Abstract
Background
Pancreatic adenocarcinoma is a highly aggressive cancer, with surgical resection and systemic therapy offering the only hope for long-term survival. Carbohydrate antigen 19-9 (CA 19-9) has been used as a prognostic marker after resection; however, the relationship between survival and pre-treatment CA 19-9 level remains unclear. This study evaluates pre-treatment serum CA 19-9 level as a predictor for long-term survival.
Methods
The U.S. National Cancer Data Base [2004–2012] was reviewed for patients with clinical stages I–III resected pancreatic adenocarcinoma with recorded pre-treatment CA 19-9 levels (U/mL). Kaplan Meier and Weibull survival analyses were performed.
Results
Four thousand seven hundred and one patients were included: 12.6% received neoadjuvant therapy (NAT), 27.4% underwent surgery, and 60.1% underwent surgery and adjuvant therapy. Amongst those who underwent initial surgery, there was no association between CA 19-9 levels ≤800 (≤100, 101–300, 301–500, 501–800) with survival (stage I P=0.7592, stage II P=0.5088, stage III P=0.9037). Levels >800 were associated with significantly worse survival in all stages (P≤0.0001, all). Amongst those who received NAT, levels >800 were associated with worse survival in early (stage I P=0.0001), but not advanced stage disease (stage II P=0.1891, stage III P=0.9316). In multivariable analyses, levels >800 demonstrated a 3.29 greater hazard of mortality with respect to patients with levels ≤100 (P<0.0001).
Conclusions
Pre-treatment CA 19-9 levels >800 appear to be associated with advanced disease, and are negatively associated with long-term survival. However, levels ≤800 had no significant association with survival. Although this study suggests an association, further study is needed to evaluate whether patients with CA 19-9 levels >800 benefit from NAT.
Keywords: Pancreatic cancer, carbohydrate antigen 19-9 (CA 19-9)
Introduction
Pancreatic adenocarcinoma is a highly aggressive cancer, with surgical resection and systemic therapy offering the only hope for long-term survival. Due to its anatomical location, the majority of patients present with advanced disease, with only 15–20% presenting as surgical candidates (1). Thus, there is a tremendous demand for prognostic markers.
One such marker is carbohydrate antigen 19-9 (CA 19-9), a sialylated Lewis blood group antigen regarded to be the most sensitive and specific serum marker for pancreatic cancer to date (2-4). While a CA 19-9 level of 37 U/mL is regarded as normal, a wide array of non-malignant elevators make the tumor marker a poor screening test in asymptomatic patients (5,6). CA 19-9 has been used as a prognostic marker after resection to monitor for recurrence and as a prognostic factor in inoperable cancer (7,8). However, correlation of CA 19-9 level at diagnosis with survival in resectable pancreatic cancer remains unclear. This study evaluates serum CA 19-9 level prior to treatment as a predictor for long-term survival.
Methods
Data
The National Cancer Data Base (NCDB) is a clinical oncology database based on hospital registry data in the United States. The data reflects over 70% of newly diagnosed cancer cases [1998–2012] from over 1,500 Commission on Cancer accredited facilities. This was a retrospective cohort study of clinical data from this registry from 2004–2012. The study was deemed exempt by the M. S. Hershey Medical Center Human Subject Protection Board and it conforms to the provisions of in accordance with the Helsinski Declaration as revised in 2013, available at: http://www.wma.net/en/30publications/10policies/b3/%20index.html.
Patient selection
The NCDB was reviewed for patients diagnosed with clinical stages I–III resected pancreatic adenocarcinoma with CA 19-9 levels prior to treatment recorded. Patients with clinical stage IV disease or unknown stage were excluded. Clinical stage is a coded variable within the NCDB which is determined per individual institution. Histologies included in this analysis were carcinoma and adenocarcinoma identified using the third edition of the International Classification of Diseases for Oncology (ICD-O-3) primary site codes: C250–C254, C257–C259 and histology ICD-O-3 codes: 8050/3 8140/3, 8144/3, 8148/3, 8160/3, 8230/3, 8255/3, 2890/3, 8310/3, 8323/3, 8342/3, 8346/3, 8380/3, 8430/3, 8460/3, 8461/3, 8480/3, 8481/3, 8500/3, 8503/3, 8504/3, 8507/3, 8510/3, 8521/3, 8523/3, 8550/3, 8560/3, 8562/3, 8570/3, 8574/3, and 8576/3. Patients who underwent an unknown surgical procedure, or local excision of tumor were excluded, in order to best capture those patients undergoing formal pancreatic resection. Patients with missing or unknown CA 19-9 levels were excluded. In the NCDB, CA 19-9 level is coded as 0–980 U/mL, with values over 980 U/mL coded simply as ≥980 U/mL.
