ABSTRACT
Burkholderia pseudomallei is a Gram-negative, facultative intracellular pathogen that causes the disease melioidosis in humans and other mammals. Respiratory infection with B. pseudomallei leads to a fulminant and often fatal disease. It has previously been shown that glycoconjugate vaccines can provide significant protection against lethal challenge; however, the limited number of known Burkholderia antigens has slowed progress toward vaccine development. The objective of this study was to identify novel antigens and evaluate their protective capacity when incorporated into a nanoglycoconjugate vaccine platform. First, an in silico approach to identify antigens with strong predicted immunogenicity was developed. Protein candidates were screened and ranked according to predicted subcellular localization, transmembrane domains, adhesive properties, and ability to interact with major histocompatibility complex (MHC) class I and class II. From these in silico predictions, we identified seven “high priority” proteins that demonstrated seroreactivity with anti-B. pseudomallei murine sera and convalescent human melioidosis sera, providing validation of our methods. Two novel proteins, together with Hcp1, were linked to lipopolysaccharide (LPS) and incorporated with the surface of a gold nanoparticle (AuNP). Animals receiving AuNP glycoconjugate vaccines generated high protein- and polysaccharide-specific antibody titers. Importantly, immunized animals receiving the AuNP-FlgL-LPS alone or as a combination demonstrated up to 100% survival and reduced lung colonization following a lethal challenge with B. pseudomallei. Together, this study provides a rational approach to vaccine design that can be adapted for other complex pathogens and provides a rationale for further preclinical testing of AuNP glycoconjugate in animal models of infection.
KEYWORDS: Burkholderia pseudomallei, melioidosis, nanoglycoconjugate, nanovaccine, reverse vaccinology
INTRODUCTION
Burkholderia pseudomallei is a Gram-negative aerobic bacterium common to tropical and subtropical climates worldwide. This saprophytic bacterium can survive in soil and water and, upon transmission to humans or other susceptible mammals, cause the disease melioidosis. Human disease can present with a wide variety of clinical manifestations, including cutaneous and soft tissue abscesses, lymphadenopathy, and sepsis (1, 2). Clinical symptoms have been correlated with the route of infection; percutaneous infection often results in a purulent lesion at the site of inoculation, whereas respiratory infection causes a more rapid fulminant disease that often includes pneumonia, fever, bacteremia, and sepsis (1, 3, 4). Melioidosis treatments are limited, as B. pseudomallei is inherently resistant to major antibiotic classes, including many β-lactams, aminoglycosides, and macrolides (5, 6). Additionally, there are currently no vaccines available, either licensed or in clinical trials. High morbidity and mortality among respiratory cases, together with the limited treatment options, have resulted in the categorization of B. pseudomallei as a tier 1 select agent. The public health threat associated with B. pseudomallei emphasizes the need for an effective vaccine that can be administered to at-risk military personnel and aid workers, together with populations living in areas where it is endemic.
For the past 2 decades, many groups have attempted to develop a vaccine against B. pseudomallei through various approaches, including live-attenuated, whole-cell killed/irradiated, and subunit vaccines (reviewed in references 7 and 8). While these experimental vaccines have been shown to provide protection against a lethal challenge, sterilizing immunity remains elusive. Subunit vaccines remain an attractive alternative to live-attenuated vaccines, because they can be administered to a more diverse population without the concern for dormancy or reversion to the wild type. Subunit vaccines also do not require the use of biosafety level 3 (BSL3) facilities, an important consideration for cost-effectiveness and personnel safety. The most common subunit vaccines today, both licensed and experimental, contain immunogenic proteins. These proteins are known to stimulate a T-cell-dependent immune response, resulting in T-cell and B-cell memory, along with antibody affinity and isotype switching (9, 10). In fact, previous studies have shown that proteins alone can provide significant, albeit incomplete, protection against Burkholderia challenge (11–14). However, more recent studies have shown that when immunogenic proteins are incorporated in a glycoconjugate vaccine in conjunction with the highly antigenic Burkholderia lipopolysaccharide (LPS) or capsular polysaccharide (CPS), a more robust immune response is generated (15–18). Our lab has previously demonstrated that the incorporation of a glycoconjugate with the surface of a gold nanoparticle can enhance the immune response and increase protection in a murine model of inhalational glanders, a disease caused by the closely related host-adapted pathogen B. mallei (17). Additionally, these nanoglycoconjugates were also shown to be safe and immunogenic in nonhuman primates (18). While these studies are highly promising, complete protection was not achieved, and further optimization is needed for increased efficacy. However, the current pool of well-characterized Burkholderia proteins is limited, impeding the progress toward vaccine optimization. To address this need, we have adapted and optimized an in silico methodology for selecting novel protein candidates based on their predictive subcellular localization, antigenicity, and affinity for major histocompatibility complex (MHC) class I and class II. The top 7 vaccine candidates, together with known Burkholderia antigen Hcp1, were selected for expression and purification. Hcp1 was chosen for comparison studies because of its well-characterized immunogenicity, reactivity with convalescent human melioidosis sera, and ability to provide protection against lethal B. pseudomallei challenge in a mouse model (11, 19, 20). To validate our in silico predictions, we confirmed the seroreactivity of these 7 novel proteins with anti-B. pseudomallei murine sera, as well as with human convalescent melioidosis sera. Upon confirmation of immunogenicity, we incorporated two of these novel proteins into a gold nanoparticle (AuNP) glycoconjugate vaccine platform and evaluated immunogenicity in mice. AuNP glycoconjugates were immunogenic, generating high protein- and LPS-specific IgG titers. Additionally, immunization with AuNP glycoconjugates containing the novel flagellar protein FlgL and a protein combination (FlgL, Hcp1, and hemagglutinin) demonstrated 90% and 100% survival following a lethal challenge, respectively. Surviving animals also demonstrated a significant reduction in lung colonization compared to groups receiving adjuvant alone. Together, these studies have confirmed and validated the use of in silico methodologies for the identification of novel vaccine candidates. The use of these methodologies abrogates the need for high-containment facilities and can easily be applied to other complex pathogens. Importantly, by using reverse vaccinology predictions, we could identify previously unknown antigens that reacted with convalescent human melioidosis sera. In this study, we have optimized the construction of an AuNP glycoconjugate vaccine and demonstrated its ability to protect against lethal respiratory challenge with B. pseudomallei. Together, these studies provide the rationale for continued testing of AuNP glycoconjugate vaccines in preclinical models of melioidosis.
RESULTS
Identification of novel Burkholderia antigens via bio- and immunoinformatics approaches.
To identify potential Burkholderia vaccine antigens, we first selected outer membrane and secreted proteins with >98% conservation between B. pseudomallei and the closely related host-adapted B. mallei pathogen. B. mallei was chosen for informatics analyses based on its smaller genome size, 99% genetic identity with B. pseudomallei (increased probability of cross-protection), and its mammalian reservoir (loss of genes involved only in environmental adaptation). From this pool, we eliminated proteins with >1 transmembrane domain to simplify downstream protein purification. To evaluate the potential immunogenicity, we selected proteins with high predicted antigenicity and similarity to known adhesins. Each protein was screened for the number and affinity of MHC-I and MHC-II epitopes. Finally, proteins were ranked against each other based on the predicted immunogenicity (e.g., adhesion, antigenicity, and number/affinity of MHC-I and MHC-II epitopes) (Fig. 1). Based on these characteristics, the top seven proteins were selected from this final pool and compared with the known Burkholderia antigenic protein Hcp1 in downstream validation and immunization studies (Table 1).
FIG 1.

Schematic representation of protein selection and validation. Protein antigens were selected based on conservation, physiochemical properties (e.g., subcellular localization, transmembrane domains), and predicted number and affinity of MHC epitopes. In silico predictions were then validated by confirming seroreactivity with convalescent human and experimental murine melioidosis sera. Proteins were then incorporated into a nanoglycoconjugate vaccine and evaluated for immunogenicity in mice.
