Abstract
The gold standard of diagnosis for nonmelanoma and melanoma skin cancer has been skin biopsy with routine paraffin embedded hematoxylin and eosin histopathology. This practice is frequently carried out on suspicious lesions to rule out a malignant process. Therefore, as a result, many biopsies are done on benign lesions. Unlike other fields of medicine that rely on noninvasive imaging modalities, the use of imaging devices in dermatology has not been as robust. This has been mainly due to the limited resolution offered by imaging devices that is needed to detect malignant changes in the cutaneous layers. However, the demand for more efficient in vivo and ex vivo imaging tools to reduce the amount of biopsies have led to new areas of investigation using noninvasive modalities to augment the clinical diagnosis of skin cancer. The use of noninvasive imaging both in vivo and ex vivo has the potential to increase efficiency of diagnosis and management, decrease healthcare cost, improve clinical care and enhance patient satisfaction.
KEYWORDS : basal cell carcinoma, fluorescent confocal microscopy, laser ablation, Mohs micrographic surgery, multispectral fluorescence lifetime tomography, noninvasive imaging, nonmelanoma skin cancer, optical coherence tomography, Raman spectroscopy, reflectance confocal microscopy
About 25% of all malignant neoplasms in humans are nonmelanoma skin cancers (NMSC). Approximately 3.5 million new cases of NMSC are diagnosed in the USA every year, and the incidence rate is increasing [1]. Between 1992 and 2006 the rate has increased by 4.2% per year. Of these cases, about 70% are basal cell carcinomas (BCCs). BCC is the most common skin malignancy and the most common malignancy worldwide. The rise of NMSC incidence is multifactorial; related to ultraviolet light exposure, recreational behavior, life expectancy and registration of skin cancer diagnosis. As such, BCC occurs most frequently on the head and neck regions. Women tend to have a greater frequency of BCC on the lower extremities while men have more occurring on the ear. The overall incidence is also increasing with rates estimated to have risen between 20 and 80% with similar increases noted worldwide and tend to increase with increasing age [2]. The mortality rates from NMSC remain low in comparison to other types of malignancies, but can cause significant morbidity for the patient.
BCC mainly causes local destruction with metastasis or spread extremely rare and can be thought of in terms of high or low risk. Mortality from BCC is quite rare and can occur in immunocompromised patients. Cases of metastatic BCC are more likely from patients with basal cell nevus syndrome or tumors with aggressive histologic patterns (morpheaform/infiltrating). Perineural invasion is also an indicator of aggressive disease [3].
Basal cell carcinoma of the integumentary system are usually detected on a routine physician clinical exam and diagnosed by histological analysis via skin biopsy. The use of skin biopsy to diagnose skin malignancies is considered the gold standard of diagnosis and a benchmark used to base other diagnostic modalities against. However, in patients with an extensive history of basal cell carcinomas or widespread actinic damage multiple biopsies may not be practical or cosmetically appreciable. Additionally a histopathologic diagnosis requires tissue processing and interpretation time.
Classification of BCC
At least 26 different subtypes of BCC have been described [4]. Six basic histopathologic subtypes of BCC include: superficial, nodular, morpheaform (sclerosing), infundibulocystic, basosquamous and fibroepithelial tumor of Pinkus. Since histologic type may influence the clinical course of the disease it can be important to differentiate between these. This can also guide treatment decisions, as more aggressive subtypes may not respond as well to topical therapies as would superficial subtypes.
Superficial shave biopsy specimens may not allow for typing or the detection of a specific histologic subtype. Therefore, a superficial growth pattern may not exclude the presence of a more aggressive deeper growth pattern. While the nodular type is considered low-risk, high-risk, or aggressive, subtypes include the infiltrative, morpheaform and multinodular types. This is due to a tendency to become more invasive and recur. However, superficial BCC is also prone to recurrence due to subclinical extension and inadequate removal.