Outcomes and covariates
The primary outcome assessed was survival from diagnosis. The highest CA 19-9 lab value documented in the medical record prior to treatment was coded with the database. In order to best capture patients with malignancy related elevations in CA 19-9, the study population was stratified at a CA 19-9 level of 800 U/mL. Covariates included sex, race, age, median income, insurance type and the Charlson/Deyo comorbidity index (CCI). The CCI is an index of 15 common comorbidities including ranging from cardiac to pulmonary, to vascular, to renal, to endocrine disorders (9,10).
Treatment facilities were characterized by geographic region and facility type. Disease was characterized by clinical and pathological variables including the American Joint Committee on Cancer (AJCC) clinical stage, number of lymph nodes sampled, number of positive lymph nodes, surgical margins, and pathological stage. Treatment was characterized by surgery type (distal pancreatectomy, whipple, total pancreatectomy and other), neoadjuvant treatment (NAT), and adjuvant treatment.
Statistical analysis
STATA software (version 12.1, StataCorp., College Station, TX, USA) was used to perform statistical analyses. Patient, disease, and treatment characteristics were compared between groups using student’s t-tests, and chi square tests, as appropriate. Survival was assessed with Kaplan-Meier analyses stratified by CA 19-9 categories and a Weibull model.
Results
Of the 4,701 patients with clinically staged I–III resected pancreatic adenocarcinoma, 592 (12.6%) received neoadjuvant therapy, 1,286 (27.4%) underwent surgical resection alone, and 2,823 (60.1%) underwent surgery followed by adjuvant therapy. The median CA 19-9 level was 888 U/mL (range, 0–980 U/mL), with a distribution demonstrating 19.9% with CA 19-9 levels ≤100 U/mL, 12.2% between 100 and 300 U/mL, 6.6% between 300 and 500 U/mL, 7.1% between 500 and 800 U/mL, and 54.2% >800 U/mL (Figure 1).
Figure 1.
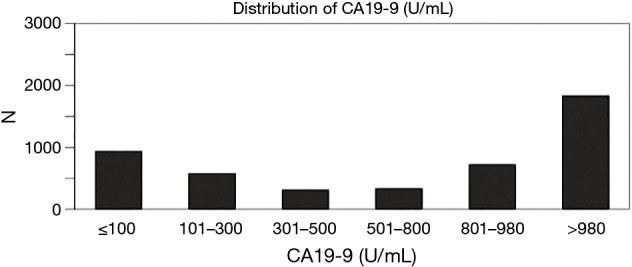
Distribution of CA 19-9 levels. CA 19-9, carbohydrate antigen 19-9.
Patient demographics stratified by treatment and CA 19-9 levels ≤800 and >800 U/mL are reported in Table 1. Within the cohort of patients who underwent surgical resection alone or received adjuvant therapy, patients with CA 19-9 levels ≤800 U/mL were younger (66.0 vs. 66.8, P=0.0174), and non-white (23.4% vs. 21.3%, P=0.002) with respect to patients with CA 19-9 levels ≤800 U/mL. Within the cohort of patients who received neoadjuvant therapy, patients with CA 19-9 levels ≤800 U/mL tended to be non-white (25.0% vs. 14.3%, P=0.007), but there was no difference in age (P=0.326) or sex (P=0.409).
Table 1. Patient demographics.