TABLE 1.
Top seven candidates (together with known antigen Hcp1) identified via bioinformatics and immunoinformatics analyses
| Protein | Locus |
Placement |
Seroreactivity | Expression | ||
|---|---|---|---|---|---|---|
| B. mallei | B. pseudomallei | MHC-I | MHC-II | |||
| Hemagglutinin | BMAA1324 | BPSS0908 | 1 | 20 | Yes | Yes (22, 23) |
| FlgD | BMA3327 | BPSL0272 | 4 | —a | Unknown | Unknown |
| OmpW | BMA2010 | BPSL2704 | 5 | 7 | Unknown | In vitrob (21) |
| FlgL | BMA3336 | BPSL0281 | 8 | 21 | Unknown | Unknown |
| Porin OpcP1 | BMAA1122 | BPSS0708 | 9 | 3 | Yes | Yes (22) |
| Porin | BMAA0599 | BPSS0757 | 12 | 4 | Unknown | Unknown |
| Porin OpcP | BMAA1353 | BPSS0879 | — | 1 | Yes | In vitroc (21, 25) |
| Hcp1 | BMAA0742 | BPSS1498 | 13 | — | Yes | Yes (14) |
—, not available.
For 5.9% of B. pseudomallei OMPs.
For 4.6% of B. pseudomallei OMPs and 2.5% of B. mallei OMPs.
Protein expression and purification.
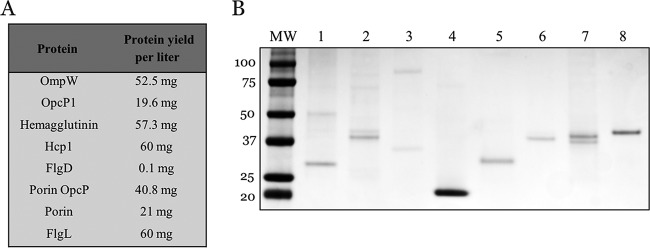
Proteins of interest were amplified with gene-specific primers (see Table S3 in the supplemental material) and inserted into a pET30a(+) expression vector. Upon induction with IPTG (isopropyl-β-D-thiogalactopyranoside), all proteins were expressed in high yields (ranging from 20 to 60 mg per liter of bacterial culture) as inclusion bodies, except for FlgD (Fig. 2A). Despite sufficient bacterial growth, FlgD was expressed in very low yields (0.1 mg per liter culture). Following inclusion body isolation and refolding, SDS-PAGE revealed the high purity of recombinant proteins (Fig. 2B).
FIG 2.
Expression of Burkholderia antigens. His-tagged (6×) recombinant proteins were expressed as inclusion bodies in a pET30a(+) IPTG-inducible expression vector. Inclusion bodies were purified, denatured, and solubilized into a refolding buffer prior to dialysis against PBS. (A) Protein yields per liter bacterial culture. (B) SDS-PAGE of top seven candidates and Hcp1. Lane 1, OmpW; 2, OpcP1; 3, hemagglutinin; 4, Hcp1; 5, FlgD; 6, OpcP porin; 7, porin; 8, FlgL.
Validation of in silico predictions.
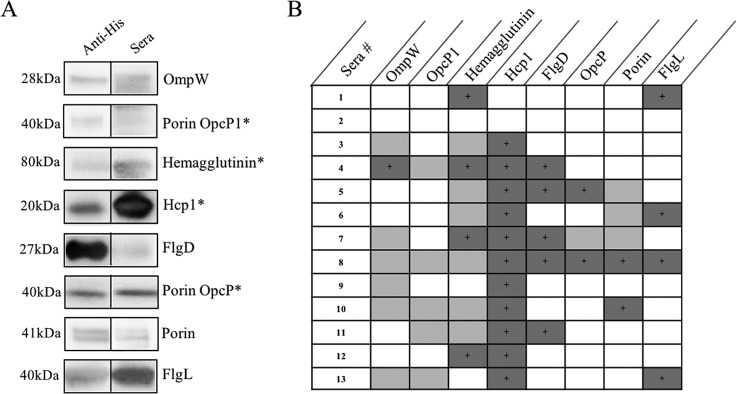
To validate the immunogenicity of these proteins, we first examined their reactivity against sera taken from chronically infected mice. When evaluated via enzyme-linked immunosorbent assay (ELISA), all recombinant proteins exhibited reactivity at dilutions of 1:50 (data not shown), with endpoint titers determined to be twice the standard deviation from those of naive sera. Further, when transferred to a polyvinylidene difluoride (PVDF) membrane, all proteins exhibited various reactivities with convalescent human melioidosis sera (Fig. 3A and B) but did not react with seronegative human sera (data not shown).
FIG 3.
Validation of in silico predictions. Purified proteins were run by 4 to 20% SDS-PAGE and Western blotting was performed using human sera (diluted 1:500 to 1:1000) or anti-His antibody (diluted 1:10,000) prior to the addition of goat anti-human or anti-mouse horseradish peroxidase (HRP)-conjugated secondary antibody, respectively. (A) Representative Western blots of purified protein with convalescent human melioidosis sera compared to anti-His antibody. *, known seroreactive antigens. (B) Chart representing the various reactivities of proteins with convalescent human sera. Dark shading, strong reactivity (e.g., FlgL); light shading, weak reactivity (e.g., Porin OpcP1); no shading, no reactivity.
Burkholderia proteins enhance immunogenicity of AuNP glycoconjugate platform.
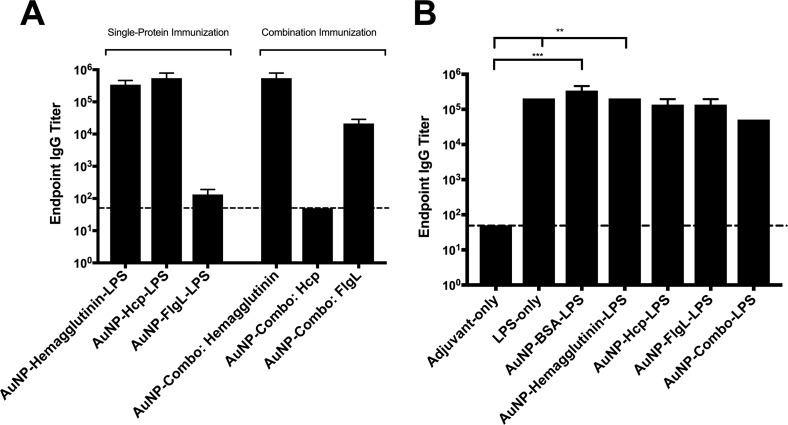
Our lab has previously shown that gold nanoparticle AuNP glycoconjugates can provide significant protection in murine and nonhuman primate models of inhalational glanders (17, 18). However, these vaccines were only partially protective against a lethal challenge and generated weak anti-LPS antibody titers. To improve the immunogenicity of this vaccine platform, we optimized a method for incorporating these novel proteins into a nanoglycoconjugate vaccine formulation. When incorporated into this AuNP glycoconjugate platform, all proteins exhibited various degrees of conjugation efficacy as determined by SDS-PAGE (not shown). To evaluate the immune response to our optimized nanoglycoconjugate platform, two novel vaccine candidates (together with Hcp1 and bovine serum albumin [BSA]) exhibiting the highest conjugation efficacies were selected for in vivo immunization studies. The mice receiving a prime and two boosts of AuNP glycoconjugate vaccine generated high protein- and LPS-specific IgG titers (Fig. 4A and B). Importantly, immunization with AuNP glycoconjugates generated anti-LPS antibody titers ranging from 1:104 to 1:105 (Fig. 4B).
FIG 4.
