ABSTRACT
Naturally acquired immunity against malaria is largely mediated by serum antibodies controlling levels of blood-stage parasites. A limited understanding of the antigenic targets and functional mechanisms of protective antibodies has hampered the development of efficient malaria vaccines. Besides directly inhibiting the growth of Plasmodium parasites, antibodies can opsonize merozoites and recruit immune effector cells such as monocytes and neutrophils. Antibodies against the vaccine candidate merozoite surface protein 1 (MSP-1) are acquired during natural infections and have been associated with protection against malaria in several epidemiological studies. Here we analyzed serum antibodies from semi-immune individuals from Burkina Faso for their potential (i) to directly inhibit the growth of P. falciparum blood stages in vitro and (ii) to opsonize merozoites and to induce the antibody-dependent respiratory burst (ADRB) activity of neutrophils. While a few sera that directly inhibited the growth of P. falciparum blood stages were identified, immunoglobulin G (IgG) from all individuals clearly mediated the activation of neutrophils. The level of neutrophil activation correlated with levels of antibodies to MSP-1, and affinity-purified MSP-1-specific antibodies elicited ADRB activity. Furthermore, immunization of nonhuman primates with recombinant full-size MSP-1 induced antibodies that efficiently opsonized P. falciparum merozoites. Reversing the function by preincubation with recombinant antigens allowed us to quantify the contribution of MSP-1 to the antiparasitic effect of serum antibodies. Our data suggest that MSP-1, especially the partially conserved subunit MSP-183, is a major target of opsonizing antibodies acquired during natural exposure to malaria. Induction of opsonizing antibodies might be a crucial effector mechanism for MSP-1-based malaria vaccines.
KEYWORDS: P. falciparum MSP-1, opsonizing antibodies, ADRB, respiratory burst, neutrophil
INTRODUCTION
Despite remarkable progress over the past years, malaria remains a major global health issue, with approximately 438,000 deaths and 214 million clinical cases worldwide in 2015, most of which occur in Africa due to infection with Plasmodium falciparum (1). The emergence of multidrug-resistant parasites emphasizes the need for effective vaccines, which are currently not available (2). For vaccine development, it is important to understand protective immune mechanisms, to identify antigenic targets, and to establish robust and reliable assays measuring correlates of protection. Individuals living in regions in which malaria is endemic naturally acquire immunity against the disease with increasing numbers of survived infections (3–6). This protection appears largely mediated by serum antibodies controlling levels of blood-stage parasites (7).
Merozoites are key targets of naturally acquired antibodies (8–10), and associations between antibody levels and protective human immunity have been reported for several merozoite antigens (10–13). Antibodies targeting merozoites can function via different pathways, such as direct growth inhibition or recruitment of immune effector cells (reviewed in reference 14). However, it remains unclear which antibody mechanisms determine protection against malaria. The direct growth inhibition assay (GIA) of Plasmodium blood stages remains the most commonly used functional assay for blood-stage vaccine candidates and merozoite antigens (15, 16), although whether direct growth inhibitory activity in vitro correlates with protection against clinical malaria is controversial (16).
Increasing evidence points toward an important role of opsonizing antibodies, which bind to merozoites and recruit effector cells, such as monocytes (17, 18) or neutrophils (19), via their Fc receptors; these eliminate the parasites, either by phagocytosis (18) or by secretion of reactive oxygen species (ROS) (19). Indeed, merozoites are mainly targeted by the cytophilic antibodies immunoglobulin G1 (IgG1) and IgG3 (20–22), which can bind to the Fc receptors of immune cells (18) or fix complement factors (23). The acquisition of opsonizing antibodies increases with age and malaria exposure (18) and correlates with protection (18, 19). Four functional assays have been developed to measure opsonizing antibodies in vitro, i.e., (i) the antibody-dependent cellular inhibition (ADCI) assay (17), (ii) the opsonic phagocytosis assay (OPA) (18, 24), (iii) the antibody-dependent complement inhibition (Ab-C) assay (23), and (iv) the antibody-dependent respiratory burst (ADRB) assay (19). Importantly, several studies using these assays show a correlation between opsonizing antibodies and protection against malaria (18, 19, 23–27).
In the ADRB assay, antibodies opsonize P. falciparum merozoites and interact with neutrophils via their Fc receptors, resulting in the production of ROS (19) (see Fig. S1 in the supplemental material). Oxidant damage mediated by ROS can kill the parasites (28–32) and is associated with protection against malaria (19, 33, 34). Interestingly, the antimalarial drugs mefloquine and artesunate cause parasite death by generating ROS (29, 35), and ROS also protect against severe malaria in sickle and fetal erythrocytes (36) as well as in thalassemic and glucose-6-phosphate dehydrogenase (G6PD)-deficient red blood cells (RBCs) (33). In regions in which malaria is endemic, higher age (37) and a more diverse antimerozoite antibody repertoire (38) promote ADRB activity, suggesting that such activity plays a role in naturally acquired immunity. Indeed, ADRB activity correlates with protection from clinical malaria in areas in Senegal in which the disease is mesoendemic or holoendemic (19).
A number of merozoite antigens that elicit opsonizing antibodies, such as merozoite surface protein 119 (MSP-119) (23, 39), MSP-1block2 (40), MSP-2 (18, 23), MSP-3 (18, 41, 42), MSP-5 (37), MSP-6 (43), merozoite surface protein Duffy binding-like-1 (MSPDBL-1) and MSPDBL-2 (27, 44), and glutamate-rich protein (GLURP) (45), have been identified. However, the antigenic targets of antibodies inducing complement fixation, opsonic phagocytosis, or neutrophil respiratory burst might differ. So far, only MSP-119, the small conserved C-terminal part of MSP-1, has been identified as an antigenic target that contributes to neutrophil respiratory burst activity (39).
MSP-1 is the major protein at the surface of Plasmodium merozoites (46). The approximately 190-kDa glycosylphosphatidylinositol (GPI)-anchored precursor protein is processed into four major subunits (MSP-183, MSP-130, MSP-138, and MSP-142) by PfSUB1 prior to invasion (47–49); MSP-142 is further processed into MSP-133 and MSP-119 by PfSUB2. MSP-1 appears mainly dimorphic, and all P. falciparum strains can be assigned to one of the two allelic types, namely, K1 (e.g., FCB1 strain) and Mad20 (e.g., 3d7 strain), corresponding to MSP-1F and MSP-1D, respectively. MSP-1 is essential for Plasmodium blood stages (50) and plays an important role during erythrocyte invasion (51), especially for the initial interaction between merozoites and RBCs (52, 53), as well as RBC rupture and parasite egress (54). Indeed, it was shown recently that MSP-1 acts as a platform for several peripheral MSPs, such as MSP-3, MSP-6, MSP-7, MSPDBL-1, and MSPDBL-2; interestingly, all of these complexes could be inhibited by targeting MSP-183 with antibodies (55). Antibodies to MSP-1 can inhibit parasite growth in vitro (51, 56) and have been associated with protection against malaria in several epidemiological studies (57–60) and in immunization experiments with animals (46, 61–63).
In this study, we used heterologously produced MSP-1 spanning the entire amino acid sequence of the 191-kDa molecule from P. falciparum strain 3d7, henceforth called MSP-1D. Sera from semi-immune individuals from Burkina Faso were employed to analyze the role of naturally acquired MSP-1-specific antibodies in mediating direct growth inhibition and neutrophil respiratory burst activity. In addition to correlation analyses of antibody levels and functional activity, affinity-purified MSP-1D antibodies were examined for their activity in GIAs and ADRB assays. Furthermore, an antigen-reversal GIA and antigen-reversal ADRB assay were established in order to quantify the contribution of single antigens to functional activity. Results show that MSP-1D and its subunit MSP-183 are major targets of opsonizing antibodies, which induce a neutrophil respiratory burst in individuals with naturally acquired immunity. Furthermore, we demonstrate that immunization with MSP-1D induces opsonizing antibodies in rhesus monkeys.
RESULTS
Growth inhibitory potential of MSP-1-specific antibodies.
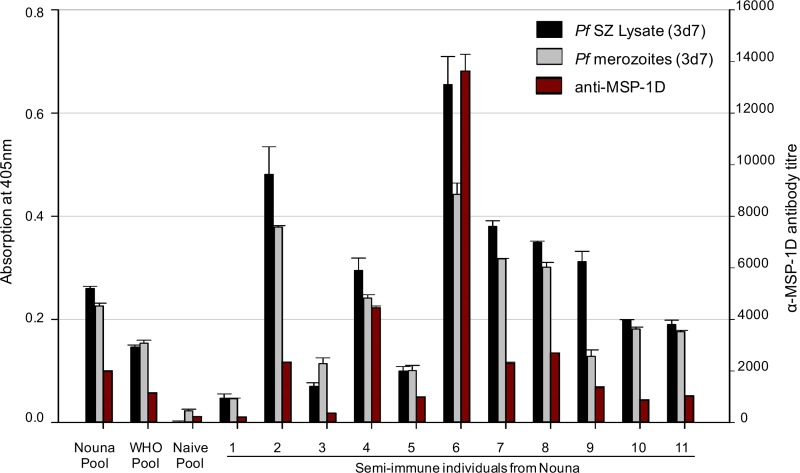
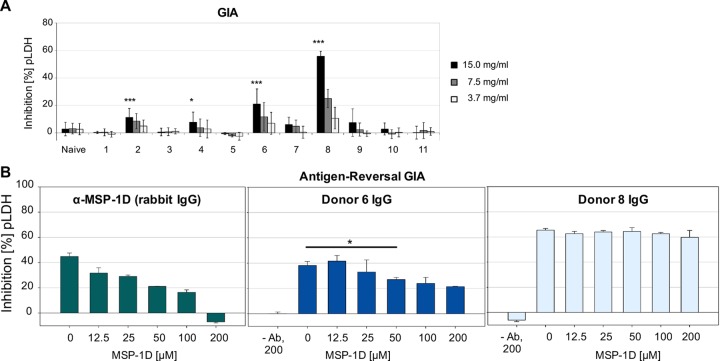
Sera from 11 healthy young adults from Nouna, Burkina Faso (an area with high rates of seasonal malaria transmission), were analyzed for (i) antibodies directed toward P. falciparum-specific antigens and (ii) their potential to inhibit parasite growth in vitro. Sera of malaria-naive Europeans served as controls. There were strong correlations between the levels of antibodies to MSP-1 and the levels of antibodies to P. falciparum schizont lysate or merozoites (Spearman correlation coefficients of r = 0.899 [P ≤ 0.001] and r = 0.886 [P ≤ 0.001], respectively) (Fig. 1). Antibodies from four semi-immune individuals directly inhibited the growth of P. falciparum blood-stage parasites in vitro (Fig. 2A). Total IgG from the two donors with the highest growth inhibition (GI) activity were analyzed for the contribution of MSP-1-specific antibodies to growth inhibitory activity by using an antigen-reversal GIA. Here, growth inhibition assays were performed in the presence of increasing concentrations of MSP-1D. While MSP-1D had no effect on IgG from donor 8, the growth inhibitory activity of IgG from donor 6 was diminished by about 50% upon addition of MSP-1 (Fig. 2B). Interestingly, donor 6 had the highest titer of antibodies to MSP-1 (Fig. 1), suggesting that high antibody concentrations are required in GI assays. Thus, naturally acquired MSP-1-specific antibodies can contribute to growth inhibitory activity in vitro.
