Abstract
Background
Selective internal radiation therapy (SIRT) with yttrium-90 resin (Y-90 resin) microspheres has been used as a locoregional therapy for patients with unresectable hepatocellular carcinoma (HCC). We examined patient and disease characteristics that might affect survival after Y-90 resin, as well as treatment tolerability.
Methods
Data from patients with unresectable HCC treated with Y-90 resin at a single institution were reviewed retrospectively. Survival was assessed with Kaplan-Meier curves and log-rank tests. Response was evaluated with the response evaluation criteria in solid tumors (RECIST) criteria. Adverse events (AEs) were noted, and laboratory values were graded with CTCAE v3.0.
Results
Data from 111 patients were analyzed. AEs occurred in 23 patients at 1 week after treatment and in 46 at 3 months. At 6 months, 13 patients had a complete response and 13 had a partial response. Factors associated with longer overall survival (OS) included early-stage disease [27.8 months for patients with Barcelona-Clinic Liver Cancer (BCLC) A vs. 9.2 months for BCLC C]; treatment with other locoregional therapies (69.0 vs. 11.4 months); and lack of bilobar disease (23.5 vs. 9.4 months), portal vein thrombosis (16.2 vs. 8.6 months), ascites (16.6 vs. 10.3 months), and treatment with sorafenib (17.2 vs. 10.3 months). In six patients, Y-90 resin was used as a bridge to liver transplantation, which greatly improved survival (69.0 vs. 12.1 months).
Conclusions
Several characteristics may prove useful for selecting patients likely to respond well to Y-90 resin. These results should be confirmed in prospective studies.
Keywords: Yttrium-90 resin (Y-90 resin), radioembolization, locoregional therapy, liver cancer, predictive factors
Introduction
In the past, the incidence of liver cancer was lower in the United States (US) than in other regions of the world (1). However, the rate in the US has more than tripled in recent decades, from 2.6 per 100,000 in 1975 to 8.8 per 100,000 in 2013 (2). In 2017, approximately 40,710 new cases of liver cancer are expected in the US, and 75% of these cases will be hepatocellular carcinoma (HCC) (3). HCC is a complex condition that has many causes, including hepatitis B virus, hepatitis C virus, excessive alcohol consumption, obesity, diabetes, and exposure to aflatoxins (4,5). The prevalence of these causes and their contribution to the course of disease can be influenced by additional factors such as geographic region, ethnicity, race, and age (4,5). The prognosis for HCC is poor, with an overall 5-year survival from diagnosis of less than 20% (6). Without treatment, patients with advanced HCC usually survive less than 6 months (7).
Treatment options for patients with HCC are multifaceted and may depend on the presence or extent of underlying liver disease, the stage at which HCC is diagnosed, and tumor characteristics (8). Patients with early-stage HCC may be treated with potentially curative surgical treatments such as resection, ablation, and liver transplantation (9,10). For unresectable intermediate-stage HCC [Barcelona-Clinic Liver Cancer (BCLC) stage B], transarterial chemoembolization (TACE) is generally the treatment of choice (9,11). Until recently, sorafenib, an angiogenesis inhibitor, was the only FDA-approved systemic therapy available for patients with unresectable HCC (12). Sorafenib provides a demonstrated survival benefit for patients with advanced HCC (BCLC C) and is also given to patients with unresectable intermediate HCC who are ineligible for TACE or who show progression despite locoregional therapies (9,11). Regorafenib, a multikinase inhibitor, was recently approved for patients with HCC previously treated with sorafenib (13,14). However, the efficacy of sorafenib and regorafenib is limited, with median overall survival (OS) of less than 11 months for each (10,13), and both are associated with substantial side effects (13,15).
Selective internal radiation therapy (SIRT) with Y-90 resin microspheres (Y-90 resin SIRT) is an intra-arterial, catheter-based locoregional therapy that has been used to treat patients with unresectable HCC. Once delivered, the microspheres largely remain in the tumors, where radiation is delivered within a limited range, and sparing normal surrounding liver tissue from damage. In a multicenter retrospective European study (ENRY), Y-90 resin SIRT produced a clinically relevant median OS of 12.8 months [95% confidence interval (CI): 10.9–15.7 months] in patients with different tumor stages, including those with advanced disease (16).
Many factors potentially affect the response to SIRT, including patient demographics, disease characteristics such as etiology and severity, performance status, previous treatments, and subsequent liver transplantation. However, limited data are available on which of these factors increase survival. The aim of this large, retrospective, single-center analysis was to investigate the effects of a wide range of factors on survival after Y-90 resin SIRT and to assess the tolerability of Y-90 resin in this heterogeneous real-life population.
Methods
Patient selection
All patients with unresectable HCC at Methodist Dallas Medical Center who underwent at least one SIRT procedure with Y-90 resin microspheres (SIR-Spheres®, Sirtex Medical Limited, Sydney, Australia) between April 1, 2004, and March 27, 2013, were included in this retrospective analysis. HCC was considered unresectable if it was multifocal or bilobar or if malignant portal vein thrombosis, portal hypertension, or decompensated liver disease (Child’s Pugh B or C) were present.
