Abstract
Background:
Previous findings suggested that bevacizumab might be able to improve response rate (RR) in colorectal cancer patients with high lactic dehydrogenase (LDH) basal levels.
Methods:
We conducted a phase II trial to prospectively ascertain whether bevacizumab in combination with FOLFIRI could have an improved clinical activity in patients with high LDH serum levels. Primary end point of the study was RR; secondary end points were median overall survival and median progression-free survival (mPFS).
Results:
A total of 81 patients were enrolled. No difference in terms of ORR (39% vs 31% for low vs high LDH level stratum, P=0.78) and mPFS (14.16 vs 10.29 months, HR: 1.07, 95% CI: 0.51–2.24, P=0.83) between the strata was observed, whereas overall survival (OS) was significantly longer for patients with low LDH (24.85 vs 15.14 months, HR: 4.08, 95% CI: 1.14–14.61, P=0.0004). In a not-pre-planned exploratory analysis using different cut-off ranges for LDH, we observed RR up to 70%, with no improvement in progression-free survival or OS.
Conclusions:
The CENTRAL trial failed to demonstrate that high LDH levels were related to a significantly improved RR in patients receiving first-line FOLFIRI and bevacizumab. The LDH serum levels should then no further be investigated as a predictive factor in this setting.
Keywords: bevacizumab, colorectal cancer, LDH, predictive factors
The introduction of different treatment options (Fakih, 2015) in the therapeutic scenario of metastatic colorectal cancer (CRC) patients (Aprile et al, 2015) led to a substantial improvement in prognosis during the past few years. To date, however, no preferred first-line therapy has been identified. On the one hand we can now exclude from anti-EGFR treatment patients with putative refractory colorectal tumours (i.e., those harbouring a RAS mutant status), but on the other hand we are still lacking any predictive marker of response in this setting. Furthermore, although tumour-driven angiogenesis is a key target for metastatic CRC, no effective clinical or biological biomarker has been yet validated for patients receiving antiangiogenic therapy (Giampieri et al, 2014). Most of the data investigating molecular predictive factors in CRC patients receiving bevacizumab in combination with chemotherapy (Hurwitz et al, 2004; Saltz et al, 2008; González-Vacarezza et al, 2015; Pinto et al, 2016) suggested a potential role for the hypoxia-induced angiogenesis model. The hypoxia-inducible factor 1-α has a crucial function as transcription factor upregulating the neoangiogenesis process and influencing the transcription of several glycolytic enzymes such as lactic dehydrogenase (LDH) (Maxwell et al, 2001). As a consequence high LDH levels might be an indirect sign of activated tumour angiogenesis (Tas et al, 2001a; Tas et al, 2001b; Faloppi et al, 2016). In fact, high LDH serum levels besides indicating a diffuse metastatic involvement affecting prognosis (Koukourakis et al, 2005; Wu et al, 2010), can also indicate VEGF-A and VEGF receptor 1 overexpression (Azuma et al, 2007).
We previously analysed the role of LDH pretreatment serum levels in metastatic CRC patients receiving first-line chemotherapy doublet with or without the addition of bevacizumab (Scartozzi et al, 2012). Median progression-free survival (mPFS) in patients treated without bevacizumab, stratified for high and low LDH levels, was 4.2 vs 8 months (P=0.0003), and median overall survival (mOS) was 19.6 and 34.9, respectively (P=0.0014). In the bevacizumab-treated group, partial responses (PRs) were seen in 14 (58%) LDH-high and 8 (14%) LDH-low patients (P=0.0243), mPFS was 7.3 and 8.5 months, respectively (P=0.2) and mOS was 22 and 26.6 months, respectively (P=0.7).
On the basis of these findings we suggested that high LDH levels were correlated with worse prognosis and that bevacizumab seemed able to improve clinical outcome in this specific group of patients that usually present with an adverse natural history. The improved response rate (RR) also suggests a role for LDH as a predictive marker. A prospective validation of these results was lacking and might be relevant for the clinical practice.
Patients and methods
The CENTRAL trial was a prospective phase II non-randomised clinical trial with a up-front biomarker stratification. Patients treated with first-line FOLFIRI and bevacizumab were prospectively stratified according to LDH serum levels. The aim of the trial was to prospectively ascertain whether bevacizumab in combination with chemotherapy could have an improved clinical activity in patients with high LDH serum levels compared to patients with normal LDH serum levels. Primary end point was RR; secondary end points were mPFS, mOS and toxicity profile.
