Abstract
Background
The placement of a metallic stent as a bridge to surgery (SBTS) could represents an option for the treatment of left-sided malignant colonic obstruction in centres with adequate skills. Several meta-analyses demonstrated better short-term outcomes after SBTS when compared with emergency surgery (ES); however, some studies reported a higher local recurrence rate. The aim of the present review is to investigate the long-term outcomes of stent bridge to surgery as compared to ES.
Methods
A systematic review was performed to retrieve studies comparing long-term oncologic outcomes of SBTS and ES. Local and overall recurrence rate, overall and disease-free survival were retrieved and results were expressed as risk ratios.
Results
Seventeen studies and a total of 1,333 patients were included in the analysis. No significant differences were reported in recurrence rate [risk ratio (RR) =1.11; 95% confidence interval (95% CI): 0.84–1.47, P=0.47], 3-year mortality (RR =0.90; 95% CI: 0.73–1.12, P=0.34) and 5-year mortality (RR =1.00; 95% CI: 0.82–1.22, P=0.99). There were no differences among randomized and observational studies.
Conclusions
SBTS has similar long-term oncologic outcomes to ES and in centres with appropriate skill should be considered the best treatment option for left-sided malignant colonic obstructions.
Keywords: Colonic stent, emergency surgery (ES), long-term outcomes, colonic obstruction
Introduction
Colorectal cancer is the most common cause of large bowel obstruction, which represents the presentation of disease in about 10% of the patients (1). This condition requires an immediate treatment with surgical intervention, with related high morbidity and mortality (2,3). In the 90s the insertion of self-expandable metallic stents (SEMS) has been proposed as an alternative to surgery, both for palliation in advanced disease and as a stent as a bridge to surgery (SBTS) in order to solve the obstruction and to allow delayed elective surgery. Several randomized trials investigated this issue and were summarized in several meta-analyses: SBTS resulted in a lower morbidity, with more favorable short-term results (4-7). The World Society of Emergency Surgery (WSES) Guidelines on left-sided malignant colonic obstruction recommended SBTS as the better option when and where skills are available (8). SBTS developed a good diffusion as a treatment option due to favorable short-term outcomes; however, the long-term prognosis of this treatment has not been well clarified.
Patients with colorectal obstructing cancer carry a worse prognosis as compared to elective patients without obstructive features (9,10). Moreover, concerns regarding the oncologic outcomes after the insertion of SEMS have been expressed: stenting is suspected to increase local and systemic neoplastic seeding. In particular, the direct effect of mechanical compression of the tumour could induce haematogenous spread and, in case of perforation, the risk of peritoneal involvement would be increased (11-16); however, the real effect on overall survival remains unclear, with contrasting results reported in literature.
The aim of this systematic review and meta-analysis is to investigate the long-term outcomes of SBTS as compared to emergency surgery (ES) in left-sided colorectal obstructing cancers, in order to clarify the real effect on disease free survival and overall survival.
Methods
Literature search and study selection
A systematic review was performed in Medline, Embase, PubMed, Cochrane Central Register of Controlled Trials (CCTR) and Cochrane Database of Systematic Reviews (CDSR) until 20th January 2017. The following terms were used combined with AND/OR: colonic stent, stent bridge to surgery, large bowel malignant obstruction, oncologic outcomes. All the titles and abstracts of retrieved references were reviewed independently by two researchers (M Ceresoli and N Allievi) and those identified as potentially relevant were included in the full-text analysis and then selected if they met the inclusion criteria.
Selection criteria
For the purposes of the current meta-analysis inclusion and exclusion criteria were defined as follows: full-text publications written in English reporting follow-up comparison between colonic SBTS and ES in left-sided and rectum obstructive cancer were selected. Either randomized trial and prospective or retrospective comparative cohort studies were initially selected, in order to maximize the number of patients. Studies were excluded if they regarded right side obstructive cancer or if they included no data about the long-term follow-up. Case series, letters, case reports and review were also excluded.
Data extraction, outcome measures
For each selected paper the following elements were retrieved: study protocol and design, period of study, number of participant centres, number of included patients and data about follow up including median follow-up, 3-year and 5-year mortality and recurrence rate, overall recurrence rate and local recurrence rate. Where available, data of subgroup of patients with potential curative resections were also analyzed. In case data about long-term outcomes were not available as proportion or percentages, we estimated the rate from the Kaplan-Meier survival curves with the highest possible accuracy and then the number of event were calculated.