| Variables | Surgery +/− adjuvant therapy | Neoadjuvant therapy | |||||
|---|---|---|---|---|---|---|---|
| ≤800 (n=1,907) | >800 (n=2,223) | P value | ≤800 (n=256) | >800 (n=336) | P value | ||
| Age | 66.0 | 66.8 | 0.0174 | 63.4 | 64.2 | 0.326 | |
| 18–59 | 27.2% | 23.5% | 36.7% | 31.8% | |||
| 60–69 | 32.2% | 33.7% | 34.5% | 36.4% | |||
| 70–79 | 31.4% | 32.1% | 24.2% | 25.5% | |||
| 80–90 | 9.3% | 10.7% | 4.5% | 6.3% | |||
| Sex | 0.893 | 0.409 | |||||
| Male | 51.2% | 51.4% | 47.3% | 50.7% | |||
| Female | 48.8% | 48.6% | 52.7% | 49.3% | |||
| Race | 0.002 | 0.007 | |||||
| White (Non-Hispanic) | 76.6% | 78.7% | 75.0% | 85.7% | |||
| Black (Non-Hispanic) | 11.1% | 7.8% | 14.4% | 8.3% | |||
| Other (Non-Hispanic) | 3.5% | 3.2% | 3.4% | 1.1% | |||
| Hispanic | 8.8% | 10.3% | 7.2% | 4.9% | |||
| Insurance | 0.013 | 0.009 | |||||
| Private | 34.8% | 34.3% | 45.1% | 46.4% | |||
| Medicare | 54.0% | 54.7% | 41.7% | 46.7% | |||
| Medicaid & other gov | 7.2% | 5.7% | 9.5% | 3.7% | |||
| Unknown | 1.2% | 2.4% | 1.9% | 0.3% | |||
| Not insured | 2.8% | 2.8% | 1.9% | 2.9% | |||
| Median income | 0.093 | 0.004 | |||||
| <58,000 | 17.4% | 15.0% | 17.4% | 12.3% | |||
| 58,000–74,000 | 23.5% | 23.6% | 26.1% | 19.5% | |||
| 74,000–93,000 | 24.2% | 26.7% | 21.2% | 33.2% | |||
| >93,000 | 32.5% | 31.0% | 32.6% | 30.1% | |||
| Comorbidities | 0.072 | 0.216 | |||||
| CDCC score 0 | 67.0% | 63.7% | 68.2% | 62.8% | |||
| CDCC score 1 | 26.3% | 28.4% | 26.5% | 33.0% | |||
| CDCC score 2 | 6.7% | 7.9% | 5.3% | 4.3% | |||
| CDCC score missing | 0.0% | 0.0% | 0.0% | 0.0% | |||
| Facility type | 0.412 | 0.772 | |||||
| Community | 4.0% | 4.1% | 1.9% | 1.4% | |||
| Comprehensive community | 38.9% | 40.8% | 29.5% | 31.8% | |||
| Academic/research | 56.8% | 55.0% | 68.6% | 66.8% | |||
| Other | 0.4% | 0.2% | 0.0% | 0.0% | |||
| Facility location | 0.031 | 0.333 | |||||
| Northeast | 21.6% | 18.4% | 22.0% | 28.4% | |||
| South | 35.6% | 35.1% | 34.8% | 31.5% | |||
| Midwest | 27.8% | 30.1% | 31.8% | 28.7% | |||
| West | 15.0% | 16.4% | 11.4% | 11.5% | |||
Disease and treatment characteristics within the two cohorts are presented in Table 2. Among patients who underwent surgery with or without adjuvant therapy, those with CA 19-9 levels >800 U/mL tended to have higher clinically staged disease (P=0.001) and pathologically staged disease (P<0.0001) relative to patients with CA 19-9 levels ≤800 U/mL. They were more likely to undergo a pancreaticoduodenectomy (P<0.001), have positive surgical margins (P<0.0001), greater numbers of positive regional lymph nodes (P<0.0001), and were less likely to receive adjuvant therapy (P<0.0001). Among patients who received neoadjuvant therapy, there was no statistically significant difference in clinical or pathological stage, surgical margins, or positive number of lymph nodes between patients with CA 19-9 levels ≤800 U/mL and >800 U/mL.
Table 2. Disease and treatment characteristics.
