCVA (logMAR) | 1.48 ± 0.26 (0.90 ~ 1.90) | 1.49 ± 0.14 (1.22 ~ 1.80) | 0.878 |
| BCVA (logMAR) | −0.11 ± 0.16 (−0.30 ~ 0.30) | −0.15 ± 0.12 (−0.30 ~ 0.15) | 0.721 |
Patient preoperative data: Artisan group (n = 11), the phakic intraocular lens (pIOL) group who were implanted with ARTISAN® pIOLs [VL (−) pIOLs]; and the Artiflex group (n = 15), the pIOL group who were implanted with ARTIFLEX® pIOLs [VL (+) pIOLs]. There were no significant differences in preoperative age, cycloplegic refractive value, axial length, uncorrected visual acuity (UCVA), and best corrected visual acuity (BCVA) between the two groups. SD, standard deviation. Data were analyzed using the Mann-Whitney U Test.
Table 2.
Difference in parameters between ARTISAN® and ARTIFLEX® pIOLs: The most notable point is the difference of transmission of VL, 360–400 nm wavelength between the two lenses.
| ARTISAN® | ARTIFLEX® | |
|---|---|---|
| Spectral transmission | VL blocked | Not VL blocked |
| Incision size during operation (mm) | 6.5 | 3.2 |
| Refractive index | 1.49 | 1.43 |
| Lens material of optical zone | Polymethyl methacrylate (PMMA) | Silicone |
| Spherical aberration (Z4 0) | — | Lower than ARTISAN®* |
*Spherical aberration (Z4 0) is not measured by the manufacturer, but based on the ref.30. pIOL, phakic intraocular lens. VL, violet light.
Table 3.
All the individual subject’s results.
| Group | Age | Changes in the refraction for approximately 5 years (5 years postoperatively minus 3 months postoperative data) (Diopter) | Changes in axial length over 5 years (5 years postoperatively minus preoperative data) (mm) |
|---|---|---|---|
| Artisan group | 58 | −1.38 | NA |
| 46 | 0.25 | 0.33 | |
| 34 | 0.00 | 0.08 | |
| 28 | −1.50 | 0.61 | |
| 38 | −1.50 | 0.65 | |
| 44 | −2.88 | 0.69 | |
| 31 | −1.38 | 0.67 | |
| 32 | −0.13 | 0.00 | |
| 46 | −1.50 | 0.03 | |
| 46 | −0.38 | 0.14 | |
| 36 | −1.63 | 0.60 | |
| Average of Artisan group | 39.9 | −1.09 | 0.38 |
| Artiflex group | 31 | −0.88 | 0.31 |
| 37 | −1.00 | NA | |
| 42 | −0.26 | NA | |
| 41 | 0.25 | 0.25 | |
| 36 | −0.63 | −0.04 | |
| 33 | −1.00 | 0.13 | |
| 42 | −0.50 | −0.26 | |
| 36 | −0.38 | 0.01 | |
| 44 | −0.75 | −0.04 | |
| 26 | −0.75 | 0.18 | |
| 28 | −0.25 | 0.08 | |
| 34 | −0.63 | 0.10 | |
| 53 | −0.25 | 0.15 | |
| 33 | −0.50 | −0.02 | |
| 28 | 0.13 | 0.34 | |
| Average of Artiflex group | 36.3 | −0.49 | 0.09 |
NA = Not Applicable.
Higher order aberrations (HOAs) and residual astigmatism
There were no significant differences in HOAs and residual astigmatism 5 years postoperatively between the two groups (Table 4).
Table 4.
Comparisons of residual astigmatism, and higher order aberrations between the Artisan and Artiflex groups 5 years postoperatively.
| Artisan Group (mean ± SD) | Artiflex Group (mean ± SD) | P Value | |
|---|---|---|---|
| Number | 11 cases 11 eyes | 15 cases 15 eyes | − |
| Residual astigmatism (diopter) | −0.68 ± 0.53 | −0.55 ± 0.40 | 0.474 |
| J0 component of residual astigmatism (diopter) | 0.10 ± 0.28 | 0.01 ± 0.27 | 0.198 |
| J45 component of residual astigmatism (diopter) | 0.11 ± 0.30 | 0.02 ± 0.22 | 0.357 |
| Cornea THOA (µm)* | 0.05 ± 0.02 | 0.05 ± 0.03 | 0.643 |
| Internal THOA (µm)* | 0.07 ± 0.03 | 0.06 ± 0.02 | 0.238 |
| Ocular THOA (µm)* | 0.08 ± 0.04 | 0.06 ± 0.02 | 0.216 |
SD = standard deviation; THOA = total higher-order aberrations, root mean square (RMS) of the third- to sixth-order Zernike coefficients. *Pupillary diameter = 4 mm (Artisan group [10 cases 10 eyes], Artiflex group [15 cases 15 eyes]). Data were analyzed using the Mann-Whitney U Test.