Assessing risk of bias
For randomized controlled trials the risk of bias was assessed comprehensively according to the guidelines of the Cochrane Collaboration (17), attributing a judgement for the following items: random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, assessment of incomplete data outcome, selective reporting and other source of bias. For the prospective and retrospective comparative cohort studies the quality of the included studies and the risk of bias were assessed using the MINORS score (18).
Statistical analysis
Data was analysed with Review Manager (RevMan) (Version 5.3 Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011). Outcomes were expressed as risk ratio (RR) and 95% confidence interval (95% CI) and were calculated with the fixed-effect and random-effect models of the Mantel-Haenszel test (19,20); statistical heterogeneity was quantified using the I2 inconsistency test and, when significant (P<0.1), the sole results of the random-effects model were reported.
Results
Selected studies
A total number of 1,083 abstract were retrieved and, after review of title and abstract, 25 were assessed for eligibility; there was good agreement between the two authors. Among them, after full text review, 17 studies were included for the analysis: 5 RCTs (21-25), 3 prospective comparative cohort studies (16,26,27) and 9 retrospective comparative cohort studies (13-15,28-33). Five studies did not report follow-up data (34-38), 2 studies reported short-term outcomes of other included trials (39,40) and were also excluded. A retrospective comparative cohort study reported unclear data about long-term follow up, therefore it was decided to exclude it from the analysis (41).
Table 1 shows the characteristics of the included studies. See the PRISMA flow diagram in Figure 1.
Table 1. Characteristics of included studies.
| Study ID | Year of publication | Country | Study design | Included patients | Centres | Included patients characteristics | ITT analysis | Years of study | ACT (%) | Median follow-up | Risk of bias/MINORS score |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | SBTS | ES | SBTS | ES | SBTS | ES | ||||||||||
| Arezzo | 2016 | Italy, Spain | RCT | 115 | 56 | 59 | 5 | Only curative resections | x | 2008–2015 | 66 | 70 | 16 | 16 | Low | |
| Alcantara | 2011 | Spain | RCT | 28 | 15 | 13 | 1 | Only curative resections | x | 2004–2006 | n/a | n/a | 37 | 37 | Medium | |
| Ghazal | 2013 | Egypt | RCT | 60 | 30 | 30 | 1 | Only curative resections | 2009–2012 | 100 | 100 | 18 | 18 | Low | ||
| Sloothaak | 2014 | Netherlands | RCT | 58 | 26 | 32 | 25 | Only curative resections | x | 2007–2009 | 50 | 46 | 41 | 45 | Low | |
| Tung | 2013 | Hong Kong, China | RCT | 48 | 24 | 24 | 1 | All patients | x | 2002–2005 | 54 | 75 | 32 | 65 | Low | |
| Dastur | 2008 | UK | RC | 42 | 19 | 23 | 1 | All patients | x | 1997–2004 | n/a | n/a | 21 | 30 | 18/24 | |
| Gianotti | 2013 | Italy | PC | 91* | 45* | 46* | 1 | Subgroup analysis of curative resections for cancer | 2004–2011 | n/a | n/a | n/a | n/a | 16/24 | ||
| Gorissen | 2013 | UK | PC | 105 | 62 | 43 | 1 | Only curative resections | x | 2006–2012 | 41 | 25 | 32 | 33 | 17/24 | |
| Ho | 2016 | Hong Kong, China | RC | 102 | 62 | 40 | 1 | Only curative resections | x | 2006–2014 | 45 | 27 | 21 | 25.5 | 18/24 | |
| Kavanagh | 2013 | Ireland | RC | 49 | 23 | 26 | 1 | Only curative resections | x | 2005–2011 | n/a | n/a | 27.4 | 26 | 18/24 | |
| Kim | 2013 | South Korea | RC | 95 | 25 | 70 | 1 | All patients | 1996–2007 | 84 | 65 | 43 | 54 | 20/24 | ||
| Kim | 2016 | South Korea | RC PS matched | 168 | 112 | 56 | 6 | Only curative resections | x | 2004–2010 | 83 | 78 | 45 | 49 | 18/24 | |
| Kwak | 2016 | South Korea | RC PS matched | 84 | 42 | 42 | 1 | All patients | x | 2005–2011 | 60 | 73 | 42.1 | 52.8 | 18/24 | |
| Quereshy | 2014 | Hong Kong, China | PC | 67 | 28 | 39 | 1 | Only curative resections | x | 1998–2008 | n/a | n/a | 26.5 | 31.3 | 17/24 | |
| Sabbagh | 2013 | France | RC PS adjusted | 87 | 48 | 39 | 2 | All patients | x | 1998–2011 | 56 | 43 | 30 | 37 | 13/24 | |
| Saida | 2003 | Japan | RC | 84 | 44 | 40 | 1 | All patients | 1986–2001 | 66 | 53 | 84 | 84 | 14/24 | ||
| Yan | 2017 | China | RC | 60 | 27 | 33 | 1 | All patients | x | 2007–2012 | 70 | 45 | n/a | n/a | 17/24 | |
*, subgroup analysis of cancer patients. ACT, adjuvant chemotherapy; PC, prospective cohort; RC, retrospective cohort; PS, propensity score.