Antibody responses following nanoglycoconjugate vaccination. Sera were collected from vaccinated animals at 2 weeks postboost and pooled (n = 10). Protein- or LPS-specific IgG titers were assessed via ELISA, with endpoint titers determined to be twice the standard deviation from those of naive sera. Bars represent the means from three replicates. (A) Protein-specific IgG responses across vaccinated groups. Sera taken from the AuNP-combo-LPS group were assessed against each individual protein (hemagglutinin, Hcp1, and FlgL). (B) LPS-specific IgG responses. Significant differences in LPS-specific IgG titers were determined via one-way ANOVA followed by Tukey's post hoc test. Levels of significance (compared to adjuvant-only): **, P < 0.005; ***, P < 0.0005.
Protection studies.
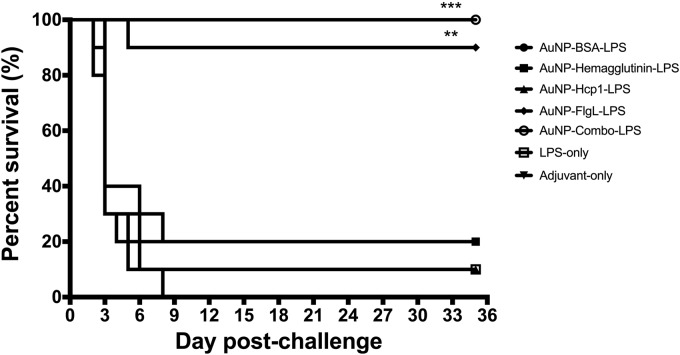
To evaluate the protective capacity of these novel AuNP glycoconjugate platforms, immunized animals were challenged intranasally (i.n.) with 3.4 times the 50% lethal dose (LD50) of B. pseudomallei K96243. Survival was monitored for 35 days postinfection. Animals receiving AuNP-FlgL-LPS and AuNP-combo-LPS demonstrated the highest protection, with 90% and 100% survival at 35 days postinfection, respectively (Fig. 5). All animals immunized with AuNP-BSA-LPS succumbed to infection by day 8 postinfection (Fig. 5). Groups receiving adjuvant only or AuNP-hemagglutinin-LPS-demonstrated 20% survival at day 35 postinfection. In addition, both AuNP-Hcp1-LPS and LPS-only immunized groups demonstrated 10% survival at day 35 postinfection.
FIG 5.
Prime and two boost vaccinations with AuNP-FlgL-LPS and AuNP-combo-LPS provide 90% and 100% protection against B. pseudomallei challenge, respectively. Survival curves for AuNP glycoconjugate groups (n = 10) after i.n. challenge with 3.4 LD50 of B. pseudomallei K96243. Prior to challenge, animals received a prime and two boost vaccinations (s.c.) with the following vaccine formulations: adjuvant only (▼), LPS only (□), AuNP-BSA-LPS (·), AuNP-hemagglutinin-LPS (■), AuNP-Hcp1-LPS (▲), AuNP-FlgL-LPS (⬥), and AuNP-combo-LPS (○). Animals were monitored for 35 days postchallenge. Statistical analyses were performed by the Kaplan-Meier method, followed by log-rank test. Levels of significance compared to the adjuvant-only group: **, P < 0.005; ***, P < 0.0005.
CFU enumeration.
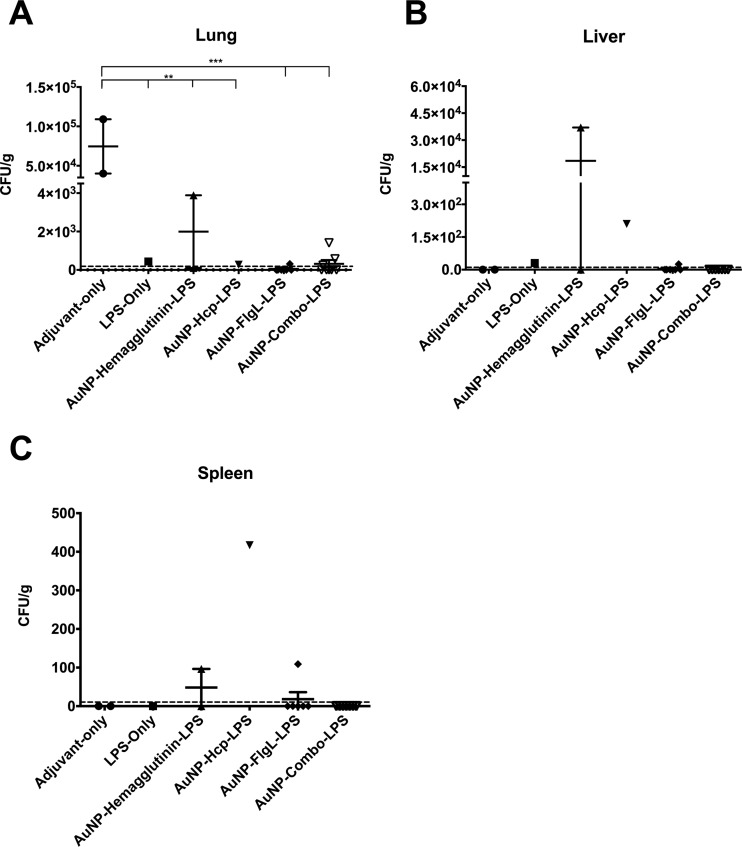
Bacterial burden in the lung, liver, and spleen from surviving mice was assessed by standard bacterial enumeration after tissue processing. Animals that received adjuvant alone had the highest lung bacterial colonization, whereas other groups receiving LPS alone or AuNP glycoconjugate vaccines demonstrated a significant reduction in bacterial burden (Fig. 6A). Importantly, animals receiving AuNP-FlgL-LPS or the combination (AuNP-combo-LPS) demonstrated the most significant reduction in lung colonization (P < 0.001) compared to those receiving adjuvant alone. However, no significant differences were observed in the livers or spleens of the control or AuNP glycoconjugate-immunized groups, except for the AuNP-Hcp1-LPS group (Fig. 6B and C). The surviving animal (n = 1) from this group demonstrated significantly increased splenic colonization (P < 0.0001) compared to those receiving adjuvant alone (n = 2).
FIG 6.
Animals immunized with AuNP-glycoconjugate vaccines exhibited reduced bacterial colonization in lungs. Bacterial colonization in the lungs (A), livers (B), and spleens (C) at 35 days postchallenge with B. pseudomallei K96243. The plots represent individual averages and standard errors of the means from individual mice. Significant differences in colonization were individually ascertained via one-way ANOVA followed by Tukey's post hoc test. Levels of significance (compared to adjuvant-only): **, P < 0.005; ***, P < 0.0005.
DISCUSSION
In this study, we optimized a reverse vaccinology approach to identify immunogenic Burkholderia proteins using publicly available informatics programs. This simplified approach to candidate identification is widely applicable to other pathogens. We first selected proteins based on the conservation (>98% identity) between B. pseudomallei and B. mallei. Because B. mallei is an obligate mammalian pathogen, the selection of these conserved antigens allowed the exclusion of genes involved only in environmental adaptation. The top seven proteins, together with Hcp1, were selected based on their predicted subcellular localization, antigenicity, adhesion, and MHC epitopes (Fig. 1; see also Table S1 in the supplemental material). The selection of protein candidates with predicted extracellular or outer membrane localization was important to increase the chance that these antigens would encounter the host's immune system. The high expression of recombinant protein upon IPTG induction (Fig. 2A) indicates that these proteins are nontoxic to bacteria, an important consideration for vaccine scalability. In contrast, the low yields of FlgD produced by Escherichia coli suggest that this protein might be unstable and easily degraded or may result from an unusual secondary structure of RNA that interferes with ribosomal binding. Future studies will evaluate whether altering the codons or expressing in a protease-deficient E. coli strain may improve protein production of FlgD.