Histologicaly BCC shows small, dark staining, cells that resemble the basal cell layer of the epidermis, with large and small nuclei. These can occur within the epidermis and the dermis. At the periphery of the basaloid island the columnar cells are often arranged in a Paddling fashion. This may be absent when the basaloid tumor cells are in cordlike arrangement or in small nests. Cystic structures may also be seen in BCC. The dermal stroma is also an important part of the BCC histology and a clue to diagnosis. The stroma is characterized as loose and fibromyxoid, with a sparse lymphoid infiltrate [5]. These characteristic histologic features are important to consider when the use of noninvasive imaging is employed as these features can also be appreciated with this modality.
Noninvasive imaging modalities
Noninvasive imaging provides a novel and rapid approach to the diagnosis of cutaneous tumors, including BCC. Early BCCs can be difficult to clinically as well as histologically diagnosis. This is very true for lesions on the face, where benign neoplasms can clinically mimic superficial BCCs. While this chapter will focus on the use of reflectance confocal microscopy (RCM) there are numerous modalities with which to image cutaneous neoplasms including: Raman spectroscopy [6], fluorescence polarization [7], multiphoton [8], fluorescence lifetime [9], fluorescence imaging [10], optical coherence tomography and reflectance confocal microscopy. In some circumstances these modalities can be combined to capture a more precise image of the cutaneous neoplasm.
• Optical coherence tomography
OCT is a noninvasive imaging modality that allows visualization of epidermal and papillary dermal structures up to approximately 2 mm in depth. OCT uses low coherence length light as a source and by exploiting the light scattering properties of skin structures, allows visualization of such. Similar to an ultrasound sonogram, OCT produces a cross-sectional view of the tissue, more akin to a traditionally sectioned histopathological view.
OCT can be utilized to diagnose BCC and other NMSC. OCT can visualize characteristic superficial nests of BCC as well as superficial dermal tumor nests. These are delineated from the surrounding stroma by a darker shadow. OCT has also been used to differentiate between BCC subtypes [11].
Mogensen et al. studied the use of OCT in the diagnosis of NMSC [12]. OCT and polarization-sensitive (PS) OCT were used in 104 patients. Observer-blinded evaluation of OCT images were done for 64 BCC samples: one basosquamous carcinoma, 39 actinic keratoses, two malignant melanomas, nine benign lesions, as well as 105 images of perilesional skin. A sensitivity of 79–94% and specificity of 85–96% in differentiating normal skin from lesions were reported. Important features for BCC diagnosis were absence of well-defined layering in OCT and PS-OCT images and the presence of dark lobules. Discrimination of AK from BCC had an error rate of 50–52%. They concluded that it was difficult to distinguish actinic keratosis and BCC. In this study OCT diagnosis was less accurate than clinical diagnosis, but had high accuracy in distinguishing lesions from normal skin.
Banzhaf et al. studied the use of OCT during imiquimod treatment of BCC [13]. Nine patients who had a diagnosis of BCC and were scanned with OCT before treatment, at 1 week, 4 weeks and then 3 months posttreatment. The lesions were identified clinically and with OCT. All BCCs were thought to be cleared, but at follow-up residual suspicious structures were seen clinically in four cases. OCT and histology both ruled out residual BCC. This group concluded that OCT was able to identify superficial BCC. However, monitoring during imiquimod treatment revealed impaired image quality, which was due to inflammation, crusting and ulceration. When residual BCC tissue was suspected, OCT was able to rule this out.
• Raman spectroscopy
Raman spectroscopy is another noninvasive imaging tool that exploits the difference in the scattering of light of cutaneous structures. Each structure generates a different vibrational energy, which corresponds to its spectral peak, called a Raman signal. Instead of a structural ‘picture’, Raman spectroscopy gives a more quantitative view. A Raman spectroscope uses a near infrared wavelength of light, and a meter to detect the spectra associated with the structure [14]. These vibrational spectra are sensitive to molecular structure, conformation and chemical interactions, and therefore it is possible to detect the chemical changes in tissue samples that can occur in cancerous scenarios. In cancerous tumors there are cellular alterations such as an increased nucleus-to-cytoplasm ratio, disordered chromatin and a higher metabolic activity that can be detected via Raman spectroscopy. Traditional Raman spectroscopy can be quite time consuming, therefore researchers have utilized it with other modalities to circumvent this.