Simulation of the peripheral refraction after pIOL implantation
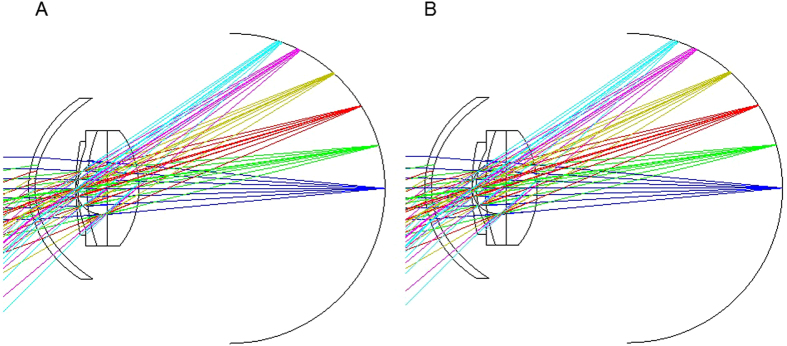
The focusing images were nearly identical. Even if the wavelength is around 400 nm, the focusing images showed the same results as Fig. 3A,B. The defocus amounts were the same and +1.18 D at the incident angle of 20 degrees for the two pIOLs. They were +2.93 D for ARTISAN® pIOL, and +3.04 D for ARTIFLEX® pIOL, at the incident angle of 30 degrees.
Figure 3.
Simulation results of peripheral refraction after ARTISAN® and ARTIFLEX® phakic intraocular lens (pIOL) implantation. (A) Simulation result of peripheral refraction after ARTISAN® pIOL implantation. (B) Simulation result of peripheral refraction after ARTIFLEX® pIOL implantation. There is no difference in the amount of peripheral hyperopic defocus between ARTISAN® and ARTIFLEX® in the eye model simulation. This indicates that the differences in myopic progression and axial length elongation between the two pIOLs did not seem to depend on the difference of peripheral hyperopic defocus.
Discussion
First, we measured the transmittance of the ARTISAN® and ARTIFLEX® pIOLs, and we confirmed that ARTISAN® was a non-VL transmitting pIOL, ARTIFLEX® was a VL transmitting pIOL (Fig. 1C). Then, we retrospectively studied human clinical data and found that VL also had the suppressive effect against adult (>25 years old) high myopia as well as school-aged (<20 years old) myopia19. It is notable that high myopic patients who were implanted with the non-VL transmitting pIOLs were almost twice as myopic in the change of refraction and four times longer in the change of axial length and more likely to suffer from myopia progression, compared to those who were implanted with the VL transmitting pIOLs. We demonstrated that the VL transmitting pIOL suppressed myopia progression and axial length elongation compared with the non-VL transmitting one.
The changes of the axial length in adult high myopia without staphyloma (the average age was 46.5 years of age and age range was from 22 to 76 years of age, axial length ranged from 26.58 to 33.17 mm, which is almost the same as our current study) was 0.06 mm/year23 and the changes of the axial length in an adult high myopia with staphyloma (the average age was 52.4 years and age range was from 29 to 84 years old, axial length ranged from 26.45 to 34.43 mm) was also 0.06 mm/year23. The history of refractive surgery was one of the exclusion criteria in that study. Therefore, the patients were assumed to have used contact lenses or eyeglasses for the refractive correction, although the details of myopic correction method were not described in the paper. According to our study19 on the spectral transmission of the most commonly used eyeglasses and contact lenses in Japan, almost all eyeglasses blocked VL, and some contact lenses blocked VL partially, although the other contact lenses were transmitting VL. In the current study, the group of non-VL transmitting pIOLs (ARTISAN®; Artisan group) showed the greater axial length elongation (0.38 mm/5 years = 0.076 mm/year) than the group of VL transmitting pIOLs (ARTIFLEX®; Artiflex group) (0.09 mm/5 years = 0.018 mm/year). Comparing this data with the previously reported ones23 for the patients with the almost the same background, the group of contact lenses or eyeglasses showed the axial length elongation (0.06 mm/year) in between the non-VL transmitting and the VL transmitting pIOLs. These results are understandable for the transmittance of VL, though this comparison was examined among different materials as well as different spectral transmissions.