FIG 1.
Antibody profiles of 11 semi-immune individuals from Burkina Faso. Levels of antibodies to P. falciparum 3d7 schizont lysate, merozoites, and MSP-1D were determined by ELISAs. Spearman's rank correlation coefficients for levels of antibodies to MSP-1 and to P. falciparum schizont (SZ) lysate or merozoites were r = 0.899 (P ≤ 0.001) and r = 0.886 (P ≤ 0.001), respectively. Nouna Pool, IgG from 11 semi-immune individuals from Nouna, Burkina Faso; WHO Pool, IgG from malaria-exposed Kenyan adults (NIBSC code 10/198 [64]); Naive Pool, IgG from malaria-naive European individuals (n = 4).
FIG 2.
Contributions of antibodies to MSP-1 to growth inhibitory activity. (A) Growth inhibitory activity of protein G-purified IgG from 11 semi-immune donors in three independent experiments, each with triplicate measurements. Statistical differences in the GI activities of the semi-immune individuals and a malaria-naive pool were assessed by t tests. *, P ≤ 0.05; ***, P ≤ 0.001. (B) Antigen-reversal GIA with increasing concentrations of MSP-1D. P. falciparum (3D7) parasites were cultured in the presence of purified IgG and increasing concentrations of competitor antigen MSP-1D for one parasite cycle (40 h). The readout is the activity of Plasmodium LDH (pLDH). Mean values are shown with standard deviations; statistical differences were assessed using paired t tests (donor 6, P = 0.024).
Correlation of MSP-1 antibody titers with neutrophil respiratory burst activity.
Antibody-dependent cellular immunity was analyzed via the respiratory burst (ADRB) assay (19), based on the killing of parasites by ROS from recruited neutrophils (see Fig. S1 in the supplemental material). The ADRB assay, in which oxygen radicals are measured by chemiluminescence monitoring using isoluminol, was established in our laboratory. A clear chemiluminescence signal was detected only if all assay components, i.e., P. falciparum merozoites, purified IgG from malaria-exposed individuals, freshly purified polymorphonuclear neutrophils (PMNs), and isoluminol, were present. Only background activity was obtained with IgG from malaria-naive individuals (Fig. S2). The ADRB index was calculated as described previously (19, 37, 39), using the chemiluminescence maximum of the curve. Interestingly, this readout correlated perfectly with the total area under the curve within the first 60 min (Pearson correlation coefficient of r = 0.996) (Fig. S3). Furthermore, the ADRB assay showed remarkable reproducibility even when different P. falciparum merozoite and PMN preparations were used (intra-assay coefficient of variation [CV] of <6% and interassay CV of <12%) (Fig. S4).
Purified IgG from all 11 semi-immune individuals from Burkina Faso could mediate the activation of neutrophils and the release of ROS (Fig. 3A); remarkably, the ADRB activity of these donors was higher than the activity measured with the WHO IgG pool prepared from malaria-exposed Kenyan adults (National Institute for Biological Standards and Control [NIBSC] code 10/198 [64]) (Fig. S5A). The level of ADRB activity correlated well with levels of antibodies to MSP-1D (Spearman's r = 0.758; P = 0.001) (Fig. 3B), MSP-1F (r = 0.615; P = 0.024) (Table S1), and P. falciparum schizont lysate (r = 0.767; P < 0.001) and merozoites (r = 0.855; P < 0.001) (Fig. 3C). Thus, MSP-1-specific antibodies may opsonize merozoites and activate neutrophils.
FIG 3.
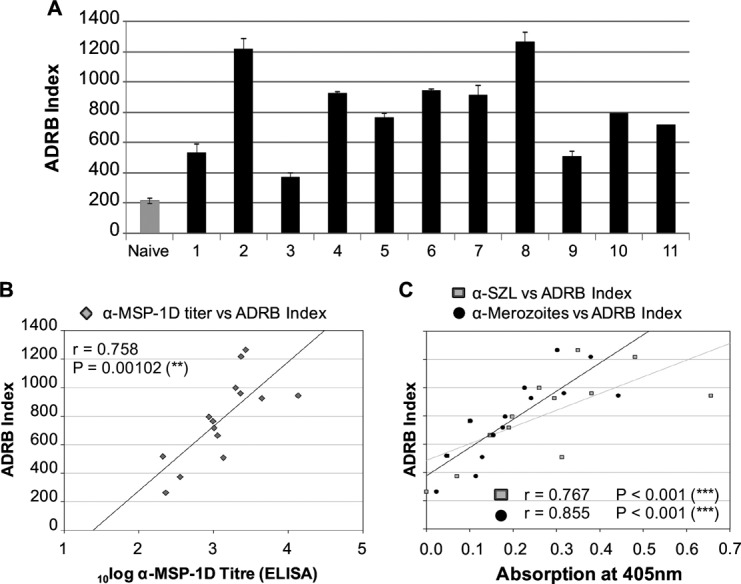
Correlation of ADRB activity with MSP-1 antibody titers. (A) ADRB activity of 11 semi-immune individuals and a malaria-naive pool (n = 4) against P. falciparum 3d7 merozoites. The means of duplicate measurements with standard errors of the mean (SEMs) are shown. (B) MSP-1 antibody titers in semi-immune sera, correlating with ADRB activity (Spearman's rank correlation coefficient of r = 0.758). (C) Levels of antibodies to P. falciparum 3d7 schizont lysate (α-SZL) and merozoites, correlating with ADRB activity (Spearman's rank correlation coefficients of r = 0.767 and r = 0.855, respectively).
Affinity-purified MSP-1 antibodies opsonize P. falciparum merozoites.
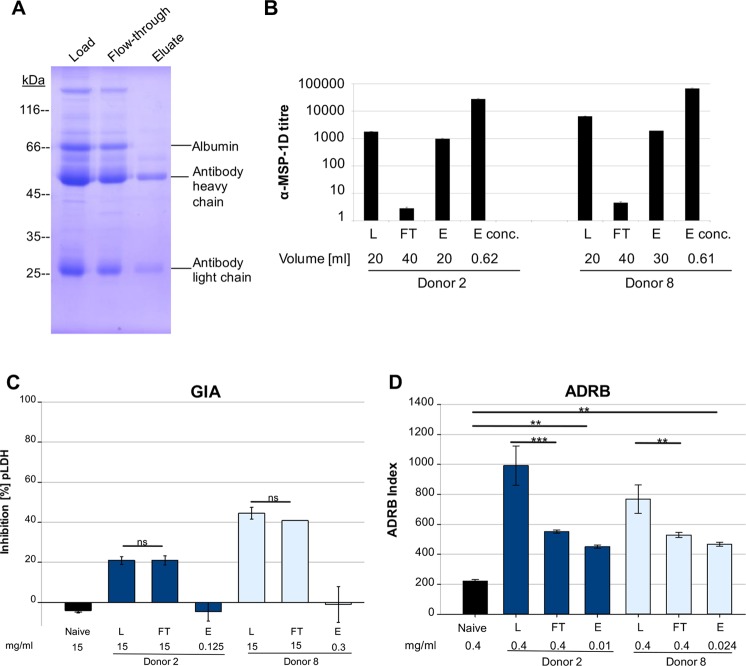
To further examine opsonizing MSP-1-specific antibodies, sera from two semi-immune individuals (donors 2 and 8) were fractionated by antigen affinity purification with MSP-1D. A preparation of MSP-1-specific antibodies was obtained (Fig. 4A and B), while the flowthrough (FT) material was efficiently depleted, as shown by enzyme-linked immunosorbent assay (ELISA) (Fig. 4B). Importantly, ADRB activity was reduced substantially in the FT fraction, compared to total IgG, and MSP-1-specific antibodies could opsonize merozoites and activate neutrophils (Fig. 4D). Interestingly, the same eluate fractions containing MSP-1-specific antibodies were not active in the GI assay, while MSP-1-depleted sera showed undiminished activity (Fig. 4C). Thus, MSP-1-specific antibodies from donors 2 and 8, although not active in the GI assay, were capable of activating neutrophils via opsonized merozoites.
FIG 4.
MSP-1 as target of opsonizing antibodies. (A and B) Sera from two semi-immune individuals were fractionated by antigen affinity purification with MSP-1D. The fractions were analyzed by Coomassie blue staining (shown for donor 2) (A) and ELISA (B). MSP-1 antibody titers were strongly reduced in MSP-1-depleted IgG (flowthrough [FT] fraction) and enriched in the eluate fractions (E and Econc.). (C) Affinity-purified MSP-1 antibodies did not neutralize the parasite. Affinity-purified MSP-1 antibodies (E) from donors 2 and 8 did not inhibit parasite growth in vitro, while MSP-1-depleted sera (FT) showed undiminished activity, similar to that of total IgG (L). (D) Affinity-purified MSP-1 antibodies opsonized merozoites. ADRB activity was reduced in MSP-1-depleted IgG (FT), compared to the total IgG fraction (L). MSP-1-specific IgG (E) could recruit neutrophils. Statistical differences were assessed using one-way repeated-measures ANOVA. **, P ≤ 0.01; ***, P ≤ 0.001.
MSP-1 and its processing fragment MSP-183 are major targets of opsonizing antibodies.