| Variables | Surgery +/− adjuvant therapy | Neoadjuvant therapy | |||||
|---|---|---|---|---|---|---|---|
| ≤800 (n=1,907) | >800 (n=2,223) | P value | ≤800 (n=256) | >800 (n=336) | P value | ||
| Clinical stage | 0.001 | 0.059 | |||||
| Stage I | 44.8% | 40.5% | 27.7% | 22.6% | |||
| Stage II | 51.5% | 55.0% | 59.1% | 57.3% | |||
| Stage III | 2.9% | 4.3% | 13.3% | 20.1% | |||
| Surgery type | <0.0001 | 0.682 | |||||
| Distal resection | 16.2% | 11.6% | 11.4% | 8.9% | |||
| Whipple | 51.0% | 54.8% | 53.8% | 55.6% | |||
| Total resection | 12.5% | 12.2% | 14.4% | 16.3% | |||
| Other | 20.3% | 21.4% | 20.5% | 19.2% | |||
| Regional lymph nodes sampled | 16.2 | 16.7 | 0.158 | 17.1 | 14.2 | 0.011 | |
| Positive regional lymph nodes | 2.2 | 3.0 | <0.0001 | 1.2 | 1.1 | 0.754 | |
| Surgical margins | <0.0001 | 0.855 | |||||
| No residual tumor | 76.0% | 70.0% | 81.1% | 78.5% | |||
| Residual tumor, NOS | 8.4% | 9.5% | 8.0% | 7.7% | |||
| Microscopic residual tumor | 12.5% | 15.4% | 7.2% | 8.6% | |||
| Macroscopic residual tumor | 0.9% | 1.2% | 0.4% | 0.9% | |||
| Indeterminate or unknown | 2.3% | 4.0% | 3.4% | 4.3% | |||
| Pathological stage | <0.0001 | 0.058 | |||||
| Stage 0 | 0.4% | 0.2% | 0.8% | 0.6% | |||
| Stage 1 | 12.1% | 7.1% | 21.2% | 18.3% | |||
| Stage 2 | 75.5% | 76.8% | 61.0% | 59.0% | |||
| Stage 3 | 2.3% | 4.3% | 3.0% | 3.7% | |||
| Stage 4 | 1.7% | 1.7% | 0.0% | 2.6% | |||
| Unknown | 5.2% | 7.2% | 9.1% | 12.6% | |||
| Treatment | <0.0001 | 1.000 | |||||
| Any NAT | 0.0% | 0.0% | 100.0% | 100.0% | |||
| Surgery only | 26.9% | 35.1% | 0.0% | 0.0% | |||
| Any adj | 73.1% | 64.9% | 0.0% | 0.0% | |||
NOS, not otherwise specified; NAT, neoadjuvant therapy; adj, adjuvant therapy.
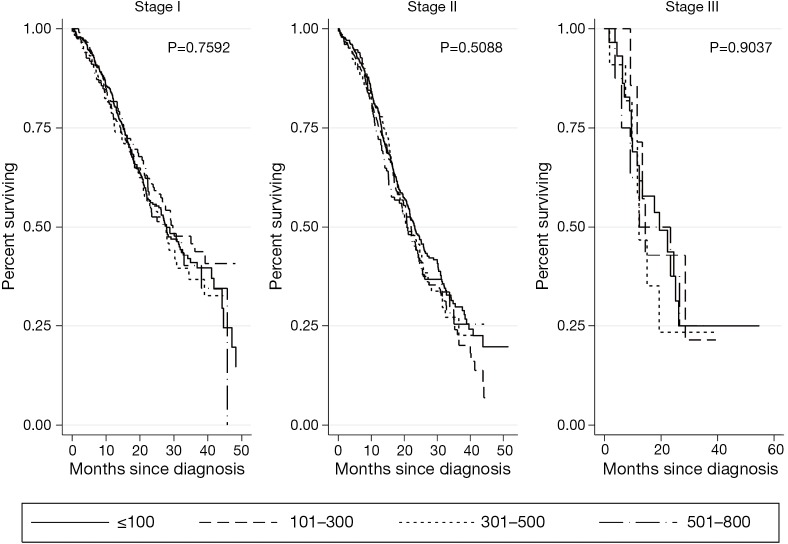
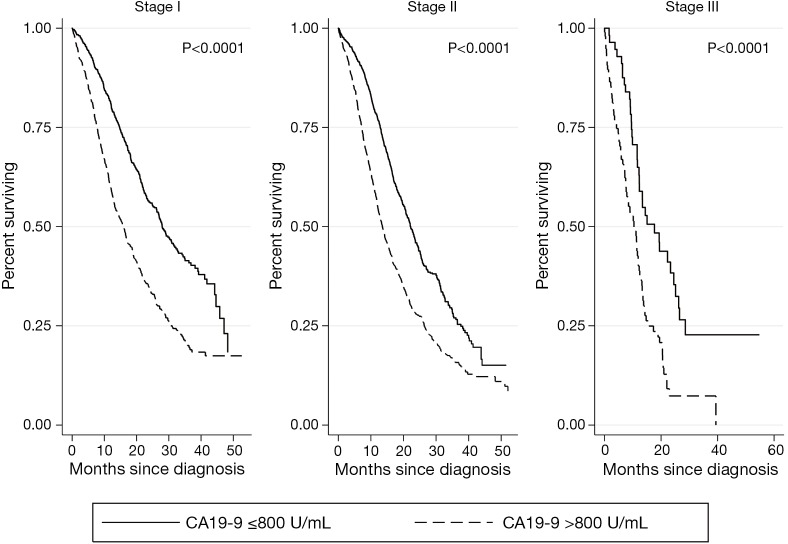
Kaplan-Meier survival analyses for patients who underwent surgical resection with or without adjuvant therapy are presented in Figure 2. There was no association of CA 19-9 levels ≤800 U/mL with survival at any stage (stage I P=0.7592, stage II P=0.5088, stage III P=0.9037). CA 19-9 levels >800 U/mL were associated with significantly worse survival in all clinical stages of disease (stage I P<0.0001, stage II P<0.0001, stage III P<0.0001) (Figure 3).