In the next step, we considered whether VL passes through the crystalline lens to the retina in order to induce the suppressive effect against myopia progression. We calculated the VL transmittance of a 41-year-old human crystalline lens from a previous study31, which is similar to the age bracket of the subjects in the current study, resulting in approximately 10% of VL being transmitted through the human crystalline lens. This data suggested that the VL spectral transmittance reaching the retina would be different between the Artisan and Artiflex groups even though VL transmission is reduced by the crystalline lens. Although we speculated that chorioretinal tissue could be one of the targets of VL exposure19, other tissues, including the crystalline lens, should also be considered. Further investigation on the target tissues of VL in addition to the choroid and retina should be conducted in the future.
Refractions in the peripheral retinal area have been considered a controversial issue for myopia progression32–34 and shapes of the eye differ by individual. We simulated the refractions in the peripheral retinal area in the same eye model (Fig. 3A,B). However, we found no differences in peripheral hyperopic defocus between the two pIOLs. Though we did not measure the real peripheral hyperopic defocus of these patients, these simulation results implicated that the differences in myopic progression and axial length elongation between the two pIOLs did not depend on the difference of the peripheral hyperopic defocus.
There are some limitations in the current study. First, the sample size is small though it is estimated sufficient according to the statistical assumption. Second, the differences in factors other than the spectral transmittance, such as postoperative pupil diameter, refractive index, lens material, and spherical aberration30 between the two pIOLs (Table 2) were not evaluated. These parameters should be investigated in the future. Third, other confounding factors in myopia such as outdoor time7,8,17, time spent in near work35, and parental myopia36 were not included in the current study. Further investigation is being considered on those confounding factors in our future studies. Fourth, the ARTISAN® pIOL was indicated when the anterior chamber depth was from 2.8 to 3.2 mm (7 out of 11 cases), which is one of the selection biases in the current study.
In summary, though there are no effective methods to retard adult high myopia and axial length elongation, the introduction of VL exposure may contribute to the reduction of myopic progression and axial length elongation even for adult humans with high myopia. Violet light may be one of the potent candidates for prevention of myopia, especially for sight-threatening high myopia.
Methods
Comparison in the myopic progression and axial length elongation of adult human between the non-violet-light transmitting pIOL (ARTISAN®) group and the violet light transmitting pIOL (ARTIFLEX®) group
We retrospectively evaluated the change of refraction and the axial length elongation after iris-fixated pIOL implantation and compared them between two pIOLs, ARTISAN® (Ophtec BV) and ARTIFLEX® (Ophtec BV) (Fig. 1A,B). The Keio University School of Medicine Ethics Committee approved the clinical study. All procedures involving human subjects were performed in accordance with the tenets of the Declaration of Helsinki. Written informed consents were obtained from all patients after they received the explanation. The ARTISAN® pIOL has a convex-concave polymethyl methacrylate (PMMA) optic with a 6.0-mm optical zone and PMMA haptics. The ARTIFLEX® pIOL has a convex-concave silicone optic with a 6.0-mm optical zone and PMMA haptics. The refractive indices were 1.49 for the ARTISAN® pIOL and 1.43 for the ARTIFLEX® pIOL. The ARTISAN® pIOL transmits minimal VL whereas the ARTIFLEX® pIOL transmits more VL (Fig. 1C). Twenty-six eyes of 26 Japanese patients with myopia exceeding −6.00 D were followed for over a five-year period after pIOL implantation at Keio University Hospital. One experienced surgeon (K.N.) performed all pIOL implantations. They were divided into two groups: the Artisan group, the pIOL group with 11 eyes of 11 patients (mean age, 39.9 ± 8.9 years) implanted with ARTISAN® pIOLs; and the Artiflex group, the pIOL group with 15 eyes of 15 patients (mean age, 36.3 ± 7.2 years) implanted with ARTIFLEX® pIOLs. The primary outcome measures were the differences in the refraction and axial length elongation over a five-year period after implantation of the pIOLs between the two groups. The patient preoperative data is shown in Table 1. There were no significant differences in preoperative age, cycloplegic