When screened against experimental murine and convalescent human melioidosis sera, all proteins exhibited seroreactivity. The wide range in reactivity with human sera (Fig. 3B) may be indicative of numerous factors, including differences in human HLA alleles or the infecting B. pseudomallei strain. Importantly, only IgG was assessed; therefore, the stage of infection may also play a role in the different seroreactivities observed. Taken together, these results indicate that these proteins are expressed during infection and are immunogenic in murine and human hosts.
To our knowledge, none of these antigens has been tested in a vaccine study; however, a few studies have examined the immunogenicity and functionality of these proteins. Of the seven proteins identified via informatics analyses, three proteins, namely, FlgD, FlgL, and a porin (BMA0599), remain entirely novel antigens and to our knowledge, have not yet been characterized in vitro or in vivo. However, some characterization has been performed on the remaining four proteins. Specifically, other groups have shown that two of these proteins, porin OpcP and OmpW, comprise 4.63% and 5.88% of B. pseudomallei total outer membrane proteins (OMPs) under in vitro growth conditions, respectively (21). Interestingly, these authors also demonstrated that supplementing the growth medium with amino acids resulted in increased expression of the porin OpcP to 11% of B. pseudomallei OMPs, suggesting a possible role for this predicted porin in amino acid transport (21). Importantly, the porins OpcP and OpcP1, together with hemagglutinin, have previously been shown to react with convalescent human melioidosis sera (22–25), with the hemagglutinin protein also demonstrating reactivity with experimental equine glanders sera (24). This protein has also been shown to stimulate interferon gamma (IFN-γ) production from whole blood isolated from seropositive donors (26). Additionally, this hemagglutinin has been shown to play a role in B. pseudomallei adhesion, internalization, and plaque formation in A549 cells (26), as well as survival in J774.2 macrophage-like cells (27). However, the exact role of this protein in B. pseudomallei virulence remains controversial, as one study demonstrated a 40-fold decrease in mean lethal dose (MLD) in a mutant strain (27), while another showed no difference in bacterial colonization compared to the wild type (26). However, this discrepancy may be the result of differences in parent bacterial strains, challenge dosages, or routes of inoculation.
To our knowledge, this study represents the first attempt to evaluate these novel Burkholderia proteins in a vaccine formulation. The incorporation of highly antigenic polysaccharides into Burkholderia vaccine formulations is important, as antipolysaccharide antibodies are associated with protection against Burkholderia in both animals and humans (28–31). However, B. pseudomallei and B. mallei are BSL3 pathogens, making the isolation of antigenic components (e.g., LPS) difficult. To avoid these complications, many researchers have turned to excluded strains of B. pseudomallei or the avirulent B. thailandensis for LPS isolation (15, 20, 32). Importantly, the structure of B. thailandensis LPS has been shown to be nearly identical to that of B. pseudomallei and is able to protect against lethal B. pseudomallei challenge (32, 33). It has been shown that the conjugation of bacterial polysaccharides to carrier proteins can elicit T-cell help, thereby generating memory responses (34). As such, there is currently considerable interest in generating glycoconjugate vaccines against various pathogens. Our nanoglycoconjugate vaccine design represents a unique approach for screening various antigen combinations to achieve increased vaccine efficacy.
When incorporated into this nanoparticle platform, all proteins exhibited various levels of conjugation efficacy. Because this conjugation is highly dependent on the availability of primary amines (e.g., lysine residues), it is likely that the three-dimensional structure of the protein is hindering the availability of these residues. To evaluate the efficacy of these AuNP glycoconjugate vaccines in vivo, we selected two novel proteins (hemagglutinin and FlgL) that demonstrated high conjugation efficacy. After a prime and two boost vaccinations with nanoglycoconjugates containing immunogenic proteins (FlgL, hemagglutinin, and Hcp1) or BSA, mice generated high LPS-specific serum IgG titers ranging from 1:104 to 1:105. Interestingly, animals receiving LPS alone also generated high anti-LPS titers of 2 × 105. Because all immunized mice received equivalent LPS (10 μg) immunizations, the wide range in anti-LPS endpoint titers could be indicative of protein immunogenicity (e.g., increased or decreased processing and presentation on MHC) or may reflect differential proportions of unconjugated LPS. To address this question, further studies will focus on elucidating the conjugation efficacy and concentration needed to achieve the most robust anti-LPS titers.
Importantly, these anti-LPS titers are evidence of increased optimization of this nanoglycoconjugate platform, as previous studies generated endpoint titers of 1:100 (17). It is important to note that differences in nanoglycoconjugate construction (e.g., incorporation of novel proteins) and vaccination strategies (e.g., LPS concentration, route of immunization, and adjuvant) may also account for this improved immunogenicity.
After the final vaccination, immunized animals were challenged i.n. with 3.4 LD50 of B. pseudomallei K96243. The AuNP-FlgL-LPS and AuNP-combo-LPS vaccination groups showed 90% and 100% protection at day 35 postinfection, respectively, whereas other groups demonstrated 20% survival (adjuvant only and AuNP-hemagglutinin-LPS), 10% survival (LPS and AuNP-Hcp1-LPS), and 0% survival (AuNP-BSA-LPS) (Fig. 5). These results suggest that the immunogenic protein plays an important role in mediating protection. The 20% survival observed in the adjuvant-only-immunized group was likely a remnant of innate stimulation following adjuvant administration. Future studies will focus on increasing the delay between immunization and challenge to more fully evaluate the longevity of this protective immune response.
In the lungs of surviving animals, all immunized groups demonstrated a significant reduction in bacterial colonization compared to those receiving adjuvant alone (Fig. 6). However, the most significant reduction (P < 0.001) was observed in the AuNP-FlgL-LPS- and AuNP-combo-LPS-vaccinated groups. A similar trend was seen in the liver and spleen, with many animals receiving AuNP-FlgL-LPS or AuNP-combo-LPS and demonstrating colonizations below the limit of detection. Meanwhile, the surviving animals from the AuNP-Hcp1-LPS (n = 1) and AuNP-hemagglutinin-LPS (n = 2) groups demonstrated both spleen and liver colonizations. However, it is important to note that the limited number of surviving animals prevents further conclusions, and additional studies are needed to fully understand the protective capacity of these vaccine formulations.
The limited survival observed in the AuNP-Hcp1-LPS group was unexpected, as this antigen has previously been shown to provide protection against both B. mallei and B. pseudomallei in several studies (11, 14, 17). However, since these studies administered Hcp1 through alternate routes (e.g., intraperitoneal and i.n.), it is possible that the protection is route dependent. Additionally, the present study used B. mallei Hcp1. While it differs from B. pseudomallei K96243 Hcp1 by only 1 amino acid, it is possible that differences in the 3-dimensional structure could have affected immune recognition. Further studies are needed to test these hypotheses.
One limitation of this study is that it only evaluates the humoral immune response. Previous studies have shown that antibodies are essential for protection against Burkholderia, as B-cell depletion results in exacerbated disease and decreased protection following vaccination (8, 35). Therefore, the incorporation of antibody-inducing proteins is important when designing a subunit vaccine against Burkholderia. Nevertheless, it is currently understood that balanced cellular and humoral immune responses will be required for vaccine-induced protective immunity against B. pseudomallei and B. mallei (29, 36–38). Interestingly, in the present study, there does not appear to be a direct correlation between antibody titers (anti-LPS or antiprotein) and protection. It is important to note that this study only evaluated total IgG, and it is possible that specific antibody isotypes are mediating the protection. However, the lack of correlation between antibody responses and protection suggests that the cellular immune response is playing a significant role in protection. Further studies will focus on elucidating the correlates of protection mediated by these AuNP glycoconjugate vaccines. Together, the results of this study represent a unique approach to identifying novel vaccine antigens and provide the foundation for continued optimization of Burkholderia glycoconjugate vaccines.