Figure 2.
Kaplan Meier analyses for patients who underwent surgery +/− adjuvant therapy by CA 19-9 level (U/mL). CA 19-9, carbohydrate antigen 19-9.
Figure 3.
Kaplan Meier analyses for patients who underwent surgery +/− adjuvant therapy by CA 19-9 level (U/mL). CA 19-9, carbohydrate antigen 19-9.
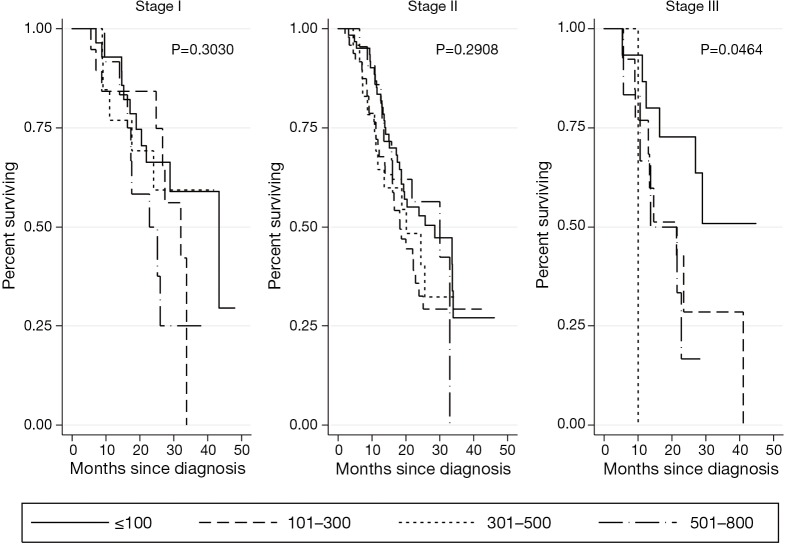
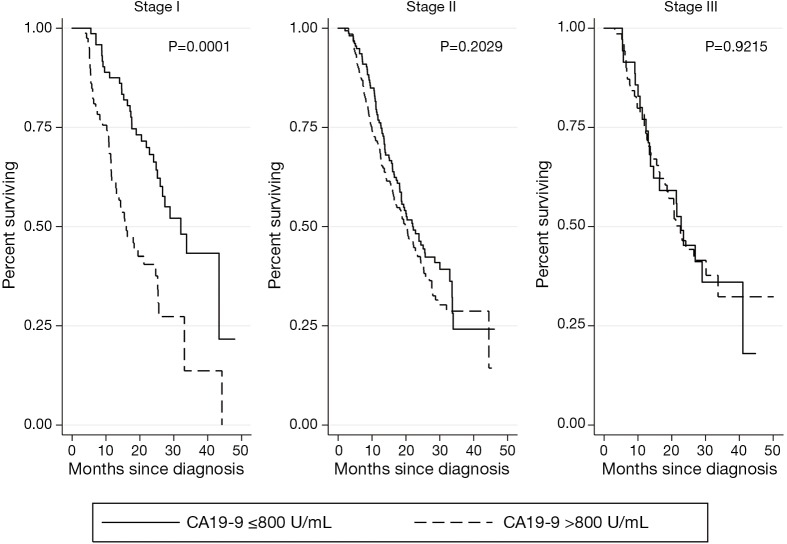
Kaplan-Meier survival analyses for patients who received neoadjuvant therapy followed by surgical resection are presented in Figure 4. There was no association of CA 19-9 levels ≤800 U/mL with survival in early stage disease (stage I P=0.3030, stage II P=0.2908). While there was a statistically significant difference in survival among CA 19-9 levels below 800 in stage III disease (P=0.0464), this analysis was limited by small sample size (n=35). CA 19-9 levels >800 U/mL were associated with significantly worse survival in stage I disease (P=0.0001), but not in stage II or III disease (P=0.2029, P=0.9215, respectively) (Figure 5).