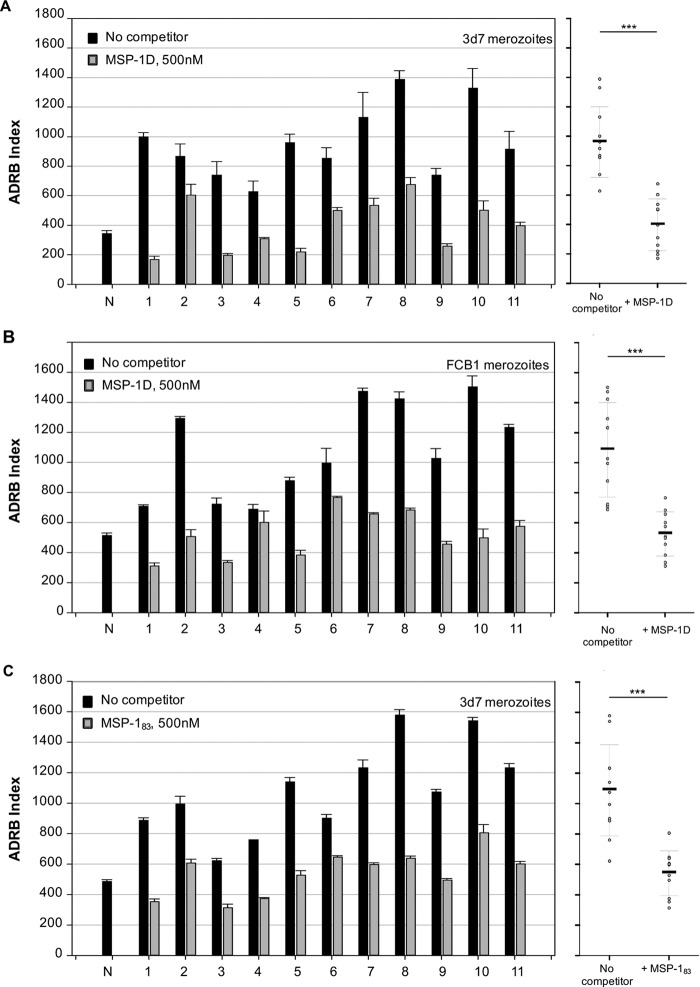
An antigen-reversal ADRB assay was established in order (i) to assess whether MSP-1-specific antibodies play a role in opsonization in all 11 semi-immune individuals from Burkina Faso, (ii) to examine potential cross-reactivity of the opsonizing antibodies between different P. falciparum strains, and (iii) to identify the MSP-1 processing fragments eliciting opsonizing antibodies. By preincubation of serum antibodies with recombinant antigens such as MSP-1, the contribution of antigen-specific antibodies to ADRB activity could be quantified. Prior to their use in antigen-reversal ADRB assays, all recombinant antigens were pretested for their chemiluminescence activity in the presence of PMNs and isoluminol but in the absence of merozoites; all competitors showed only background chemiluminescence activity (Fig. S5B). Furthermore, preincubation of human IgG from semi-immune individuals together with control proteins such as bovine serum albumin (BSA) did not reduce ADRB activity (Fig. S5A), indicating that the potential decrease of ADRB activity by preincubation of antibodies with MSPs is specific. After identifying an appropriate IgG concentration for the antigen-reversal ADRB assay (200 μg/ml for human IgG) (Fig. S6), we determined an adequate competitor antigen concentration by preincubation of IgG from semi-immune individuals with increasing concentrations of MSP-1D, followed by the ADRB assay. Neutrophil respiratory burst activity decreased substantially even at very low MSP-1D concentrations (2 nM) and followed a hyperbolic decay curve (Fig. S7); to ensure that nearly all antigen-specific antibodies were removed, a competitor antigen concentration of 500 nM was chosen.
In the presence of 500 nM MSP-1D antigen, ADRB activity was strongly reduced in all 11 semi-immune donors from Burkina Faso. The mean reductions were 58.6% using P. falciparum (3d7) merozoites and 51.7% using P. falciparum (FCB1) merozoites (Fig. 5A and B), indicating strong cross-reactivity of MSP-1-specific opsonizing antibodies. Levels of antibodies to the partially conserved MSP-1D processing fragments MSP-183 and MSP-142 were comparable in the 11 individuals from Burkina Faso (Fig. S8). Interestingly, preincubation of antibodies from the semi-immune individuals with 500 nM MSP-183 greatly reduced ADRB activity; the mean reduction was 50.2% (Fig. 5C). Thus, MSP-1, especially its processing fragment MSP-183, was a major target of opsonizing antibodies in individuals with naturally acquired immunity.
FIG 5.
MSP-1 as target of opsonizing antibodies (antigen-reversal ADRB assay) and importance of conserved regions. ADRB activity of protein G-purified IgG from 11 semi-immune donors was analyzed against P. falciparum 3d7 (A and C) and FCB-1 (B) merozoites in the absence and presence of MSP-1D (A and B) or its processing fragment MSP-183 (C). Mean values of duplicate measurements are shown as individual columns (left) and in vertical point plots (right); statistical differences were calculated using paired t tests. The average reductions of the means in the presence of competitor were 58.6% (MSP-1D with 3d7 merozoites), 50.2% (MSP-183 with 3d7 merozoites), and 51.7% (MSP-1D with FCB1 merozoites). N, IgG from malaria-naive European donors (n = 4). ***, P ≤ 0.001.
Immunization with MSP-1D induces opsonizing antibodies in rhesus monkeys.
Purified IgGs from rhesus monkeys (n = 5) that had been immunized three times with formulated MSP-1D were examined with the antigen-reversal ADRB assay for opsonizing antibodies directed against MSP-1D and MSP-183. Sera were sampled 2 weeks (day 70) and >4 months (day 182) after the third immunization. Levels of antibodies against MSP-1D were comparable in immunized rhesus monkeys (day 70) and in the Nouna IgG pool (Fig. S8B). Interestingly, the observed high level of ADRB activity induced by immunization with MSP-1D was similar to the activity found for the Nouna IgG pool (Fig. 6) and was efficiently competed out by preincubating the IgGs with 500 nM MSP-1D. A substantial decrease was also observed after preincubation with 500 nM MSP-183 (Fig. 7). Comparable results were obtained with P. falciparum 3d7 or FCB1 strains. Remarkably, ADRB activities were similar at day 70 and day 182 (Fig. 6), indicating that immunization of nonhuman primates with MSP-1D apparently induces a long-lasting antibody response capable of opsonizing P. falciparum merozoites. In summary, both natural exposure to malaria and immunization of rhesus monkeys with recombinant MSP-1D elicited antibodies against MSP-1 and its processing fragment MSP-183 that were effective in merozoite opsonization and neutrophil activation.
FIG 6.
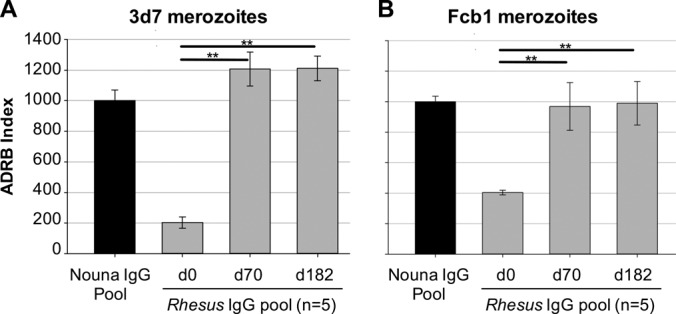
Immunization with MSP-1D eliciting opsonizing antibodies in rhesus macaques. Rhesus monkeys (n = 5) were immunized three times (days 0, 28, and 56) with recombinant MSP-1D and adjuvant (CoVaccine HT). Blood was sampled on days 0, 70, and 182. The ADRB activity of protein G-purified IgG from blood was analyzed against P. falciparum 3d7 (A) and FCB1 (B) merozoites. d0, preimmune control IgG; Nouna IgG Pool, protein G-purified IgG from 11 semi-immune individuals from Nouna, Burkina Faso. Statistical differences were assessed by one-way repeated-measures ANOVA. **, P ≤ 0.01.
FIG 7.
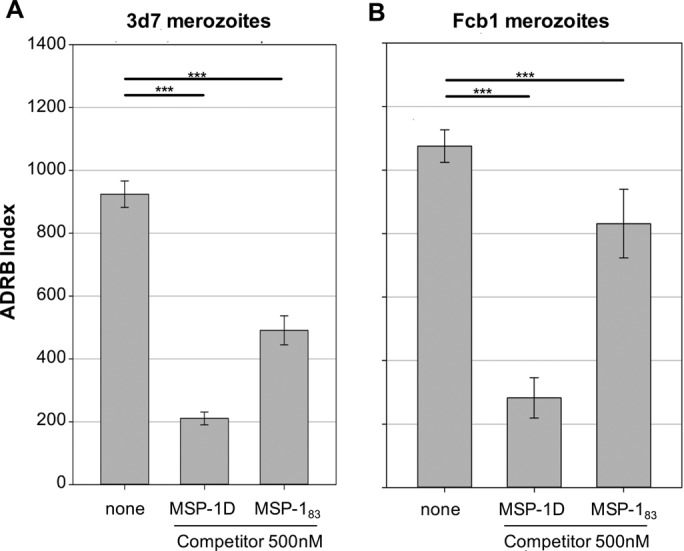
Antigen-reversal ADRB assay with rhesus MSP-1D antibodies and MSP-1 competitors. Protein G-purified IgG from rhesus monkeys (n = 5) that had been immunized three times with MSP-1D (day 70) was analyzed for opsonizing activity with 3d7 (A) and FCB1 (B) merozoites, in the presence of 500 nM MSP-1D or MSP-183. Shown are the means of two independent experiments, each with duplicate measurements. Statistical differences were assessed using one-way ANOVA. ***, P ≤ 0.001.
DISCUSSION
The importance of antibodies for protection against malaria was established in 1961 by passive transfer experiments with immunoglobulins (7). Their antigenic targets and protective mechanisms remain unclear, but such information is urgently needed for malaria vaccine development. Correlations between direct growth inhibition of Plasmodium blood stages in vitro, as measured in the GIA, and protection against malaria have been weak and inconsistent (reviewed in reference 16), suggesting that protective antibodies may act via other functional mechanisms. Indeed, recent studies strongly implicated the importance of opsonizing antibodies for naturally acquired immunity (18, 19, 23–27). Antibodies that opsonize P. falciparum merozoites and mediate the release of reactive oxygen species from neutrophils can be measured in the ADRB assay. ADRB activity is associated with protection against clinical malaria in different transmission areas in Senegal (19), as well as with protection against severe malaria in Kenyan children within the first 6 months of life (65). Despite a poor correlation between ADRB activity and GIA activity (18, 38), total IgG active in both assays is associated with protection against severe malaria in Kenyan children (38). Thus, protective immunity may result from a combination of antibody-mediated mechanisms.