Patients who were eligible to undergo Y-90 resin SIRT had Eastern Cooperative Oncology Group (ECOG) performance status 0–2; platelets >60,000; creatinine <2 mg/dL; bilirubin <2 mg/dL; and international normalized ratio (INR) <1.2. Patients were not eligible for SIRT if they had any extrahepatic disease; contraindication to hepatic artery catheterization such as vascular abnormalities, bleeding diathesis, allergy to contrast dye, concurrent malignancy, refractory ascites, previous external beam radiation, or evidence of any uncorrectable flow to the gastrointestinal tract; or greater than 30 Gy of radiation estimated to be delivered to the lung based on angiography or Tc-99 microaggregated albumin scan (shunt fraction of 20% or greater).
Data were de-identified, and an IRB exemption was granted for this HIPAA-compliant study.
Y-90 resin SIRT protocol
All patients were treated by a multidisciplinary team led by transplant hepatologists and consisting of oncologists, surgeons, hepatologists, and interventional and cross-sectional radiologists. The pretreatment evaluation included a medical history and physical examination. Key data were documented, including demographics, presence or absence of cirrhosis, etiology of HCC, evidence of portal hypertension, HCC characteristics, MELD score, and prior HCC treatment such as radiofrequency ablation (RFA), hepatic resection, chemotherapy, or radiation therapy. All patients underwent the following baseline evaluation: complete blood count, prothrombin time, serum creatinine, liver aminotransferases, bilirubin, chest X-ray, and chest computed tomography (CT).
Patients underwent imaging with multiphasic CT or hepatic MRI to determine portal vein patency, tumor and non-tumor volume, and extrahepatic tumor burden. This imaging was used to confirm whether patients were appropriate for SIRT, assisted in planning for whole-liver or selective radioembolization, and allowed the appropriate activity of Y-90 resin to be calculated. Preparation for and administration of Y-90 resin were performed in accordance with a standard protocol (17). One author (IS), in conjunction with a group of radiologists at the medical center where the procedures were carried out, read all results from the radiographic data collected for this retrospective analysis.
Adverse events (AEs)
Data on AEs at 1 week and 3 months after the Y-90 resin SIRT procedure were extracted from the patient charts. Signs of radiation hepatitis generally develop at 1 week, and radiation injury peaks at 1 to 3 months. Laboratory values were graded in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) v3.0. We do not report the severity of other AEs by grade because this information was infrequently reported in the patient charts.
Tumor response
Where the information was available, we recorded the longest diameter of each target tumor on MRI or dynamic CT as its size. Tumor size was recorded at baseline (before SIRT) and 6 weeks, 3 months, and 6 months after Y-90 resin SIRT. The response evaluation criteria in solid tumors (RECIST) criteria (18) were used to determine tumor response.
Statistical methods
OS was estimated by the Kaplan-Meier method, and the corresponding median survival times and 95% CI of the medians were determined. The inverse Kaplan-Meier method was used to determine the median follow-up. Log-rank tests were used to compare survival curves. The Wilcoxon signed-rank test was used to evaluate the change from baseline in laboratory values. Alpha was set at 0.05, and all tests were two-tailed. Data were analyzed by using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
A total of 115 patients received at least one Y-90 resin SIRT treatment during the study period. Of these, four were excluded from the analysis because the duration of follow-up was unknown. Most patients were male (85/111, 76.6%), about half were white (59/111, 53.2%), and about one-third were 70 years or older (38/111, 34.2%) (Table 1). Hepatitis C was reported as an HCC etiology for 65 of 110 patients (59.1%). Half the patients had bilobar disease (54/108, 50.0%), and most patients had early- or intermediate-stage disease, as assessed with several scales (Table 1). No patients exhibited extrahepatic disease at time of Y-90 resin treatment.
Table 1. Demographic and disease characteristics.
| Characteristic | Value |
|---|---|
| Sex (N=111)a, n (%) | |
| Female | 26 (23.4) |
| Male | 85 (76.6) |
| Race (N=111), n (%) | |
| Caucasian | 59 (53.2) |
| Asian | 30 (27.0) |
| Hispanic | 20 (18.0) |
| Indian | 1 (0.9) |
| Other | 1 (0.9) |
| Age (N=111), years | |
| Mean ± SD | 65.8±9.6 |
| Range | 42.1–84.0 |
| <70, n (%) | 73 (67.0) |
| ≥70, n (%) | 38 (34.2) |
| Y-90 resin treatments (N=111), n (%) | |
| 1 | 95 (85.6) |
| 2 | 14 (12.6) |
| 3 | 2 (1.8) |
| Bilobar disease (N=108), n (%) | 54 (50.0) |
| HCC etiology (N=110)b, n (%) | |
| Hepatitis B | 9 (8.2) |
| Hepatitis C | 65 (59.1) |
| Alcohol | 27 (24.5) |
| NASH | 12 (10.9) |
| Cryptogenic | 8 (7.3) |
| MELD (N=111), n (%) | |
| 6 | 16 (14.4) |
| 7 | 25 (22.5) |
| 8 | 18 (16.2) |
| 9 | 20 (18.0) |
| ≥10 | 32 (28.8) |
| Child-Pugh grade (N=111), n (%) | |
| A | 82 (73.9) |
| B | 26 (23.4) |
| C | 3 (2.7) |
| Performance status (ECOG) (N=107), n (%) | |
| 0 | 96 (89.7) |
| 1 | 9 (8.4) |
| 2 | 2 (1.9) |
| Okuda stage (N=111), n (%) | |
| I | 86 (77.5) |
| II | 25 (22.5) |
| PVT (N=111), n (%) | 17 (15.3) |
| BCLC (N=111), n (%) | |
| A | 38 (34.2) |
| B | 51 (45.9) |
| C | 22 (19.8) |
| Radiation dose (GBq, N=111), median (IQR) | 1.14 (0.56) |
| Ascites (N=111), n (%) | 23 (20.7) |
| Gastrointestinal bleeding (N=111), n (%) | 3 (2.7) |
a, number of patients with available data is indicated; b, some patients had more than one etiology. HCC, hepatocellular carcinoma; NASH, nonalcoholic steatohepatitis; MELD, model for end-stage liver disease; ECOG, Eastern Cooperative Oncology Group; PVT, portal vein thrombosis; BCLC, Barcelona-Clinic Liver Cancer staging system.
