Abstract
Habituation of wild great apes for tourism and research has had a significant positive effect on the conservation of these species. However, risks associated with such activities have been identified, specifically the transmission of human respiratory viruses to wild great apes, causing high morbidity and, occasionally, mortality. Here, we investigate the source of bacterial-viral co-infections in wild and captive chimpanzee communities in the course of several respiratory disease outbreaks. Molecular analyses showed that human respiratory syncytial viruses (HRSV) and human metapneumoviruses (HMPV) were involved in the etiology of the disease. In addition our analysis provide evidence for coinfection with Streptococcus (S.) pneumoniae. Characterisation of isolates from wild chimpanzees point towards a human origin of these bacteria. Transmission of these bacteria is of concern because – in contrast to HRSV and HMPV - S. pneumoniae can become part of the nasopharyngeal flora, contributing to the severity of respiratory disease progression. Furthermore these bacteria have the potential to spread to other individuals in the community and ultimately into the population. Targeted vaccination programs could be used to vaccinate habituated great apes but also human populations around great ape habitats, bringing health benefits to both humans and wild great apes.
Subject terms: Zoology, Conservation biology, Bacterial infection
Introduction
Due to their close genetic relationship, humans and other great apes share susceptibility to a considerable number of pathogens1,2 with the transmission of human respiratory pathogens to captive apes being a well-documented concern3–11. In this context, it had been long suspected that pathogen transmission from humans might account for disease outbreaks observed in wild great apes habituated to human presence for research and tourism7,12–14. This suspicion was confirmed when common human respiratory paramyxoviruses (human respiratory syncytial virus (HRSV) and human metapneumovirus (HMPV)) were identified in chimpanzee (Pan troglodytes verus) lung tissue and faeces collected during separate disease outbreaks in Taï National Park (Côte d’Ivoire)15,16. Further studies on wild chimpanzees in Tanzania (Pan troglodytes schweinfurthii) and gorillas (Gorilla beringei) in Rwanda and the Central African Republic have demonstrated additional incidences in which human respiratory paramyxoviruses have been transmitted to great apes indicating that these events are neither isolated nor a feature of a particular region (Table 1)17–19.
Table 1.
Characteristics and analyses of respiratory disease outbreaks among chimpanzees in Taï National Park (Côte d’Ivoire) and Limbe Wildlife Center (Cameroon).
| Location | Group | Year | Morbidity | Mortality | Samples collected | HRSV | HMPV | S. pneumoniae (Screening PCR) | S. pneumoniae (Culture) | S. pneumoniae (MLST) |
|---|---|---|---|---|---|---|---|---|---|---|
| Taï National Park | South Group | 2009 | 86% | 6*/37 | Lung tissue (n = 6) | Type A | negative | Positive | accomplished (n = 2) | accomplished (n = 2; ST 8485) |
| Limbe Wildlife Center | Group 1 | 2006 | 100% | None | Pharyngeal swab (n = 2) | negative | Type A2 | Positive | not tested | not tested |
| 2007 | 23% | None | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| Group 2 | 2006 | 29% | None | Pharyngeal swab (n = 3) | negative | Type A2 | Positive | not tested | not tested | |
| 2007 | 100% | None | Pharyngeal swab (n = 1) | Type B | negative | Positive | not tested | not tested | ||
| Group 3 | 2007 | 100% | 1/4 | Lung tissue (n = 1) | Type B | negative | Positive | not tested | accomplished (n = 1; ST 8949) |
*Refers to the number of individuals where necropsies had been performed. Further five animals were found dead but necropsies were not performed due to the destruction of the carcass by scavengers or logistical reasons. Additionally three animals disappeared during the outbreak and are considered victims of the epidemic.
n.s. not sampled.
In addition to human viruses, the bacterium Streptococcus pneumoniae (or pneumococcus) was also detected during outbreaks where samples of lung tissue had been collected15,18,20, leading to the conclusion that it was the combination of respiratory viruses and pneumococci, and not the viral infection alone, that caused the fatalities15,16. Given that invasive pneumococcal infections are also a primary cause of severe pneumonia in humans worldwide21,22, these bacterial co-infections are a cause for concern since they could lead to increased mortality and persist in the great ape populations.
However the provenance of the pneumococci strains from the great ape cases mentioned above remains uncertain. Sequence analyses have so far only been performed on S. pneumoniae positive chimpanzee samples from Taï National Park and these showed differences to known human sequences20,23. Unfortunately, there is a lack of data on regional human pneumococci sequence types and therefore transmission from humans could not be confirmed20.