Here, we provide further support for opsonizing antibodies mediating respiratory bursts of neutrophils in individuals with naturally acquired immunity against malaria and we identify P. falciparum MSP-183 as a major target antigen of ADRB activity. By using sera from semi-immune individuals from Burkina Faso, we show that (i) MSP-1-specific antibodies partly contribute to direct growth inhibitory activity (Fig. 2), (ii) the titers of antibodies to MSP-1 correlate with ADRB activity (Fig. 3), (iii) antibodies eliciting respiratory burst activity are mainly cross-reactive (Fig. 5), and (iv) MSP-1 and its partially conserved subunit MSP-183 are important targets of opsonizing antibodies (Fig. 4 and 5). Furthermore, we could induce in nonhuman primates antibodies that were functional in ADRB assays by immunization with recombinant MSP-1D (Fig. 6 and 7).
So far, only one antigenic target of ADRB activity has been identified, namely, the conserved C-terminal part of MSP-1, P. falciparum MSP-119 (39). Antibodies to P. falciparum MSP-119 were shown to contribute about 33% of the total neutrophil respiratory burst activity in two Senegalese villages, using isogenic P. falciparum parasites with the non-cross-reactive MSP-119 orthologue from Plasmodium chabaudi or sera after removal of P. falciparum MSP-119-specific antibodies (39). Since we used alternative experimental approaches, including an ADRB protocol that differs in certain respects, such as the use of sera versus purified IgG and the use of sera from a different African country, direct comparison of our results with those of the previous study is not possible. However, we show that antibodies, either acquired by natural exposure to malaria or by immunization of rhesus monkeys with recombinant MSP-1D, opsonize P. falciparum merozoites of different strains equally well and ADRB activity against the P. falciparum FCB1 strain can be efficiently reduced with MSP-1 from the 3d7 strain (MSP-1D). Thus, cross-reactive regions within MSP-1 appear to be the main targets of opsonizing antibodies that elicit neutrophil respiratory burst activity. Interestingly, opsonizing antibodies that recruit monocytes for merozoite phagocytosis, as measured in the OPA, are effective against 15 different parasite strains, indicating high strain transcendence (26). Thus, opsonizing antibodies that are functional in the ADRB assay or the OPA seem to target mainly cross-reactive regions within merozoite antigens, which would be beneficial for vaccine development.
Here, two experimental approaches were employed to quantify the contributions of single antigens to ADRB activity, namely, affinity purification of MSP-1-specific antibodies and the antigen-reversal ADRB assay. Several studies have tested affinity-purified antibodies against several merozoite antigens for their opsonizing capacity, using different assays such as the ADCI assay (44, 66) and the OPA (18, 27). However, this approach is quite time-consuming, requires special equipment for immunoaffinity chromatography, and uses relatively large amounts of serum and antigen. Thus, the antigen-reversal ADRB methodology, in which preincubation of serum antibodies with recombinant antigens allows us to quantify the contributions of certain antigens to ADRB activity, was developed. Since this relatively easy approach requires only small amounts of serum and recombinant antigen, it could be readily adapted to other merozoite antigens.
Importantly, we could induce opsonizing antibodies that cause neutrophil respiratory burst activity in rhesus monkeys by immunization with recombinant full-length MSP-1 (Fig. 6). This finding has several major implications. First, we demonstrate here for the first time that antibodies raised in nonhuman primates are compatible with human Fcγ receptors on neutrophils. To date, this has been one of the major limitations of the ADRB assay, since no animal species in which compatible antibodies can be generated has been known and neutrophils from other species, such as mice, differ substantially from human neutrophils regarding their Fcγ receptor expression and binding abilities (67). Second, we show for the first time that antibodies functional in ADRB activity can be induced by immunization with a recombinant protein. In a recent study, ADRB-effective antibodies could not be induced by vaccination of mice with AdHu5-PyMSP-142 but could be induced by primary infection with Plasmodium yoelii (68). Opsonizing activity of antibodies raised in mice or rabbits by immunization with different merozoite antigens was recently reported for opsonic phagocytosis (69) and complement-dependent inhibition (23). However, this is the first report on opsonizing antibodies induced in primates. Third, we reveal that immunization with recombinant MSP-1D generates antibodies that are (i) long-lasting, with undiminished ADRB activity more than 4 months after the last immunization, (ii) cross-reactive, with similar activities against P. falciparum 3d7 and FCB1 parasites, and (iii) active via the same functional mechanisms as naturally acquired antibodies, thus supporting full-length MSP-1 as a promising vaccine candidate.
Indeed, protective potential of MSP-1 has been suggested by various epidemiological studies in regions in which malaria is endemic (57–60), as well as by immunization experiments with mice (46) and monkeys (61–63, 70, 71). However, the epitopes within MSP-1 that elicit protective antibodies and their immune mechanisms have not been identified. Most studies to date have analyzed the direct growth inhibitory potential of MSP-1-specific antibodies in vitro, although a correlation between this mechanism and protective immunity remains elusive. Furthermore, it is controversial whether and under what circumstances MSP-1 contributes to direct growth inhibition of Plasmodium blood stages, since some publications reported MSP-1-specific GI activity (50, 51, 56, 71–75) and others presented contradicting data (17, 63, 76–78). The different results regarding MSP-1-specific GI activity both in previous studies and in our study could have several explanations, i.e., (i) there may be different IgG isotypes or affinities of MSP-1 antibodies that partly inhibit the growth of P. falciparum in vitro, (ii) antibodies may target various MSP-1 epitopes and only some are growth inhibitory, and (iii) the MSP-1 antibody titer may be too low for detectable GI activity in the in vitro assay. The latter is a very reasonable possibility, since high antibody levels are necessary for direct growth inhibitory activity in vitro (76), although this might not reflect the in vivo situation. We could measure a contribution of MSP-1 antibodies to GI activity only for the African donor with the highest MSP-1 antibody titer (Fig. 2). Interestingly, affinity-purified antibodies against MSP-183 from the same individual efficiently inhibited the growth of P. falciparum in vitro (56), supporting our results with the antigen-reversal GIA.
Importantly, besides direct growth inhibition, MSP-1 also elicits opsonizing antibodies that can act at much lower antibody levels, compared to the GIA. Different immune mechanisms mediated by MSP-1-specific opsonizing antibodies have been described, including (i) the recruitment of monocytes by MSP-1block 2 antibodies, as measured via the ADCI assay (40), (ii) the fixation of complement factors by antibodies directed against MSP-119 and MSP-1block 2, as detected via antibody-mediated complement-dependent inhibition (23), and (iii) the induction of neutrophil respiratory burst activity, as analyzed via the ADRB assay and reported for antibodies to MSP-119 (39) and MSP-183 (this work). However, MSP-119 antibodies did not seem to play a role in monocyte opsonic phagocytosis, since the OPA response for transgenic P. falciparum merozoites with the MSP-119 orthologue from P. chabaudi did not differ from that for wild-type parasites (26). Furthermore, we observed a high level of cross-reactivity between different parasite strains in the ADRB assay, suggesting that antibodies to the highly polymorphic block 2 of MSP-1, which is located within MSP-183, likely play no role in neutrophil respiratory burst activity. Thus, opsonizing antibodies elicited by different regions within MSP-1 appear to act by various independent immune mechanisms. Interestingly, higher antibody levels and their actions via multiple mechanisms are associated with naturally acquired immunity (79, 80), suggesting that several antibody-mediated mechanisms contribute to protection against malaria. Since the antigenic epitopes within MSP-1 are distributed over the whole protein and elicit a variety of immune responses, we propose full-length MSP-1 as a malaria vaccine candidate.
In summary, we show that the malaria antigen MSP-1 elicits not only neutralizing but also opsonizing antibodies against P. falciparum blood-stage parasites. Our data suggest that full-length MSP-1 is a major target of opsonizing antibodies, which elicit neutrophil respiratory burst activity and are acquired during natural exposure to malaria. Furthermore, we show for the first time that opsonizing antibodies can be induced in primates by immunization with a recombinant antigen, and we provide further support for the ADRB assay as a robust functional assay of humoral immunity. Finally, our data strengthen the idea of full-length MSP-1 as a promising vaccine candidate, with the induction of opsonizing antibodies as an important immune mechanism.
MATERIALS AND METHODS
Study population.
Blood samples were obtained from 11 healthy young adults (18 to 31 years of age) from Nouna, an area in Burkina Faso with high rates of seasonal malaria transmission. Blood donors (i) had experienced no fever episodes within the previous 15 days, (ii) had not been vaccinated within the previous month, (iii) had received no medication within the previous 2 weeks, and (iv) were HIV, hepatitis B virus (HBV), and syphilis (VDRL test) negative. Blood donations of 100 ml each were taken at the end of the dry season (with low rates of malaria transmission). Lymphocytes and sera were prepared via Ficoll centrifugation and were transported to Heidelberg. Ethical approval was granted by the Ethical Committee of the Medical Faculty, University of Heidelberg, and B. Kouyaté, director of the Centre de Recherche en Santé de Nouna (CRSN).
Rhesus monkey immunizations.
Rhesus monkey immunizations and antibody preparation were carried out at the Biomedical Primate Research Centre (BPRC) (Rijswijk, Netherlands) in 2010. The BPRC is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and is compliant with recommendations of the Weatherall report on the use of nonhuman primates in research (81). The study was approved by an independent ethics committee at BPRC, constituted in accordance with Dutch law (DEC598) and European Acts (directive 2010/63/EU) on animal experimentation. To minimize animal discomfort, immunization and blood sampling were all performed under ketamine sedation. Animals were assigned in a manner that ensured that age, weight, and sex distributions were similar among groups, and treatments were randomly assigned to groups. Captive-bred rhesus macaques (n = 5 [2 female and 3 male]; age, 4.6 to 12.5 years) were immunized intramuscularly 3 times (on days 0, 28, and 56) with 100 μg MSP-1D per dose, formulated with CoVaccine HT (82, 83), in a 500-μl volume. From each animal, 29 ml blood was collected by venous puncture on days 0, 28, 56, 70, 126, and 182. IgGs from sera were purified by protein G affinity purification and were stored at −80°C.
Rabbit immunizations.