Additional treatments
Of the 111 patients in the analysis, 65% had one or more additional treatments concurrent with or before Y-90 resin SIRT. The most common therapies were sorafenib (38/111, 34.2%) and TACE (33/111, 29.7%). Surgery and RFA had each been performed in 9 patients (8.1%).
Tumor response
The number of patients with responses evaluable with the RECIST criteria declined from 97 at baseline to 43 at 6 months after Y-90 resin SIRT (Table 2). For those with evaluable lesions, tumor diameter decreased over time from a median of 6.0 cm (IQR 5.5) at baseline to 2.6 cm (IQR 5.8) 6 months after the Y-90 resin SIRT was initiated (Table 2). At 6 months, 13 patients had a complete response, and an additional 13 had a partial response.
Table 2. Tumor response based on RECIST guidelines.
| Time point | Lesion diameter (cm), median (IQR) | Number with available data (N=111) | RECIST, n (%) | |||
|---|---|---|---|---|---|---|
| CR | PR | SD | PD | |||
| Baseline | 6.0 (5.5) | 97 | – | – | – | – |
| 6 weeks | 4.3 (5.3) | 80 | 13 (11.7) | 17 (15.3) | 37 (33.3) | 13 (11.7) |
| 3 months | 3.7 (4.0) | 58 | 12 (10.8) | 16 (14.4) | 22 (19.8) | 8 (7.2) |
| 6 months | 2.6 (5.8) | 43 | 13 (11.7) | 13 (11.7) | 11 (9.9) | 6 (5.4) |
RECIST, Response Evaluation Criteria In Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Survival analyses
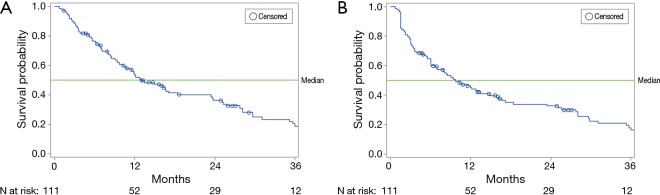
At the time of the analysis, 80 patients (72.1%) had died, with a median follow-up of 46.9 months (95% CI: 0.7–69.0). The median OS for all patients was 13.1 months (95% CI: 10.3–18.4), and the median survival without progression in the liver (liver PFS) for all patients was 9.8 months (95% CI: 6.8–14.8) (Figure 1). Log-rank analyses identified several factors associated with significantly longer OS, liver PFS, or both (Table 3). In addition, those patients who received a liver transplant after SIRT lived significantly longer (median 69.0 months) than those who did not receive a transplant (median 12.1 months) (Table 3). Survival data for all variables analyzed are shown in Table S1.
Figure 1.
Kaplan-Meier survival curves. (A) Median overall survival was 13.1 months (95% CI: 10.3–18.4); (B) median progression-free survival in the liver was 9.8 months (95% CI: 6.8–14.8).
Table 3. Significant predictors of survival.
| Baseline characteristic | n | Median OS (95% CI) (months) | Pa | Median liver PFS (95% CI) (months) | Pa |
|---|---|---|---|---|---|
| Bilobar disease | 0.002 | 0.057 | |||
| No | 54 | 23.5 (12.2–29.7) | 13.5 (7.9–27.8) | ||
| Yes | 54 | 9.4 (6.9–12.1) | 6.9 (5.2–10.3) | ||
| PVT | 0.001 | 0.006 | |||
| No | 94 | 16.2 (11.4–25.7) | 12.0 (7.1–25.1) | ||
| Yes | 17 | 8.6 (3.6–13.5) | 5.6 (3.0–10.6) | ||
| PST | 0.024 | 0.032 | |||
| 0 | 96 | 13.1 (10.6–23.5) | 9.8 (6.8–15.8) | ||
| 1 | 9 | 6.9 (2.1–)b | 5.6 (2.1–13.5) | ||
| 2 | 2 | 5.1 (3.8–6.4) | 2.7 (1.5–3.8) | ||
| BCLC | 0.011 | 0.002 | |||
| A | 38 | 27.8 (12.8–35.3) | 28.2 (12.8–35.8) | ||
| B | 51 | 11.4 (8.3–16.7) | 8.1 (3.4–11.4) | ||
| C | 22 | 9.2 (3.6–17.2) | 6.0 (3.6–10.6) | ||
| Ascites | 0.053 | 0.023 | |||
| No | 88 | 16.6 (10.6–25.3) | 10.6 (6.8–17.2) | ||
| Yes | 23 | 10.3 (7.0–13.5) | 9.4 (2.6–13.5) | ||
| Sorafenib | 0.022 | 0.123 | |||
| No | 73 | 17.2 (12.0–25.7) | 12.8 (7.9–18.4) | ||
| Yes | 38 | 10.3 (5.3–12.2) | 6.0 (3.6–12.2) | ||
| Liver-directed treatmentc | <0.001 | <0.001 | |||
| No | 95 | 11.4 (8.5–14.8) | 8.4 (6.0–11.4) | ||
| Yes | 16 | 69.0 (–)e | 69.0 (–)e | ||
| Liver transplantd | 0.001 | <0.001 | |||
| No | 103 | 12.1 (9.4–16.6) | 9.0 (6.0–12.8) | ||
| Yes | 6 | 69.0 (–)e | 69.0 (–)e | ||
| Year of treatment | 0.029 | 0.067 | |||
| 2004–2009 | 52 | 17.8 (11.9–28.1) | 12.7 (6.9–25.8) | ||
| 2010–2013 | 59 | 10.8 (6.8–14.8) | 8.1 (4.8–12.8) |
a, Log-rank test; b, upper bound of the 95% CI was not reached; c, liver-directed treatments were locoregional therapies such as microwave ablation, RFA, TACE, and transarterial embolization; d, liver transplant was performed after Y-90 resin SIRT; e, upper and lower bounds of the 95% CI were not reached. OS, overall survival; Liver PFS, liver-progression-free survival; PST, performance status test (Eastern Cooperative Oncology Group); BCLC, Barcelona-Clinic Liver Cancer staging system; RFA, radiofrequency ablation; TACE, transarterial chemoembolization.