In this article we investigate respiratory disease outbreaks causing mortalities among wild and captive wild-borne great apes in West and western-Central Africa. Again, HRSV and HMPV were detected together with pneumococci and we provide evidence that all were of human origin. These results are important since, in contrast to human respiratory viruses, pneumococci may become an established part of the great ape oropharyngeal flora and could severely impact the outcome of subsequent respiratory infections or, as in humans, become the primary cause of severe pneumonia.
Results and Discussion
Samples were collected in the course of several respiratory disease outbreaks that occurred at two different locations: in the Limbe Wildlife Center (LWC) situated in Cameroon, and in Taï National Park (TNP), Côte d’Ivoire.
The LWC is a wildlife rehabilitation project in which rescued great apes are kept in naturalistic enclosures. Here, a total of five distinct outbreaks of respiratory disease occurred over a period of two years (2006/2007), in three separate chimpanzee groups (see Table 1): group 1 consisted of 22 individuals (age >5years), group 2 of 14 individuals between two and five years of age and group 3 consisted of four individuals of less than two years of age. The chimpanzees showed varying clinical symptoms (coughing, serous to mucopurulent nasal discharge, lethargy, inappetence) over the course of the outbreaks. Symptoms indicative of lower respiratory tract infection, such as dyspnoea and wheezing sounds, were noted on auscultation of the lungs of 12 individuals. Duration of the outbreaks ranged from several days up to nine weeks. Morbidity ranged from 23 to 100% (with 100% in three of the five outbreaks; see Table 1). During these outbreaks pharyngeal swabs from six chimpanzees were taken under anesthesia. During one outbreak an infant chimpanzee (referred to as LWC infant) died, a necropsy was performed and tissue samples were taken. Throughout the outbreaks, animals with severe symptoms were placed on antiobiotic therapy (see Supplementary Information).
In TNP, wild chimpanzees are habituated to the presence of human observers and individually known as a result of a project focusing on wild chimpanzee behaviour24. Here, three chimpanzee communities (so called North, East and South community) are under observation. In November 2009 the South community was affected by a respiratory disease outbreak. Morbidity and mortality were high: 32/37 chimpanzees developed severe respiratory symptoms (including high frequency of heavy cough, lethargy, nasal discharge). Four adults (>15 years; hereinafter referred to as TNP adult 1–4) and two infants (0–5 years, referred to as TNP infant 1 and 2) were found shortly after death and necropsies were performed. In addition, for one chimpanzee (adult) only one arm and the head was found due to scavenging. Four infants were found dead but necropsies were not performed and three further adults disappeared in the course of the outbreak and are likely victims of the outbreak as well. In total likely 8 adults and 6 infant chimpanzees died in this outbreak. Twelve out of the 37 individuals who showed severe symptoms of respiratory disease were treated with Extencilline (Benzathine benzylpenicillin, Sanofi-Aventis France), a long acting antibiotic administered by remote injection. Of these nine survived.
Histopathology
Histopathological examination of lung tissue samples from TNP and LWC chimpanzees revealed a severe purulent bronchopneumonia as cause of disease. The bronchoalveolar tissue was diffusely infiltrated with neutrophilic granulocytes and macrophages. Furthermore, all lung tissue samples collected during the TNP outbreak showed multinucleated cell syncytia, pointing towards an HRSV infection (Fig. 1). Additionally, gram-positive cocci could be demonstrated within the altered tissue giving evidence for a secondary bacterial infection. In addition, the lung tissue of one animal (TNP adult 3) revealed signs of a lung mite infection (likely Pneumonyssus simicola). The lung tissue from the LWC infant showed a multifocal to diffuse purulent bronchopneumonia with marginal alveolar histiocytosis. The periodic acid schiff (PAS) reaction was negative for all samples excluding a fungal infection.
Figure 1.
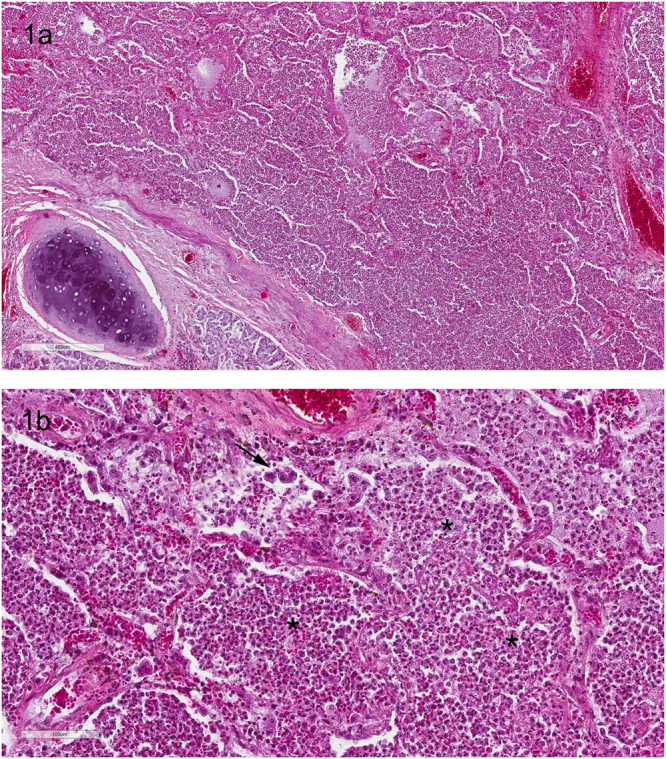
(a,b) Histopathology from lung of a chimpanzee from TNP, purulent bronchopneumonia. (1a) overview showed parts of a bronchus in the lower left and lung alveoli completely filled with exudate. Scale bare 400 µm. (1b) The exudate in the alveoli (asterisk) is composed of neutrophilic granulocytes, histiocytes and fibrin, the arrow points on cell syncytia, scale bar 100 µm. Hematoxylin and Eosin stain.