Rabbit immunizations were performed at Confarma France SARL (Hombourg, France), in compliance with animal health legal regulations and according to European Commission good manufacturing practice (GMP) and current GMP guidelines. New Zealand White rabbits (n = 6) were immunized intramuscularly with lyophilized full-length P. falciparum MSP-1D (50 μg) and Infectious Disease Research Institute (IDRI) soluble emulsion (SE) adjuvant plus glucopyranosyl lipid adjuvant (GLA) (100 μg), in a total volume of 500 μl, on days 0, 28, and 56. Two weeks after the last immunization (day 70), blood was sampled by heart puncture and serum was prepared and transported to Heidelberg. There, IgGs were purified from serum via protein A affinity chromatography and were tested for growth inhibitory activity.
P. falciparum merozoite antigens.
MSP-1D was produced in Escherichia coli and purified under GMP-compatible conditions by Biomeva GmbH (Heidelberg, Germany), as will be described elsewhere. Recombinant MSP-1F (183.4 kDa, from the FCB1 strain) and the MSP-1D fragments MSP-142, MSP-138, and MSP-130 were produced and purified as described by Kauth et al. (84). MSP-183 (81.8 kDa, from the 3d7 strain) was expressed and purified as described previously (85). All proteins except MSP-1D contain a N-terminal hexahistidine tag. BSA (66 kDa) was obtained commercially (product number 8076.2; Carl Roth, Karlsruhe, Germany).
P. falciparum (3d7) schizont extract was produced from a highly synchronous late-schizont-stage culture with high levels of parasitemia via saponin lysis (0.2% saponin in 7.5 mM NaCl plus 0.25 mM sodium citrate [pH 7.0]) of RBCs, followed by complete lyses of parasite schizonts and protein solubilization using radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich). The parasite protein extract was stored at −80°C.
Antibody controls.
Positive controls included (i) a serum pool from malaria-semi-immune individuals (n = 11) from Nouna, Burkina Faso, called the Nouna IgG pool, and (ii) a WHO reference reagent for anti-malaria (P. falciparum) human serum, i.e., a serum pool from malaria-exposed individuals from Kisumu, Kenya (NIBSC code 10/198 [64]). Negative controls were two pools of sera from malaria-naive individuals (each n = 4) from Europe.
Purification of antibodies from human sera.
Sera were heat inactivated for 30 min at 56°C. Total IgG was purified by protein G affinity chromatography (Pierce, Thermo Scientific), according to the manufacturer's instructions. Briefly, 5 ml serum was diluted with 10 ml binding buffer, incubated overnight at 4°C, and centrifuged at 2,400 × g for 10 min at 4°C, and the supernatant was passed over 5 ml of washed and equilibrated resin (protein G immobilized to agarose). After washing with binding buffer (Per-Bio) and phosphate-buffered saline (PBS), IgG was eluted with 10 ml elution buffer (0.1 M glycine [pH 2.5]) and immediately neutralized with 1.5 ml 1 M Tris (pH 8.0). IgG was then dialyzed against RPMI 1640 medium and concentrated using Amicon Ultra centrifugal filters (Millipore), followed by sterile filtration (0.22 μm) and adjustment of the concentration to 30 mg/ml with RPMI 1640 medium. The IgG preparations were stored at −20°C.
Chromatographic affinity purification of MSP-1-specific antibodies from human serum was performed using the Äkta Purifier 100 system (GE Healthcare). MSP-1D was covalently coupled to a preactivated resin (Ultra Link Biosupport; Thermo Scientific) according to the manufacturer's instructions. In total, 44 mg MSP-1D was coupled to 625 mg dry beads, corresponding to 5 ml resin. Human serum (20 ml) was diluted 1:1 with PBS, incubated overnight at 4°C, and centrifuged at 2,400 × g for 10 min, and the supernatant was applied to the column with a flow rate of 0.5 ml/min. After washing with PBS, anti-MSP-1 antibodies were eluted with an acidic buffer (75 mM glycine, 0.5 M NaCl [pH 2.8]) and immediately neutralized with 0.1 volume of 1 M Tris (pH 8.0). The eluate was sterile filtered (0.22 μm), concentrated, and dialyzed against RPMI 1640 medium using Amicon Ultra centrifugal filters (Millipore). All fractions from the affinity purification were analyzed via Coomassie blue-stained SDS gels and a MSP-1D ELISA.
ELISA.
The ELISA was performed as described previously (86, 87), with minor modifications; 96-well microtiter plates (Nunc MaxiSorp; Thermo Fisher) were coated with 100 nM recombinant protein in 0.1 ml coating buffer (34 mM Na2CO3, 16 mM NaHCO3 [pH 10.6]) or with 0.5 μg P. falciparum merozoites or schizont extract in 0.1 ml PBS. Goat anti-human IgG-alkaline phosphatase conjugate (Sigma-Aldrich, Germany), diluted 1:30,000 in blocking buffer, was used as the secondary antibody. The reaction was stopped with 0.1 ml NaOH (0.2 M), and the optical density at 405 nm (OD405) was determined.
Antibody titers against recombinant antigens were calculated using a trendline generated with optical density (OD) values of serial dilutions in the linear range; antibody endpoint titers corresponded to the antibody dilutions at OD405 values of 0.2. Since a close correlation between single-point OD values at appropriate dilutions and antibody endpoint titers has been reported (88, 89), single-point OD values in the linear range were used as proxies for levels of antibodies against merozoites and the schizont lysate. To define the linear range, a standard curve was generated using the IgG pool from malaria-exposed individuals from Kenya (WHO standard), in serial dilutions. For quality control, samples were analyzed in duplicate and pools from malaria-naive donors (n = 4) and malaria-exposed donors from Burkina Faso (n = 11) and Kenya (NIBSC code 10/198 [64]) were included for each measurement.
Growth inhibition assay.
Protein G-purified IgG from human sera was analyzed for its ability to inhibit growth of P. falciparum (3d7) parasites based on the activity of Plasmodium lactate dehydrogenase (LDH), according to previously described protocols (56, 90) with modifications. Briefly, 25 μl (total volume) IgG at different concentrations and 25 μl schizont-stage parasites (0.6% parasitemia and 4% hematocrit) were added in triplicate to a sterile 96-well F-bottom plate (Greiner). Controls, i.e., (i) 25 μl RPMI 1640 medium plus 25 μl parasites (parasite growth control), (ii) 25 μl culture medium plus 25 μl 4% hematocrit (RBC control), (iii) rabbit anti-AMA-1 antibodies (BG98 GIA standard from BPRC) (positive control), and (iv) IgG pool from malaria-naive individuals (n = 4) (negative control), were included. Following 40 h of incubation, parasite growth was detected using a biochemical assay based on Plasmodium LDH activity, measured as OD650. Growth inhibition was calculated as follows: inhibition (%) = 100% − [(OD650 for IgG sample − OD650 for RBC control)/(OD650 for parasite growth control − OD650 for RBC control)] × 100.
The antigen-reversal GIA was developed in order to quantify the contribution of antigen-specific antibodies to growth inhibitory activity. A total volume of 10 μl/well competitor antigen (MSP-1D, 1 mg/ml) in serial dilutions and 15 μl IgG (stock concentrations of 40 mg/ml for human IgG and 20 mg/ml for rabbit IgG) were preincubated in a sterile 96-well plate (Greiner) for 1 h at 37°C, in 5% CO2 and 3% O2 with 95% humidity. Then 25 μl schizont-stage parasites (0.6% parasitemia and 4% hematocrit) were added and incubated for 40 h. In addition to the controls described above, a competitor control (highest competitor concentration used, with no IgG) and an IgG control (no competitor) were included. The following procedure was performed as described above.
P. falciparum culture and merozoite preparation.
P. falciparum 3d7 and FCB1 laboratory strains were cultured at 37°C, in 5% CO2 and 3% O2 with 95% humidity, in culture medium (RPMI 1640 medium supplemented with l-glutamine, 25 mM HEPES, 0.1 mM hypoxanthine, 20 μg/ml gentamicin, and 10% human serum) at 4% hematocrit. Parasite cultures were synchronized with 5% d-sorbitol at the ring stage.
Merozoite extracts were prepared from synchronized schizont-stage cultures with about 5% parasitemia. Schizonts were ruptured by mechanical force (pipetting) and centrifuged twice at 400 × g for 20 min to remove erythrocytes (pellet), and merozoites were recovered from the supernatant by centrifugation for 15 min at 1,500 × g. The merozoite pellet was resuspended in RPMI 1640 medium and stored at −20°C. The quality of the merozoite preparation was assessed via Giemsa-stained smears, and merozoite numbers were estimated by fluorescence-activated cell sorting (FACS) measurements. Prior to use, all merozoite preparations were tested in parallel with the NA pool in the ADRB assay and their concentration was adjusted to show the same ADRB activity.
PMN purification.
Whole-blood samples from malaria-naive healthy adults were collected in lithium-heparin-containing tubes (Sarstedt). Written informed consent was obtained from all participants, and ethical approval was granted by the Ethical Committee of the Medical Faculty, University of Heidelberg. Blood from 3 donors (12 ml each) was pooled, mixed 1:1 with 3% dextran (Carl Roth) in 0.9% NaCl, and incubated for 18 min at room temperature to pellet RBCs. The supernatant was centrifuged (500 × g for 10 min at 4°C), and the thin white layer of PMNs was resuspended in 0.9% NaCl. This suspension was layered carefully on top of Ficoll-Histopaque (Sigma-Aldrich) and centrifuged at 400 × g for 35 min at room temperature without break. The thin PMN layer above the erythrocytes was resuspended in ice-cold double-distilled water, incubated for 30 s to lyse remaining erythrocytes, and neutralized with an equal volume of 1.8% NaCl. After centrifugation (500 × g for 5 min at 4°C), the pellet was washed with Hanks' balanced salt solution (Thermo Fisher Scientific) and centrifuged, and the PMN pellet was resuspended in cold PBS. The quality of the preparation and PMN numbers were determined in a hemacytometer after trypan blue staining. The PMN concentration was adjusted to 1.3 × 107 cells/ml (ADRB assay) or 2.5 × 107 cells/ml (antigen-reversal ADRB assay) with sterile PBS; the purity and viability of the cells were >95%. PMNs were stored at 4°C and used for ADRB assays within <1 h after isolation.
ADRB assay.