AEs
One week after the Y-90 resin SIRT treatment, 23 of 107 patients (21.5%) experienced selected AEs (Table 4). The percentage increased to 43.4% (46/106) after 3 months. Mean albumin concentration decreased significantly from baseline to 1 month, whereas the INR and total bilirubin both increased significantly over this time (P<0.001 for all three comparisons, Table 5). At 3 months, most patients had normal (grade 0) or grade 1 laboratory values for albumin (41/72, 57%), creatinine (67/72, 93%), INR (57/67, 85%), and total bilirubin (40/72, 56%).
Table 4. Adverse events after Y-90 resin.
| Adverse events | One week after Y-90 resina, n (%) | Three months after Y-90 resinb, n (%) |
|---|---|---|
| Any adverse event | 23 (21.5) | 46 (43.4) |
| Abdominal pain | 8 (7.5) | 15 (14.2) |
| Ascites | 6 (5.6) | 20 (18.9) |
| Nausea | 4 (3.7) | 6 (5.7) |
| Edema | 3 (2.8) | 7 (6.6) |
| Fatigue | 3 (2.8) | 3 (2.8) |
| Vomiting | 2 (1.9) | 5 (4.7) |
| Ulcer | – | 5 (4.7) |
| Jaundice | – | 4 (3.8) |
| GI bleeding | – | 8 (7.5) |
a, adverse events were available for 107 patients at 1 week; b, adverse events were available for 106 patients at 3 months. Y-90 resin, yttrium-90 resin.
Table 5. Changes in laboratory values from baseline to one month after Y-90 resin SIRT (N=111).
| Laboratory test | n | Baseline | Month 1 | Change | P value |
|---|---|---|---|---|---|
| AFP (ng/dL) | 55 | 4,738.13±16,097.26 | 4,172.15±17,195.45 | −565.98 | 0.552 |
| Albumin (g/dL) | 90 | 3.55±0.59 | 3.37±0.68 | −0.18 | <0.001 |
| Creatinine (mg/dL) | 91 | 0.95±0.26 | 0.95±0.32 | 0.00 | 0.190 |
| INR | 88 | 1.14±0.24 | 1.25±0.35 | 0.11 | <0.001 |
| Bilirubin, total (mg/dL) | 90 | 1.06±0.50 | 2.00±2.02 | 0.94 | <0.001 |
Y-90 resin, yttrium-90 resin; SIRT, selective internal radiation therapy; AFP, alpha fetoprotein; INR, international normalized ratio.
Discussion
Y-90 resin SIRT has been shown to increase median OS in patients with HCC (16,19), but which of the many specific patient and disease characteristics might predict response is not known. In this retrospective study, we examined survival outcomes in a large number of patients with unresectable HCC who were treated with Y-90 resin SIRT at a single tertiary referral center. The procedure was generally well tolerated. The median OS for all patients in this analysis was 13.1 months, similar to the median OS of 13.2 months in a retrospective study of 30 Asian patients with inoperable HCC who were treated with Y-90 resin SIRT (19) and longer than the median OS of 10.7 months for patients with unresectable HCC treated with sorafenib (15) and 10.6 months for patients with unresectable HCC treated with regorafenib after progression on sorafenib (13) seen in phase 3 trials of these drugs.
We found that several factors were associated with significantly longer survival. Patient factors such as performance status, and disease factors such as BCLC stage, the presence of portal vein thrombosis, and the absence of ascites, could all potentially be used to identify patients who might benefit the most from treatment with Y-90 resin SIRT. In addition, our results indicate that Y-90 resin SIRT can be used to downstage tumors or otherwise serve as a bridge to liver transplantation. As expected (10,11), transplantation provided a strong survival benefit (69.0 vs. 12.1 months, P=0.001), although this population was small (n=6).