Gniadecka et al. reported the use of Raman spectroscopy to detect BCC and the associated biochemical changes. These included protein and lipid alterations such as changes in the amide bands and changes in collagen [15]. In one study, a sensitivity of 94% and specificity of 98% were reported when Raman spectroscopy was used to discern BCC and melanoma from normal skin, nevi and actinic keratosis [16].
To reduce the time needed for the Raman spectroscopic evaluation of BCC tissue samples Notingher et al. integrated tissue autofluorescence imaging, which has a high sensitivity and high speed but low specificity, with Raman scattering, which has a high sensitivity and high specificity but low speed [17]. First autofluorescence images were used to select out sampling points that were suspicious for BCC. Then these areas were subjected to Raman spectroscopy, which was used to establish the diagnosis based on a spectral classification model (100% sensitivity, 92% specificity per spectrum). This combination was able to diagnose BCC while reducing the overall time needed.
• Multispectral fluorescence lifetime tomography
Multiphoton tomography is an optical imaging technique that has been utilized for dermatologic purposes in vivo and specifically BCC evaluation. This technology acts by exciting fluorescence from the target through the simultaneous absorption of two or more photons of infrared light. Images are then generated in two dimensions by raster-scanning the excitation area across the specimen. The spatial resolution has been reported to be similar to histopathologic (1 mm lateral, 2 mm axial) resolution [18].
This technique exploits the endogenous flurophores contained in the skin. These include: melanin, elsatin, collagen, porphyrins, flavins and NADPH. To discriminate between fluorophores and the corresponding tissue, fluorescence lifetime imaging (FLIM) is often added. FLIM measures the rate of decay of the fluorescence signal following a short pulse of excitation light. Patalay et al. reported on the use of multispectral FLIM multiphoton tomograpy to detect BCCs from normal skin. Fluorescence intensity and FLIM images were collected from 27 normal skin samples and 19 excised BCCs, using four emission spectral channels: blue, green, yellow and red. They reported that with this technique, detecting BCCs was accomplished with a sensitivity and specificity of 79 and 93%, respectively [19].
• Confocal laser scanning microscopy
Confocal laser scanning can be used in the reflectance mode (RCM) or a fluorescence mode. In vivo RCM produces a real time en face, horizontal section, image of the skin being scanned. It is capable of producing cellular resolution from the epidermis to the upper, papillary, dermal layers. There is an inherent trade off with this modality between higher resolution and increased depth of visualization [20].
The mechanism of confocal scanning involves a single point illumination, via a laser source of a specific wavelength. This light is transmitted through the skin to illuminate a point within the tissue. This light is then reflected back and goes through a pinhole in an optically conjugate plane to form an image in the detector. The pinhole only allows reflected light from the focal point to enter, hence the term confocal. This produces a 2D horizontal image. The contrast in RCM images is dependent upon the differences in reflectivity of the tissue, which is an inherent property of the tissue due to differences in molecular structures. Structures with a high refractive index appear bright. For skin, melanin and melanosomes have the highest refractive index and therefore appear white in RCM images. Commercially available microscopes, such as the RCM Vivascope 1500 (Caliber Imaging & Diagnostics Inc., NY, USA), utilizes a diode laser source with a near infrared wavelength at 830 nm. At 830 nm the depth of visualization is limited to a depth of about 200–300 μm and is a wavelength that is nonharmful to human skin. This device allows an 8 × 8 mm field of view and utilizing software can be visualized as a mosaic of images. There are also a commercially available handheld Vivascope 3000, as well as a Multilaser, which combines fluorescence and reflectance confocal microscopy. For ex vivo tissue, incubation of the specimen in acetic acid before imaging induces compaction of chromatin, which increases light backscatter and renders nuclei bright and more easily detectable [21]. In the fluorescence mode, imaging relies on endogenous and exogenous flurophores. Exogenous sources are usually fluorescent dyes.