refractive value, axial length, uncorrected visual acuity and best-corrected visual acuity between the two groups. The inclusion criteria for both groups were a minimum age of 25 years with myopia exceeding −6.00 D; no previous ocular surgery, uveitis, cataracts, diabetic retinopathy, corneal diseases, glaucoma, or a history of ocular trauma. The inclusion criteria for pIOL implantation was a sufficiently deep anterior chamber (2.8 mm or more for the ARTISAN® pIOL and 3.2 mm or more for the ARTIFLEX® pIOL), and an adequate endothelial cell count (2,000 cells/mm2 or higher). Regarding the selection of a pIOL, the ARTISAN® pIOL was indicated when the anterior chamber depth was from 2.8 to 3.2 mm (7 out of 11 cases). When both pIOLs were indicated, the patients selected the pIOL after they received an explanation of the differences in the incision size, lens materials, long-term results, and complications between the ARTISAN® and ARTIFLEX® pIOLs. The exclusion criteria for both groups were active ocular or systemic disease likely to affect wound healing, pregnancy, and nursing mothers. No intraoperative or postoperative complications developed and no enhancements were performed. Objective refraction was measured by autorefractor (ARK-700A, NIDEK) five times, and we used the mean data for analysis. The axial length was measured five times preoperatively and postoperatively using the phakic mode of the IOLMaster (Carl Zeiss Meditec), and we used the mean data for analysis. Surgical technique and preoperative and postoperative treatment were the same as previously described37.
Spectral transmission of pIOLs
The UV-visible absorption spectra of these samples were recorded with a UV-2600 spectrophotometer (Shimadzu Corporation, Kyoto, Japan) (Fig. 1C).
HOAs and residual astigmatism
Each measurement of the corneal, internal, and ocular HOAs were recorded 5 years post-operatively using the OPD-Scan (Nidek Co., Aichi, Japan). The most reliable data (i.e., the well-delineated line of the pupillary edge, clear Placido rings, and the absence of the eyelid edge on the cornea in the map of the eye image) were adopted as the final results based on the image quality. Corneal HOAs originate from the anterior cornea; internal HOAs originate from the lens, the pIOL, and the posterior corneal surface. All wavefront aberrations were calculated and plotted with respect to the corneal vertex. The corneal topography was measured using Placido disk technology, and the ocular wavefront was measured using the principle of skiascopic phase difference.
The coefficients of Zernike polynomials were determined up to the sixth order for 4-mm optical zones from the wavefront data. All measurements were performed under maximal mydriasis induced by eye drops containing 0.5% tropicamide and 0.5% phenylephrine hydrochloride (Mydrin-P; Santen Pharmaceutical Co., Osaka, Japan); the pupillary diameters during measurements exceeded 4 mm in all patients. The root mean squares (RMSs) of the total HOAs from the third- to sixth-order Zernike coefficients were calculated.
Residual astigmatism was the subjective astigmatism 5 years postoperatively. The astigmatism vector was decomposed into vertical/horizontal (J0) and oblique (J45) components using the power vector analysis described by Thibos et al.38. The conversion to power vector notation was performed using the following equations38, where C is the negative cylindrical power and α is the cylinder axis: J0 = (−C/2) cos (2α), J45 = (−C/2) sin (2α).
Simulation of peripheral refraction after pIOL implantation
The focusing images of the pIOLs on the peripheral parts of the retina were calculated using Liou-Brennan eye model39 and ZEMAX optics design software (Radiant Zemax, Washington, USA). The paraxial focus position of the pIOLs with −10 D power was obtained and was 23.05 mm from the back surface of the crystalline lens at the wavelength of 546.1 nm. The retinal spherical surface with the radius of −12.0 mm was set and the defocus amounts for the incident angles of 0, 10, 20, 30, 40, 45 degrees were calculated at the pupil of 4 mm diameter. Each focus is presented by the seven light rays as shown in Fig. 3A,B.
Statistical analysis
A Mann-Whitney U Test was used to compare data between the two groups for the clinical study. A P value of less than 0.05 was considered significant. All statistical analyses were performed using IBM SPSS Statistics version 21.0 (IBM Corp, New York, USA).
The sample size calculation indicated that a sample size of at least 8 cases would be required (a minimum acceptable reduction of a difference of 0.25 mm over the five-year period, so 8 cases in each group were needed to have a 5% alpha level and 95% power).