In the ENRY study, a prospective European multicenter trial of Y-90 resin SIRT in 325 patients with unresectable HCC, the median OS was 12.8 months (95% CI: 10.9–15.7) (16). Similar to the results in this investigation, OS varied significantly by disease stage, performance status, liver health, and tumor burden. More recently, a sub-analysis of the ENRY database was conducted to evaluate the clinical outcomes among elderly patients (70 years or older) compared with younger patients (20). Radioembolization was as well tolerated and effective in the elderly as it was in younger patients with unresectable HCC. Similarly, we found no difference in survival between those 70 years or older and those younger than 70 (Table S1).
Sorafenib became the standard treatment for patients with advanced HCC therapy after the SHARP trial showed a higher median OS with sorafenib (10.7 months) than with placebo (7.9 months) (15). Thus, it was surprising that sorafenib was associated with shorter survival in our cohort. The reason for this is not clear, although patient selection may have been a contributing factor, because patients with less-advanced disease were less likely to have received sorafenib than those with intermediate or more-advanced disease [BCLC A, 5/38 (13%) received sorafenib; BCLC B, 23/51 (45%); BCLC C 10/22 (45%); P=0.003]. This finding may also have contributed to the shorter survival in 2010–2013, after the introduction of sorafenib, than in the earlier years. Further analyses will be needed to confirm this result and to understand the mechanisms involved.
Recently announced results from a head-to-head comparison of Y-90 resin SIRT and sorafenib for HCC, the phase 3 SARAH trial (21), did not show a significant difference in OS between the groups, although patients in the Y-90 resin arm had significantly fewer AEs and higher quality of life. Similar results were reported from a recent investigator-initiated multicenter clinical trial in Asia, which compared Y-90 with standard-dose (400 mg bid) sorafenib (22). As in the SARAH trial, the OS was similar in the two groups (Y-90: 8.54 months, sorafenib: 10.58 months), and serious AEs occurred in a much smaller percentage of patients in the Y-90 arm (27%) than in the sorafenib arm (>50%). Notably, however, a much higher percentage of patients in the Y-90 arm (27%) than in the sorafenib arm (9%) did not receive the planned therapy, which may have affected the results. In addition, the tumor response rate was substantially better in the Y-90 arm (16.5%) than in the sorafenib arm (1.7%). One difference from our study was that patients only received one injection of Y-90, whereas in our clinical protocol patients received up to 3 treatments with Y-90, depending on tolerability and efficacy. One arm of an ongoing phase 3 studies in patients with inoperable HCC (SORAMIC, NCT01126645) is examining whether adding Y-90 resin SIRT to sorafenib will improve survival over treatment with sorafenib alone. This may be more comparable to our study, in which the treatments were often given concomitantly.
TACE is the standard of care and first-line treatment for intermediate-stage unresectable HCC (9,11). In a recent investigation comparing SIRT with TACE (SIRTACE), a single treatment session with Y-90 resin appeared to be as safe, and had a similar impact on health-related quality of life, as multiple sessions of TACE (23), suggesting that SIRT might be an option for patients eligible for TACE. These two treatment modalities have different benefits and drawbacks. SIRT requires two separate procedures; the first maps the hepatic vasculature to identify and embolize any extrahepatic branches that could allow for dispersal of Y-90 microspheres to nontarget organs (24), and the second delivers the Y-90 microspheres. Both are generally performed as outpatient procedures (25). TACE only requires one procedure but is associated with a postembolization syndrome characterized by pain, fever, nausea, and vomiting. This syndrome can be severe and requires that patients receiving TACE be hospitalized to manage symptoms. Major complications for TACE are uncommon but include liver infarction, biliary duct injury, and gastric or duodenal ulcers (25,26). The dominant side effect reported for SIRT is fatigue (25). Major complications for SIRT are rare and generally result from irradiation of nontarget tissue rather than from embolic effects (27). These complications include radiation-induced ulcers of the gastrointestinal tract, gastroduodenal injury, radiation pneumonitis, and radiation-induced liver disease (25,28).
A cost-effectiveness analysis by Rostambeigi et al. of SIRT versus TACE has concluded that, taking into account the costs of hospitalization required for TACE and the costs of preliminary arterial mapping procedures for SIRT, the overall costs for SIRT are more than twice those of TACE (29). Because studies have not shown a clear survival advantage for SIRT or TACE when applied to patients with intermediate HCC (BCLC B) (23,29), the choice of treatment modality is one that must take into account a number of individual patient considerations.
Strengths and limitations of the study
This relatively large study enabled us to examine the associations of many patient, disease, and treatment factors with survival outcomes after Y-90 resin SIRT. The retrospective nature necessarily limits the conclusions that we can draw from our findings. In particular, we were not able to analyze the severity of non-laboratory AEs because CTCAE grade was not included in the patient charts. Furthermore, the small number of patients in some subgroups, such as those with ≥2 SIRT procedures, limits statistical power. However, our results can be used to design prospective studies to further examine the effects of specific characteristics, such as performance status, the presence of portal vein thrombosis, BCLC stage, or prior treatment, on the outcomes of Y-90 resin SIRT.
Conclusions
The use of Y-90 resin SIRT may provide an important tool for improving OS in patients with unresectable HCC, particularly those with certain patient and disease characteristics. The results from recent and upcoming phase 3 clinical trials, in conjunction with increasing worldwide experience, will clarify the efficacy and survival benefits of SIRT in this patient population.