Molecular analysis
All samples collected from the TNP and LWC outbreaks were analysed molecularly by screening PCR and tested positive for either HMPV- or HRSV-specific genes as well as for the lytA gene harboured by S. pneumoniae (see Table 1). Phylogenetic analyses of generated paramyxovirus sequences revealed that both HRSV and HMPV sequences (Genbank accession no. JX489496- JX489498) belonged to recent pandemic waves (see Supplementary Figs 1–3). Informing a relaxed molecular clock model with tip dates25 we estimated that the LWC chimpanzee-infecting HMPV diverged from its closest human-infecting relatives in 2004 (95% highest posterior density: 2003–2004). The date of divergence between the HRSV-A strain from TNP chimpanzees and humans was 2007 (95% highest posterior density: 2006–2008) and for HRSV-B strains infecting LWC chimpanzees and humans 2007 (95% highest posterior density: 2007) These results mirror findings from previous outbreaks15–19 and emphasize the high incidence of anthropozoonotic transmission of human respiratory paramyxoviruses.
In order to gain new insights on the bacterial community present in the lung samples collected during the outbreak in TNP, we performed 16S rRNA PCR. Characterization of the sequences using a BLAST search in GenBank indicated the presence of Streptococcus species in all samples analysed. In addition, for one individual (TNP adult 3) Pasteurella (P.) multocida was also among the predominant BLAST hits. P. multocida is a commensal in the upper respiratory tracts of many domestic and wild animal species worldwide and coinfection with P. multocida and S. pneumoniae among TNP chimpanzees has been reported previously16,20,26. In addition, for the samples from TNP adult 1, the BLAST search revealed Clostridium sp. Since most species of the genus Clostridium are saprophytic, we conclude that this finding was due to the tissue samples being partly decayed.
Molecular analysis of the S. pneumoniae is considered separately in the next section.
Phenotypic and molecular characterisation of S. pneumoniae
Isolation of pneumococci was achieved for TNP adult 1 and 2 (isolate IMT 113 and 115) from whom lung tissue samples had been stored adequately in Skim Milk-Tryptone-Glucose-Glycerol (STGG). Antibiotic susceptibility testing (Table 2) showed low resistance: isolates were resistant against sulfamethoxazole/trimethoprim (TMP-SMX) only. Further characterisation by Multi Locus Sequence Typing (MLST, see Table 3) indicated that alleles from isolates termed IMT 113 and 115 were identical and matched known alleles from human strains. Their allelic profile revealed 6 matches to various published serotype 19 A strains from Kenya and the Gambia (>100 isolates) but harbour a variant ddl allele. Therefore a new sequence type (ST 8485) was assigned (see Table 2). Serotyping of the TNP samples by PCR also revealed capsular type 19 A.
Table 2.
Resistance pattern of pneumococcus isolates present in lung tissue of deceased TNP chimpanzees (adults 1 and 2).
| Drug tested | IMT 113 | IMT 115 |
|---|---|---|
| chloramphenicol | S | S |
| enrofloxacin | S | S |
| oxacillin | S | S |
| sulfamethoxazole/trimethoprime | R | R |
| clarithromycin | S | S |
| erythromycin | S | S |
| vancomycin | S | S |
| clindamycin | S | S |
| tetracycline | S | S |
Table 3.