All consecutive histologically proven metastatic CRC patients receiving first-line chemotherapy with FOLFIRI and bevacizumab, as per Italian label, were eligible for our study. All patients were prospectively stratified according to LDH serum levels upon study entry. The cut-off value for high vs low LDH serum levels was set at 1.2 ULN based on our previous report (Scartozzi et al, 2012). Treatment was administered as follows: irinotecan 180 mg m−2 IV infusion on day 1; leucovorin 200 mg m−2 IV followed by 5-fluorouracil 400 mg m−2 IV bolus and 5-fluorouracil 600 mg m−2 IV continuous infusion over 22 h on days 1 and 2, every 2 weeks in combination with bevacizumab 5 mg kg−1 on IV infusion on day 1 every 2 weeks. Dose reductions and supportive care as for local guidelines. Response rate was assessed every 12 weeks according to the Response Evaluation Criteria in Solid Tumors 1.1 (RECIST 1.1) criteria. Treatment was administered until progression, patients’ withdrawal of consent and unacceptable toxicity. Toxicities were evaluated according to National Cancer Institute-Common Toxicity Criteria 4.0 at every chemotherapy cycle. All patients receiving at least one cycle were considered evaluable for toxicity.
This study was performed in accordance with the study protocol, the ethical principles stated in the Declaration of Helsinki as well as those indicated in the International Conference on Harmonization (ICH) Note for Guidance on Good Clinical Practice (GCP; ICH E6, 1995), and all applicable regulatory requirements. All patients had to sign a written informed consent before study entry. Adequate information was given to eligible patients by the principal investigator or co-investigators at each participating centres and in accordance with local regulations. Written informed consent to participate in the clinical study had to be given before any study-related activities were carried out. The declaration of informed consent was personally signed and dated by the subject, and by the investigator/person designated by the investigator to conduct the informed consent discussion.
Statistical considerations
To detect a difference in terms of RR among patients with high LDH serum levels (estimated around 15–20%) and patients with normal LDH serum levels (estimated around 50–55%), assuming a probability alpha of 0.10 and beta of 0.10, required sample size was 80 patients.
Statistical analysis was performed with the MedCalc Statistical Software version 14.10.2 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014). For statistical analysis, RR was defined as the proportion of patients who achieved a complete response (CR) or PR according to the RECIST criteria, overall survival (OS) was defined as the time interval between the treatment start date and death, or last follow-up visit for patients lost at follow-up, whereas progression-free survival (PFS) was defined as the interval between the treatment start date and death, first sign of clinical progression or last follow-up visit for patients lost at follow-up. The association between categorical variables was estimated by χ2-test. Survival distribution was estimated by the Kaplan–Meier method. Significant differences in probability of survival between the strata were evaluated by log-rank test. A significant level of 0.05 was chosen to assess the statistical significance.
For exploratory analyses receiver-operating characteristic (ROC) curve analysis was used to individuate the optimal LDH cut-off value in our population.
Results
Patients’ baseline characteristics
A total of 81 patients were enrolled at 18 sites in Italy: 49 males and 32 females, median age was 65 years (34–80); 80% (65/81) had Eastern Cooperative Group Performance Status (ECOG PS) 0, whereas 20% (16/81) had ECOG PS 1; 67% (54/81) had metastatic disease at diagnosis, whereas 33% (27/81) had locally advanced disease at the time of diagnosis. Seventy-five per cent (61 out of 81) and 25% (20 out of 81) had colon and rectal primary tumours, respectively (Table 1).
Table 1. Patients and tumour baseline characteristics.