The ADRB assay measures the generation of ROS via chemiluminescence; it was performed as described previously (19) with modifications. Briefly, 40 μl P. falciparum merozoites (∼2.5 × 105 merozoites) and 10 μl protein G-purified IgG (10 mg/ml) were incubated for 1 h at 37°C in opaque white 96-well Lumitrac microplates (Greiner Bio-One). Freshly purified human PMNs (100 μl/well, 1.3 × 107 cells/ml PBS) and isoluminol (4-aminophthalhydrazide) (100 μl/well of a 1:100 dilution in PBS of a 4-mg/ml stock solution in dimethyl sulfoxide; Santa Cruz Biotechnology) were added rapidly using an Eppendorf multichannel pipette. Chemiluminescence detection started immediately using a FLUOstar Optima microplate reader (BMG Labtech), with 1-s measurements every minute for 2 h.
For the antigen-reversal ADRB assay, 50 μl competitor protein (2.5 μM in PBS, sterile filtered) or PBS and 10 μl protein G-purified IgG (human IgG, 5 mg/ml; rhesus IgG, 1.25 mg/ml) were incubated for 1.5 h at 37°C in opaque white 96-well Lumitrac microplates. P. falciparum merozoites (40 μl) were added to each well and incubated for 1.5 h at 37°C. Freshly purified human PMNs (50 μl/well, 2.5 × 107 cells/ml PBS) and isoluminol were added rapidly and the subsequent procedure was performed as described above.
For calculation of the ADRB index, the chemiluminescence activity (in light units [LU]) of a sample was normalized to the chemiluminescence activity of a semi-immune IgG pool from Burkina Faso by calculating the ADRB index with the following formula: ADRB index = (LU for the sample maximum/LU for the semi-immune IgG pool maximum) × 1,000. The semi-immune IgG pool from Burkina Faso (n = 11), an isoluminol-only control, and at least one malaria-naive pool (n = 4) were included on each plate. Competitor proteins were pretested (i) without merozoites and (ii) without merozoites plus IgG. To enable rapid handling, less than 60 wells per plate were used. All samples were measured in duplicate.
Statistical analysis.
SigmaPlot version 12.3 (Systat Software) was used for data analysis. Antibody levels (ELISA), GIA values, and ADRB activity levels were compared by Spearman's rank correlation. Paired data were analyzed for statistical significance using paired t tests (for two samples) or one-way repeated-measures analysis of variance (ANOVA) (for >2 samples); unpaired data were examined using t tests (for two samples) or one-way ANOVA (for >2 samples).
Supplementary Material
ACKNOWLEDGMENTS
We are very grateful to the volunteers from Burkina Faso for participating in this study. Furthermore, the continuous interest and generous support of Michael Lanzer are gratefully acknowledged.
This work was supported by the Deutsche Forschungsgemeinschaft (grant SFB 544), the Bujard Fund within the assets of a foundation at the University of Heidelberg, the German Centre for Infectious Diseases, and the BB-Bank Karlsruhe, Germany. The rhesus monkey study was supported by an EC Small and Medium-sized Enterprises grant (Framework Programme 6, project LSHP-CT-2006-018918), and the BPRC.
We declare we have no conflicting interests relevant to the study.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CVI.00155-17.
REFERENCES
- 1.World Health Organization. 2016. World malaria report 2015. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Halbroth BR, Draper SJ. 2015. Recent developments in malaria vaccinology. Adv Parasitol 88:1–49. doi: 10.1016/bs.apar.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Marsh K, Kinyanjui S. 2006. Immune effector mechanisms in malaria. Parasite Immunol 28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 4.Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. 1999. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med 5:340–343. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 5.Snow RW, Omumbo JA, Lowe B, Molyneux CS, Obiero JO, Palmer A, Weber MW, Pinder M, Nahlen B, Obonyo C, Newbold C, Gupta S, Marsh K. 1997. Relation between severe malaria morbidity in children and level of Plasmodium falciparum transmission in Africa. Lancet 349:1650–1654. doi: 10.1016/S0140-6736(97)02038-2. [DOI] [PubMed] [Google Scholar]
- 6.Aponte JJ, Menendez C, Schellenberg D, Kahigwa E, Mshinda H, Vountasou P, Tanner M, Alonso PL. 2007. Age interactions in the development of naturally acquired immunity to Plasmodium falciparum and its clinical presentation. PLoS Med 4:e242. doi: 10.1371/journal.pmed.0040242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen S, McGregor IA, Carrington S. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 8.Crompton PD, Kayala MA, Traore B, Kayentao K, Ongoiba A, Weiss GE, Molina DM, Burk CR, Waisberg M, Jasinskas A, Tan X, Doumbo S, Doumtabe D, Kone Y, Narum DL, Liang X, Doumbo OK, Miller LH, Doolan DL, Baldi P, Felgner PL, Pierce SK. 2010. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci U S A 107:6958–6963. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osier FH, Fegan G, Polley SD, Murungi L, Verra F, Tetteh KK, Lowe B, Mwangi T, Bull PC, Thomas AW, Cavanagh DR, McBride JS, Lanar DE, Mackinnon MJ, Conway DJ, Marsh K. 2008. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun 76:2240–2248. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richards JS, Arumugam TU, Reiling L, Healer J, Hodder AN, Fowkes FJ, Cross N, Langer C, Takeo S, Uboldi AD, Thompson JK, Gilson PR, Coppel RL, Siba PM, King CL, Torii M, Chitnis CE, Narum DL, Mueller I, Crabb BS, Cowman AF, Tsuboi T, Beeson JG. 2013. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol 191:795–809. doi: 10.4049/jimmunol.1300778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeson JG, Drew DR, Boyle MJ, Feng G, Fowkes FJ, Richards JS. 2016. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol Rev 40:343–372. doi: 10.1093/femsre/fuw001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowkes FJI, Richards JS, Simpson JA, Beeson JG. 2010. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med 7:e1000218. doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osier FH, Mackinnon MJ, Crosnier C, Fegan G, Kamuyu G, Wanaguru M, Ogada E, McDade B, Rayner JC, Wright GJ, Marsh K. 2014. New antigens for a multicomponent blood-stage malaria vaccine. Sci Transl Med 6:247ra102. doi: 10.1126/scitranslmed.3008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teo A, Feng G, Brown GV, Beeson JG, Rogerson SJ. 2016. Functional antibodies and protection against blood-stage malaria. Trends Parasitol 32:887–898. doi: 10.1016/j.pt.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Crompton PD, Miura K, Traore B, Kayentao K, Ongoiba A, Weiss G, Doumbo S, Doumtabe D, Kone Y, Huang C-Y, Doumbo OK, Miller LH, Long CA, Pierce SK. 2010. In vitro growth-inhibitory activity and malaria risk in a cohort study in Mali. Infect Immun 78:737–745. doi: 10.1128/IAI.00960-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duncan CJ, Hill AV, Ellis RD. 2012. Can growth inhibition assays (GIA) predict blood-stage malaria vaccine efficacy? Hum Vaccin Immunother 8:706–714. doi: 10.4161/hv.19712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouharoun-Tayoun H, Attanath P, Sabchareon A, Chongsuphajaisiddhi T, Druilhe P. 1990. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med 172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osier FH, Feng G, Boyle MJ, Langer C, Zhou J, Richards JS, McCallum FJ, Reiling L, Jaworowski A, Anders RF, Marsh K, Beeson JG. 2014. Opsonic phagocytosis of Plasmodium falciparum merozoites: mechanism in human immunity and a correlate of protection against malaria. BMC Med 12:108. doi: 10.1186/1741-7015-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joos C, Marrama L, Polson HEJ, Corre S, Diatta A-M, Diouf B, Trape J-F, Tall A, Longacre S, Perraut R. 2010. Clinical protection from falciparum malaria correlates with neutrophil respiratory bursts induced by merozoites opsonized with human serum antibodies. PLoS One 5:e9871. doi: 10.1371/journal.pone.0009871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanisic DI, Richards JS, McCallum FJ, Michon P, King CL, Schoepflin S, Gilson PR, Murphy VJ, Anders RF, Mueller I, Beeson JG. 2009. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun 77:1165–1174. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiling L, Richards JS, Fowkes FJ, Barry AE, Triglia T, Chokejindachai W, Michon P, Tavul L, Siba PM, Cowman AF, Mueller I, Beeson JG. 2010. Evidence that the erythrocyte invasion ligand PfRh2 is a target of protective immunity against Plasmodium falciparum malaria. J Immunol 185:6157–6167. doi: 10.4049/jimmunol.1001555. [DOI] [PubMed] [Google Scholar]
- 22.Richards JS, Stanisic DI, Fowkes FJ, Tavul L, Dabod E, Thompson JK, Kumar S, Chitnis CE, Narum DL, Michon P, Siba PM, Cowman AF, Mueller I, Beeson JG. 2010. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis 51:e50–e60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- 23.Boyle MJ, Reiling L, Feng G, Langer C, Osier FH, Aspeling-Jones H, Cheng YS, Stubbs J, Tetteh KK, Conway DJ, McCarthy JS, Muller I, Marsh K, Anders RF, Beeson JG. 2015. Human antibodies fix complement to inhibit Plasmodium falciparum invasion of erythrocytes and are associated with protection against malaria. Immunity 42:580–590. doi: 10.1016/j.immuni.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill DL, Eriksson EM, Li Wai Suen CS, Chiu CY, Ryg-Cornejo V, Robinson LJ, Siba PM, Mueller I, Hansen DS, Schofield L. 2013. Opsonising antibodies to P. falciparum merozoites associated with immunity to clinical malaria. PLoS One 8:e74627. doi: 10.1371/journal.pone.0074627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tiendrebeogo RW, Adu B, Singh SK, Dziegiel MH, Nebie I, Sirima SB, Christiansen M, Dodoo D, Theisen M. 2015. Antibody-dependent cellular inhibition is associated with reduced risk against febrile malaria in a longitudinal cohort study involving Ghanaian children. Open Forum Infect Dis 2:ofv044. doi: 10.1093/ofid/ofv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill DL, Wilson DW, Sampaio NG, Eriksson EM, Ryg-Cornejo V, Harrison GL, Uboldi AD, Robinson LJ, Beeson JG, Siba P, Cowman AF, Hansen DS, Mueller I, Schofield L. 2016. Merozoite antigens of Plasmodium falciparum elicit strain-transcending opsonizing immunity. Infect Immun 84:2175–2184. doi: 10.1128/IAI.00145-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu CY, Hodder AN, Lin CS, Hill DL, Li Wai Suen CS, Schofield L, Siba PM, Mueller I, Cowman AF, Hansen DS. 2015. Antibodies to the Plasmodium falciparum proteins MSPDBL1 and MSPDBL2 opsonize merozoites, inhibit parasite growth, and predict protection from clinical malaria. J Infect Dis 212:406–415. doi: 10.1093/infdis/jiv057. [DOI] [PubMed] [Google Scholar]
