Abstract
Bovine viral diarrhea virus (BVDV) is a significant pathogen associated with gastrointestinal, respiratory, and reproductive diseases of cattle worldwide. It causes continuous economic losses to the cattle industry primarily due to decreased reproductive performance. The ability of virus to cross the placenta during early pregnancy can result in the birth of persistently infected (PI) calves. Persistently infected animals are generally much more efficient transmitters of BVDV than transiently or acutely infected animals because they are capable of shedding large quantities of virus throughout their lives and are considered the primary reservoirs for BVDV. Due to the nature of viral infections, there is no treatment to fully cure an animal of a viral infection. All control programs which are in use in many countries of the world, mainly depend upon the detection of PI animals, eliminating them and preventing their return into the herds. Detection of PI animals at early stage, particularly soon after birth is of significant benefit to implement BVDV control programs. Available diagnostic tests such as virus isolation (VI), immunohistochemistry (IHC), Antigen-Capture ELISA (ACE), and reverse transcriptase polymerase chain reaction (RT-PCR) are used for detection of PI cattle. Each method to detect BVDV has advantages, disadvantages, and applicability for different diagnostic situations. The reliability of diagnostic tests is optimized by choosing the appropriate sampling strategy on the basis of animal age.
Key Words: Antigen-Capture ELISA, BVD, Immunohistochemistry, Persistent infection, RT-PCR
Introduction
Bovine viral diarrhea virus (BVDV) as a small, enveloped single-stranded positive sense RNA virus, about 12.5 kb, is a member of the genus Pestivirus, family Flaviviridae. BVDV is a common infection of cattle worldwide so that most herds are at risk for infection. The broad nature of the disease, transmittance, and lack of treatment have made it a globally enzootic, and one of the most significant cattle diseases (Tautz et al., 2003 ▶; Gunn et al., 2005 ▶; Uzal et al., 2016 ▶).
The infection can cause severe economic losses due to decreased fertility and milk production, slow fetal growth, diarrhea, respiratory symptoms, reproductive dysfunctions such as abortion, teratogenesis, embryonic resorption, fetal mummification and stillbirth, immuno-logical dysfunctions, concurrent infections, impaired herd performance, and the dreaded state of persistent infection (PI) in calves (Deregt and Loewen, 1995 ▶; Brock, 2004 ▶). Although BVDV is named for its primary host, its prevalence in non-bovine species has become increasingly recognized. To date, the virus has been isolated in over 40 species and serological evidence indicates that most wild ruminants are susceptible to BVDV infection. In addition to wildlife, multiple domestic non-bovid species have also been reported to carry and spread the disease. There is evidence of transient infection (TI) within most of these species, resulting in the familiar BVDV syndromes of reproductive insufficiency, respiratory disease, and immunosuppression (Nielsen et al., 2000; Vilcek and Nettleton, 2006 ▶).
With regard to potential of BVDV infection in farmed and free-ranging wildlife, the risk of transmission of the disease from wildlife to cattle remains unknown (Uzal et al., 2016 ▶). Bovine viral diarrhea virus has been found in sheep, goats, pigs, buffaloes and wildlife, although the chance of transmission to or from cattle has not been fully established. Transmission between sheep and cattle has been experimentally proven (Deregt et al., 2005 ▶; Vilcek and Nettleton, 2006 ▶; Lamm et al., 2009 ▶). Isolation of the virus in wild ruminant animals such as deer and elk in North America has been reported (Vilcek et al., 2000 ▶; Grooms and Keilen, 2002 ▶).
Epidemiological investigations have shown that demographic factors such as herd size and density are significant predictors for the prevalence of infection in populations where BVDV is endemic (Ezanno et al., 2008 ▶; Talafha et al., 2009 ▶; Van Campen, 2010 ▶). Higher seroprevalences are observed in herds with purchased animals from different sources (Lindberg and Houe, 2005 ▶; Ezanno et al., 2008 ▶). Because of the error-prone nature of the RNA polymerases responsible for replication of viral RNA, BVDV is highly mutable. Therefore, there is a range of virulence among BVDV isolates, varying from subclinical infections or mild clinical disease to severe fatal syndromes. Persistently infected cattle are an important reservoir of virus and shed large amounts of virus throughout their lives spreading virus among cattle herds. All control programs which are in use in many countries of the world, largely depend on the detection and removal of PI animals, and prevention of introduction of PI animals in the herds with biosecurity programs and/or vaccination. This paper aims to review various aspects and complications of detection and control of persistent BVDV infections in cattle herds.