| Patients | 81 |
| Gender | |
| Male | 49 |
| Female | 32 |
| Mean age (years) | 62.86 |
| Median age (range) | 65 (34–80) |
| Performance status | |
| 0 | 80% (65 out of 81) |
| 1 | 20% (16 out of 81) |
| Site of primary tumour | |
| Sigma-colon | 75% (61 out of 81) |
| Rectum | 25% (20 out of 81) |
| Metastases (M) | |
| 0 | 33% (27 out of 81) |
| 1 | 67% (54/81) |
| Site of metastasis | |
| Liver | 38 |
| Liver+other | 18 |
| Lymph nodes | 6 |
| Lung | 10 |
| Other sites | 9 |
Treatment outcome and safety
In the global population 9% of the patients (7 out of 81) achieved CR, 30% (24 out of 81) PR, 43% (35 out of 81) stable disease, 11% (9/81) experienced progressive disease (PD) at 12 weeks, and 7% (6 out of 81) did not undergo radiological evaluation (1 patient died for causes other than cancer, 2 patients withdrew their consent after study enrolment, 1 patient stopped treatment for unacceptable toxicity and 2 patients stopped treatment for medical causes other than cancer). At 2-year follow-up, 52 out of 81 (64%) patients enrolled in the study had progressed and mPFS was 12.98 months (95% CI: 8.0–16.06); 24 out of 81 (30%) patients had died and mOS was 24.52 months (95% CI:20.75–25.47). Most common grade 3–4 adverse events reported were diarrhoea (11.4%), neutropenia (17% febrile neutropenia: 7%) and others 11%.
Stratification according to LDH levels
Among enrolled patients 47% (38 out of 81) had high LDH levels, whereas 43% (35 out of 81) had low LDH levels. In 8 patients (10%) LDH level was not assessed at the time of study entry.
Primary end point
Overall RR in the high LDH level stratum and in the low LDH level stratum was 39% (15 out of 38) vs 31% (11 out of 35) (P=0.78).
Secondary end points
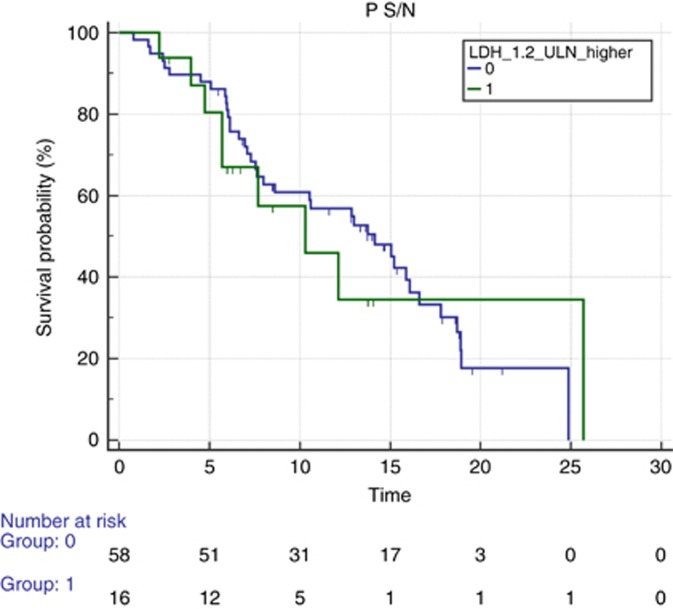
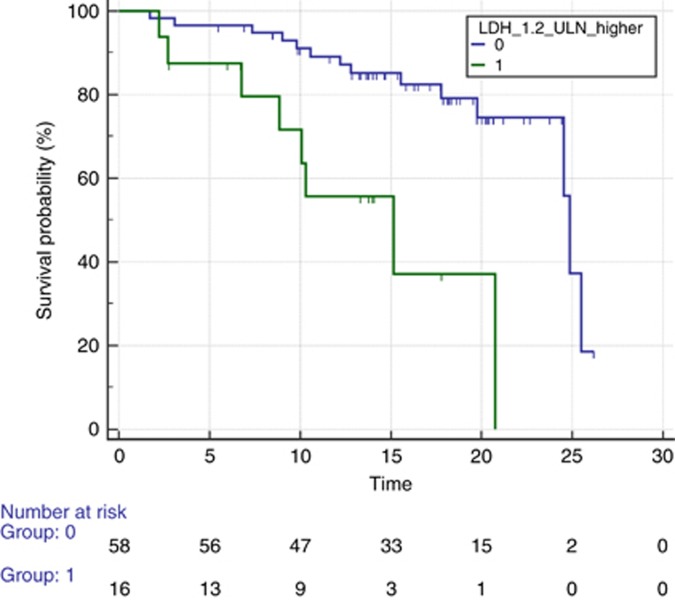
There was no difference in terms of PFS between the two groups (mPFS 14.16 vs 10.29 months for low vs high, HR: 1.07, 95% CI: 0.51–2.24, P=0.83; Figure 1). On the contrary, we observed a significantly longer median post-progression survival after first line (3.14 vs 17.4 months, HR: 2.61, 95% CI: 0.74–9.20, P=0.004), and OS was significantly longer for patients with low LDH vs those who had high LDH (mOS 24.85 vs 15.14, HR: 4.08, 95% CI: 1.14–14.61, P=0.0004; Figure 2).