Acknowledgements
We thank S. Shioya for assistance with measuring spectral transmission of the phakic intraocular lenses; C. Oshima for editing the manuscript; Y. Shigeno, H. Nakayama, and M. Kato for assistance in inputting the data.
Author Contributions
H.T., K.N., and K.T. designed the study. K.N. performed all refractive surgeries in this study. K.O. performed simulation results of peripheral refraction after pIOL implantation. H.T. analyzed the data. H.T., K.O., T.K., K.T., and K.N. wrote and revised the manuscript.
Competing Interests
The authors (H.T., T.K., K.T., K.N.) are in the process of applying for a patent (PCT/JP2015/65997) for potential products for myopia suppression.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Holden BA, et al. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology. 2016;11:00025–00027. doi: 10.1016/j.ophtha.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Dolgin E. The myopia boom. Nature. 2015;519:276–278. doi: 10.1038/519276a. [DOI] [PubMed] [Google Scholar]
- 3.Ip JM, et al. Role of near work in myopia: findings in a sample of Australian school children. Invest Ophthalmol Vis Sci. 2008;49:2903–2910. doi: 10.1167/iovs.07-0804. [DOI] [PubMed] [Google Scholar]
- 4.Read SA, Collins MJ, Vincent SJ. Light exposure and physical activity in myopic and emmetropic children. Optom Vis Sci. 2014;91:330–341. doi: 10.1097/OPX.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 5.French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013;114:58–68. doi: 10.1016/j.exer.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Guggenheim JA, et al. Time outdoors and physical activity as predictors of incident myopia in childhood: a prospective cohort study. Invest Ophthalmol Vis Sci. 2012;53:2856–2865. doi: 10.1167/iovs.11-9091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rose KA, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology. 2008;115:1279–1285. doi: 10.1016/j.ophtha.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Jones LA, et al. Parental history of myopia, sports and outdoor activities, and future myopia. Invest Ophthalmol Vis Sci. 2007;48:3524–3532. doi: 10.1167/iovs.06-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones-Jordan LA, et al. The contributions of near work and outdoor activity to the correlation between siblings in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study. Invest Ophthalmol Vis Sci. 2014;55:6333–6339. doi: 10.1167/iovs.14-14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.French AN, Morgan IG, Mitchell P, Rose KA. Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study. Ophthalmology. 2013;120:2100–2108. doi: 10.1016/j.ophtha.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 11.Jin JX, et al. Effect of outdoor activity on myopia onset and progression in school-aged children in northeast China: the Sujiatun Eye Care Study. BMC ophthalmology. 2015;15:73. doi: 10.1186/s12886-015-0052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tkatchenko TV, et al. Photopic visual input is necessary for emmetropization in mice. Exp Eye Res. 2013;115:87–95. doi: 10.1016/j.exer.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norton TT, Siegwart JTJ. Light levels, refractive development, and myopia–a speculative review. Exp Eye Res. 2013;114:48–57. doi: 10.1016/j.exer.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith EL, III, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012;53:421–428. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karouta C, Ashby RS. Correlation between light levels and the development of deprivation myopia. Invest Ophthalmol Vis Sci. 2015;56:299–309. doi: 10.1167/iovs.14-15499. [DOI] [PubMed] [Google Scholar]
- 16.Stone RA, Pardue MT, Iuvone PM, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013;114:35–47. doi: 10.1016/j.exer.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He M, et al. Effect of Time Spent Outdoors at School on the Development of Myopia Among Children in China: A Randomized Clinical Trial. Jama. 2015;314:1142–1148. doi: 10.1001/jama.2015.10803. [DOI] [PubMed] [Google Scholar]
- 18.Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120:1080–1085. doi: 10.1016/j.ophtha.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Torii H, et al. Violet Light Exposure Can Be a Preventive Strategy Against Myopia Progression. Ebiomedicine. 2017;15:210–219. doi: 10.1016/j.ebiom.2016.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CIE. International Standard CEI/IEC 62471 (CIE S 009: 2002) First edition 2006–07 edn, 89 (International Electrotechnical Commission, 2006).