Figure 4.
Kaplan Meier analyses for patients who received neoadjuvant therapy followed by surgery, by CA 19-9 level (U/mL). CA 19-9, carbohydrate antigen 19-9.
Figure 5.
Kaplan Meier analyses for patients who received neoadjuvant therapy followed by surgery, by CA 19-9 level (U/mL). CA 19-9, carbohydrate antigen 19-9.
After controlling for patient, disease, and treatment characteristics, patients who received neoadjuvant or adjuvant therapy had a lower hazard of mortality relative to patients who underwent surgical resection alone (HR 0.60, P<0.001, HR 0.52, P<0.001) (Table 3). Patients with CA 19-9 levels 801–980 U/mL or >980 U/mL had significantly greater hazards of mortality relative to patients with CA 19-9 levels ≤100 U/mL (HR 3.29, P<0.001, HR 1.51, P<0.001, respectively).
Table 3. Multivariate survival analysis.
| Variables | Coefficient | 95% confidence interval | P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Treatment | ||||
| Surgery | Reference | |||
| Neoadjuvant | 0.60 | 0.53 | 0.68 | <0.001 |
| Adjuvant | 0.52 | 0.48 | 0.56 | <0.001 |
| Age (years) | ||||
| 18–59 | Reference | |||
| 60–69 | 1.12 | 1.00 | 1.25 | 0.043 |
| 70–79 | 1.23 | 1.08 | 1.40 | 0.001 |
| 80–90 | 1.35 | 1.15 | 1.59 | <0.001 |
| Sex | ||||
| Male | Reference | |||
| Female | 0.96 | 0.89 | 1.04 | 0.309 |
| Race | ||||
| White | Reference | |||
| Black | 0.89 | 0.83 | 1.37 | 0.606 |
| Other | 0.89 | 0.79 | 0.99 | 0.033 |
| Hispanic | 1.00 | 0.80 | 1.00 | 0.050 |
| Insurance | ||||
| Medicare | Reference | |||
| Private | 0.91 | 0.82 | 1.01 | 0.065 |
| Medicaid & other gov | 1.01 | 0.86 | 1.20 | 0.866 |
| Unknown | 0.40 | 0.29 | 0.55 | <0.001 |
| Not Insured | 1.07 | 0.83 | 1.37 | 0.606 |
| Median income | ||||
| <58,000 | Reference | |||
| 58,000–74,000 | 0.88 | 0.79 | 0.99 | 0.033 |
| 74,000–93,000 | 0.89 | 0.80 | 1.00 | 0.050 |
| >93,000 | 0.81 | 0.72 | 0.91 | <0.001 |
| Comorbidities | ||||
| CDCC score 0 | Reference | |||
| CDCC score 1 | 1.03 | 0.95 | 1.12 | 0.462 |
| CDCC score 2 | 1.24 | 1.08 | 1.43 | 0.002 |
| Facility type | ||||
| Community | Reference | |||
| Comprehensive community | 0.91 | 0.75 | 1.09 | 0.301 |
| Academic/research | 0.79 | 0.65 | 0.95 | 0.013 |
| Other | 1.54 | 0.75 | 3.18 | 0.243 |
| Facility location | ||||
| Northeast | Reference | |||
| South | 1.04 | 0.93 | 1.16 | 0.501 |
| Midwest | 1.12 | 1.00 | 1.25 | 0.046 |
| West | 0.87 | 0.76 | 0.99 | 0.036 |
| Surgery type | ||||
| Distal resection | Reference | |||
| Whipple | 1.17 | 1.04 | 1.32 | 0.010 |
| Total resection | 1.10 | 0.95 | 1.28 | 0.203 |
| Other | 1.10 | 0.96 | 1.26 | 0.171 |
| Surgical margins | ||||
| No residual tumor | Reference | |||
| Residual tumor, NOS | 1.57 | 1.39 | 1.78 | <0.001 |
| Microscopic residual tumor | 1.62 | 1.46 | 1.80 | <0.001 |
| Macroscopic residual tumor | 1.50 | 1.06 | 2.11 | 0.021 |
| Indeterminate or unknown | 1.16 | 0.94 | 1.43 | 0.177 |
| Pathological stage | ||||
| Stage 0 | Reference | |||
| Stage 1 | 0.66 | 0.51 | 0.86 | 0.002 |
| Stage 2 | 1.08 | 0.86 | 1.35 | 0.521 |
| Stage 3 | 1.24 | 0.92 | 1.66 | 0.153 |
| Stage 4 | 1.91 | 1.37 | 2.68 | <0.001 |
| Unknown | 1.08 | 0.83 | 1.41 | 0.556 |
| CA 19-9 (U/mL) | ||||
| ≤100 | Reference | |||
| 101–300 | 1.10 | 0.95 | 1.27 | 0.184 |
| 301–500 | 1.08 | 0.90 | 1.29 | 0.393 |
| 501–800 | 1.08 | 0.91 | 1.28 | 0.409 |
| 801–980 | 3.29 | 2.89 | 3.74 | <0.001 |
| >980 | 1.51 | 1.36 | 1.69 | <0.001 |
NOS, not otherwise specified.