MATERIALS AND METHODS
Bioinformatics and immunoinformatics.
To identify potential vaccine candidates, we screened for desirable physiochemical properties and predicted immunogenicity. First, we evaluated the gene conservation between B. pseudomallei and the closely related host-adapted pathogen B. mallei. Using the B. mallei proteome of >5,000 proteins (obtained from the Burkholderia Genome Database [39]), we used BLAST analyses to select proteins that exhibited >98% conservation between the two Burkholderia species. Using the Vaxign (40) program, we eliminated any proteins exhibiting sequence homology with host (human and mouse) proteins. Next, we used pSORTb (41) to screen for outer membrane and extracellular (e.g., secreted) proteins, selecting proteins with prediction scores of >9.5. We used the transmembrane prediction programs TMHMM (42), Phobius (43), and HMMTOP (44) to select proteins with ≤1 transmembrane domain. Following this analysis, we examined the predicted antigenicity (threshold = 0.4) and adhesive properties via VaxiJen (45) and Vaxign, respectively. Finally, we evaluated the predicted stability of the protein via ProtParam (46). Following these physiochemical analyses, we screened for predicted immunogenicity based on the binding to MHC-I and MHC-II molecules. To evaluate potential MHC-I and MHC-II epitopes and their corresponding affinities, we used the NetCTL 1.2 (47) and NetMHCII 2.2 (48) programs, respectively. A summary of these analyses is included in Table S1 in the supplemental material. Finally, to downselect to the most ideal vaccine candidates, proteins were ranked against each other based on their adhesion probability and predicted antigenicity, as well as the number and affinity of MHC epitopes. The top seven protein candidates (together with the known Burkholderia antigen Hcp1) were selected for further validation studies.
Cloning and expression of Burkholderia antigens.
B. mallei ATCC 23344 DNA was isolated via the Qiagen DNeasy blood and tissue kit, according to the manufacturer's directions. Sequences encoding OmpW (BMA2010 or BPSL2704), porin OpcP1 (BMAA1122 or BPSS0708), hemagglutinin (BMAA1324 or BPSS0908), Hcp1 (BMAA0742 or BPSS1498), FlgD (BMA3327 or BPSL0272), OpcP porin (BMAA1353 or BPSS0879), porin (BMAA0599 or BPSS0757), and FlgL (BMA3336 or BPSL0281) were amplified via Phusion polymerase (New England BioLabs) and cloned into a pET30a(+) expression vector using NdeI and XhoI or HindIII-HF (New England BioLabs) restriction sites (see Table S2). The open reading frame (ORF) for each protein was inserted in-frame with a 6-histidine (His) tag at the C terminus (see Table S3). Ligation, transformation, and expression were performed according to the manufacturer's directions (pET system; Novagen) with some modifications. Upon confirmation of successful gene insertion via gel electrophoresis and directional sequencing (UTMB Genomics Core), plasmids were transformed into BL21(DE3) competent E. coli (New England BioLabs) via heat shock treatment. To induce protein expression, overnight cultures were diluted 1:100 in 40 ml of Luria-Bertani (LB) broth, grown to an optical density at 600 nm (OD600) of ∼0.5, and induced with a 1 mM final concentration of IPTG. At 3 h postinduction, cultures were centrifuged (4,000 × g for 15 min), and the resulting bacterial pellets were frozen at −20°C. To confirm protein expression, bacterial pellets were resuspended in cold 20 mM Tris-HCl (pH 7.5) and sonicated with a 750-W ultrasonic processor on ice for 10 pulses (30 s on, 30 s off). The resulting material was centrifuged (14,000 × g for 10 min) to separate soluble and insoluble fractions. Following the addition of 2× Laemmli buffer (Bio-Rad), samples were heated at 95°C for 5 min and run on 10% gels for SDS-PAGE (Bio-Rad). Protein bands were cut out, digested, and analyzed via matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (UTMB Mass Spectrometry Core) to confirm the protein identity.
Recombinant protein purification and refolding.
To induce the expression of protein inclusion bodies, overnight cultures were diluted 1:100 in 500 ml LB broth, grown to an OD600 of ∼0.5 and induced with 1 mM IPTG. Cultures were grown for 3 h at 37°C with agitation and pelleted (6,000 × g for 15 min). Pellets were frozen at −20°C prior to protein purification. To purify inclusion bodies, 1 g of the cell pellets was resuspended in 10 ml 1× CelLytic B (Sigma) in phosphate-buffered saline (PBS) together with 0.2 mg/ml lysozyme and 1 pellet EDTA-free protease inhibitor cocktail (Roche Diagnostics). This solution was incubated with shaking for 15 min and centrifuged (16,000 × g for 15 min) to pellet insoluble material. The insoluble pellets were resuspended in 10 ml 1× CelLytic B (Sigma) and vortexed for 2 min prior to the addition of 0.2 mg/ml lysozyme (Sigma). This solution was incubated for 10 min at room temperature and centrifuged (16,000 × g for 5 min). To fully remove all soluble material from the inclusion bodies, the insoluble pellets were washed 10 times with CelLytic B (Sigma) diluted 1:100 in PBS, with centrifuging (16,000 × g for 5 min) between washes. To solubilize inclusion bodies, 1 g of insoluble material was resuspended in 8 ml of CelLytic B (Sigma) and incubated with shaking for 30 min at room temperature. After incubation, the solutions were centrifuged (16,000 × g for 15 min) to pellet cell debris. Supernatants containing solubilized inclusion bodies were assayed via bicinchoninic acid (BCA) (Pierce) to determine protein concentrations and stored at −20°C until use. Solubilized inclusion bodies were adjusted to 1 mg/ml in buffer containing 8 M urea, 100 mM NaH2PO4, and 10 mM Tris-HCl (pH 7.3). Adjusted proteins were added dropwise to a 50× volume of refolding buffer containing 50 mM Tris-HCl (pH 8.5), 240 mM NaCl, 10 mM KCl, 1 mM EDTA, 0.5 M arginine, 0.75 M guanidine HCl, 0.5% Triton X-100, and 1 mM dithiothreitol (DTT; Athena ES). These solutions were dialyzed against 4 to 6 liters of 2× PBS for 2 h and 1× PBS for 2 h, followed by eight exchanges with 1× PBS performed at 8-h intervals. All proteins were dialyzed against PBS, except for Hcp1 (BMAA0742 or BPSS1498), which was dialyzed against decreasing concentrations of Tris-HCl from 50 mM to 20 mM, with exchanges performed as described above. The resulting protein solutions were concentrated via filter centrifugation (EMD Millipore Amicon Ultra-15, 10-kDa molecular mass cutoff), assayed via BCA to determine the protein concentrations, and stored at −80°C until use.
Validation of immunogenicity predictions.
To validate our in silico predictions, we confirmed seroreactivities with both anti-B. pseudomallei murine sera and convalescent human melioidosis sera. Convalescent-phase sera were obtained from seropositive volunteers (Northeastern Thailand) with informed written consent according to the Khon Kaen University ethics committee for human research. Deidentified human sera were received, and studies at UTMB were excluded from IRB review. Anti-B. pseudomallei sera were obtained from C57BL/6 mice 35 days postinfection with a sublethal dose of B. pseudomallei K96243, and sera from nonimmunized control mice were used as a control. To determine IgG antibody titers and evaluate seroreactivity, Corning high-binding polystyrene plates (Fisher) were coated overnight with 10 μg/ml recombinant protein in PBS. After coating, plates were washed twice with wash buffer (PBS containing 0.05% Tween 20) and blocked in blocking solution (0.1% Tween 20 and 2% bovine serum albumin) for 2 h. After blocking, plates were washed twice prior to the addition of serum samples. Sera were diluted 1:25 in sample diluent (PBS solution containing 0.01% Tween 20 and 1% BSA), added to the plate, and serially diluted. After the addition of sera, goat anti-mouse IgG polyclonal antibody (1:500; Abcam) was added to the plate and incubated for 3 h at room temperature with shaking. After incubating, the plate was washed four times with wash solution prior to the addition of a tetramethylbenzidine (TMB; eBioscience) substrate for 15 min at room temperature. The reactions were stopped with 2 N H2SO4, and the plates were read with an Epoch nanospectrophotometer at 450 to 570 nm. Endpoint titers were determined to be the OD450–570 value equivalent to twice the standard deviation from those of naive sera.