Figure 1.
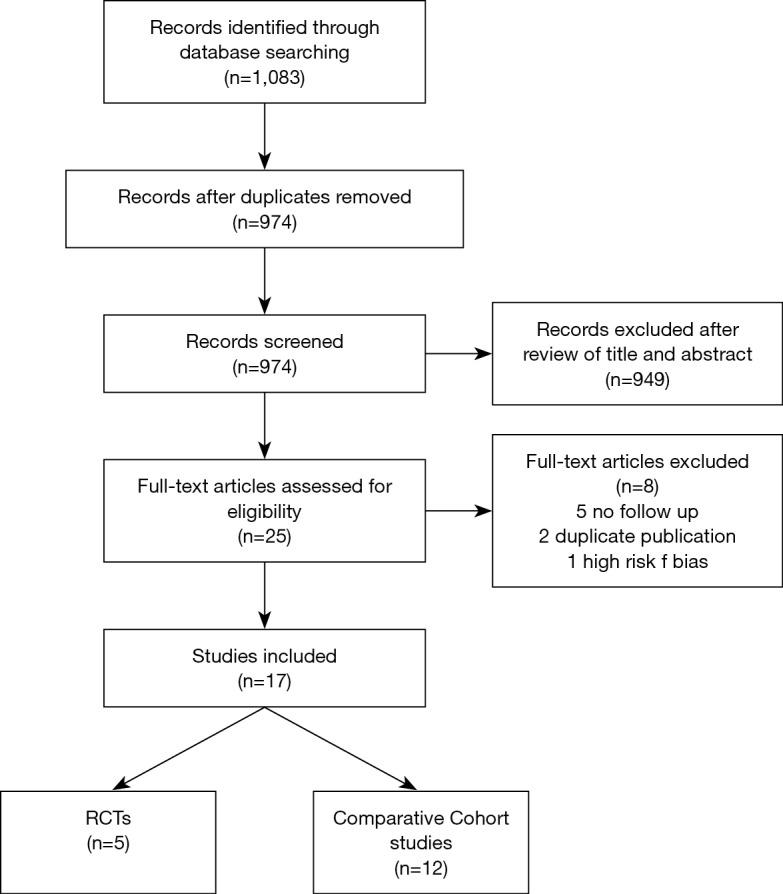
PRISMA flow diagram.
All the included studies had similar study protocols, with the exception of the study design; all but two (26,32) reported the median follow-up, ranging between 16 and 84 months. Eleven studies reported the oncologic treatment and the pooled rate of patients receiving adjuvant chemotherapy after surgery was similar for both the groups (63% vs. 54%, RR =1.08; 95% CI: 0.95–1.23, P=0.26). Table 1 shows the characteristics of included studies.
Risk of bias and quality of the studies
All the included RCT were judged as at low risk of bias, according to the Cochrane collaboration tool. Two trials were discontinued prematurely due to the high rate of anastomotic leak within the ES group (22) and to the high comorbidity rate in the stent group (SBTS) (24). Moreover, the trial by Ghazal did not report data as intention-to-treat analysis but as per protocol analysis (23) (Table 2).
Table 2. Risk of bias assessment of RCT included.
| Study ID | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Alcantara 2011 | Unclear | Unclear | Adequate | Adequate | Adequate | Adequate | Premature ending |
| Arezzo 2016 | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | None |
| Tung 2016 | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | None |
| Ghazal 2013 | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | None |
| Sloothaak 2014 | Adequate | Adequate | Adequate | Adequate | Adequate | Adequate | Premature ending |
The quality of comparative cohort studies was assessed using the MINORS score; no study obtained the maximum score (Table 1).