Discussion
While a CA 19-9 level of 37 U/mL is widely regarded as normal, there are varying reports of levels associated with malignancy prognosis (5). This study found an association between pre-treatment CA 19-9 levels >800 U/mL and advanced stage disease. A CA 19-9 level >800 U/mL was associated with worse survival in all clinical stages of disease, and was found to be an independent poor prognostic factor. Some have used CA 19-9 levels to characterize resectability. A study of 244 patients with pancreatic adenocarcinoma, reported a 70% R0 resection in patients with both negative preoperative CA 19-9 and CEA levels (11). A number of small single institution studies have evaluated the predictive value of CA 19-9 levels in resectability. The CA 19-9 level reported as predictive of resectability has ranged from 92.8 to 622 U/mL (11-14). To our knowledge, this is the first study to evaluate the prognostic impact of a wide range of CA 19-9 levels.
In this study, neoadjuvant therapy was associated with a survival benefit in patients with CA 19-9 levels >800 U/mL. A broad study by Bergquist et al. evaluating patients with stage I and II disease, reported that though elevations in CA 19-9 levels >37 U/mL were associated with poorer survival, administration of neoadjuvant chemotherapy mitigated this effect (15). While surgery and systemic adjuvant therapy are standard of care, as few as half may actually receive intended adjuvant therapy (16,17). In this study, nearly one third underwent surgical resection, but received no form of systemic therapy. Receipt of neoadjuvant therapy has been associated with survival benefits in advanced pancreatic cancer, and appears to offer survival benefits in patients with elevated CA 19-9 (18,19). Elevations in the biomarker, particularly >800 U/mL should prompt clinicians to consider neoadjuvant therapy.
To our knowledge, this is the largest and most contemporary analysis of CA 19-9 levels at diagnosis of pancreatic adenocarcinoma, and the only to examine the impact of CA 19-9 elevations at multiple levels in resectable pancreatic cancer. However, there are some important limitations which should be acknowledged, including those inherent to retrospective reviews of large databases. The NCDB codes only for the highest CA 19-9 value documented in the medical record prior to treatment. These values are derived from a heterogenous population of hospitals with non-uniform biomarker assays. Additionally, the NCDB does not code for potential confounders of CA 19-9 elevation such as PPI use, or biliary obstruction. Given the retrospective nature of the study, the treatment cohorts likely contain a selection bias.
Conclusions
An elevated CA 19-9 level >800 U/mL appears to be associated with more advanced stage disease; however, CA 19-9 ≤800 drawn prior to treatment had no significant association with long-term survival. CA 19-9 levels >800 U/mL were negatively associated with long-term survival. Although this study suggests an association, further study is needed to evaluate whether patients with CA 19-9 levels > 800 U/mL benefit from NAT.
Acknowledgements
None.
Ethical Statement: The study was deemed exempt by the M. S. Hershey Medical Center Human Subject Protection Board and it conforms to the provisions of in accordance with the Helsinski Declaration as revised in 2013, available at: http://www.wma.net/en/30publications/10policies/b3/%20index.html.