The en face mosaic that is produced is called a VivaBlock and additionally a vertical plane of images can be obtained, called a VivaStack. Real-time videos can also be obtained allowing the user to capture events such as blood flow, or cellular migration.
RCM has proven to be a very valuable tool for evaluating BCC. RCM imaging has shown the ability to detect BCCs in vivo with sensitivity of 92–100% and specificity of 97–88% [22]. It can be used for diagnostic purposes as well as for monitoring treatment efficacy, resolution, or recurrence. Ziefle et al. compared the use of confocal laser microscopy versus histological imaging in detecting excised BCC [23]. The sensitivity and specificity of BCC detection varied across different histological sections including midsections, lateral margins, muffins and bread loaf sections. The overall sensitivity of confocal laser microscopy was 94.0% in midsections. It was noted that midsection portions had a high probability of showing large portions of BCC nests. The overall sensitivity of confocal laser scanning was 73.7% in lateral margins with smaller tumor strands. The accuracy of diagnoses varied based on the section type and the observer. This study soaked the tissue in 10% acetic acid for 90 s and then stained it in toluidine blue for 2 min before imaging to help improve detection of tumor. This study showed that confocal preparation and diagnosis was quicker the traditional processing and histologic diagnosis however only the midsectioning diagnosis reached over 90% sensitivity. Criteria defined for the diagnosis of BCC include the following: tumor islands, polarization of nuclei, clefting and blood vessels (Table 1) [24]. This is akin to features seen on traditional histology as well as dermoscopy (Box 1; see Figure 1).
Table 1. . Reflectance confocal microscopy diagnostic criteria for basal cell carcinoma.
| BCC features | RCM findings |
| Tumor islands | Round/oval structures at the level of the dermal–epidermal junction that are darker or lighter than the surrounding epidermis or dermis |
| Polarization of nuclei | ‘Streaming’ cells within the tumor islands or overlying keratinocytes whose nuclei are elongated along the same axis |
| clefting | Slit-like spaces between the tumor island and surrounding stroma. These areas darker in contrast |
| Blood vessels | Elongated and thickened blood vessels appear darker often oriented parallel to the epidermis; can contain round white blood cells that appear cleft |
BCC: Basal cell carcinoma; RCM: Reflectance confocal microscopy.
Box 1. Correlation of features of basal cell carcinomas between histopathology, dermoscopy and reflectance confocal microscopy.
Histopathology
Vasculature between tumor islands and epidermis
Basaloid tumor islands
Pigmented tumor islands
Melanophages and melanin
Polarization of basaloid cells
Cleft-like spaces with mucin deposition
Dermoscopy
Arborizing vessels
Nonpigmented pink structures
Maple leaf structures with blue ovoid nests
Gray structures
Reflectance confocal microscopy
Hyporeflective (dark) tumor islands
Refractile tumor islands
Refractile cells and bleft dots
Paddling nuclei
Hyporeflective (dark) clefting around tumor islands
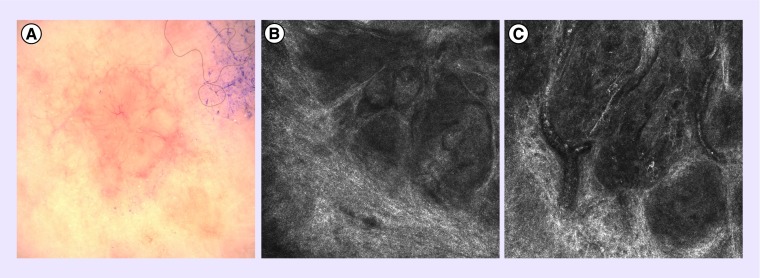
Figure 1. . Nodular basal cell carcinoma.