Figure 1.
Median progression-free survival according to LDH levels. Median progression-free survival (PFS) for patients receiving first-line FOLFIRI and bevacizumab prospectively stratified according to LDH low (blue line) or high (green line) serum levels. Median PFS was 14.16 vs 10.29 months for patients with low vs high LDH (HR: 1.07, 95% CI: 0.51–2.24, P=0.83). A full colour version of this figure is available at the British Journal of Cancer journal online.
Figure 2.
Median overall survival according to LDH levels. Median overall survival (OS) for patients receiving first-line FOLFIRI and bevacizumab prospectively stratified according to LDH low (blue line) or high (green line) serum levels. Median OS was 24.85 vs 15.14 for patients with low LDH vs high LDH (HR: 4.08, 95% CI: 1.14–14.61, P=0.0004). A full colour version of this figure is available at the British Journal of Cancer journal online.
Unplanned exploratory analysis according to different LDH cut-off levels
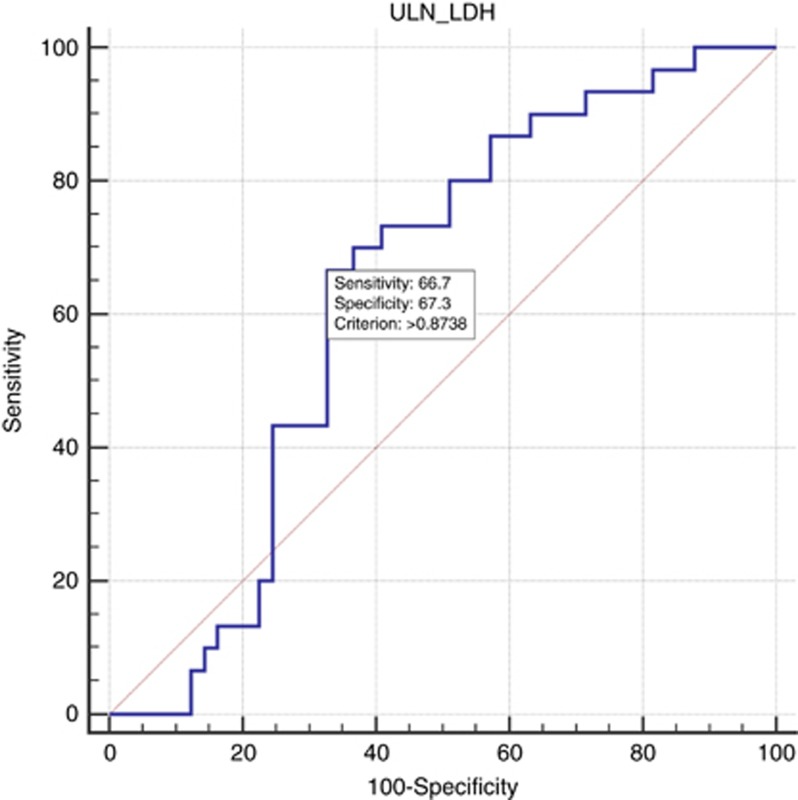
The ROC curve analysis indicated that ULN × 0.87 had the best Youden index (0.34) with a sensitivity of 67% and a specificity of 67% (Figure 3).
Figure 3.
Receiver-operating characteristics analysis based on pretreatment LDH serum levels. Receiver-operating characteristic (ROC) analysis based on pretreatment LDH serum levels results with OS as end point. In this model, sensitivity was 66.7% (95% CI: 40.7–82.8) and specificity was 67.3% (95% CI: 71.9–93.1). The area under the curve was 0.610, P=0.0942. A full colour version of this figure is available at the British Journal of Cancer journal online.