Overall and local recurrence
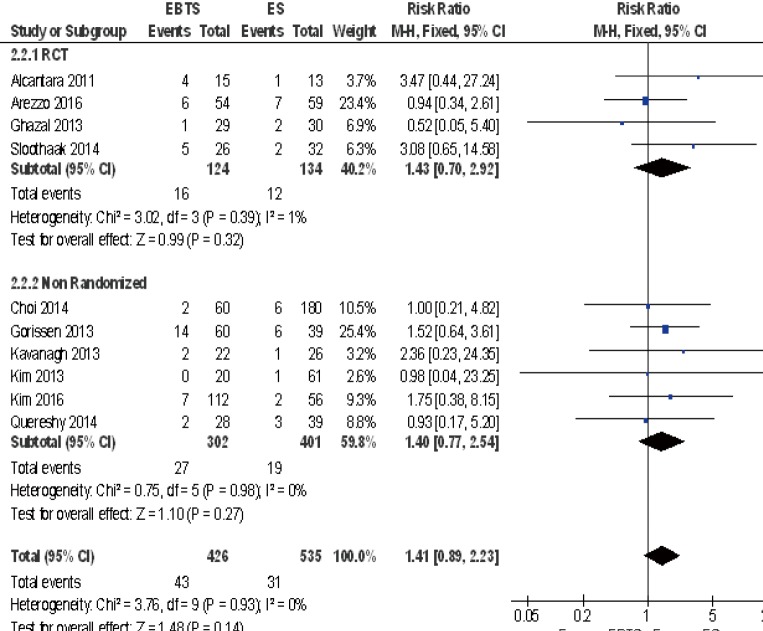
A total of 13 studies reported the recurrence rate and 1089 patients were included in the analysis: there were no significant differences between SBTS and ES (RR =1.11; 95% CI: 0.84–1.47, P=0.47). There were no differences among randomized and observational studies. Ten studies reported the rate of local recurrence and no significant differences were depicted (RR =1.41; 95% CI: 0.89–2.23, P=0.14). There were no differences among RCT and observational studies (Figure 2).
Figure 2.
Forest plot of local recurrences between patients treated with SBTS or ES. SBTS, stent as a bridge to surgery; ES, emergency surgery.
3-year mortality
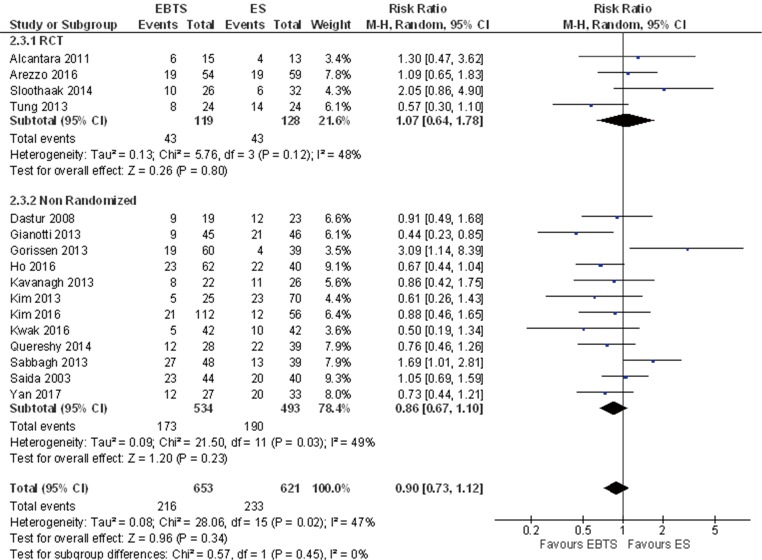
Sixteen studies reported data about 3-year mortality and 1,274 patients were included in the analysis: there were no significant differences among the two groups (RR =0.90; 95% CI: 0.73–1.12, P=0.34) without differences among different study designs. Including only patients who underwent potentially curative resection, no differences were found (RR =1.04; 95% CI: 0.78–1.39, P=0.78) (Figure 3).
Figure 3.
Forest plot of 3-year mortality between patients treated with SBTS or ES. SBTS, stent as a bridge to surgery; ES, emergency surgery.