(A) Clinical image of a nodular basal cell carcinoma with pearly borders and telangiectasias. (B) Reflectance confocal microscopy image of tumor islands. (C) Reflectance confocal microscopy image showing dilated blood vessels.
Clinical applications of RCM for BCC
RCM has been utilized as a tool to noninvasively diagnose BCC but its clinical applications also include use during Mohs micrographic surgery, as well as nonsurgical removal of malignancies.
Larson et al. studied the use of fluorescence confocal mosaicing for the rapid detection of BCC margins in Mohs micrographic surgical sections [25]. Acridine orange was used as staining and imaging was done under fluorescence at 500–700 nm. This represents a novel approach to the diagnosis of BCC during Mohs compared with the traditional frozen section pathology that is employed. They compared 17 tissue samples, each divided into two halves and these 34 submosaics were imaged and assessed for the presence or absence of tumor. These were then compared with the gold standard histology. Of the 34 submosaics, 15 represented true positives for BCC, 16 were true negatives, and there was one false positive, false negative, as well as once true positive for a squamous cell carcinoma. This group reported a 94% sensitivity and 94% specificity using two graders, one Mohs surgeon and one experienced confocal reader. Multiple different types of BCC were represented, including 11 nodular BCC, five micronodular, four infiltrative and three superficial BCC; all were correctly identified. Gareau et al. also showed its usefulness in detecting BCC within Mohs micrographic surgical specimens [26].
Longo et al. studied the use of RCM to assess the efficacy of photodynamic therapy (PDT) in the treatment of BCC and to evaluate the skin changes following the PDT [27]. They included 12 BCCs that were treated. Dermoscopy and RCM imaging were performed at baseline, 7 days, 30 days and 18 months after PDT. They found that at 7 days post-treatment RCM showed the presence of dendritic-shaped cells within the epidermis, which they correlated to activated Langerhans cells. At 1 month, the RCM images showed persistence BCC in two cases. This was not appreciated on clinical or dermoscopic exam. At the 18-month follow-up the group did not report signs of BCC persistence or recurrence. They concluded that RCM was a valuable additional tool for PDT treatment monitoring of BCC.
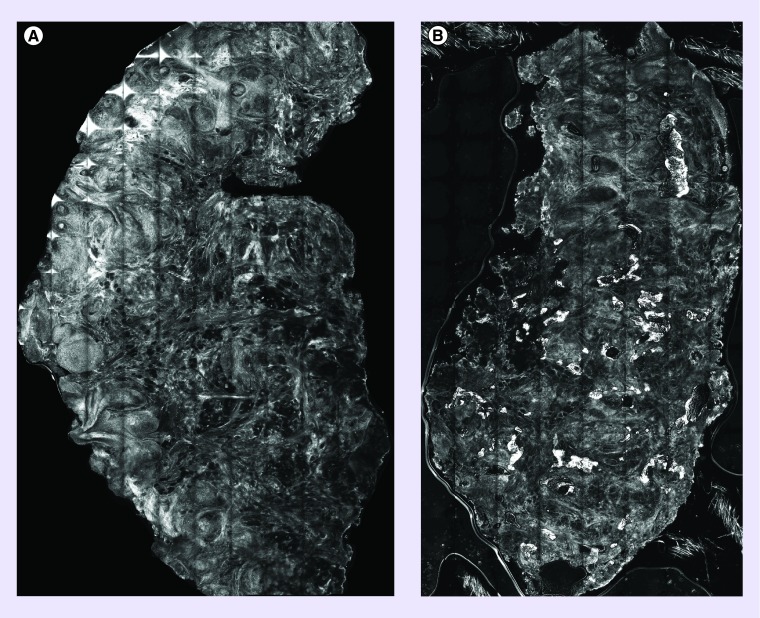
Sierra et al. [28] and Rossi et al. [29] have utilized RCM both in vivo and ex vivo to help guide the laser ablation of BCC. By utilizing RCM, areas of BCC were indentified in tissue sections and then ablated using either an Erbium-YAG or carbon dioxide laser. Then these areas were reimaged using RCM to detect any residual BCC tumor or complete clearance. The ex vivo studies were able to define which parameters of the laser would be suitable for tumor destruction that was subsequently used for the in vivo (see Figure 2).