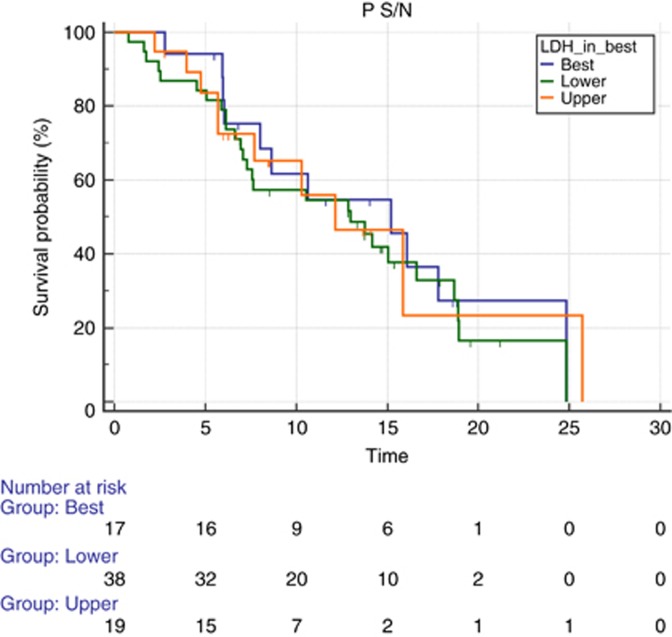
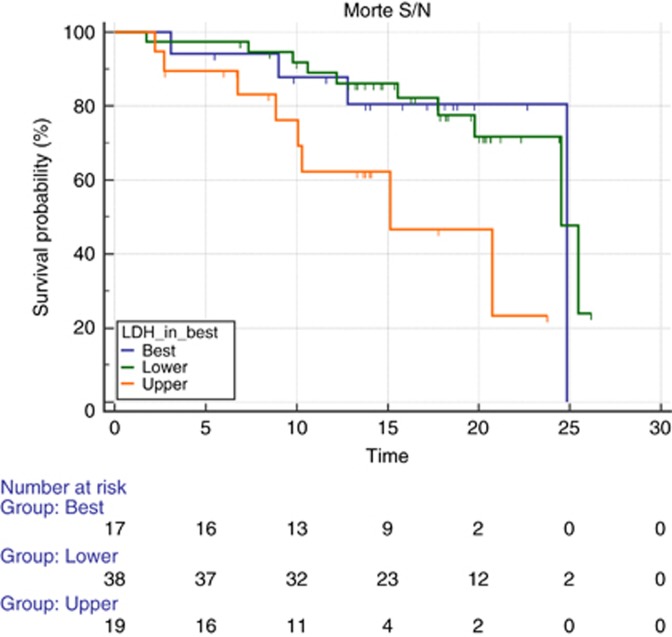
In all, 12 out of 17 patients (70%) with LDH serum levels between ULN × 0.87 and ULN × 1 (17 out of 81, 21% of the global population) showed a response to treatment (CR and PR). However, this did not translate into a significantly different PFS among patients showing LDH levels below ULN × 0.87 vs ULN × 0.87–ULN × 1 vs those showing LDH levels above ULN × 1 (mPFS 12.98 vs 15.21 vs 12.13 months, P=0.87; Figure 4). Patients with LDH-high serum levels (>ULN × 1) experienced a worse survival than patients with LDH serum levels <0.87 ULN or patients with LDH between ULN × 0.87 and ULN × 1 (mOS 24.52 vs 24.85 vs 15.14 months, P=0.02; Figure 5).
Figure 4.
Median PFS according to different levels of LDH. Median PFS according to three different levels of LDH: lower (green line) vs intermediate (blue line) vs upper (orange line). Progression-free survival was 12.98 for lower vs 15.21 for intermediate vs 12.13 for upper LDH (P=0.87). A full colour version of this figure is available at the British Journal of Cancer journal online.
Figure 5.
Median OS according to different levels of LDH. Median OS according to three different levels of LDH: lower (green line) vs intermediate (blue line) vs upper (orange line). Overall survival was 24.52 and 24.58 months for patients with lower levels of LDH and intermediate values of LDH vs 15.14 months for patients with LDH greater than ULN × 1 (P=0.02). A full colour version of this figure is available at the British Journal of Cancer journal online.
Discussion
Our trial failed to prospectively confirm that LDH serum levels above ULN × 1.2 were related to significantly improved RR in metastatic colorectal patients receiving first-line treatment with FOLFIRI and bevacizumab.