Biotypes and strains of BVDV
The genus Pestivirus is composed of four recognized species, BVDV-1 and BVDV-2 (previously referred to as genotypes 1 and 2), classical swine fever virus and border disease virus (Tautz et al., 2003 ▶; Uzal et al., 2016 ▶).
There are hundreds of different strains of the virus, characterized by viral nucleotide sequence comparison or by monoclonal antibody (MAb) serotyping, which can also be categorized under two biotypes based on their growth characteristics in cell cultures (Ridpath et al., 2010 ▶). The rare cytopathic (CP) biotype will damage tissue cultures and the much more common non-cytopathic (NCP) will not. Biotypes apparently behave differently in vivo. Non-cytopathic strains have a tropism for leukocytes, lymphoid organs and the respiratory tract, while CP strains are more restricted to the digestive tract (Bezek et al., 1994 ▶).
The syndromes caused by the two biotypes differ mainly in the occurrence and severity of disease that they cause upon infection. Both biotypes can cause disease in cattle, however, in the great majority of viruses (about 90%) isolated in the laboratory, all of the PI, and the more severe forms of the disease are caused by the NCP biotype (Kelling, 2004 ▶; Fulton et al., 2006 ▶; Birk et al., 2008 ▶; Neill et al., 2008 ▶). Cytopathic biotypes have only been isolated in connection with outbreaks of mucosal disease (MD) and the NCP biotype is commonly found in nature and causes PI in animals (Bezek et al., 1994 ▶; Peterhans et al., 2003 ▶; Schweizer et al., 2006 ▶). Cytopathic BVDV arises from rare mutations of the NCP strains. Non-cytopathic viruses are associated with the majority of BVDV infections (>90%) and can cause mild to severe TI as well as PI. Cytopathic biotypes cause MD when PI cattle become superinfected with a CP BVDV (Bolin et al., 1985 ▶).
In general, transient BVDV infections can be divided into five categories: acute, severe acute, hemorrhagic infection, bovine respiratory disease, and immuno-suppression-only. In addition to these five syndromes, BVDV can also cause chronic disease and MD in PI animals (Evermann and Ridpath, 2002 ▶). The importance of acute TI in the transmission and maintenance of BVDV within a population of animals (domestic and wild) should not be underestimated. These TI animals are responsible for up to 93% of all in utero infections that result in the birth of PI calves (Wittum et al., 2001 ▶). Therefore, most all PI animals come from TI dams, but the source of the virus for the TI infection is a PI animal.
The BVDV can be divided into two species or genotypes (BVDV-1 and BVDV-2), which may be differentiated from each other and from other Pestiviruses by MAb directed against the E2 protein, or by genetic analysis of different regions of the genome (Pellerin et al., 1994 ▶; Paton et al., 1995 ▶; Ridpath, 2010 ▶). Furthermore, both genotypes are divided into subtypes. At least fifteen subgenotypes of BVDV-1 and two subgenotypes of BVDV-2 have been identified (Ridpath et al., 2010 ▶).