Figure 2. . Reflectance confocal microscopy of ex vivo basal cell carcinoma tissue.
(A) Pre-Laser reflectance confocal microscopy image showing basal cell carcinoma tumor islands; (B) post-Laser ablation reflectance confocal microscopy images showing nuclear destruction and clearance of tumor.
Rajadhyaksha et al. [30] has also reported on the use of real time video mosaicing using a handheld confocal microscope (Vivascope 3000). A total of 13 RCM videos were acquired in vivo including five residual margins after BCC excisions. Imaging focused on the dermoepidermal junction to detect any positivity in the margins. The individual frames of the video were then extracted and identification tags were cropped. The cropped frames were then stitched using videomosaicing software (Microsoft Image Composite Editor [ICE]). They reported that acquisition of RCM videos covering 5.0–16.0 mm2 was performed in about 20–60 s. These mosaics were deemed to be high quality for resolution, contrast and, cellular-level morphology. This represents a future direction for the acquisition and capturing of in vivo samples.
Conclusion & future perspective
Noninvasive imaging is becoming a rapidly emerging adjuvant tool for the evaluation and diagnosis of skin tumors, especially for basal cell carcinoma. The features of BCC make it quite suitable for these imaging modalities and its histological features can be translated to such. As the incidence of BCC continues to increase the need for rapid and efficient diagnosis does as well. Noninvasive imaging modalities such as RCM as well as Raman spectroscopy and OCT are proving to be both sensitive and specific, and possibly cost effective. As the technology continually improves, resolution, depth of view and area visualized will continue to improve. The future of noninvasive imaging will likely be rapid bedside evaluation and diagnosis. Additionally these modalities will be used in conjunction with Mohs micrographic surgery, laser ablation, as well as topical agents used for destruction of BCC.
EXECUTIVE SUMMARY.
The rate of nonmelanoma skin cancer is steadily increasing annually with a majority of these represented by basal cell carcinoma (BCC).
Noninvasive imaging techniques such as optical coherence tomography, Raman spectroscopy and confocal microscopy are becoming increasingly utilized for the diagnosis of BCC.
Histologic and dermoscopic features can be translated to reflectance confocal microscopy images allowing clinicians as basis to interpret images.
Confocal microscopy, both reflectance and fluorescent, has been utilized ex vivo as well as in vivo with and emphasis on detection of residual lesion or monitoring during treatment.
Multiple imaging modalities are being combined to augment the detection of BCC as well as the time needed to image a lesion, creating a more rapid and efficient diagnosis.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Rigel DS, Friedman RJ, Kopf AW. Lifetime risk for development of skin cancer in the U.S. population: current estimate is now 1 in 5. J. Am. Acad. Dermatol. 1996;35(6):1012–1013. doi: 10.1016/s0190-9622(96)90139-5. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch. Dermatol. 2010;146(3):283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 3.Blixt E, Nelsen D, Stratman E. Recurrence rates of aggressive histologic types of basal cell carcinoma after treatment with electrodesiccation and curettage alone. Dermatol. Surg. 2013;39(5):719–725. doi: 10.1111/dsu.12122. [DOI] [PubMed] [Google Scholar]