Table S1. Kaplan-Meier estimates of survival by baseline variable.
| Category | n | Median OSa (95% CI) (months) | P value log-rank | Median liver PFSb (95% CI) (months) | P value log-rank |
|---|---|---|---|---|---|
| All patients | 111 | 13.1 (10.3–18.4) | 9.8 (6.8–14.8) | ||
| Sex | 0.498 | 0.638 | |||
| Female | 26 | 10.3 (5.3–27.8) | 7.0 (3.3–16.6) | ||
| Male | 85 | 14.8 (10.6–23.8) | 11.4 (6.9–16.2) | ||
| Race | 0.387 | 0.057 | |||
| Asian | 30 | 11.9 (3.6–25.1) | 6.0 (3.1–14.8) | ||
| Hispanic | 20 | 9.8 (5.7–31.1) | 8.2 (3.2–13.5) | ||
| Indian | 1 | 7.0 | 3.0 | ||
| Other | 1 | – | 1.5 | ||
| White | 59 | 16.7 (10.6–27.8) | 15.8 (7.1–25.8) | ||
| Race by category | 0.135 | ||||
| White | 59 | 16.7 (10.6–27.8) | 0.502 | 15.8 (7.1–25.8) | |
| Non-White | 52 | 11.4 (7.9–14.8) | 6.9 (3.4–11.4) | ||
| Age (years) | 0.176 | 0.207 | |||
| <65 | 50 | 14.8 (10.6–27.8) | 12.2 (6.9–17.2) | ||
| ≥65 | 61 | 12.0 (7.1–23.6) | 8.1 (5.2–13.5) | ||
| Age (years) | 0.510 | 0.622 | |||
| <70 | 73 | 13.1 (10.3–18.4) | 9.4 (5.6–14.8) | ||
| ≥70 | 38 | 13.5 (7.1–29.7) | 12.8 (6.8–29.7) | ||
| Number of SIRT treatments | 0.850 | 0.783 | |||
| 1 | 94 | 12.1 (9.0–16.6) | 9.0 (6.0–13.1) | ||
| 2 | 14 | 18.4 (7.1–29.7) | 17.2 (6.9–29.7) | ||
| 3 | 2 | 33.1 (23.6–42.5) | 24.3 (6.0–42.5) | ||
| Bilobar disease | 0.002 | 0.057 | |||
| No | 54 | 23.5 (12.2–29.7) | 13.5 (7.9–27.8) | ||
| Yes | 54 | 9.4 (6.9–12.1) | 6.9 (5.2–10.3) | ||
| Etiology hepatitis B | 0.266 | 0.188 | |||
| No | 101 | 12.2 (9.4–17.2) | 9.4 (6.0–13.5) | ||
| Yes | 9 | 25.1 (0.9–)c | 25.1 (0.9–)c | ||
| Etiology hepatitis C | 0.436 | 0.611 | |||
| No | 45 | 17.2 (8.1–27.8) | 13.1 (6.0–23.5) | ||
| Yes | 65 | 11.9 (9.4–16.7) | 9.4 (5.3–12.8) | ||
| Etiology alcohol | 0.784 | 0.169 | |||
| No | 83 | 12.8 (9.8–23.6) | 10.3 (6.8–15.8) | ||
| Yes | 27 | 13.5 (5.7–28.1) | 7.9 (2.8–23.5) | ||
| Etiology NASH | 0.649 | 0.705 | |||
| No | 98 | 12.8 (9.4–18.4) | 9.4 (6.0–13.5) | ||
| Yes | 12 | 27.8 (2.3–35.3) | 15.8 (2.3–35.3) | ||
| Etiology cryptogenic | 0.847 | 0.490 | |||
| No | 102 | 13.5 (9.8–23.6) | 9.4 (6.0–14.8) | ||
| Yes | 8 | 13.1 (3.6–42.5) | 13.1 (3.6–42.5) | ||
| MELD | 0.087 | 0.080 | |||
| 6 | 16 | 23.6 (9.4–)c | 12.0 (6.0–35.8) | ||
| 7 | 25 | 10.8 (6.8–17.2) | 6.8 (3.0–12.2) | ||
| 8 | 18 | 28.1 (5.7–)c | 28.2 (3.6–)c | ||
| 9 | 20 | 11.9 (3.6–28.1) | 9.0 (2.2–28.2) | ||
| ≥10 | 32 | 12.8 (7.0–16.7) | 7.1 (3.7–14.8) | ||
| Child-Pugh grade | 0.183 | 0.135 | |||
| A | 82 | 16.6 (11.6–25.3) | 12.8 (8.1–18.4) | ||
| B | 26 | 10.3 (3.8–25.1) | 3.7 (2.1–12.2) | ||
| C | 3 | 8.5 (8.3–9.4) | 8.4 (6.0–9.4) | ||
| PST | 0.024 | 0.032 | |||
| 0 | 96 | 13.1 (10.6–23.5) | 9.8 (6.8–15.8) | ||
| 1 | 9 | 6.9 (2.1–)c | 5.6 (2.1–13.5) | ||
| 2 | 2 | 5.1 (3.8–6.4) | 2.7 (1.5–3.8) | ||
| Okuda | 0.061 | 0.427 | |||
| I | 86 | 16.6 (11.4–25.3) | 10.6 (6.0–17.2) | ||
| II | 25 | 9.4 (5.6–13.5) | 9.4 (3.8–13.5) | ||
| PVT | 0.001 | 0.006 | |||
| No | 94 | 16.2 (11.4–25.7) | 12.0 (7.1–25.1) | ||
| Yes | 17 | 8.6 (3.6–13.5) | 5.6 (3.0–10.6) | ||
| BCLC | 0.011 | 0.002 | |||