Genotypes 1 include the classic isolates, which are commonly used in laboratory reference and vaccine strains. Genotype 2 includes BVDV strains associated with high mortality acute and peracute infections, thrombocytopenia and hemorrhaging (Vilcek et al., 2001 ▶). Although both genotypes cause disease, severe cases of clinical disease may be more commonly seen with the BVDV-2 genotype (Kelling, 2004 ▶). BVDV-1 strains are predominant in most part of the world, whereas BVDV-2 was recognized as the cause of severe acute haemorragic disease in North America (Pellerin et al., 1994 ▶), being more recently reported in Europe and Asia with low virulence (Letellier et al., 1999 ▶; Luzzago et al., 2006 ▶; Ridpath, 2010 ▶; Khodakaram-Tafti et al., 2016 ▶). Furthermore, a new Pestivirus species, tentatively called HoBi-like (BVDV3), or atypical Pestivirus, was recently identified in fetal bovine serum imported from Brazil to Europe. These viruses are genetically and antigenically related to BVDV-1 and 2 and cause disease similar to that traditionally associated with BVDV infections. HoBi-like viruses may not be detected by conventional BVDV diagnostic techniques. These viruses have been identified in Brazil, Southeast Asia, and Europe (Schirrmeier et al., 2004 ▶; Uzal et al., 2016 ▶).
Transmission
The main transmission route in infected herds is direct contact with a PI animal. The horizontal transmission of BVDV may be direct or indirect via inhalation or ingestion of virus contaminated materials (Lindberg, 2003 ▶). Horizontal transmission occurs mainly by contacts with virus-shedding animals, but PI and TI animals excrete the virus in different amounts (Houe, 1995 ▶). Transmission between small ruminants and cattle, both ways, has been demonstrated (Carlsson, 1991 ▶; Carlsson and Belak, 1994 ▶; Paton et al., 1995 ▶) and BVDV has been isolated from many other captive and free-living ruminants which are considered a potential source of virus (Lùken, 1995 ▶). Bovine viral diarrhea virus has also been isolated from pigs (Terpstra and Wensvoort, 1988 ▶); but their importance in transmission is unclear. Although the prevalence among pigs has been related to contact with cattle (Lùken, 1995 ▶), BVDV infection in pigs with no indication of virus transmission from cattle has also been described.
Common mechanisms of horizontal transmission include: fomites (feed, water, and equipment such as nose tongs, milk bottle nipples, and needles), palpations (if the same pair of gloves are worn for all exams), secretions and excretions (urine, faeces, mucus, milk), crowding (can also increase transmission if animals are infected with the respiratory type of BVDV), and vectors (horse flies, stable flies, head flies, face flies) have also been shown to transmit BVDV (Niskanen et al., 2000 ▶; Niskanen and Lindberg, 2003 ▶; Bolin and Grooms, 2004 ▶; Schirrmeier et al., 2004 ▶; Stringfellow et al., 2005 ▶; Lindberg et al., 2006 ▶).
If a cow is PI, its fetus will become infected. The virus has the ability to cause transplacental infection resulting in different outcomes depending on the stage of gestation at which the acute infection takes place, leading to fetal death, malformations, acute syndromes of the neonate, immune tolerance and lifelong viral persistence (Peterhans et al., 2003 ▶). Recently, BVDV antigen was detected in two neonate calves with clinical signs of congenital tremor (Taghipour Bazargani et al., 2011 ▶).
Other mechanisms of vertical transmission include: contaminated semen, embryo transfer, and contaminated modified live vaccines. Infected bulls can shed BVDV in semen for prolonged periods, and cattle have been infected following insemination with frozen semen from these animals (Schlafer et al., 1990 ▶; Houe, 1995 ▶; Falcone et al., 1999 ▶; Givens et al., 2003 ▶; Niskanen et al., 2003 ▶; Stringfellow et al., 2005 ▶; Bielanski et al., 2009 ▶).
Transient infection and classical BVD
Bovine viral diarrhea virus gains access to the oropharyngeal mucosa by ingestion or inhalation. Following contact with the mucosal lining of the mouth or nose, initial replication occurs in epithelial cells with a predilection for the palatine tonsils and newly assembled viruses egress via exocytosis. The virus is able to spread systemically through the blood stream. Spread can occur through both free virus in the serum and virus infected leucocytes. In males, BVDV replicates in the seminal vesicles and the prostate gland.
The outcome of TI and ensuing viremia is probably related to several factors including genotype and virulence of the virus, age of host, immune and physiologic status of the host, and whether or not the animal is pregnant, and if so, the age of pregnancy, and also the presence of other pathologic agents (Brodersen, 2004 ▶).