Allelic profile of S. pneumoniae originating from chimpanzee lung tissue compared to closely related human isolates from the MLST data base.
| Isolate/DNA sample (country of origin) | Allel number for gene fragment | ST | Serotype | ||||||
|---|---|---|---|---|---|---|---|---|---|
| aroE | gdh | gki | recP | spi | xpt | ddl | |||
| IMT113/115 (TNP, Côte d’Ivoire) | 7 | 11 | 4 | 1 | 6 | 112 | 8 | 8485* | 19 A |
| 107 isolates (Kenia and Gambia) | 7 | 11 | 4 | 1 | 6 | 112 | 14 | 847 | 19 A |
| PVT0032 (Gambia) | 7 | 11 | 4 | 1 | 6 | 112 | 15 | 3326 | 19 A |
| LWC infant (LWC, Cameroon) | 177 | 134 | 4 | 18 | 9 | 83 | 18 | 8949 | n.d. |
| KSA08 (Chad) | 177 | 134 | 4 | 18 | 9 | 83 | 18 | 8949 | 9 V |
Samples analysed in this study are written in bold.
n.d. not detected.
*New ST.
Lung samples from the LWC infant had not been stored adequately for cultivation. Therefore, a full DNA extract of this sample was used for further characterization. Hence, MLST results are based on “virtual clones” (see methods). For the LWC sample all seven alleles matched known alleles from human strains and indeed, allelic profiles were identical to a serotype 9 V strain isolated from a human of African origin (see Table 2). DNA from the LWC sample was tested for capsular type 9 V27 but tested negative. In addition we tried another 9 V/9 A primer combination described by Carvalho et al.28 but again the DNA tested negative. Unfortunately due to lack of DNA material it was not possible to test for further serotypes.
In summary, the MLST profiles of the strains described here showed high similarities or were even identical to human isolates, especially in Africa. In addition, results from the antibiotic susceptibility testing (Table 2) showed that the isolates from TNP chimpanzees were resistant to TMP-SMX. Resistance to TMP-SMX, which has been used widely in developing countries, is observed frequently and globally in human strains of S. pneumoniae29. The fact that this drug has never been used in the chimpanzees of TNP supports the assumption that the S. pneumoniae strain found originated in humans.
S. pneumoniae infection, especially in combination with respiratory viruses, can lead to invasive lower respiratory disease with fatal outcomes. Furthermore, and in contrast to respiratory virus infections, which are usually temporary, S. pneumonia infection in humans can result in an asymptomatic colonization of the nasopharynx. Additionally this bacterium can spread horizontally and remain silent within a population30. In fact, pneumococcal disease in humans will not occur without preceding nasopharyngeal colonization30, hence an increased rate of colonization within a population will increase the incidence of invasive pneumococcal disease. To investigate whether the TNP chimpanzee population are ‘carriers’ of S. pneumoniae, we investigated a throat swab obtained in 2011 during a necropsy on an adult female chimpanzee living in TNP (named “XENA”; East group). This chimpanzee had died from an anthrax-like disease31 independent of any respiratory disease outbreak. Since no isolate of S. pneumoniae could be obtained, we tested full DNA for the presence of the new variant of the capsule 3 (cps3Taï) described in chimpanzees that died between 2004 and 200623 and performed MLST analyses. Indeed, the sample was positive for cps3Taï. MLST revealed several sequences with close matches to S. pneumoniae but also S. oralis and S. mitis. The finding of mixed sequences of oral commensal bacteria was not unexpected since we used a throat swab (instead of a pure culture of S. pneumoniae) for examination. However, exact matches to all sequences characterizing the ‘South clone’ ST 2309 (found in the lung tissue of deceased TNP chimpanzees which died in respiratory disease outbreaks in TNP in 2004 and 2006), have been amongst the sequences obtained. This provides preliminary evidence that this ST may have circulated within the chimpanzee community for at least five years.
Conclusion
Here we provide the first evidence that S. pneumoniae can be transmitted and that the origin of this pathogen might be human. S. pneumoniae has the potential to circulate over long periods of time in a given population (a finding supported by our data on the chimpanzee XENA) and may cause severe disease once an individual is weakened by further primary viral infections or through other circumstances30,32. Given that adolescent female chimpanzees migrate to other communities and that other inter-troop encounters occur, the reported findings could have serious implications, not only in respect to the clinical outcome of individuals infected, but also for populations of wild chimpanzees33. In addition, the risk of ex-captive chimps transmitting human S. pneumoniae into wild populations is a serious concern for reintroduction programs.
These results underline the importance of hygiene measures, as established in TNP and described in the new IUCN guidelines, in limiting the risk of pathogen transmission between humans and great apes34. Moreover these results underscore the importance of developing non-invasive sample collection methods to assess the risk of the spread of such pathogens to wild chimpanzee and other great ape communities. We note that, characterisation of S. pneumoniae present in human communities in contact to or neighbouring wild great ape populations are needed to allow further conclusions regarding naturally occurring, and introduced, infections. Given the availability of vaccines that provide protection against the serotypes most frequently associated with pneumococcal disease in humans, characterisation of the distribution of serotypes would be of great interest. Such data will inform the design of vaccination campaigns targeting human-habituated great apes but also human populations in contact with wild great apes, providing enormous benefits to both human and wild animal health.