5-year mortality
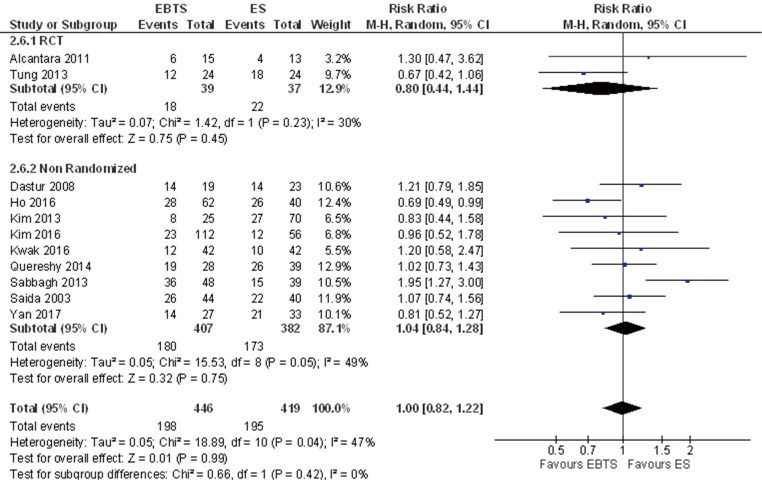
A total of 865 patients from eleven studies were included in the analysis for 5-year mortality: extrapolated data showed no significant differences (RR =1.00; 95% CI: 0.82–1.22, P=0.99). Including in the analysis only patients with potentially curative resections, data from 8 studies were available and no significant differences were reported (RR =0.95; 95% CI: 0.53–1.69, P=0.86) (Figure 4).
Figure 4.
Forest plot of 5-year mortality between patients treated with SBTS or ES. SBTS, stent as a bridge to surgery; ES, emergency surgery.
3- and 5-year recurrence
Data regarding recurrence at three years was available from only seven studies: there was no significant difference among the two groups (RR =1.15; 95% CI: 0.95–1.39, P=0.14); data for the five-year outcome was available from six studies and no differences were found with the meta-analysis (RR =1.05; 95% CI: 0.88–1.25, P=0.59).
Discussion
The results of the present meta-analysis show that long-term oncological outcomes of patients treated with SBTS are comparable with those of patients treated with ES in case of left-sided malignant colonic obstruction. In particular, the pooled results of all the included studies did not show any differences in terms of local or systemic recurrence and no differences in 3- and 5-year survival.
The rationale of SEMS positioning as a bridge to surgery is to resolve the acute situation with colonic decompression, therefore transforming ES into scheduled or elective. The clinical success of the SEMS allows stabilization of comorbidities, improvement of nutritional status, accurate staging and definition of a tailored treatment for the patients, in the best conditions available. Patients with obstructing colonic tumour carry a worse prognosis than patients presenting without the obstructive picture (10). The increased interstitial pressure in the neoplastic mass can play a pivotal role in cells dissemination and it has been associated with cell shedding and tumour embolisation into lymphatic vessels (42,43).
The role of SEMS in neoplastic dissemination is an issue of great debate among surgeons, as demonstrated by concerns raising from several published studies. Maruthachalam et al. (11) demonstrated that circulating mRNAs of CEA and CK20 were significantly higher after colonic stenting: the authors identified the tumor manipulation during guidewire insertion and the tumor dilatation during stent deployment as possible culprits of this phenomenon. Moreover, the dilatation and the manipulation could induce shedding and dissemination of cancer cells into the peritoneal cavity.
Gorissen et al. (16) reported a higher rate of local recurrence in patients treated with SBTS, especially in younger subjects (32% vs. 8%); however, at a multivariate analysis stenting was not correlated to this augmented local recurrence and no effect on overall survival was noted. Kim et al. (14) demonstrated a higher rate of perineural invasion at histopathological examination of tumours treated with SBTS compared to those operated in emergency (76% vs. 51%): also in this case, no differences in survival rates were detected.
The role of circulating tumour cells still represents an issue of difficult interpretation with not clear results reported in the Literature: a large multicenter study demonstrated the presence of circulating cells in patients with stage III colorectal cancer but no clinical effects on long-term survivals and recurrences was detected (44). Despite the evidence of neoplastic cell spread from the primary site during the stenting procedure, the results of the current meta-analysis demonstrate that no clinical effects could be detected in long-term survival and prognosis and that SBTS did not affect negatively the oncological outcomes.
A hypothesis may be drawn to explain this phenomenon: post-operative complications affects negatively the oncologic outcomes and survivals (45). As previously demonstrated by several meta-analyses, SBTS has better short-term outcomes in term of post-operative morbidity (4-6); this could result in a higher rate of patients receiving full dose adjuvant chemotherapy with the appropriate timing, with better results in term of survival, as evidenced in the literature (46). Although not significantly, patients treated with SBTS had a higher rate of adjuvant chemotherapy, as compared to those treated with ES (63% vs. 54%) in the included studies of the present meta-analysis. Unfortunately, no data regarding the timing of chemotherapy and the adherence to the protocol is available, hindering definitive evidence-based judgement on this hypothesis.