The majority of TI is caused by NCP viruses. Infected animals shed virus in nasal and oral secretions, less so in feces and urine. This form of infection is important in pregnant cattle because of the ability of the virus to cross the placenta and cause intrauterine infections of the fetus (Brodersen, 2004 ▶; Smith et al., 2008 ▶).
Infection of immunocompetent, seronegative, non-pregnant animals in 70-90% of cases results in subclinical infection or mild clinical disease. In a few situations, animals, mainly more than 6 months old, a clinical syndrome as classical BVD develops. After an incubation period of 5-7 days, the affected animals develop fever, leukopenia and viremia that may persist up to 15 days. The virus is present in leukocytes (buffy coat), especially lymphocytes and monocytes, and in plasma. The clinical symptoms include lethargy, anorexia, mild oculonasal discharge, diarrhea, mild oral erosions and ulcers (Uzal et al., 2016 ▶).
Severe acute BVD
Since the early 1990s, a syndrome of severe acute BVD has been recognized with high morbidity and mortality in susceptible animals. Primary infections with a few highly virulent BVDV-2 strains caused this syndrome with peracute to acute course and signs of fever, sudden death, diarrhea, or pneumonia. The pathogenesis of BVDV-2 is most frequently linked to increased strain virulence (Luzzago et al., 2001 ▶; Fulton et al., 2006 ▶). Production of inflammatory cytokines, in response to widespread infection of mononuclear phagocytes has been postulated as a cause of this severe disease (Chase et al., 2004 ▶). In some cases, a thrombocytopenic syndrome with clinical symptoms including epistaxis, hyphema, mucosal hemorrhages, bleeding at injection sites and bloody diarrhea, is superimposed on the alimentary syndrome. The mechanism of thrombocytopenia is not completely defined, although infected megakaryocytes in the bone marrow undergo necrosis (Peterhans et al., 2003 ▶; Ridpath, 2005 ▶).
Fetal infections
During pregnancy, BVDV has the ability to cross the placenta and cause intrauterine infections. The outcome of BVDV fetal infections in susceptible heifers and cows is dependent on the age of the fetus when exposed (Brock, 2003 ▶). When a pregnant seronegative cow is infected with a NCP BVDV biotype, the virus can be easily transferred to the fetus. Fetal infection during the first trimester of gestation can result in abortion, fetal mummification and formation of several different types of congenital anomalies such as cerebellar hypoplasia, cataracts, retinal degeneration, optic neuritis, skeletal malformations, hypotrichosis, and general growth retardation (Deregt and Loewen, 1995 ▶; Brock, 2003 ▶; Brodersen, 2004 ▶; Grooms, 2004 ▶; Smith and Grotelueschen, 2004 ▶; Khodakaram-Tafti and Ikede, 2005 ▶). Some researchers believe that abortions may appear at any time during pregnancy and are not necessarily associated with the time of infection (Lindberg, 2003 ▶). If the fetus survives the early infection, they invariably become PI (Grooms, 2004 ▶; Khodakaram-Tafti and Ikede, 2005 ▶; Uzal et al., 2016 ▶). In fact, most new PI detected in an infected herd will be the result of TI in dams with a normal immune response (Moennig et al., 2005 ▶). Persistently infected calves remain viremic for life, and are immunotolerant to homologous NCP BVD viruses.