| A | 38 | 27.8 (12.8–35.3) | 28.2 (12.8–35.8) | ||
| B | 51 | 11.4 (8.3–16.7) | 8.1 (3.4–11.4) | ||
| C | 22 | 9.2 (3.6–17.2) | 6.0 (3.6–10.6) | ||
| Liver transplant | 0.001 | <0.001 | |||
| No | 103 | 12.1 (9.4–16.6) | 9.0 (6.0–12.8) | ||
| Yes | 6 | 69.0 (–)c | 69.0 (–)c | ||
| Ascites | 0.053 | 0.023 | |||
| No | 88 | 16.6 (10.6–25.3) | 10.6 (6.8–17.2) | ||
| Yes | 23 | 10.3 (7.0–13.5) | 9.4 (2.6–13.5) | ||
| PSE | 0.153 | 0.147 | |||
| No | 102 | 14.8 (10.8–23.6) | 10.6 (6.8–16.2) | ||
| Yes | 9 | 9.4 (2.6–27.8) | 8.4 (2.6–10.3) | ||
| GI bleeding | 0.235 | 0.511 | |||
| No | 108 | 14.8 (10.6–23.6) | 9.8 (6.0–15.8) | ||
| Yes | 3 | 9.4 (7.1–13.5) | 9.4 (7.1–13.5) | ||
| Cancer treatment surgery | 0.864 | 0.479 | |||
| No | 102 | 13.5 (9.8–23.6) | 9.0 (6.0–14.8) | ||
| Yes | 9 | 12.2 (5.3–42.5) | 12.2 (5.3–42.6) | ||
| Cancer treatment sorafenib | 0.022 | 0.123 | |||
| No | 73 | 17.2 (12.0–25.7) | 12.8 (7.9–18.4) | ||
| Yes | 38 | 10.3 (5.3–12.2) | 6.0 (3.6–12.2) | ||
| Cancer treatment TACE | 0.227 | 0.626 | |||
| No | 78 | 12.0 (8.6–16.7) | 9.4 (6.0–13.5) | ||
| Yes | 33 | 23.5 (11.6–29.7) | 12.2 (3.4–28.2) | ||
| Cancer treatment RFA | 0.691 | 0.895 | |||
| No | 102 | 13.5 (9.4–23.5) | 9.4 (6.0–14.8) | ||
| Yes | 9 | 12.8 (1.6–35.3) | 12.8 (1.6–35.3) | ||
| Cancer treatment chemo-embolization | 0.643 | 0.696 | |||
| No | 108 | 12.8 (9.8–18.4) | 9.8 (6.8–14.8) | ||
| Yes | 3 | 25.3 (16.7–47.4) | 1.5 (1.5–47.5) | ||
| Albumin, CTC grade | 0.222 | 0.149 | |||
| 0 | 61 | 18.4 (12.1–27.8) | 13.1 (8.1–25.8) | ||
| 1 | 31 | 10.3 (6.4–14.8) | 6.0 (3.0–10.3) | ||
| 2 | 19 | 9.0 (5.6–31.1) | 9.0 (3.6–31.1) | ||
| Albumin CTC grade 0 vs. 1+ | 0.185 | 0.309 | |||
| 0 | 61 | 18.4 (12.1–27.8) | 13.1 (8.1–25.8) | ||
| 1+ | 50 | 9.4 (7.0–13.5) | 6.8 (3.6–10.3) | ||
| Total bilirubin CTC grade | 0.195 | 0.530 | |||
| 0 | 58 | 18.4 (12.2–25.7) | 13.1 (8.1–18.4) | ||
| 1 | 28 | 8.6 (5.7–28.1) | 8.6 (2.8–28.2) | ||
| 2 | 25 | 8.5 (5.6–15.8) | 6.0 (3.2–9.0) | ||
| Total bilirubin CTC grade 0 vs. 1+ | 0.071 | 0.292 | |||
| 0 | 58 | 18.4 (12.2–25.7) | 13.1 (8.1–18.4) | ||
| 1+ | 53 | 8.6 (6.4–13.5) | 6.9 (3.4–11.4) | ||
| Creatinine | 0.515 | 0.717 | |||
| ≤1.2 | 98 | 13.1 (9.8–23.6) | 9.4 (6.0–16.2) | ||
| >1.2 | 13 | 13.8 (2.3–25.1) | 12.8 (2.3–25.1) |
a, OS time is the time (months) to death, which is defined as the time from first Y-90 resin treatment until recorded date of death; b, PFS in the liver is defined as the time (months) until death or liver progression as determined by RECIST criteria; c, blank values indicate that the bound of the 95% CI was not reached. OS, overall survival; PFS, progression-free survival; RECIST, response evaluation criteria in solid tumors; BCLC, Barcelona-Clinic Liver Cancer staging system; CTC, common terminology criteria for adverse events; PSE, portosystemic encephalopathy ECOG; MELD, model for end-stage liver disease; NASH, nonalcoholic steatohepatitis; PST, performance status test; PVT, portal vein thrombosis; RFA, radiofrequency ablation; TACE, transarterial chemoembolization; ECOG, Eastern Cooperative Oncology Group.