A selection bias deriving from factors such as tumour burden, RAS/B-RAF status and site of primary metastatic involvement (liver vs lung vs both vs others) might have confounded the impact of high LDH levels as predictor of improved RR during chemotherapy in combination with bevacizumab. In particular, the relatively high number of patients experiencing CR during treatment in our study was mainly dependant on the fraction of patients (10 out of 81, 12%) with isolated small-lung metastases, a subset of patients reportedly to have a particularly favourable outcome. Furthermore, the reported mOS for the whole population (24.5 months) was not completely in line with recent studies suggesting more encouraging figures for this parameter. The mPFS (12.98 months) was on the contrary comparable to available data in similar case series (Stintzing et al, 2016; Bennouna et al, 2017). Accordingly, to our findings a recently published meta-analysis (Li et al, 2016) showed that high LDH levels might be related to an adverse impact on OS, with a small positive predictive impact on PFS. Similar results were also published by Silvestris et al (2015) in metastatic CRC patients receiving first-line bevacizumab in combination with FOLFIRI. On the one hand the authors of this study confirmed that high LDH levels were related to a worse OS and on the other hand they also suggested an OS improvement in patients showing a decrease in LDH serum levels during treatment.
A recent analysis (Marmorino et al, 2017) indicated that high LDH levels seemed more likely to predict a worse outcome in patients receiving beyond-progression administration of bevacizumab in combination with chemotherapy, thus suggesting a more evident negative impact of LDH values on post-progression survival, such as in our study.
Participating centres contributed to patients’ enrolment blindly (except for the coordinating centre) in accordance to the pre-defined cut-off value. Therefore, we believe that the hypothesis of a process of ‘selection’ of patients with an increased/decreased likelihood of response based on their disease status, rather than effectively on the LDH level, can be excluded.
The trial might have failed its primary end point as a consequence of a sub-optimal LDH serum levels’ cut-off value choice (for LDH: to solve this issue, we compared our results to other trials that tried to assess the role of high LDH and potential efficacy of antiangiogenic drugs).
In the CONFIRM-1 (Hecht et al, 2011) and CONFIRM-2 (Van Cutsem et al, 2011) trials, although the addition of PTK/ZK to first- and second-line FOLFOX failed to improve OS and PFS in metastatic CRC patients, patients with levels of LDH above ULN × 1.5 also had improved survival outcomes.
In our unplanned exploratory analysis we observed an impressively high RR in patients showing LDH serum levels between ULN × 0.87 and ULN × 1. In addition, when we performed a ROC curve analysis for PD in this population and used ULN × 1.2 as cut-off, sensitivity for PD increased. Taken together these findings might suggest that the role of LDH as potential predictive marker is likely to be offset by its prognostic function particularly when high levels of LDH are observed.
In a previously published analysis from our group (Del Prete et al, 2015) treatment outcome was adversely affected by high LDH levels in patients receiving third- and forth-line regorafenib for metastatic CRC, thus suggesting a potential negative prognostic effect in heavily pretreated patients as well. This seems in line with the results of our study in terms of OS, suggesting once again a much stronger role of LDH as negative prognostic factor rather than as positive predictive factor.
Finally, different LDH isoforms might have different functions in this setting. Serum LDH is the result of the sum of circulating levels of at least five different isoforms. Although LDH is an enzyme that catalyses the same chemical reaction of H+ release from lactic acid molecule, not all isoforms are inducible as a result of a hypoxic insult and not all isoforms catalyse the reaction similarly. These considerations may partly explain some of the results of our analysis. We can hypothesise that for patients with relevant tumour burden but with an adequate tumour blood (and then oxygen) supply, high LDH levels are not dependent on hypoxia but may better reflect the overall tumour burden with consequential prognostic implications. On this basis, it would be interesting to verify whether LDH-5 isoform levels, which have been more closely linked to hypoxia, are related to patients outcome during antiangiogenic treatment. Intriguingly data from the CONFIRMS trials suggested that LDH-5 expression was significantly related to worse prognosis, but this prognostic effect was reverted in patients receiving the antiangiogenic drug vatalanib (Koukourakis et al, 2011).
The present prospective study failed to confirm retrospective data suggesting that LDH serum levels might have a predictive role during bevacizumab treatment in metastatic CRC patients.
As a consequence LDH serum levels should not be further investigated as a predictive factor.