Persistent infection
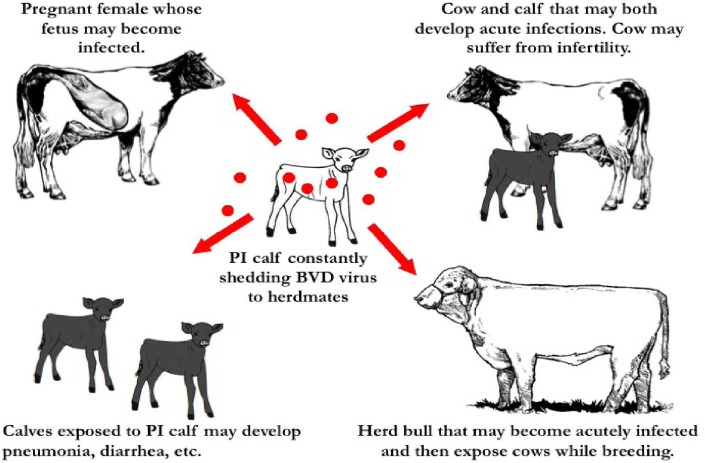
Several factors have influenced the persistence of BVDV in cattle. A non-lytic infection produced by NCP BVDV strains and the ability to evade the host immune response is the primary mechanism of persistence. When NCP biotype of BVDV infects the dam during the first trimester, the immature immune system of fetus is not able to develop a sufficient immune response yet and the virus produces the PI. Because the immune system of the fetus now recognizes the virus as part of its make-up, the virus will remain in the calf as long as it lives. Persistently infected animals are viremic (virus-positive and antibody-negative or seronegative), continually shed large amounts of BVDV in all body secretions including: nasal discharge, saliva, semen, faeces, etc. These animals serve as a major reservoir of virus for within the herd as well as the mechanism for maintaining BVDV in the cattle population (Fig. 1) (Brock, 2003 ▶; Grooms, 2004 ▶; Zimmer et al., 2004 ▶). Because the PI calves serve as one of the main reservoirs that maintain BVDV within the cattle population and BVDV spreads through most organs in the animal, but no lesions are present, it is exceedingly important to identify and remove these animals from the herd. Persistently infected cattle excrete the virus throughout their, life being a source of infection for other animals in a herd (Houe, 1999 ▶; Fray et al., 2000 ▶).
Fig. 1.
PI cattle excrete the virus throughout the life being a source of infection for other animals in a herd
The age-specific prevalence of PI is greatest at birth and decreases with age. Approximately 50% of BVDV PI calves will die during the first year of life due to other pathogens that affect PI animals more severely (Smirnova et al., 2008 ▶). Some PI calves can survive until maturity and if they are retained for breeding, their offspring is always PI but often fails to survive. PI bulls can produce semen of an acceptable quality, but may be associated with infertility (Moennig et al., 2005 ▶). Voges et al. (1998) ▶ have reported a case of a bull that was strongly sero-positive and non-viraemic but persistently shed the virus in the semen.
Calves that are born PI are sometimes weak. Once an animal is PI, it is always infected. PI calves may appear normal, but are frequently poor doers having reduced growth rates, immunosuppression, increased morbidity and mortality because they are more susceptible to many calfhood diseases, such as pneumonia. Most PI calves succumb to MD usually between the ages of 6 months and 2 years (Odeon et al., 2003 ▶; Uzal et al., 2016 ▶).
The prevalence of BVDV PI cattle has typically been observed in the range of 0.5% to 2% (Brock, 2003 ▶; Peterhans et al., 2003 ▶; Smith et al., 2008 ▶), although the prevalence of PI within herds is variable and may be as high as 25-30% when a large number of naïve cows, early in pregnancy, have been exposed to NCP BVDV. The success of any program to eradicate BVDV from a cattle population depends on the ability to detect all PI animals (Peterhans et al., 2003 ▶; Zimmer et al., 2004 ▶; Smith et al., 2008 ▶; Van Campen, 2010 ▶; Nelson et al., 2015 ▶).
Mucosal disease
This clinicopathologic syndrome occurs when PI animals become infected with a closely related CP strain of BVDV, or probably more commonly, when the virus causing the persistent congenital infection spontaneously develops a recombination encoding NS3. The result is an overwhelming infection that destroys cells and to which the animal is incapable of responding (Uzal et al., 2016 ▶). Mucosal disease is characterized by a high mortality rate with animals dying usually within 1-2 weeks after the onset of clinical signs. Post-mortem examinations reveal erosions and ulcers in the mucosa at various sites along the gastrointestinal tract (Baker, 1987 ▶).
Mucosal disease in nature is probably a rare event because several factors have influenced the occurrence of MD cases. First, an animal must be PI with BVDV. Second, superinfection of the PI animal with an antigenically similar CP BVDV or the generation of a mutant virus is required for it to occur (Tautz et al., 2003 ▶). Both NCP and CP biotypes are consistently found in animals that come down with MD (Kummerer et al., 2000 ▶; Bolin et al., 2004 ▶). However, post mortem examination of PI calves that succumbed to MD revealed high levels of CP virus in enteric tissues (Brownlie, 1990 ▶).