To evaluate seroreactivity with convalescent human sera, 1 to 2 μg of recombinant proteins were diluted in Laemmli buffer (Bio-Rad) and heated at 95°C for 5 min. Samples were run by SDS-PAGE (10% or 4 to 20% gels; Bio-Rad) at 100 V for 1.5 h. Proteins were transferred to Immobilon PVDF membranes (EMD Millipore) using a Trans-Blot SD semi-dry transfer cell (Bio-Rad) at 15 V for 30 min. The membranes were blocked for 1 h at room temperature in Tris-buffered saline containing 0.001% Tween 20 (TBST) solution containing 5% powdered milk and 1% BSA. After blocking, the membranes were probed with human sera (diluted 1:500 to 1:1000) or goat anti-His antibody (diluted 1:10,000) and incubated overnight at 4°C with rocking. After incubating, the membranes were washed three times with TBST (10 min each) prior to the addition of goat anti-mouse IgG diluted 1:5,000 to 1:10,000 for 1 h at room temperature. The membranes were washed three times with TBST prior to the addition of ECL 2 chemiluminescent substrate (Pierce) and were imaged with an ImageQuant LAS 4000 system.
Lipopolysaccharide purification.
Lipopolysaccharide was purified from Burkholderia thailandensis as previously described (20). Briefly, B. thailandensis E264 was inoculated into 2.5 liters of LB broth and allowed to grow for ∼20 h at 37°C with agitation (200 rpm). Then, the bacterial pellet was obtained via centrifugation (6,000 × g for 15 min) and resuspended in a 1:1 mixture of phenol and water. After heating to 80°C, the solution was cooled and dialyzed against 4 to 5 exchanges of water. This solution was clarified via centrifugation (6,000 × g for 15 min) and lyophilized. The lyophilized substance was resuspended in an aqueous solution containing 10 mM Tris-HCl (pH 7.5), 1 mM MgCl2, 1 mM CaCl2 and digested with RNase, DNase I, and proteinase K (50 μg/ml each). A gel-like pellet of LPS was obtained via ultracentrifugation (100,000 × g for 3 h) and lyophilized. For additional purification, the sample was washed 4 to 5 times with 90% ethanol and lyophilized. The final pellet was weighed, resuspended in PBS or water to 1 mg/ml, and stored at −80°C until use. LPS purity was assessed via SDS-PAGE and Western blotting (not shown).
Construction of AuNP glycoconjugates.
Gold nanoparticle (AuNP) glycoconjugates were synthesized as previously described (17), with modifications. First, 15-nm spherical AuNPs were synthesized via the reduction of 1 mM gold(III) chloride trihydrate with 90 mM sodium citrate dihydrate, according to the method described by Turkevich et al. (49). Particle sizes and shapes were confirmed via transmission electron microscopy (TEM). To immobilize antigens to the AuNP surface, 0.1 mM 16-mercaptohexadecanoic acid (16-MHDA) and 0.1% Triton X-100 were added to a solution of AuNPs. After 2 h of incubation, this solution was filter centrifuged (EMD Millipore Amicon Ultra-15, 3-kDa molecular mass cutoff) and the process repeated to ensure complete coverage. The attachment of 16-MHDA was confirmed by measuring plasmon resonance via UV-Vis spectroscopy (data not shown). To facilitate conjugation, 0.1 mM 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM; Sigma), 0.1% Tween 20, and 20 μg recombinant protein were added to a 1-ml solution of gold nanoparticles (AuNPs) and allowed to incubate overnight at room temperature with agitation. To prevent the aggregation of AuNPs, conjugation reactions were performed in either 1× PBS (pH 7.5) or 0.1 M borate (pH 8.6) buffer. LPS was conjugated to AuNPs using a modified thiol-maleimide coupling approach. First, 4.3 μl of 40 mM N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and 17.3 μl of 10 mM N-hydroxysuccinimide (NHS) were combined with 0.2 mg LPS and allowed to incubate for 15 min. Next, 10.9 μl of 800 μM 6-maleimidocaproic acid hydrazide, trifluoroacetic acid salt (EMCH) was added, and the mixture was incubated an additional 15 min. Then, the pH of the LPS solution was adjusted to 7.0 with weak NaOH and incubated for 1 h at room temperature with rocking, followed by filter centrifugation and desalting into 5 mM EDTA. In the meantime, 25.4 μl of 250 μM S-acetylthioglycolic acid N-hydroxysuccinimide ester (SATA; Sigma) was added to protein-conjugated AuNPs and incubated for 1 h at room temperature. The reaction was quenched with 50 μl 50% (wt/vol) hydroxylamine and 50 μl of 5 mM EDTA. Then, the protein-conjugated AuNPs were filter centrifuged and resuspended in an LPS-EDTA solution for 4 h, at which time the reaction was quenched with 10 μl of 5 mM N-ethylmaleimide. Prior to immunization, the AuNP glycoconjugates were washed twice with PBS (with filter centrifuging between washes) and resuspended to a final desired volume. Conjugation was confirmed by SDS-PAGE (not shown).
Mouse immunization studies.
Six- to eight-week-old female C57BL/6 mice (Charles River) were housed in microisolator cages under pathogen-free conditions, were provided with rodent feed and water ad libitum, and were maintained on a 12-h light cycle. To allow adequate acclimation, mice were housed within the animal facility for 1 week prior to experimentation. This study was carried out in strict accordance with the recommendations in the guide for the care and use of laboratory animals of the National Institutes of Health. The protocol was approved by the animal care and use committee of the University of Texas Medical Branch (protocol number 0503014B). To evaluate immunogenicity in an animal model, mice were immunized subcutaneously (s.c.) at 2-week intervals with a prime and 2 boosts of AuNP glycoconjugate vaccine formulation containing 10 μg of protein (BSA, hemagglutinin, Hcp1, or FlgL) and 10 μg LPS in PBS, together with 500 μg Alhydrogel and 30 μg poly(I·C) adjuvants (InvivoGen). For the combination group (AuNP-combo-LPS), mice received equal parts AuNP-hemagglutinin-LPS, AuNP-FlgL-LPS, and AuNP-Hcp1-LPS (3.33 μg each for 10 μg total protein concentration). Control groups received adjuvant alone or in combination with 10 μg LPS. To evaluate antibody titers, blood was drawn retro-orbitally 2 weeks following the last boost. To isolate sera, blood was incubated for 30 min at room temperature to allow for clotting and was centrifuged (10,000 × g for 10 min). Sera were removed and stored at −20°C until use. For ELISAs and serum assays, individual sera from each immunization group were pooled (n = 10).
Bacterial growth conditions.
All bacterial infections were performed with B. pseudomallei K96243 or B. mallei ATCC 23344 in CDC-approved and registered biosafety level 3 (BSL3) and animal biosafety level 3 (ABSL3) facilities at the University of Texas Medical Branch. All experiments were performed in accordance with select agent standard operating practices. Prior to the animal challenge, B. pseudomallei K96243 freezer stocks were streaked onto an LB agar plate and incubated for 36 h at 37°C. For liquid cultures, 3 to 5 colonies were selected and inoculated in 20 ml LB broth and grown for 12 h at 37°C with agitation. Challenge doses were prepared from overnight cultures diluted in PBS.
LD50 determination.