The results of this meta-analyses should be interpreted at the light of a great limitation: none of the included studies was designed for long-term follow up. As a direct consequence, median follow up times were limited and heterogeneous and survival rates were estimated with the Kaplan Meier method rather than observed. Further accurate studies are needed to investigate the long-term outcomes with a proper design and number of included patients.
Conclusions
The results of the present meta-analysis demonstrate that long-term oncologic outcomes are comparable in patients treated with SBTS or ES for left-sided malignant colonic obstructions. At the light of the favorable short-term outcomes and the absence of long-term clinical effects of the procedure, SBTS should be considered as a valid treatment option in centres with adequate technical skills.
Acknowledgements
We would like to give special thanks to Mrs. Franca Boschini, Papa Giovanni XXIII Hospital Library for the bibliographic research.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Yeo HL, Lee SW. Colorectal emergencies: review and controversies in the management of large bowel obstruction. J Gastrointest Surg 2013;17:2007-12. 10.1007/s11605-013-2343-x [DOI] [PubMed] [Google Scholar]
- 2.Tekkis PP, Kinsman R, Thompson MR, et al. The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg 2004;240:76-81. 10.1097/01.sla.0000130723.81866.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carraro PG, Segala M, Cesana BM, et al. Obstructing colonic cancer: failure and survival patterns over a ten-year follow-up after one-stage curative surgery. Dis Colon Rectum 2001;44:243-50. 10.1007/BF02234300 [DOI] [PubMed] [Google Scholar]
- 4.Cirocchi R, Farinella E, Trastulli S, et al. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: A systematic review and meta-analysis. Surg Oncol 2013;22:14-21. 10.1016/j.suronc.2012.10.003 [DOI] [PubMed] [Google Scholar]
- 5.Huang X, Lv B, Zhang S, et al. Preoperative Colonic Stents Versus Emergency Surgery for Acute Left-Sided Malignant Colonic Obstruction: A Meta-analysis. J Gastrointest Surg 2014;18:584-91. 10.1007/s11605-013-2344-9 [DOI] [PubMed] [Google Scholar]
- 6.Tan CJ, Dasari BV, Gardiner K. Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg 2012;99:469-76. 10.1002/bjs.8689 [DOI] [PubMed] [Google Scholar]
- 7.Allievi N, Ceresoli M, Fugazzola P, et al. Endoscopic Stenting as Bridge to Surgery versus Emergency Resection for Left-Sided Malignant Colorectal Obstruction: An Updated Meta-Analysis. Int J Surg Oncol 2017;2017:2863272. 10.1155/2017/2863272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansaloni L, Andersson RE, Bazzoli F, et al. Guidelenines in the management of obstructing cancer of the left colon: consensus conference of the world society of emergency surgery (WSES) and peritoneum and surgery (PnS) society. World J Emerg Surg 2010;5:29. 10.1186/1749-7922-5-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korenaga D, Ueo H, Mochida K, et al. Prognostic factors in japanese patients with colorectal cancer: The significance of large bowel obstruction—univariate and multivariate analyses. J Surg Oncol 1991;47:188-92. 10.1002/jso.2930470310 [DOI] [PubMed] [Google Scholar]
- 10.Runkel NS, Schlag P, Schwarz V, et al. Outcome after emergency surgery for cancer of the large intestine. Br J Surg 1991;78:183-8. 10.1002/bjs.1800780216 [DOI] [PubMed] [Google Scholar]
- 11.Maruthachalam K, Lash GE, Shenton BK, et al. Tumour cell dissemination following endoscopic stent insertion. Br J Surg 2007;94:1151-4. 10.1002/bjs.5790 [DOI] [PubMed] [Google Scholar]