- 4.Wade TR, Ackerman AB. The many faces of basal-cell carcinoma. J. Dermatol. Surg. Oncol. 1978;4(1):23–28. doi: 10.1111/j.1524-4725.1978.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 5.Kazlouskaya V, Malhotra S, Navarro R, et al. Dermal changes in superficial basal cell carcinoma, melanoma in situ and actinic keratosis and their implications. J. Cutan. Pathol. 2013;40(12):1014–1020. doi: 10.1111/cup.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nijssen A, Maquelin K, Santos LF, et al. Discriminating basal cell carcinoma from perilesional skin using high wave-number Raman spectroscopy. J. Biomed. Opt. 2007;12(3):034004. doi: 10.1117/1.2750287. [DOI] [PubMed] [Google Scholar]
- 7.Yaroslavsky AN, Salomatina EV, Neel V, Anderson R, Flotte T. Fluorescence polarization of tetracycline derivatives as a technique for mapping nonmelanoma skin cancers. J. Biomed. Opt. 2007;12(1):014005. doi: 10.1117/1.2435710. [DOI] [PubMed] [Google Scholar]
- 8.Paoli J, Smedh M, Wennberg AM, Ericson MB. Multiphoton laser scanning microscopy on non-melanoma skin cancer: morphologic features for future non-invasive diagnostics. J. Invest. Dermatol. 2008;128(5):1248–1255. doi: 10.1038/sj.jid.5701139. [DOI] [PubMed] [Google Scholar]
- 9.Galletly NP, Mcginty J, Dunsby C, et al. Fluorescence lifetime imaging distinguishes basal cell carcinoma from surrounding uninvolved skin. Br. J. Dermatol. 2008;159(1):152–161. doi: 10.1111/j.1365-2133.2008.08577.x. [DOI] [PubMed] [Google Scholar]
- 10.Ericson MB, Uhre J, Strandeberg C, et al. Bispectral fluorescence imaging combined with texture analysis and linear discrimination for correlation with histopathologic extent of basal cell carcinoma. J. Biomed. Opt. 2005;10(3):034009. doi: 10.1117/1.1925650. [DOI] [PubMed] [Google Scholar]
- 11.Maier T, Braun-Falco M, Hinz T, Schmid-Wendtner MH, Ruzicka T, Berking C. Morphology of basal cell carcinoma in high definition optical coherence tomography: en-face and slice imaging mode, and comparison with histology. J. Eur. Acad. Dermatol. Venereol. 2013;27(1):e97–e104. doi: 10.1111/j.1468-3083.2012.04551.x. [DOI] [PubMed] [Google Scholar]
- 12.Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists and pathologists. Dermatol. Surg. 2009;35(6):965–972. doi: 10.1111/j.1524-4725.2009.01164.x. [DOI] [PubMed] [Google Scholar]
- 13.Banzhaf CA, Themstrup L, Ring HC, Mogensen M, Jemec GB. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res. Technol. 2013;20(2):170–176. doi: 10.1111/srt.12102. [DOI] [PubMed] [Google Scholar]
- 14.Mcintosh LM, Jackson M, Mantsch HH, Stranc MF, Pilavdzic D, Crowson AN. Infrared spectra of basal cell carcinomas are distinct from non-tumor-bearing skin components. J. Invest. Dermatol. 1999;112(6):951–956. doi: 10.1046/j.1523-1747.1999.00612.x. [DOI] [PubMed] [Google Scholar]
- 15.Gniadecka M, Wulf HC, Nielsen OF, Christensen DH, Hercogova J. Distinctive molecular abnormalities in benign and malignant skin lesions: studies by Raman spectroscopy. Photochem. Photobiol. 1997;66(4):418–423. doi: 10.1111/j.1751-1097.1997.tb03167.x. [DOI] [PubMed] [Google Scholar]
- 16.Lieber CA, Majumder SK, Billheimer D, Ellis DL, Mahadevan-Jansen A. Raman microspectroscopy for skin cancer detection in vitro . J. Biomed. Opt. 2008;13(2):024013. doi: 10.1117/1.2899155. [DOI] [PubMed] [Google Scholar]