Material and Methods
Sampling
Necropsies on chimpanzees were conducted under high safety standards and precautions such as protection suits, gloves, and face masks were used to avoid contamination of samples with human pathogens. At LWC pharyngeal swabs were collected from six animals under sedation. All samples were preserved in RNAlater (LWC) or liquid nitrogen (TNP) and shipped to Germany for detailed analyses. No experiments on live animals were performed for this study, samples were collected from animals found dead or sedated for treatment necessary to save their lives, and therefore no animal ethics permission is required according to the national laws of Ivory Coast and Cameroon.
Histology
Tissue samples from five of the six deceased chimpanzees from TNP on which necropsies had been performed, and from the deceased chimpanzee from LWC were submitted to the German Primate Centre (Göttingen, Germany) for histopathological examination. All samples were fixed in 4% formalin, embedded in paraffin, cut in 2–4 µm tissue sections and stained with hematoxylin and eosin for microscopic examination. Tissue was also stained with Giemsa and Gram stain and to visualize fungal infection furthermore PAS reaction was performed.
DNA/RNA extraction and cDNA synthesis
Nucleic acids from lung tissue were extracted using the Nucleo Spin Tissue - and Nucleo Spin RNA II Kit (Macherey-Nagel, Düren, Germany), according to the manufacturer’s instructions. Swab samples stored in RNAlater were vortexed vigorously and RNA and DNA were extracted simultaneously from 280 µl of RNAlater using the QIAamp viral Mini Kit (Qiagen, Hilden, Germany). cDNA was synthesized by using the Superscript Kit (Invitrogen, Karlsruhe, Germany) and random hexamer primers (TIB Molbiol, Berlin, Germany).
PCR screening for respiratory pathogens and phylogenetic analysis
DNA or cDNA respectively were screened for influenza A&B, HMPV, HRSV, Adeno-, Picornavirus and S. pneumoniae using PCR assays as described previously35–40. For phylogenetic analysis, the G gene of HMPV or HRSV was amplified using published PCR assays17,41 PCR products were purified using ExoSAP (USB Europe GmBH, Staufen, Germany) and sequenced subsequently using the ABI Big Dye Termination Kit (Applied Biosystems, Weiterstadt, Germany). Phylogenetic analyses of the paramyxovirus sequences were performed in a Bayesian framework. First, data sets were assembled that comprised one exemplary sequence per outbreak (-year) generated in this study (see supplementary information for details) and human HMPV or HRSV G gene sequences extracted from publicly availably full genomes with known isolation dates (retrieved from NCBI). Both data sets were aligned using MUSCLE42, as implemented in SeaView, version 443, and reduced to only include unique sequences using FaBox44. Conserved alignment blocks were selected using gblocks (also implemented in SeaView). Three final alignments were generated: HMPV with 129 sequences and 711 positions, HRSV-A with 284 sequences and 968 positions and HRSV-B with 139 sequences and 954 positions. To determine the appropriate nucleotide substitution model, we estimated model likelihoods with jModeltest v2.1.1045 and compared them using the Bayesian information criterion (BIC). The selected models were GTR + G for HMPV, GTR + I + G for HRSV-A and HKY + G for HRSV-B. Bayesian analyses were run in BEAST v1.8.246 assuming a longnormal-relaxed clock and a constant population size. Three chains were run for 100 million generations; convergence of the runs and appropriate sampling of the posterior were assessed using Tracer v1.647. Post burn-in trees from the three chains were combined using LogCombiner v1.8.2 before being summarized onto the maximum clade credibility tree identified with TreeAnnotator v1.8.2 (both software programs are distributed with BEAST). Branch robustness was assessed through posterior probabilities.
16s rDNA Libraries from lung tissues
For a first screening for relevant bacteria, DNA from lung tissue samples from the TNP cases were tested using a 16s PCR48. Insufficient material was available for these analyses to be performed on the samples from the LWC infant. Amplicons were cloned into a pCR™ 2.1-TOPO® vector (Invitrogen, Karlsruhe, Germany) and colony PCR was performed on ten different clones (per sample) using M13 primers. PCR products were purified using ExoSAP (USB Europe GmBH, Staufen, Germany) and sequenced using the ABI Big Dye Termination Kit (Applied Biosystems, Weiterstadt, Germany). Sequences were compared to sequences contained in Genbank using the BLAST algorithm (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Characterization of S. pneumoniae
Isolation
Lung tissue preserved in STGG had been collected from TNP adult 1 and 2 and was streaked on tryptic soy yeast extract (TSYE) agar supplemented with 5% of defibrinated sheep blood (Oxoid, Wesel, Germany) and incubated overnight in 5% CO2. S. pneumoniae was identified by colony morphology, α-hemolysis and optochin sensitivity and confirmed by PCR analysis as described below. Isolates from TNP adult 1 are registered under the number IMT 113 and the ones from TNP adult 2 as IMT 115.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed using the agar diffusion test according to the standards given by the Clinical and Laboratory Standards Institute49,50. The antimicrobial agents tested included chloramphenicol (30 μg), clindamycin (2 μg), oxacillin (1 µg, sulfamethoxazole/trimethoprime (1, 25/23, 75 μg), tetracycline (30 μg), enrofloxacin (5 µg), erythromycin (15 µg), clarithromycin (15 µg) vancomycin (30 µg) (Becton Dickinson, Heidelberg, Germany).