- 12.Suárez J, Jimenez-Pérez J. Long-term outcomes after stenting as a "bridge to surgery" for the management of acute obstruction secondary to colorectal cancer. World J Gastrointest Oncol 2016;8:105-12. 10.4251/wjgo.v8.i1.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JS, Hur H, Min BS, et al. Oncologic outcomes of self-expanding metallic stent insertion as a bridge to surgery in the management of left-sided colon cancer obstruction: Comparison with nonobstructing elective surgery. World J Surg 2009;33:1281-6. 10.1007/s00268-009-0007-5 [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Choi GS, Park JS, et al. Higher rate of perineural invasion in stent-laparoscopic approach in comparison to emergent open resection for obstructing left-sided colon cancer. Int J Colorectal Dis 2013;28:407-14. 10.1007/s00384-012-1556-x [DOI] [PubMed] [Google Scholar]
- 15.Sabbagh C, Browet F, Diouf M, et al. Is stenting as “a bridge to surgery” an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg 2013;258:107-15. 10.1097/SLA.0b013e31827e30ce [DOI] [PubMed] [Google Scholar]
- 16.Gorissen KJ, Tuynman JB, Fryer E, et al. Local recurrence after stenting for obstructing left-sided colonic cancer. Br J Surg 2013;100:1805-9. 10.1002/bjs.9297 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JP, Green S. editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011:Table 7.7. [Google Scholar]
- 18.Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712-6. 10.1046/j.1445-2197.2003.02748.x [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 20.Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med 1987;6:341-50. 10.1002/sim.4780060325 [DOI] [PubMed] [Google Scholar]
- 21.Arezzo A, Balague C, Targarona E, et al. Colonic stenting as a bridge to surgery versus emergency surgery for malignant colonic obstruction: results of a multicentre randomised controlled trial (ESCO trial). Surg Endosc 2017;31:3297-305. 10.1007/s00464-016-5362-3 [DOI] [PubMed] [Google Scholar]
- 22.Alcántara M, Serra-Aracil X, Falcó J, et al. Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg 2011;35:1904-10. 10.1007/s00268-011-1139-y [DOI] [PubMed] [Google Scholar]
- 23.Ghazal AH, El-Shazly WG, Bessa SS, et al. Colonic Endolumenal Stenting Devices and Elective Surgery Versus Emergency Subtotal/Total Colectomy in the Management of Malignant Obstructed Left Colon Carcinoma. J Gastrointest Surg 2013;17:1123-9. 10.1007/s11605-013-2152-2 [DOI] [PubMed] [Google Scholar]
- 24.Sloothaak DA, Van Den Berg MW, Dijkgraaf MG, et al. Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br J Surg 2014;101:1751-7. 10.1002/bjs.9645 [DOI] [PubMed] [Google Scholar]
- 25.Tung KL, Cheung HY, Ng LW, et al. Endo-laparoscopic approach versus conventional open surgery in the treatment of obstructing left-sided colon cancer: long-term follow-up of a randomized trial. Asian J Endosc Surg 2013;6:78-81. 10.1111/ases.12030 [DOI] [PubMed] [Google Scholar]
- 26.Gianotti L, Tamini N, Nespoli L, et al. A prospective evaluation of short-term and long-term results from colonic stenting for palliation or as a bridge to elective operation versus immediate surgery for large-bowel obstruction. Surg Endosc 2013;27:832-42. 10.1007/s00464-012-2520-0 [DOI] [PubMed] [Google Scholar]
- 27.Quereshy FA, Poon JT, Law WL. Long-term outcome of stenting as a bridge to surgery for acute left-sided malignant colonic obstruction. Colorectal Dis 2014;16:788-93. 10.1111/codi.12666 [DOI] [PubMed] [Google Scholar]
- 28.Dastur JK, Forshaw MJ, Modarai B, et al. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol 2008;12:51-5. 10.1007/s10151-008-0399-5 [DOI] [PubMed] [Google Scholar]
- 29.Ho KM, Chan KM, Kwok SY, et al. Colonic self-expanding metal stent (SEMS) as a bridge to surgery in left-sided malignant colonic obstruction: an 8-year review. Surg Endosc 2017;31:2255-62. 10.1007/s00464-016-5227-9 [DOI] [PubMed] [Google Scholar]
- 30.Kavanagh DO, Nolan B, Judge C, et al. A Comparative Study of Short- and Medium-term Outcomes Comparing Emergent Surgery and Stenting as a Bridge to Surgery in Patients With Acute Malignant Colonic Obstruction. Dis Colon Rectum 2013;56:433-40. 10.1097/DCR.0b013e3182760506 [DOI] [PubMed] [Google Scholar]