- 17.Kong K, Rowlands CJ, Varma S, et al. Diagnosis of tumors during tissue-conserving surgery with integrated autofluorescence and Raman scattering microscopy. Proc. Natl Acad. Sci. USA. 2013;110(38):15189–15194. doi: 10.1073/pnas.1311289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denk W, Strickler JH, Webb WW. Two-photon laser scanning fluorescence microscopy. Science. 1990;248(4951):73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 19.Patalay R, Talbot C, Alexandrov Y, et al. Multiphoton multispectral fluorescence lifetime tomography for the evaluation of basal cell carcinomas. PLoS ONE. 2012;7(9):e43460. doi: 10.1371/journal.pone.0043460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez S, Tannous Z. Real-time, in vivo confocal reflectance microscopy of basal cell carcinoma. J. Am. Acad. Dermatol. 2002;47(6):869–874. doi: 10.1067/mjd.2002.124690. [DOI] [PubMed] [Google Scholar]
- 21.Drezek RA, Collier T, Brookner CK, et al. Laser scanning confocal microscopy of cervical tissue before and after application of acetic acid. Am. J. Obstet. Gynecol. 2000;182(5):1135–1139. doi: 10.1067/mob.2000.104844. [DOI] [PubMed] [Google Scholar]
- 22.Nori S, Rius-Diaz F, Cuevas J, et al. Sensitivity and specificity of reflectance-mode confocal microscopy for in vivo diagnosis of basal cell carcinoma: a multicenter study. J. Am. Acad. Dermatol. 2004;51(6):923–930. doi: 10.1016/j.jaad.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Ziefle S, Schule D, Breuninger H, Schippert W, Moehrle M. Confocal laser scanning microscopy vs 3-dimensional histologic imaging in basal cell carcinoma. Arch. Dermatol. 2010;146(8):843–847. doi: 10.1001/archdermatol.2010.191. [DOI] [PubMed] [Google Scholar]
- 24.Astner S, Dietterle S, Otberg N, Rowert-Huber HJ, Stockfleth E, Lademann J. Clinical applicability of in vivo fluorescence confocal microscopy for noninvasive diagnosis and therapeutic monitoring of nonmelanoma skin cancer. J. Biomed. Opt. 2008;13(1):014003. doi: 10.1117/1.2837411. [DOI] [PubMed] [Google Scholar]
- 25.Larson B, Abeytunge S, Seltzer E, Rajadhyaksha M, Nehal K. Detection of skin cancer margins in Mohs excisions with high-speed strip mosaicing confocal microscopy: a feasibility study. Br. J. Dermatol. 2013;169(4):922–926. doi: 10.1111/bjd.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gareau DS, Karen JK, Dusza SW, Tudisco M, Nehal KS, Rajadhyaksha M. Sensitivity and specificity for detecting basal cell carcinomas in Mohs excisions with confocal fluorescence mosaicing microscopy. J. Biomed. Opt. 2009;14(3):034012. doi: 10.1117/1.3130331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Longo C, Casari A, Pepe P, et al. Confocal microscopy insights into the treatment and cellular immune response of Basal cell carcinoma to photodynamic therapy. Dermatology. 2012;225(3):264–270. doi: 10.1159/000345106. [DOI] [PubMed] [Google Scholar]
- 28.Sierra H, Larson BA, Chen CS, Rajadhyaksha M. Confocal microscopy to guide erbium:yttrium aluminum garnet laser ablation of basal cell carcinoma: an ex vivo feasibility study. J. Biomed. Opt. 2013;18(9):095001. doi: 10.1117/1.JBO.18.9.095001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sierra H, Damanpour S, Hibler B, Nehal K, Rajadhyaksha M, Rossi A. Confocal imaging of carbon dioxide laser-ablated basal cell carcinomas: an ex-vivo study on the uptake of contrast agent and ablation parameters. Lasers Surg. Med. 2015 doi: 10.1002/lsm.22415. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kose K, Cordova M, Duffy M, et al. Video-mosaicing of reflectance confocal images for rapid examination of large areas of skin in vivo . Br. J. Dermatol. 2014;171(5):1239–1241. doi: 10.1111/bjd.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]