Multi Locus Sequence Typing (MLST)
For MLST analysis, DNA was extracted from overnight cultures (TNP isolate IMT 113 and 115) or directly from lung tissue from the LWC sample using the Nucleo Spin Tissue Kit (Macherey & Nagel, Stadt, Land) according to the manufacturer’s instructions. PCR analyses were performed using standard primers and protocols given on the Streptococcus pneumoniae MLST website (http://pubmlst.org/spneumoniae) using a proof reading polymerase (Promega, Berlin, Germany). PCR fragments of the seven housekeeping genes adh, gdh, gki, spi, recP, xpt and ddl were obtained for all samples.
Amplicons from the TNP isolates were purified directly using ExoSAP (USB Europe GmBH, Staufen, Germany) and sequenced using the ABI Big Dye Termination Kit (Applied Biosystems, Weiterstadt, Germany). For the sample from LWC (DNA from lung tissue instead of a pure culture) MLST results were generated based on “virtual clones”: to minimize the possibility that two or multiple pneumococcus strains were involved, each of the seven amplicons was cloned into a pCR™ 2.1-TOPO® vector (Invitrogen, Karlsruhe, Germany). For every cloning reaction, ten clones were picked and colony PCR was performed using M13 primers. PCR products were purified and sequenced as described above. For every allele, sequences were checked for consistency using Genious version 7.1.4 (http://www.geneious.com)51.
Sequences obtained were entered in the MLST database and allelic numbers were assigned (all alleles were already present in the database). Since the TNP isolates showed a new allelic combination i.e. differed in one locus (ddl), a new ST was assigned by the curator (Table 2).
Serotyping PCR
Serotyping was done by PCR according to Pai et al.27. However, due to a shortage of DNA material, we did not follow the sequential multiplex PCR assay described, but rather chose serotype specific primers according to the serotype information given for human strains in the MLST database that matched our clones the most. DNA from TNP bacterial isolates were tested using the primers specific for capsule type 19 A. DNA from the LWC lung sample was tested for serotype 9 V but tested negative. In addition a 9 V/9 A primer combination described by Carvalho et al.28 was tried however the DNA tested negative. Further serotype testing was not possible due to a lack of DNA.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on request.
Accessions code
Sequences data on the HRSV and HMPV strains are available in Genbank under the accession number JX489496 - JX489498.
Electronic supplementary material
Acknowledgements
We thank the Ivorian authorities for their long-term support, especially the Ministry of the Environment and Forests as well as the Ministry of Research, the Office Ivorien des Parcs et Reserves, and the director of the Taï National Park. We are grateful to the Centre Suisse de Recherches Scientifiques and the staff of the Taï Chimpanzee Project. We also thank the according authorities from Cameroon. For sequencing we thank the staff of the RKIs sequencing laboratory. This work was supported by the ARCUS Foundation, the Robert Koch-Institute and the Max-Planck-Institute for evolutionary anthropology.
Author Contributions
S.K. performed the molecular experiments and analysed the data. K.G. and V.K. assisted in the molecular characterisation of the pneumococci. K.N. and A.L.-B. carried out the bacteriological analyses. S.K. and S.C. analysed the phylogenetic data. K.M.R. performed the histopathology. K.N., S.M., J.K., T.D., and F.L. participated in outbreak investigation and sample collection. S.K., F.L. and F.H.L. wrote the paper with input from all co-authors.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/29/2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-14769-z.