Six- to eight-week-old female C57BL/6 mice (Charles River) were acclimated for 1 week prior to the bacterial challenge. The 50% lethal dose (LD50) for B. pseudomallei K96243 was determined by administering i.n. doses of 3 × 104, 1.5 × 105, and 3 × 105 CFU/ml to groups of 10 mice. Mice were monitored for survival and the LD50 was calculated as previously described (50).
Survival study.
Three weeks after final immunization, mice (n = 10 per vaccination group) were anesthetized and challenged i.n. with 3.4 LD50 (1.06 × 105 CFU/ml) of B. pseudomallei K96243 in a total volume of 50 μl PBS. Animals were monitored daily for 35 days postinfection; then, surviving animals were euthanized and their lungs, livers, and spleens were harvested for CFU enumeration. The survival curve was generated using the Kaplan-Meier method.
CFU enumeration.
The lungs, livers, and spleens of surviving animals were weighed and homogenized in 1 ml PBS using a tissue grinder (Covidien, Mansfield, MA). Homogenate samples were serially diluted in PBS, plated on LB agar plates, and incubated for 48 h at 37°C. Bacterial colonies were counted and reported as CFU per gram of tissue. The limit of detection for bacterial detection was approximately 10 CFU per organ.
Statistical analyses.
Survival and organ colonization graphs were generated using GraphPad Prism V7.0. Significant differences in survival data were analyzed using a log-rank test (Mantel-Cox). Bacterial colonization data and LPS-specific IgG titers were analyzed via one-way analyses of variance (ANOVAs) followed by Tukey's post hoc tests.
Supplementary Material
ACKNOWLEDGMENTS
L.A.M. was supported by a McLaughlin predoctoral fellowship (no. FY2015-FY2017). This study was supported by NIH NIAID grant no. R01 AI12660101 and UTMB funds awarded to A.G.T. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIAID or NIH.
The authors thank Thomas Wood for supplying the pET30a(+) expression vector, Vsevolod Popov for performing transmission electron microscopy, Marco Marradi for assistance with glycoconjugate optimization, and Ganjana Lertmemongkolchai for providing sera.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CVI.00206-17.
REFERENCES
- 1.Currie BJ, Ward L, Cheng AC. 2010. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20-year Darwin prospective study. PLoS Negl Trop Dis 4:e900. doi: 10.1371/journal.pntd.0000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ip M, Osterberg LG, Chau PY, Raffin TA. 1995. Pulmonary melioidosis. Chest 108:1420–1424. doi: 10.1378/chest.108.5.1420. [DOI] [PubMed] [Google Scholar]
- 3.Yeager JJ, Facemire P, Dabisch PA, Robinson CG, Nyakiti D, Beck K, Baker R, Pitt ML. 2012. Natural history of inhalation melioidosis in rhesus macaques (Macaca mulatta) and African green monkeys (Chlorocebus aethiops). Infect Immun 80:3332–3340. doi: 10.1128/IAI.00675-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie BJ, Jacups SP.. 2003. Intensity of rainfall and severity of melioidosis, Australia. Emerg Infect Dis 9:1538–1542. doi: 10.3201/eid0912.020750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rholl DA, Papp-Wallace KM, Tomaras AP, Vasil ML, Bonomo RA, Schweizer HP. 2011. Molecular investigations of PenA-mediated beta-lactam resistance in Burkholderia pseudomallei. Front Microbiol 2:139. doi: 10.3389/fmicb.2011.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harris P, Engler C, Norton R. 2011. Comparative in vitro susceptibility of Burkholderia pseudomallei to doripenem, ertapenem, tigecycline and moxifloxacin. Int J Antimicrob Agents 37:547–549. doi: 10.1016/j.ijantimicag.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Hatcher CL, Muruato LA, Torres AG. 2015. Recent advances in Burkholderia mallei and B. pseudomallei research. Curr Trop Med Rep 2:62–69. doi: 10.1007/s40475-015-0042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva EB, Dow SW. 2013. Development of Burkholderia mallei and pseudomallei vaccines. Front Cell Infect Microbiol 3:10. doi: 10.3389/fcimb.2013.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zubler RH. 2001. Naive and memory B cells in T-cell-dependent and T-independent responses. Springer Semin Immunopathol 23:405–419. doi: 10.1007/s281-001-8167-7. [DOI] [PubMed] [Google Scholar]
- 10.Goldblatt D. 2000. Conjugate vaccines. Clin Exp Immunol 119:1–3. doi: 10.1046/j.1365-2249.2000.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitlock GC, Deeraksa A, Qazi O, Judy BM, Taylor K, Propst KL, Duffy AJ, Johnson K, Kitto GB, Brown KA, Dow SW, Torres AG, Estes DM. 2010. Protective response to subunit vaccination against intranasal Burkholderia mallei and B. pseudomallei challenge. Procedia Vaccinol 2:73–77. doi: 10.1016/j.provac.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey WT, Spink N, Cia F, Collins C, Romano M, Berisio R, Bancroft GJ, McClean S. 2016. Identification of an OmpW homologue in Burkholderia pseudomallei, a protective vaccine antigen against melioidosis. Vaccine 34:2616–2621. doi: 10.1016/j.vaccine.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 13.Champion OL, Gourlay LJ, Scott AE, Lassaux P, Conejero L, Perletti L, Hemsley C, Prior J, Bancroft G, Bolognesi M, Titball RW. 2016. Immunisation with proteins expressed during chronic murine melioidosis provides enhanced protection against disease. Vaccine 34:1665–1671. doi: 10.1016/j.vaccine.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 14.Burtnick MN, Brett PJ, Harding SV, Ngugi SA, Ribot WJ, Chantratita N, Scorpio A, Milne TS, Dean RE, Fritz DL, Peacock SJ, Prior JL, Atkins TP, Deshazer D. 2011. The cluster 1 type VI secretion system is a major virulence determinant in Burkholderia pseudomallei. Infect Immun 79:1512–1525. doi: 10.1128/IAI.01218-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scott AE, Burtnick MN, Stokes MG, Whelan AO, Williamson ED, Atkins TP, Prior JL, Brett PJ. 2014. Burkholderia pseudomallei capsular polysaccharide conjugates provide protection against acute melioidosis. Infect Immun 82:3206–3213. doi: 10.1128/IAI.01847-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott AE, Ngugi SA, Laws TR, Corser D, Lonsdale CL, D'Elia RV, Titball RW, Williamson ED, Atkins TP, Prior JL. 2014. Protection against experimental melioidosis following immunisation with a lipopolysaccharide-protein conjugate. J Immunol Res 2014:392170. doi: 10.1155/2014/392170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregory AE, Judy BM, Qazi O, Blumentritt CA, Brown KA, Shaw AM, Torres AG, Titball RW. 2015. A gold nanoparticle-linked glycoconjugate vaccine against Burkholderia mallei. Nanomedicine 11:447–456. doi: 10.1016/j.nano.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres AG, Gregory AE, Hatcher CL, Vinet-Oliphant H, Morici LA, Titball RW, Roy CJ. 2015. Protection of non-human primates against glanders with a gold nanoparticle glycoconjugate vaccine. Vaccine 33:686–692. doi: 10.1016/j.vaccine.2014.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pumpuang A, Dunachie SJ, Phokrai P, Jenjaroen K, Sintiprungrat K, Boonsilp S, Brett PJ, Burtnick MN, Chantratita N. 2017. Comparison of O-polysaccharide and hemolysin co-regulated protein as target antigens for serodiagnosis of melioidosis. PLoS Negl Trop Dis 11:e0005499. doi: 10.1371/journal.pntd.0005499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burtnick MN, Heiss C, Schuler AM, Azadi P, Brett PJ. 2012. Development of novel O-polysaccharide based glycoconjugates for immunization against glanders. Front Cell Infect Microbiol 2:148. doi: 10.3389/fcimb.2012.