- 21.Krutmann J, et al. Towards standardization of UV eye protection: what can be learned from photodermatology? Photodermatology, photoimmunology & photomedicine. 2014;30:128–136. doi: 10.1111/phpp.12089. [DOI] [PubMed] [Google Scholar]
- 22.McBrien NA, Adams DW. A longitudinal investigation of adult-onset and adult-progression of myopia in an occupational group. Refractive and biometric findings. Invest Ophthalmol Vis Sci. 1997;38:321–333. [PubMed] [Google Scholar]
- 23.Saka N, et al. Changes of axial length measured by IOL master during 2 years in eyes of adults with pathologic myopia. Graefes Arch Clin Exp Ophthalmol. 2013;251:495–499. doi: 10.1007/s00417-012-2066-9. [DOI] [PubMed] [Google Scholar]
- 24.Dick HB, et al. Foldable Artiflex phakic intraocular lens for the correction of myopia: two-year follow-up results of a prospective European multicenter study. Ophthalmology. 2009;116:671–677. doi: 10.1016/j.ophtha.2008.12.059. [DOI] [PubMed] [Google Scholar]
- 25.Huang D, et al. Phakic intraocular lens implantation for the correction of myopia: a report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:2244–2258. doi: 10.1016/j.ophtha.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 26.Stulting, R. D. et al. Three-year results of Artisan/Verisyse phakic intraocular lens implantation. Results of the United States Food And Drug Administration clinical trial. Ophthalmology115, 464–472.e461, Epub 2007 Nov 2026 (2008). [DOI] [PubMed]
- 27.Tahzib, N. G., Nuijts, R. M., Wu, W. Y. & Budo, C. J. Long-term study of Artisan phakic intraocular lens implantation for the correction of moderate to high myopia: ten-year follow-up results. Ophthalmology114, 1133–1142, Epub 2007 Feb 1131 (2007). [DOI] [PubMed]
- 28.Benedetti S, Casamenti V, Benedetti M. Long-term endothelial changes in phakic eyes after Artisan intraocular lens implantation to correct myopia: five-year study. J Cataract Refract Surg. 2007;33:784–790. doi: 10.1016/j.jcrs.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 29.Coullet, J. et al. Iris-supported phakic lenses (rigid vs foldable version) for treating moderately high myopia: randomized paired eye comparison. Am J Ophthalmol142, 909–916 Epub 2006 Aug 2031 (2006). [DOI] [PubMed]
- 30.Torii H, et al. Changes in Higher-Order Aberrations After Iris-Fixated Phakic Intraocular Lens Implantation. Journal of refractive surgery (Thorofare, NJ: 1995) 2013;29:693–700. doi: 10.3928/1081597X-20130816-01. [DOI] [PubMed] [Google Scholar]
- 31.Artigas JM, Felipe A, Navea A, Fandino A, Artigas C. Spectral transmission of the human crystalline lens in adult and elderly persons: color and total transmission of visible light. Invest Ophthalmol Vis Sci. 2012;53:4076–4084. doi: 10.1167/iovs.12-9471. [DOI] [PubMed] [Google Scholar]
- 32.Smith EL, 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49:2386–2392. doi: 10.1016/j.visres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sankaridurg P, et al. Spectacle lenses designed to reduce progression of myopia: 12-month results. Optom Vis Sci. 2010;87:631–641. doi: 10.1097/OPX.0b013e3181ea19c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hasebe S, Jun J, Varnas SR. Myopia control with positively aspherized progressive addition lenses: a 2-year, multicenter, randomized, controlled trial. Invest Ophthalmol Vis Sci. 2014;55:7177–7188. doi: 10.1167/iovs.12-11462. [DOI] [PubMed] [Google Scholar]
- 35.Lee YY, Lo CT, Sheu SJ, Lin JL. What factors are associated with myopia in young adults? A survey study in Taiwan Military Conscripts. Invest Ophthalmol Vis Sci. 2013;54:1026–1033. doi: 10.1167/iovs.12-10480. [DOI] [PubMed] [Google Scholar]
- 36.Gong, Y., Zhang, X., Tian, D., Wang, D. & Xiao, G. Parental myopia, near work, hours of sleep and myopia in Chinese children. Health2014 (2014).
- 37.Torii, H. et al. Myopic Regression After Phakic Intraocular Lens Implantation and LASIK. Optom Vis Sci, doi:10.1097/opx.0000000000000136 (2013). [DOI] [PubMed]
- 38.Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74:367–375. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 39.Liou HL, Brennan NA. Anatomically accurate, finite model eye for optical modeling. J Opt Soc Am A Opt Image Sci Vis. 1997;14:1684–1695. doi: 10.1364/JOSAA.14.001684. [DOI] [PubMed] [Google Scholar]