Disclaimer: The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Footnotes
Conflicts of Interest: This study was selected and presented as a poster presentation at the European Society of Medical Oncology 18th World Congress on Gastrointestinal Cancer, June 29–July 2, 2016, Barcelona, Spain.
References
- 1.Bilimoria KY, Bentrem DJ, Ko CY, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer 2007;110:738-44. [DOI] [PubMed] [Google Scholar]
- 2.Pleskow DK, Berger HJ, Gyves J, et al. Evaluation of a serologic marker, CA 19-9, in the diagnosis of pancreatic cancer. Ann Intern Med 1989;110:704-9. 10.7326/0003-4819-110-9-704 [DOI] [PubMed] [Google Scholar]
- 3.Cwik G, Wallner G, Skoczylas T, et al. Cancer antigens 19-9 and 125 in the differential diagnosis of pancreatic mass lesions. Arch Surg 2006;141:968-73. 10.1001/archsurg.141.10.968 [DOI] [PubMed] [Google Scholar]
- 4.van den Bosch RP, van Eijck CH, Mulder PG, et al. Serum CA19-9 determination in the management of pancreatic cancer. Hepatogastroenterology 1996;43:710-3. [PubMed] [Google Scholar]
- 5.Kim JE, Lee KT, Lee JK, et al. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol 2004;19:182-6. 10.1111/j.1440-1746.2004.03219.x [DOI] [PubMed] [Google Scholar]
- 6.Chang CY, Huang SP, Chiu HM, et al. Low efficacy of serum levels of CA 19-9 in prediction of malignant diseases in asymptomatic population in Taiwan. Hepatogastroenterology 2006;53:1-4. [PubMed] [Google Scholar]
- 7.Maisey NR, Norman AR, Hill A, et al. CA19-9 as a prognostic factor in inoperable pancreatic cancer: the implication for clinical trials. Br J Cancer 2005;93:740-3. 10.1038/sj.bjc.6602760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger AC, Garcia M, Jr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol 2008;26:5918-22. 10.1200/JCO.2008.18.6288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-9. 10.1016/0895-4356(92)90133-8 [DOI] [PubMed] [Google Scholar]
- 10.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 11.Fujioka S, Misawa T, Okamoto T, et al. Preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels for the evaluation of curability and resectability in patients with pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg 2007;14:539-44. 10.1007/s00534-006-1184-3 [DOI] [PubMed] [Google Scholar]
- 12.Karachristos A, Scarmeas N, Hoffman JP. CA 19-9 levels predict results of staging laparoscopy in pancreatic cancer. J Gastrointest Surg 2005;9:1286-92. 10.1016/j.gassur.2005.06.008 [DOI] [PubMed] [Google Scholar]
- 13.Maithel SK, Maloney S, Winston C, et al. Preoperative CA 19-9 and the yield of staging laparoscopy in patients with radiographically resectable pancreatic adenocarcinoma. Ann Surg Oncol 2008;15:3512-20. 10.1245/s10434-008-0134-5 [DOI] [PubMed] [Google Scholar]
- 14.Kiliç M, Göçmen E, Tez M, et al. Value of preoperative serum CA 19-9 levels in predicting resectability for pancreatic cancer. Can J Surg 2006;49:241-4. [PMC free article] [PubMed] [Google Scholar]
- 15.Bergquist JR, Puig CA, Shubert CR, et al. Carbohydrate antigen 19-9 elevation in anatomically resectable, early stage pancreatic cancer is independently associated with decreased overall survival and an indication for neoadjuvant therapy: a national cancer database study. J Am Coll Surg 2016;223:52-65. 10.1016/j.jamcollsurg.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 16.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. 10.1001/jama.2013.279201 [DOI] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Bentrem DJ, Ko CY, et al. Multimodality therapy for pancreatic cancer in the U.S.: utilization, outcomes, and the effect of hospital volume. Cancer 2007;110:1227-34. 10.1002/cncr.22916 [DOI] [PubMed] [Google Scholar]
- 18.Mirkin KA, Hollenbeak CS, Wong J. Survival impact of neoadjuvant therapy in resected pancreatic cancer: a prospective cohort study involving 18,332 patients from the national cancer data base. Int J Surg 2016;34:96-102. 10.1016/j.ijsu.2016.08.523 [DOI] [PubMed] [Google Scholar]
- 19.Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. 10.1371/journal.pmed.1000267 [DOI] [PMC free article] [PubMed] [Google Scholar]