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schell MA, Zhao P, Wells L. 2011. Outer membrane proteome of Burkholderia pseudomallei and Burkholderia mallei from diverse growth conditions. J Proteome Res 10:2417–2424. doi: 10.1021/pr1012398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felgner PL, Kayala MA, Vigil A, Burk C, Nakajima-Sasaki R, Pablo J, Molina DM, Hirst S, Chew JS, Wang D, Tan G, Duffield M, Yang R, Neel J, Chantratita N, Bancroft G, Lertmemongkolchai G, Davies DH, Baldi P, Peacock S, Titball RW. 2009. A Burkholderia pseudomallei protein microarray reveals serodiagnostic and cross-reactive antigens. Proc Natl Acad Sci U S A 106:13499–13504. doi: 10.1073/pnas.0812080106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suwannasaen D, Mahawantung J, Chaowagul W, Limmathurotsakul D, Felgner PL, Davies H, Bancroft GJ, Titball RW, Lertmemongkolchai G. 2011. Human immune responses to Burkholderia pseudomallei characterized by protein microarray analysis. J Infect Dis 203:1002–1011. doi: 10.1093/infdis/jiq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tiyawisutsri R, Holden MT, Tumapa S, Rengpipat S, Clarke SR, Foster SJ, Nierman WC, Day NP, Peacock SJ. 2007. Burkholderia Hep_Hag autotransporter (BuHA) proteins elicit a strong antibody response during experimental glanders but not human melioidosis. BMC Microbiol 7:19. doi: 10.1186/1471-2180-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harding SV, Sarkar-Tyson M, Smither SJ, Atkins TP, Oyston PC, Brown KA, Liu Y, Wait R, Titball RW. 2007. The identification of surface proteins of Burkholderia pseudomallei. Vaccine 25:2664–2672. doi: 10.1016/j.vaccine.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Campos CG, Byrd MS, Cotter PA. 2013. Functional characterization of Burkholderia pseudomallei trimeric autotransporters. Infect Immun 81:2788–2799. doi: 10.1128/IAI.00526-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazar Adler NR, Stevens MP, Dean RE, Saint RJ, Pankhania D, Prior JL, Atkins TP, Kessler B, Nithichanon A, Lertmemongkolchai G, Galyov EE. 2015. Systematic mutagenesis of genes encoding predicted autotransported proteins of Burkholderia pseudomallei identifies factors mediating virulence in mice, net intracellular replication and a novel protein conferring serum resistance. PLoS One 10:e0121271. doi: 10.1371/journal.pone.0121271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Titball RW, Burtnick MN, Bancroft GJ, Brett P. 21 March 2017 Burkholderia pseudomallei and Burkholderia mallei vaccines: are we close to clinical trials? Vaccine doi: 10.1016/j.vaccine.2017.03.022. [DOI] [PubMed] [Google Scholar]
- 29.Silva EB, Goodyear A, Sutherland MD, Podnecky NL, Gonzalez-Juarrero M, Schweizer HP, Dow SW. 2013. Correlates of immune protection following cutaneous immunization with an attenuated Burkholderia pseudomallei vaccine. Infect Immun 81:4626–4634. doi: 10.1128/IAI.00915-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho M, Schollaardt T, Smith MD, Perry MB, Brett PJ, Chaowagul W, Bryan LE. 1997. Specificity and functional activity of anti-Burkholderia pseudomallei polysaccharide antibodies. Infect Immun 65:3648–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charuchaimontri C, Suputtamongkol Y, Nilakul C, Chaowagul W, Chetchotisakd P, Lertpatanasuwun N, Intaranongpai S, Brett PJ, Woods DE. 1999. Antilipopolysaccharide II: an antibody protective against fatal melioidosis. Clin Infect Dis 29:813–818. doi: 10.1086/520441. [DOI] [PubMed] [Google Scholar]
- 32.Ngugi SA, Ventura VV, Qazi O, Harding SV, Kitto GB, Estes DM, Dell A, Titball RW, Atkins TP, Brown KA, Hitchen PG, Prior JL. 2010. Lipopolysaccharide from Burkholderia thailandensis E264 provides protection in a murine model of melioidosis. Vaccine 28:7551–7555. doi: 10.1016/j.vaccine.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 33.Heiss C, Burtnick MN, Roberts RA, Black I, Azadi P, Brett PJ. 2013. Revised structures for the predominant O-polysaccharides expressed by Burkholderia pseudomallei and Burkholderia mallei. Carbohydr Res 381:6–11. doi: 10.1016/j.carres.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avci FY, Li X, Tsuji M, Kasper DL. 2011. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat Med 17:1602–1609. doi: 10.1038/nm.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitlock GC, Lukaszewski RA, Judy BM, Paessler S, Torres AG, Estes DM. 2008. Host immunity in the protective response to vaccination with heat-killed Burkholderia mallei. BMC Immunol 9:55. doi: 10.1186/1471-2172-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mott TM, Vijayakumar S, Sbrana E, Endsley JJ, Torres AG. 2015. Characterization of the Burkholderia mallei tonB mutant and its potential as a backbone strain for vaccine development. PLoS Negl Trop Dis 9:e0003863. doi: 10.1371/journal.pntd.0003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hatcher CL, Mott TM, Muruato LA, Sbrana E, Torres AG. 2016. Burkholderia mallei CLH001 attenuated vaccine strain is immunogenic and protects against acute respiratory glanders. Infect Immun 84:2345–2354. doi: 10.1128/IAI.00328-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choh LC, Ong GH, Vellasamy KM, Kalaiselvam K, Kang WT, Al-Maleki AR, Mariappan V, Vadivelu J. 2013. Burkholderia vaccines: are we moving forward? Front Cell Infect Microbiol 3:5. doi: 10.3389/fcimb.2013.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Winsor GL, Khaira B, Van Rossum T, Lo R, Whiteside MD, Brinkman FS. 2008. The Burkholderia Genome Database: facilitating flexible queries and comparative analyses. Bioinformatics 24:2803–2804. doi: 10.1093/bioinformatics/btn524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He Y, Xiang Z, Mobley HL. 2010. Vaxign: the first web-based vaccine design program for reverse vaccinology and applications for vaccine development. J Biomed Biotechnol 2010:297505. doi: 10.1155/2010/297505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, Dao P, Sahinalp SC, Ester M, Foster LJ, Brinkman FS. 2010. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26:1608–1615. doi: 10.1093/bioinformatics/btq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 43.Käll L, Krogh A, Sonnhammer EL. 2004. A combined transmembrane topology and signal peptide prediction method. J Mol Biol 338:1027–1036. doi: 10.1016/j.jmb.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 44.Tusnády GE, Simon I. 2001. The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849–850. doi: 10.1093/bioinformatics/17.9.849. [DOI] [PubMed] [Google Scholar]
- 45.Doytchinova IA, Flower DR. 2007. VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics 8:4. doi: 10.1186/1471-2105-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. 2005. Protein identification and analysis tools on the ExPASy server, p 571–607. In Walker JM. (ed), The proteomics protocols handbook. Springer Science & Business Media, Berlin, Germany. [Google Scholar]
- 47.Larsen MV, Lundegaard C, Lamberth K, Buus S, Lund O, Nielsen M. 2007. Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC Bioinformatics 8:424. doi: 10.1186/1471-2105-8-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nielsen M, Lund O. 2009. NN-align. An artificial neural network-based alignment algorithm for MHC class II peptide binding prediction. BMC Bioinformatics 10:296. doi: 10.1186/1471-2105-10-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turkevich J, Stevenson PC, Hillier J. 1951. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc 11:55–75. doi: 10.1039/df9511100055. [DOI] [Google Scholar]
- 50.Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am J Epidemiol 27:493–497. doi: 10.1093/oxfordjournals.aje.a118408. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.