On the basis of our findings and those from other authors we confirm the strong correlation between high LDH serum levels and prognosis in this group of patients. We then suggest that LDH serum levels besides representing a potentially relevant stratification factor for clinical trials for metastatic CRC patients, should also be part of the basic clinical assessment in the clinical practice with the aim to better define overall prognosis.
Acknowledgments
The study is sponsored by Fondazione GISCAD that provided the economical support for costs related to data management, statistical analysis and the other activities of central and group coordinating centres. Roche S.p.A supported in minor part and partial refunded the study expenses, providing economical support for costs related to data management. RG, MP, BD, DF, AZ, LC, GR, NP, MGZ, PS, DG, CC, ML, RL, SC and MS are all part of the Italian Group for the Study of Gastrointestinal Cancer (GISCAD).
Disclaimer
Roche S.p.A had no role in the design of the study, data collection, data analysis or interpretation of the findings.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The authors declare no conflict of interest.
References
- Aprile G, Rihawi K, Scartozzi M, Bordonaro R (2015) Steps ahead in the treatment of advanced colorectal cancer: past, current and possible future scenarios. Future Oncol 11: 2625–2628. [DOI] [PubMed] [Google Scholar]
- Azuma M, Shi M, Danenberg KD, Gadner H, Barrett C, Jacques CJ, Sherod A, Igbal S, El-Khoueiry A, Yand D, Zhang W (2007) Serum lactate dehydrogenase levels and glycolysis significantly correlate with tumor VEGFA and VEGFR expression in metastatic CRC patients. Pharmaco-genomics 8: 1705–1713. [DOI] [PubMed] [Google Scholar]
- Bennouna J, Phelip JM, André T, Asselain B, Sébastien K, Ducreux M (2017) Observational cohort study of patients with metastatic colorectal cancer initiating chemotherapy in combination with bevacizumab (CONCERT). Clin Colorectal Cancer 16: 129–140. [DOI] [PubMed] [Google Scholar]
- Del Prete M, Giampieri R, Loupakis F, Tiziana Prochilo T, Salvatore L, Faloppi, Bianconi M, Bittoni A, Aprile G, Zaniboni A, Falcone A, Scartozzi M, Cascinu S (2015) Prognostic clinical factors in pretreated colorectal cancer patients receiving regorafenib: implications for clinical management. Oncotarget 6: 33982–33992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakih MG (2015) Metastatic colorectal cancer: current state and future directions. J Clin Oncol 33: 1809–1824. [DOI] [PubMed] [Google Scholar]
- Faloppi L, Bianconi M, Memeo R, Casadei Gardini A, Giampieri R, Bittoni A, Andrikou K, Del Prete M, Cascinu S, Scartozzi M (2016) Lactate dehydrogenase in hepatocellular carcinoma: something old, something new. Biomed Res Int 2016: 7196280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampieri R, Scartozzi M, Del Prete M, Fulli A, Faloppi L, Bianconi M, Maccaroni E, Cascinu S (2014) The ‘angiogenetic ladder’, step-wise angiogenesis inhibition in metastatic colorectal cancer. Cancer Treat Rev 40: 934–941. [DOI] [PubMed] [Google Scholar]
- González-Vacarezza N, Alonso I, Arroyo G, Martínez J, De Andrés F, LLerena A, Estévez-Carrizo F (2015) Predictive biomarkers candidates for patients with metastatic colorectal cancer treated with bevacizumab-containing regimen. Drug Metab Pers Ther 31: 2363–8907. [DOI] [PubMed] [Google Scholar]
- Hecht JR, Trarbach T, Hainsworth JD, Major P, Jager E, Wolff RA, Lloyd- Salvant K, Bodoky G, Pendergrass K, Berg W, Chen BL, Jalava T, Meinhardt G, Laurent D, Lebwohl D, Kerr D (2011) Randomized, placebo-controlled, phase III study of first-line oxaliplatin-based chemotherapy plus PTK787/ ZK222584, an oral vascular endothelial growth factor receptor inhibitor, in patients with metastatic colorectal adenocarcinoma. J Clin Oncol 20: 1997–2003. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F (2004) Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 350: 2335–2342. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E (2005) Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metast 22: 25–30. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Giatromanolaki A, Sivridis E, Gatter KC, Trarbach T, Folprecht G (2011) Prognostic and predictive role of lactate dehydrogenase 5 expression in colorectal cancer patients treated with PTK787/ZK 222584 (vatalanib) antiangiogenic therapy. Clin Cancer Res 15: 4892–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Wang Z, Xu J, Wu H, Cai S, He Y (2016) The prognostic value of lactate dehydrogenase levels in colorectal cancer: a meta-analysis. BMC Cancer 16: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmorino F, Salvatore L, Barbara C, Allegrini G, Antonuzzo L, Masi G, Loupakis F, Borelli B, Chiara S, Banzi MC, Miraglio E, Amoroso D, Dargenio F, Bonetti A, Martignetti A, Paris M, Tomcikova D, Boni L, Falcone A, Cremolini C (2017) Serum LDH predicts benefit from bevacizumab beyond progression in metastatic colorectal cancer. Br J Cancer 116: 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH, Pugh CW, Ratcliffe PJ (2001) Activation of the HIF pathway in cancer. Curr Opin Genet Dev 11: 293–299. [DOI] [PubMed] [Google Scholar]
- Pinto C, Antonuzzo L, Porcu L, Aprile G, Maiello E, Masi G, Petrelli F, Scartozzi M, Torri V, Barni S (2016) Efficacy and safety of bevacizumab combined with fluoropyrimidine monotherapy for unfit or older patients with metastatic colorectal cancer: a systematic review and meta-analysis. Clin Colorectal Cancer 16: e61–e72. [DOI] [PubMed] [Google Scholar]
- Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J (2008) Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 26: 2013–2019, Erratum in J Clin Oncol (2009) 27:653. [DOI] [PubMed] [Google Scholar]
- Scartozzi M, Giampieri R, Maccaroni E, Del Prete M, Faloppi L, Bianconi M, Galizia E, Loretelli C, Belvederesi L, Bittoni A, Cascinu S (2012) Pre-treatment lactate dehydrogenase levels as predictor of efficacy of first-line bevacizumab-based therapy in metastatic colorectal cancer patients. Br J Cancer 106: 799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestris N, Scartozzi M, Graziano G, Santini D, Lorusso V, Maiello E, Barni S, Cinieri S, Loupakis F, Pisconti S, Brunetti AE, Palasciano G, Palmieri VO, Del Prete M, Dell’Aquila E, Latiano TP, Petrelli F, Lutrino S, Rossini D, Giampieri R, Lotesoriere C, Cascinu S (2015) Basal and bevacizumab-based therapy-induced changes of lactate dehydrogenases and fibrinogen levels and clinical outcome of previously untreated metastatic colorectal cancer patients: a multicentric retrospective analysis. Expert Opin Biol Ther 15: 155–162. [DOI] [PubMed] [Google Scholar]
- Stintzing S, Modest DP, Rossius L, Lerch MM, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G, Kullmann F, Stauch M, Scheithauer W, Held S, Giessen-Jung C, Moehler M, Jagenburg A, Kirchner T, Jung A, Heinemann V (2016) FIRE-3 investigators. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 17: 1426–1434. [DOI] [PubMed] [Google Scholar]
- Tas F, Aydiner A, Demir C, Topuz E (2001. b) Lactate dehydrogenase levels at presentation predict outcome of patients with limited stage small-cell lung cancer. Am J Clin Oncol 24: 376–378. [DOI] [PubMed] [Google Scholar]
- Tas F, Aykan F, Alici S, Kaytan E, Aydiner A, Topuz E (2001. a) Prognostic factors in pancreatic carcinoma: serum LDH levels predict survival in metastatic disease. Am J Clin Oncol 24: 547–550. [DOI] [PubMed] [Google Scholar]
- Van Cutsem E, Bajetta E, Valle J, Kohne CH, Hecht JR, Moore M, Germond C, Berg W, Chen BL, Jalava T, Lebwohl D, Meinhardt G, Laurent D, Lin E (2011) Randomized, placebo-controlled, phase III study of oxaliplatin, fluorouracil, and leucovorin with or without PTK787/ZK 222584 in patients with previously treated metastatic colorectal adenocarcinoma. J Clin Oncol 29: 2004–2010. [DOI] [PubMed] [Google Scholar]
- Wu XZ, Ma F, Wang XL (2010) Serological diagnostic factors for liver metastasis in patients with colorectal cancer. World J Gastroenterol 16: 4084–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]