- 31.Kwak MS, Kim WS, Lee JM, et al. Does Stenting as a Bridge to Surgery in Left-Sided Colorectal Cancer Obstruction Really Worsen Oncological Outcomes? Dis Colon Rectum 2016;59:725-32. 10.1097/DCR.0000000000000631 [DOI] [PubMed] [Google Scholar]
- 32.Yan FH, Lou Z, Liu X, et al. Long-Term Oncological Outcomes of Endoscopic Stenting as A Bridge to Surgery Versus Emergency Surgery for Malignant Colorectal Obstruction: A Comparative Study. J Laparoendosc Adv Surg Tech A 2017;27:611-7. 10.1089/lap.2016.0529 [DOI] [PubMed] [Google Scholar]
- 33.Saida Y, Sumiyama Y, Nagao J, et al. Long-term prognosis of preoperative “bridge to surgery” expandable metallic stent insertion for obstructive colorectal cancer: comparison with emergency operation. Dis Colon Rectum 2003;46:S44-9. [DOI] [PubMed] [Google Scholar]
- 34.van den Berg MW, Sloothaak DA, Dijkgraaf MG, et al. Bridge-to-surgery stent placement versus emergency surgery for acute malignant colonic obstruction. Br J Surg 2014;101:867-73. 10.1002/bjs.9521 [DOI] [PubMed] [Google Scholar]
- 35.Pirlet IA, Slim K, Kwiatkowski F, et al. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: A multicenter randomized controlled trial. Surg Endosc 2011;25:1814-21. 10.1007/s00464-010-1471-6 [DOI] [PubMed] [Google Scholar]
- 36.Consolo P, Giacobbe G, Cintolo M, et al. Colonic acute malignant obstructions : effectiveness of self-expanding metallic stent as bridge to surgery. Turk J Gastroenterol 2017;28:40-5. 10.5152/tjg.2016.0249 [DOI] [PubMed] [Google Scholar]
- 37.Ho KS, Quah HM, Lim JF, et al. Endoscopic stenting and elective surgery versus emergency surgery for left-sided malignant colonic obstruction: A prospective randomized trial. Int J Colorectal Dis 2012;27:355-62. 10.1007/s00384-011-1331-4 [DOI] [PubMed] [Google Scholar]
- 38.Ng KC, Law W, Lee Y, et al. Self-Expanding Metallic Stent as a Bridge to Surgery Versus Emergency Resection for Obstructing Left-Sided Colorectal Cancer: A Case-Matched Study. J Gastrointest Surg 2006;10:798-803. 10.1016/j.gassur.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 39.van Hooft JE, Bemelman WA, Oldenburg B, et al. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: A multicentre randomised trial. Lancet Oncol 2011;12:344-52. 10.1016/S1470-2045(11)70035-3 [DOI] [PubMed] [Google Scholar]
- 40.Cheung HY, Chung CC, Tsang WW, et al. Endolaparoscopic approach vs. conventional open surgery in the treatment of obstructing left-sided colon cancer: a randomized controlled trial. Arch Surg 2009;144:1127-32. 10.1001/archsurg.2009.216 [DOI] [PubMed] [Google Scholar]
- 41.Choi JM, Lee C, Han YM, et al. Long-term oncologic outcomes of endoscopic stenting as a bridge to surgery for malignant colonic obstruction: Comparison with emergency surgery. Surg Endosc 2014;28:2649-55. 10.1007/s00464-014-3517-7 [DOI] [PubMed] [Google Scholar]
- 42.Lemoine L, Sugarbaker P, Van der Speeten K. Pathophysiology of colorectal peritoneal carcinomatosis: Role of the peritoneum. World J Gastroenterol 2016;22:7692. 10.3748/wjg.v22.i34.7692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayashi K, Jiang P, Yamauchi K, et al. Real-time Imaging of Tumor-Cell Shedding and Trafficking in Lymphatic Channels. Cancer Res 2007;67:8223-8. 10.1158/0008-5472.CAN-07-1237 [DOI] [PubMed] [Google Scholar]
- 44.Sotelo MJ, Sastre J, Maestro ML, et al. Role of circulating tumor cells as prognostic marker in resected stage III colorectal cancer. Ann Oncol 2015;26:535-41. 10.1093/annonc/mdu568 [DOI] [PubMed] [Google Scholar]
- 45.Artinyan A, Orcutt ST, Anaya DA, et al. Infectious Postoperative Complications Decrease Long-term Survival in Patients Undergoing Curative Surgery for Colorectal Cancer: A Study of 12,075 Patients. Ann Surg 2015;261:497-505. 10.1097/SLA.0000000000000854 [DOI] [PubMed] [Google Scholar]
- 46.Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA 2011;305:2335-42. 10.1001/jama.2011.749 [DOI] [PubMed] [Google Scholar]