- 28.Golenser J, Kamyl M, Tsafack A, Marva E, Cohen A, Kitrossky N, Chevion M. 1992. Correlation between destruction of malarial parasites by polymorphonuclear leucocytes and oxidative stress. Free Radic Res Commun 17:249–262. doi: 10.3109/10715769209079517. [DOI] [PubMed] [Google Scholar]
- 29.Gopalakrishnan AM, Kumar N. 2015. Antimalarial action of artesunate involves DNA damage mediated by reactive oxygen species. Antimicrob Agents Chemother 59:317–325. doi: 10.1128/AAC.03663-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown J, Smalley ME. 1981. Inhibition of the in vitro growth of Plasmodium falciparum by human polymorphonuclear neutrophil leucocytes. Clin Exp Immunol 46:106–109. [PMC free article] [PubMed] [Google Scholar]
- 31.Clark IA, Hunt NH. 1983. Evidence for reactive oxygen intermediates causing hemolysis and parasite death in malaria. Infect Immun 39:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allison AC, Eugui EM. 1983. The role of cell-mediated immune responses in resistance to malaria, with special reference to oxidant stress. Annu Rev Immunol 1:361–392. doi: 10.1146/annurev.iy.01.040183.002045. [DOI] [PubMed] [Google Scholar]
- 33.Friedman MJ. 1979. Oxidant damage mediates variant red cell resistance to malaria. Nature 280:245–247. doi: 10.1038/280245a0. [DOI] [PubMed] [Google Scholar]
- 34.Greve B, Lehman LG, Lell B, Luckner D, Schmidt-Ott R, Kremsner PG. 1999. High oxygen radical production is associated with fast parasite clearance in children with Plasmodium falciparum malaria. J Infect Dis 179:1584–1586. doi: 10.1086/314780. [DOI] [PubMed] [Google Scholar]
- 35.Gunjan S, Singh SK, Sharma T, Dwivedi H, Chauhan BS, Imran Siddiqi M, Tripathi R. 2016. Mefloquine induces ROS mediated programmed cell death in malaria parasite: Plasmodium. Apoptosis 21:955–964. doi: 10.1007/s10495-016-1265-y. [DOI] [PubMed] [Google Scholar]
- 36.Cyrklaff M, Srismith S, Nyboer B, Burda K, Hoffmann A, Lasitschka F, Adjalley S, Bisseye C, Simpore J, Mueller AK, Sanchez CP, Frischknecht F, Lanzer M. 2016. Oxidative insult can induce malaria-protective trait of sickle and fetal erythrocytes. Nat Commun 7:13401. doi: 10.1038/ncomms13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perraut R, Joos C, Sokhna C, Polson HE, Trape JF, Tall A, Marrama L, Mercereau-Puijalon O, Richard V, Longacre S. 2014. Association of antibody responses to the conserved Plasmodium falciparum merozoite surface protein 5 with protection against clinical malaria. PLoS One 9:e101737. doi: 10.1371/journal.pone.0101737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murungi LM, Sonden K, Llewellyn D, Rono J, Guleid F, Williams AR, Ogada E, Thairu A, Farnert A, Marsh K, Draper SJ, Osier FH. 2016. Targets and mechanisms associated with protection from severe Plasmodium falciparum malaria in Kenyan children. Infect Immun 84:950–963. doi: 10.1128/IAI.01120-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joos C, Varela ML, Mbengue B, Mansourou A, Marrama L, Sokhna C, Tall A, Trape JF, Toure A, Mercereau-Puijalon O, Perraut R. 2015. Antibodies to Plasmodium falciparum merozoite surface protein-1p19 malaria vaccine candidate induce antibody-dependent respiratory burst in human neutrophils. Malar J 14:409. doi: 10.1186/s12936-015-0935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Galamo CD, Jafarshad A, Blanc C, Druilhe P. 2009. Anti-MSP1 block 2 antibodies are effective at parasite killing in an allele-specific manner by monocyte-mediated antibody-dependent cellular inhibition. J Infect Dis 199:1151–1154. doi: 10.1086/597426. [DOI] [PubMed] [Google Scholar]
- 41.Druilhe P, Spertini F, Soesoe D, Corradin G, Mejia P, Singh S, Audran R, Bouzidi A, Oeuvray C, Roussilhon C. 2005. A malaria vaccine that elicits in humans antibodies able to kill Plasmodium falciparum. PLoS Med 2:e344. doi: 10.1371/journal.pmed.0020344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lundquist R, Nielsen LK, Jafarshad A, Soesoe D, Christensen LH, Druilhe P, Dziegiel MH. 2006. Human recombinant antibodies against Plasmodium falciparum merozoite surface protein 3 cloned from peripheral blood leukocytes of individuals with immunity to malaria demonstrate antiparasitic properties. Infect Immun 74:3222–3231. doi: 10.1128/IAI.00928-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh S, Soe S, Roussilhon C, Corradin G, Druilhe P. 2005. Plasmodium falciparum merozoite surface protein 6 displays multiple targets for naturally occurring antibodies that mediate monocyte-dependent parasite killing. Infect Immun 73:1235–1238. doi: 10.1128/IAI.73.2.1235-1238.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh S, Soe S, Weisman S, Barnwell JW, Perignon JL, Druilhe P. 2009. A conserved multi-gene family induces cross-reactive antibodies effective in defense against Plasmodium falciparum. PLoS One 4:e5410. doi: 10.1371/journal.pone.0005410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Theisen M, Soe S, Jessing SG, Okkels LM, Danielsen S, Oeuvray C, Druilhe P, Jepsen S. 2000. Identification of a major B-cell epitope of the Plasmodium falciparum glutamate-rich protein (GLURP), targeted by human antibodies mediating parasite killing. Vaccine 19:204–212. doi: 10.1016/S0264-410X(00)00181-X. [DOI] [PubMed] [Google Scholar]
- 46.Holder AA, Freeman RR. 1981. Immunization against blood-stage rodent malaria using purified parasite antigens. Nature 294:361–364. doi: 10.1038/294361a0. [DOI] [PubMed] [Google Scholar]
- 47.Trucco C, Fernandez-Reyes D, Howell S, Stafford WH, Scott-Finnigan TJ, Grainger M, Ogun SA, Taylor WR, Holder AA. 2001. The merozoite surface protein 6 gene codes for a 36 kDa protein associated with the Plasmodium falciparum merozoite surface protein-1 complex. Mol Biochem Parasitol 112:91–101. doi: 10.1016/S0166-6851(00)00350-9. [DOI] [PubMed] [Google Scholar]
- 48.Pachebat JA, Kadekoppala M, Grainger M, Dluzewski AR, Gunaratne RS, Scott-Finnigan TJ, Ogun SA, Ling IT, Bannister LH, Taylor HM, Mitchell GH, Holder AA. 2007. Extensive proteolytic processing of the malaria parasite merozoite surface protein 7 during biosynthesis and parasite release from erythrocytes. Mol Biochem Parasitol 151:59–69. doi: 10.1016/j.molbiopara.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Holder AA, Lockyer MJ, Odink KG, Sandhu JS, Riveros-Moreno V, Nicholls SC, Hillman Y, Davey LS, Tizard ML, Schwarz RT, Freeman RR. 1985. Primary structure of the precursor to the three major surface antigens of Plasmodium falciparum merozoites. Nature 317:270–273. doi: 10.1038/317270a0. [DOI] [PubMed] [Google Scholar]
- 50.O'Donnell RA, Saul A, Cowman AF, Crabb BS. 2000. Functional conservation of the malaria vaccine antigen MSP-119 across distantly related Plasmodium species. Nat Med 6:91–95. doi: 10.1038/71595. [DOI] [PubMed] [Google Scholar]
- 51.Blackman MJ, Heidrich HG, Donachie S, McBride JS, Holder AA. 1990. A single fragment of a malaria merozoite surface protein remains on the parasite during red cell invasion and is the target of invasion-inhibiting antibodies. J Exp Med 172:379–382. doi: 10.1084/jem.172.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baldwin MR, Li X, Hanada T, Liu SC, Chishti AH. 2015. Merozoite surface protein 1 recognition of host glycophorin A mediates malaria parasite invasion of red blood cells. Blood 125:2704–2711. doi: 10.1182/blood-2014-11-611707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyle MJ, Richards JS, Gilson PR, Chai W, Beeson JG. 2010. Interactions with heparin-like molecules during erythrocyte invasion by Plasmodium falciparum merozoites. Blood 115:4559–4568. doi: 10.1182/blood-2009-09-243725. [DOI] [PubMed] [Google Scholar]
- 54.Das S, Hertrich N, Perrin AJ, Withers-Martinez C, Collins CR, Jones ML, Watermeyer JM, Fobes ET, Martin SR, Saibil HR, Wright GJ, Treeck M, Epp C, Blackman MJ. 2015. Processing of Plasmodium falciparum merozoite surface protein MSP1 activates a spectrin-binding function enabling parasite egress from RBCs. Cell Host Microbe 18:433–444. doi: 10.1016/j.chom.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin CS, Uboldi AD, Epp C, Bujard H, Tsuboi T, Czabotar PE, Cowman AF. 2016. Multiple Plasmodium falciparum merozoite surface protein 1 complexes mediate merozoite binding to human erythrocytes. J Biol Chem 291:7703–7715. doi: 10.1074/jbc.M115.698282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Woehlbier U, Epp C, Kauth CW, Lutz R, Long CA, Coulibaly B, Kouyaté B, Arevalo-Herrera M, Herrera S, Bujard H. 2006. Analysis of antibodies directed against merozoite surface protein 1 of the human malaria parasite Plasmodium falciparum. Infect Immun 74:1313–1322. doi: 10.1128/IAI.74.2.1313-1322.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tolle R, Früh K, Doumbo O, Koita O, N′Diaye M, Fischer A, Dietz K, Bujard H. 1993. A prospective study of the association between the human humoral immune response to Plasmodium falciparum blood stage antigen gp190 and control of malarial infections. Infect Immun 61:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Egan AF, Morris J, Barnish G, Allen S, Greenwood BM, Kaslow DC, Holder AA, Riley EM. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J Infect Dis 173:765–769. doi: 10.1093/infdis/173.3.765. [DOI] [PubMed] [Google Scholar]