References
- 1.Leendertz FH, et al. Pathogens as drivers of population declines: The importance of systematic monitoring in great apes and other threatened mammals. Biol. Cons. 2006;131:325–337. [Google Scholar]
- 2.Calvignac-Spencer S, Leendertz SA, Gillespie TR, Leendertz FH. Wild great apes as sentinels and sources of infectious disease. Clin. microbiol. Infect. 2012;18:521–527. doi: 10.1111/j.1469-0691.2012.03816.x. [DOI] [PubMed] [Google Scholar]
- 3.Dick EC. Experimental infections of chimpanzees with human rhinovirus types 14 and 43. Proc. Soc. Exp. Biol.Med. 1968;127:1079–1081. doi: 10.3181/00379727-127-32875. [DOI] [PubMed] [Google Scholar]
- 4.Kalter, S. S. Primates viruses–their significance in Viral and immunological diseases in nonhuman primates: proceedings of a symposium, use of nonhuman primates in exotic viral and immunological disease, Feb 28-Mar 31982, 67–89 (1983).
- 5.Jones EE, et al. Predisposition to invasive pneumococcal illness following parainfluenza type 3 virus infection in chimpanzees. J. Am. Vet. Med. Assoc. 1984;185:1351–1353. [PubMed] [Google Scholar]
- 6.Kalter SS. Infectious diseases of nonhuman primates in a zoo setting. Zoo Biol. 1989;8:61–76. [Google Scholar]
- 7.Bennett, B. T., Abee, C. R. & Henrickson, R. Diseases in Nonhuman primates in biomedical research. (London: Academic Press, 1998).
- 8.Skiadopoulos MH, et al. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J. Virol. 2004;78:6927–6937. doi: 10.1128/JVI.78.13.6927-6937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szentiks CA, Köndgen S, Silinski S, Speck S, Leendertz FH. Lethal pneumonia in a captive juvenile chimpanzee (Pan troglodytes) due to human-transmitted human respiratory syncytial virus (HRSV) and infection with Streptococcus pneumoniae. J. Med. Primatol. 2009;38:236–240. doi: 10.1111/j.1600-0684.2009.00346.x. [DOI] [PubMed] [Google Scholar]
- 10.Unwin S, Chatterton J, Chantrey J. Management of severe respiratory tract disease caused by human respiratory syncytial virus and Streptococcus pneumoniae in captive chimpanzees (Pan troglodytes) J. Zoo. Wildl. Med. 2013;44:105–115. doi: 10.1638/1042-7260-44.1.105. [DOI] [PubMed] [Google Scholar]
- 11.Slater OM, et al. Human metapneumovirus infection in chimpanzees, United States. Emerg. Infect. Dis. 2014;20:2115–2118. doi: 10.3201/eid2012.140408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe ND, et al. Wild primate populations in emerging infectious disease research: the missing link? Emerg. Infect. Dis. 1998;4:149–158. doi: 10.3201/eid0402.980202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallis J, Rick Lee D. Primate Conservation: The Prevention of Disease Transmission. Int. J. Primatol. 1999;20:803–826. [Google Scholar]
- 14.Woodford MH, Butynski TM, Karesh WB. Habituating the great apes: the disease risks. Oryx. 2002;36:153–160. [Google Scholar]
- 15.Köndgen S, et al. Pandemic human viruses cause decline of endangered great apes. Curr. Biol. CB. 2008;18:260–264. doi: 10.1016/j.cub.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Köndgen S, Schenk S, Pauli G, Boesch C, Leendertz FH. Noninvasive monitoring of respiratory viruses in wild chimpanzees. EcoHealth. 2010;7:332–341. doi: 10.1007/s10393-010-0340-z. [DOI] [PubMed] [Google Scholar]
- 17.Kaur T, et al. Descriptive epidemiology of fatal respiratory outbreaks and detection of a human-related metapneumovirus in wild chimpanzees (Pan troglodytes) at Mahale Mountains National Park, Western Tanzania. Am. J. Primatol. 2008;70:755–765. doi: 10.1002/ajp.20565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palacios G, et al. Human metapneumovirus infection in wild mountain gorillas, Rwanda. Emerg. Infect. Dis. 2011;17:711–713. doi: 10.3201/eid1704.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grützmacher KS, et al. Codetection of Respiratory Syncytial Virus in Habituated Wild Western Lowland Gorillas and Humans During a Respiratory Disease Outbreak. EcoHealth. 2016;13:499–510. doi: 10.1007/s10393-016-1144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi F, et al. New Streptococcus pneumoniae clones in deceased wild chimpanzees. J. Bacteriol. 2007;189:6085–6088. doi: 10.1128/JB.00468-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasi F, Mantero M, Santus P, Tarsia P. Understanding the burden of pneumococcal disease in adults. Clin. Microbiol. Infect. 2012;18(Suppl 5):7–14. doi: 10.1111/j.1469-0691.2012.03937.x. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien KL, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 23.Denapaite D, Hakenbeck R. A new variant of the capsule 3 cluster occurs in Streptococcus pneumoniae from deceased wild chimpanzees. PloS one. 2011;6:e25119. doi: 10.1371/journal.pone.0025119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boesch, C. & Boesch-Achermann, H. The chimpanzees of the Tai Forest: behavioural ecology and evolution. (Oxford University Press, 2000).