Acknowledgements
Writing assistance for this manuscript was provided by Christine Kuepfer, Vanessa Fogg, and Naomi Ruff of Eubio Medical Communications and statistical support was provided by Mark Van Buskirk of Data Reduction (funded by Sirtex Medical Inc.).
Ethical Statement: Data were de-identified and an IRB exemption was granted for this HIPAA-compliant study. The exemption was granted by the Methodist Dallas Medical Center IRB. Informed consent was obtained from all patients before they underwent the indicated procedures.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis 2011;15:223-43, vii-x. 10.1016/j.cld.2011.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cancer Stat Facts: Liver and Intrahepatic Bile Duct Cancer. Available online: http://seer.cancer.gov/statfacts/html/livibd.html (Accessed March 3 2016).
- 3.American Cancer Society. Cancer Facts & Figures 2017. Atlanta: American Cancer Society 2017. [Google Scholar]
- 4.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology 2014;60:1767-75. 10.1002/hep.27222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am 2015;24:1-17. 10.1016/j.soc.2014.09.001 [DOI] [PubMed] [Google Scholar]
- 6.SEER Cancer Statistics Review 1975-2012: Section 14 Liver and Intrahepatic Bile Duct. Available online: https://seer.cancer.gov/archive/csr/1975_2012/results_merged/sect_14_liver_bile.pdf
- 7.Adult Primary Liver Cancer Treatment (PDQ®)–Health Professional Version. Available online: https://www.cancer.gov/types/liver/hp/adult-liver-treatment-pdq
- 8.Akoad ME, Pomfret EA. Surgical resection and liver transplantation for hepatocellular carcinoma. Clin Liver Dis 2015;19:381-99. 10.1016/j.cld.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 9.Forner A, Gilabert M, Bruix J, et al. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol 2014;11:525-35. 10.1038/nrclinonc.2014.122 [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Hepatobiliary Cancers Version 2. 2016.
- 11.European Association For The Study Of The Liver, European Organisation For Research and Treatment Of Cancer EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 12.Bupathi M, Kaseb A, Meric-Bernstam F, et al. Hepatocellular carcinoma: Where there is unmet need. Mol Oncol 2015;9:1501-9. 10.1016/j.molonc.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. 10.1016/S0140-6736(16)32453-9 [DOI] [PubMed] [Google Scholar]
- 14.Regorafenib. Available online: https://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm555548.htm (Accessed May 18 2017).
- 15.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 16.Sangro B, Carpanese L, Cianni R, et al. Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluation. Hepatology 2011;54:868-78. 10.1002/hep.24451 [DOI] [PubMed] [Google Scholar]
- 17.SIR-Spheres® microspheres (Yttrium-90 Microspheres). North Sydney, New South Wales, Australia: Sirtex Medical Limited, 2017. [Google Scholar]
- 18.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 19.Lee EW, Thakor AS, Tafti BA, et al. Y90 selective internal radiation therapy. Surg Oncol Clin N Am 2015;24:167-85. 10.1016/j.soc.2014.09.011 [DOI] [PubMed] [Google Scholar]
- 20.Golfieri R, Bilbao JI, Carpanese L, et al. Comparison of the survival and tolerability of radioembolization in elderly vs. younger patients with unresectable hepatocellular carcinoma. J Hepatol 2013;59:753-61. 10.1016/j.jhep.2013.05.025 [DOI] [PubMed] [Google Scholar]
- 21.Sirtex. SIR-Spheres® Y-90 resin microspheres Substantially Improves Quality of Survival in Primary Liver Cancer, New Study against Standard Treatment Shows [press release]. 2017.
- 22.Chow PH, Gandhi M. Phase III multi-centre open-label randomized controlled trial of selective internal radiation therapy (SIRT) versus sorafenib in locally advanced hepatocellular carcinoma: The SIRveNIB study. J Clin Oncol 2017;35:abstr 4002.
- 23.Kolligs FT, Bilbao JI, Jakobs T, et al. Pilot randomized trial of selective internal radiation therapy vs. chemoembolization in unresectable hepatocellular carcinoma. Liver Int 2015;35:1715-21. 10.1111/liv.12750 [DOI] [PubMed] [Google Scholar]
- 24.Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23. 10.1016/j.ijrobp.2006.11.060 [DOI] [PubMed] [Google Scholar]
- 25.Salem R, Lewandowski RJ. Chemoembolization and radioembolization for hepatocellular carcinoma. Clin Gastroenterol Hepatol 2013;11:604-11; quiz e43-4. [DOI] [PMC free article] [PubMed]
- 26.Rammohan A, Sathyanesan J, Ramaswami S, et al. Embolization of liver tumors: Past, present and future. World J Radiol 2012;4:405-12. 10.4329/wjr.v4.i9.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Facciorusso A, Serviddio G, Muscatiello N. Transarterial radioembolization vs chemoembolization for hepatocarcinoma patients: A systematic review and meta-analysis. World J Hepatol 2016;8:770-8. 10.4254/wjh.v8.i18.770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riaz A, Awais R, Salem R. Side effects of yttrium-90 radioembolization. Front Oncol 2014;4:198. 10.3389/fonc.2014.00198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rostambeigi N, Dekarske AS, Austin EE, et al. Cost effectiveness of radioembolization compared with conventional transarterial chemoembolization for treatment of hepatocellular carcinoma. J Vasc Interv Radiol 2014;25:1075-84. 10.1016/j.jvir.2014.04.014 [DOI] [PubMed] [Google Scholar]