- 59.al-Yaman F, Genton B, Kramer KJ, Chang SP, Hui GS, Baisor M, Alpers MP. 1996. Assessment of the role of naturally acquired antibody levels to Plasmodium falciparum merozoite surface protein-1 in protecting Papua New Guinean children from malaria morbidity. Am J Trop Med Hyg 54:443–448. doi: 10.4269/ajtmh.1996.54.443. [DOI] [PubMed] [Google Scholar]
- 60.Conway DJ, Cavanagh DR, Tanabe K, Roper C, Mikes ZS, Sakihama N, Bojang KA, Oduola AM, Kremsner PG, Arnot DE, Greenwood BM, McBride JS. 2000. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat Med 6:689–692. doi: 10.1038/76272. [DOI] [PubMed] [Google Scholar]
- 61.Siddiqui WA, Tam LQ, Kramer KJ, Hui GS, Case SE, Yamaga KM, Chang SP, Chan EB, Kan SC. 1987. Merozoite surface coat precursor protein completely protects Aotus monkeys against Plasmodium falciparum malaria. Proc Natl Acad Sci U S A 84:3014–3018. doi: 10.1073/pnas.84.9.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Etlinger HM, Caspers P, Matile H, Schoenfeld HJ, Stueber D, Takacs B. 1991. Ability of recombinant or native proteins to protect monkeys against heterologous challenge with Plasmodium falciparum. Infect Immun 59:3498–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar S, Yadava A, Keister DB, Tian JH, Ohl M, Perdue-Greenfield KA, Miller LH, Kaslow DC. 1995. Immunogenicity and in vivo efficacy of recombinant Plasmodium falciparum merozoite surface protein-1 in Aotus monkeys. Mol Med 1:325–332. [PMC free article] [PubMed] [Google Scholar]
- 64.Bryan DSN, Rigsby P, Dougall T, Ho MM. 2014. International collaborative study to evaluate and establish the 1st WHO reference reagent for anti-malaria (Plasmodium falciparum) human serum. WHO/BS/2014.2235. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 65.Murungi LM, Sonden K, Odera D, Oduor LB, Guleid F, Nkumama IN, Otiende M, Kangoye DT, Fegan G, Farnert A, Marsh K, Osier FH. 2017. Cord blood IgG and the risk of severe Plasmodium falciparum malaria in the first year of life. Int J Parasitol 47:153–162. doi: 10.1016/j.ijpara.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oeuvray C, Bouharoun-Tayoun H, Gras-Masse H, Bottius E, Kaidoh T, Aikawa M, Filgueira MC, Tartar A, Druilhe P. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594–1602. [PubMed] [Google Scholar]
- 67.Bruhns P. 2012. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 119:5640–5649. doi: 10.1182/blood-2012-01-380121. [DOI] [PubMed] [Google Scholar]
- 68.Llewellyn D, de Cassan SC, Williams AR, Douglas AD, Forbes EK, Adame-Gallegos JR, Shi J, Pleass RJ, Draper SJ. 2014. Assessment of antibody-dependent respiratory burst activity from mouse neutrophils on Plasmodium yoelii malaria challenge outcome. J Leukoc Biol 95:369–382. doi: 10.1189/jlb.0513274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quintana Mdel P, Angeletti D, Moll K, Chen Q, Wahlgren M. 2016. Phagocytosis-inducing antibodies to Plasmodium falciparum upon immunization with a recombinant PfEMP1 NTS-DBL1α domain. Malar J 15:416. doi: 10.1186/s12936-016-1459-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Perrin LH, Merkli B, Loche M, Chizzolini C, Smart J, Richle R. 1984. Antimalarial immunity in Saimiri monkeys: immunization with surface components of asexual blood stages. J Exp Med 160:441–451. doi: 10.1084/jem.160.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singh S, Miura K, Zhou H, Muratova O, Keegan B, Miles A, Martin LB, Saul AJ, Miller LH, Long CA. 2006. Immunity to recombinant Plasmodium falciparum merozoite surface protein 1 (MSP1): protection in Aotus nancymai monkeys strongly correlates with anti-MSP1 antibody titer and in vitro parasite-inhibitory activity. Infect Immun 74:4573–4580. doi: 10.1128/IAI.01679-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Blackman MJ, Scott-Finnigan TJ, Shai S, Holder AA. 1994. Antibodies inhibit the protease-mediated processing of a malaria merozoite surface protein. J Exp Med 180:389–393. doi: 10.1084/jem.180.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bergmann-Leitner ES, Duncan EH, Mullen GE, Burge JR, Khan F, Long CA, Angov E, Lyon JA. 2006. Critical evaluation of different methods for measuring the functional activity of antibodies against malaria blood stage antigens. Am J Trop Med Hyg 75:437–442. [PubMed] [Google Scholar]
- 74.Bergmann-Leitner ES, Duncan EH, Angov E. 2009. MSP-1p42-specific antibodies affect growth and development of intra-erythrocytic parasites of Plasmodium falciparum. Malar J 8:183. doi: 10.1186/1475-2875-8-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Egan AF, Burghaus P, Druilhe P, Holder AA, Riley EM. 1999. Human antibodies to the 19kDa C-terminal fragment of Plasmodium falciparum merozoite surface protein 1 inhibit parasite growth in vitro. Parasite Immunol 21:133–139. doi: 10.1046/j.1365-3024.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- 76.Wilson DW, Fowkes FJ, Gilson PR, Elliott SR, Tavul L, Michon P, Dabod E, Siba PM, Mueller I, Crabb BS, Beeson JG. 2011. Quantifying the importance of MSP1-19 as a target of growth-inhibitory and protective antibodies against Plasmodium falciparum in humans. PLoS One 6:e27705. doi: 10.1371/journal.pone.0027705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McCallum FJ, Persson KE, Mugyenyi CK, Fowkes FJ, Simpson JA, Richards JS, Williams TN, Marsh K, Beeson JG. 2008. Acquisition of growth-inhibitory antibodies against blood-stage Plasmodium falciparum. PLoS One 3:e3571. doi: 10.1371/journal.pone.0003571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Malkin E, Long CA, Stowers AW, Zou L, Singh S, MacDonald NJ, Narum DL, Miles AP, Orcutt AC, Muratova O, Moretz SE, Zhou H, Diouf A, Fay M, Tierney E, Leese P, Mahanty S, Miller LH, Saul A, Martin LB. 2007. Phase 1 study of two merozoite surface protein 1 (MSP142) vaccines for Plasmodium falciparum malaria. PLoS Clin Trials 2:e12. doi: 10.1371/journal.pctr.0020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murungi LM, Kamuyu G, Lowe B, Bejon P, Theisen M, Kinyanjui SM, Marsh K, Osier FH. 2013. A threshold concentration of anti-merozoite antibodies is required for protection from clinical episodes of malaria. Vaccine 31:3936–3942. doi: 10.1016/j.vaccine.2013.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rono J, Osier FH, Olsson D, Montgomery S, Mhoja L, Rooth I, Marsh K, Farnert A. 2013. Breadth of anti-merozoite antibody responses is associated with the genetic diversity of asymptomatic Plasmodium falciparum infections and protection against clinical malaria. Clin Infect Dis 57:1409–1416. doi: 10.1093/cid/cit556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weatherall D. 2006. The use of non-human primates in research. Academy of Medical Sciences, London, England. [Google Scholar]
- 82.Mahdi Abdel Hamid M, Remarque EJ, van Duivenvoorde LM, van der Werff N, Walraven V, Faber BW, Kocken CH, Thomas AW. 2011. Vaccination with Plasmodium knowlesi AMA1 formulated in the novel adjuvant co-vaccine HT protects against blood-stage challenge in rhesus macaques. PLoS One 6:e20547. doi: 10.1371/journal.pone.0020547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cowan GJ, Creasey AM, Dhanasarnsombut K, Thomas AW, Remarque EJ, Cavanagh DR. 2011. A malaria vaccine based on the polymorphic block 2 region of MSP-1 that elicits a broad serotype-spanning immune response. PLoS One 6:e26616. doi: 10.1371/journal.pone.0026616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kauth CW, Epp C, Bujard H, Lutz R. 2003. The merozoite surface protein 1 complex of human malaria parasite Plasmodium falciparum: interactions and arrangements of subunits. J Biol Chem 278:22257–22264. doi: 10.1074/jbc.M302299200. [DOI] [PubMed] [Google Scholar]
- 85.Kauth CW, Woehlbier U, Kern M, Mekonnen Z, Lutz R, Mücke N, Langowski J, Bujard H. 2006. Interactions between merozoite surface proteins 1, 6, and 7 of the malaria parasite Plasmodium falciparum. J Biol Chem 281:31517–31527. doi: 10.1074/jbc.M604641200. [DOI] [PubMed] [Google Scholar]
- 86.Epp C, Kauth CW, Bujard H, Lutz R. 2003. Expression and purification of Plasmodium falciparum MSP-142: a malaria vaccine candidate. J Chromatogr B Analyt Technol Biomed Life Sci 786:61–72. doi: 10.1016/S1570-0232(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 87.Woehlbier U, Epp C, Hackett F, Blackman MJ, Bujard H. 2010. Antibodies against multiple merozoite surface antigens of the human malaria parasite Plasmodium falciparum inhibit parasite maturation and red blood cell invasion. Malar J 9:77. doi: 10.1186/1475-2875-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tongren JE, Drakeley CJ, McDonald SL, Reyburn HG, Manjurano A, Nkya WM, Lemnge MM, Gowda CD, Todd JE, Corran PH, Riley EM. 2006. Target antigen, age, and duration of antigen exposure independently regulate immunoglobulin G subclass switching in malaria. Infect Immun 74:257–264. doi: 10.1128/IAI.74.1.257-264.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Drakeley CJ, Corran PH, Coleman PG, Tongren JE, McDonald SL, Carneiro I, Malima R, Lusingu J, Manjurano A, Nkya WM, Lemnge MM, Cox J, Reyburn H, Riley EM. 2005. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci U S A 102:5108–5113. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malkin EM, Diemert DJ, McArthur JH, Perreault JR, Miles AP, Giersing BK, Mullen GE, Orcutt A, Muratova O, Awkal M, Zhou H, Wang J, Stowers A, Long CA, Mahanty S, Miller LH, Saul A, Durbin AP. 2005. Phase 1 clinical trial of apical membrane antigen 1: an asexual blood-stage vaccine for Plasmodium falciparum malaria. Infect Immun 73:3677–3685. doi: 10.1128/IAI.73.6.3677-3685.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.