- 25.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Köndgen S, et al. Pasteurella multocida involved in respiratory disease of wild chimpanzees. PloS one. 2011;6:e24236. doi: 10.1371/journal.pone.0024236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J. Clin. Microbiol. 2006;44:124–131. doi: 10.1128/JCM.44.1.124-131.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.da Gloria Carvalho M, et al. Revisiting pneumococcal carriage by use of broth enrichment and PCR techniques for enhanced detection of carriage and serotypes. J. Clin. Microbiol. 2010;48:1611–1618. doi: 10.1128/JCM.02243-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karpanoja P, et al. Connection between trimethoprim-sulfamethoxazole use and resistance in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob. Agents Chemother. 2008;52:2480–2485. doi: 10.1128/AAC.01118-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann C, et al. Persistent anthrax as a major driver of wildlife mortality in a tropical rainforest. Nature. 2017;548:82–86. doi: 10.1038/nature23309. [DOI] [PubMed] [Google Scholar]
- 32.Zhou H, et al. Invasive pneumococcal pneumonia and respiratory virus co-infections. Emerg. Infect. Dis. 2012;18:294–297. doi: 10.3201/eid1802.102025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nunn CL, Thrall PH, Stewart K, Harcourt AH. Emerging infectious diseases and animal social systems. Evol. Ecol. 2008;22:519–543. [Google Scholar]
- 34.Gilardi, K. V. et al. Best practice guidelines for health monitoring and disease control in great ape populations. Occasional Papers of the IUCN Species Survival Commission56 pp. (2015).
- 35.Chmielewicz B, Nitsche A, Schweiger B, Ellerbrok H. Development of a PCR-based assay for detection, quantification, and genotyping of human adenoviruses. Clin. Chem. 2005;51:1365–1373. doi: 10.1373/clinchem.2004.045088. [DOI] [PubMed] [Google Scholar]
- 36.McAvin JC, et al. Sensitive and specific method for rapid identification of Streptococcus pneumoniae using real-time fluorescence PCR. J. Clin. Microbiol. 2001;39:3446–3451. doi: 10.1128/JCM.39.10.3446-3451.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pusch D, et al. Detection of enteric viruses and bacterial indicators in German environmental waters. Arch. Virol. 2005;150:929–947. doi: 10.1007/s00705-004-0467-8. [DOI] [PubMed] [Google Scholar]
- 38.Reiche J, et al. Human metapneumovirus: insights from a ten-year molecular and epidemiological analysis in Germany. PloS one. 2014;9:e88342. doi: 10.1371/journal.pone.0088342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiche J, Schweiger B. Genetic variability of group A human respiratory syncytial virus strains circulating in Germany from 1998 to 2007. J. Clin. Microbiol. 2009;47:1800–1810. doi: 10.1128/JCM.02286-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulze M, Nitsche A, Schweiger B, Biere B. Diagnostic approach for the differentiation of the pandemic influenza A(H1N1)v virus from recent human influenza viruses by real-time PCR. PloS one. 2010;5:e9966. doi: 10.1371/journal.pone.0009966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato M, et al. Molecular epidemiology of respiratory syncytial virus infections among children with acute respiratory symptoms in a community over three seasons. J. Clin. Microbiol. 2005;43:36–40. doi: 10.1128/JCM.43.1.36-40.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gouy M, Guindon S, Gascuel O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 44.Villesen P. FaBox: an online toolbox for fasta sequences. Mol. Ecol. Notes. 2007;7:965–968. [Google Scholar]
- 45.Posada D. jModelTest: phylogenetic model averaging. Mol. Biol. Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- 46.Drummond AJ, Suchard MA, Xie D, Rambaut A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rambaut, A., Suchard, M. A., Xie, D. & Drummond, A. J. Tracer v1.6, Available from http://beast.bio.ed.ac.uk/Tracer. (2014).
- 48.Mugisha L, Köndgen S, Kaddu-Mulindwa D, Gaffikin L, Leendertz FH. Nasopharyngeal colonization by potentially pathogenic bacteria found in healthy semi-captive wild-born chimpanzees in Uganda. Am. J. Primatol. 2014;76:103–110. doi: 10.1002/ajp.22212. [DOI] [PubMed] [Google Scholar]
- 49.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated From Animals, 3rd Edition. CLSI document VET01SEd3. Wayne, PA (2015).
- 50.Clinical and Laboratory Standards Institute. M100-S26. Performance standards for antimicrobial susceptiblity testing: 26th informational supplement. Wayne, PA (2016).
- 51.Kearse M, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on request.