This syndrome affects all ages of PI cattle but often occurs between the ages of 6 months and 2 years. Extensive ulceration of the gastrointestinal tract is the most prominent lesion. Characteristic clinical signs of MD include anorexia, fever, diarrhea, dehydration, presence of lesions in the mucous of the digestive tract, necrosis of lymphoid tissue, hoof inflammation, and loss of condition and death (Wilhelmsen et al., 1991 ▶; Kelling, 2004 ▶). Dermatitis is a sign frequently present in MD and is the common finding of BVDV in skin biopsy specimens in PI cattle, confirming the tropism of the virus for the epithelial cells (Wilhelmsen et al., 1991 ▶; Dabak et al., 2007 ▶).
Pathology
Gross pathology
At necropsy, it is often difficult or impossible to differentiate cases of severe acute BVD caused by BVDV-1 or BVDV-2 and cases of MD. Perhaps the only exception is severe acute BVD associated with thrombocytopenic syndrome which has remarkable hemorrhagic lesions caused by highly virulent strains of BVDV-2. Fulminant severe acute BVD or MD closely resembles rinderpest clinically and grossly. At the onset the animal is febrile, with serous to mucoid nasal discharge (Uzal et al., 2016 ▶). The pathological lesions are confined in several body systems. The main pathological findings include widespread mucosal congestion, deep and extensive ulcerations in dorsal and lateral epithelia of the tongue, gums, hard palate, mucosa of the oesophagus, pillars of the rumen, mucosa of the abomasum and small intestine. It is common for the presence of blood clots of several diameters to attach to the mucosa of the ileum, some of them anatomically associated with the Peyer’s patches. In general, the mesenteric lymph nodes are large, edematous and hemorrhagic (Campbell, 2004 ▶; Liebler-Tenorio et al., 2006 ▶; Lunardi et al., 2008 ▶; khodakaram-Tafti et al., 2015 ▶). Interstitial emphysema, pneumonia and fibrinous pleural adherences are commonly found in the respiratory tract. Petechial haemorrhages can be present in epicardium and myocardium. In aborted fetuses, the principal lesions include conjunctivitis, pneumonia, thymus hypoplasia and non-specific myocarditis. Placental lesions consist mainly vasculitis, edema, congestion and haemorrhage with some degeneration and necrosis (Liebler-Tenorio et al., 2006 ▶).
Histopathology
The principal microscopic lesions reveal severe lymphocyte depletion and haemorrhages in peripheral and general lymph nodes and lymphoid follicles of Peyer’ s patches (Odeon et al., 2003 ▶; Chase et al., 2004 ▶).
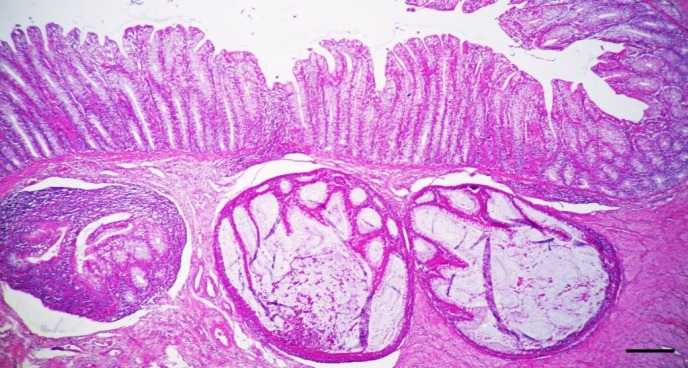
Microscopical examinations confirm the epithelial cell necrosis and vacuolation are present in the basal stratum and spinosum stratum of the squamous epithelia of the tongue and oesophagus. The epithelia of rumen could present cell necrosis and a mild non-suppurative inflammatory reaction. Lymphocyte infiltration, hyaline degeneration and fibrinoid necrotizing vasculitis of mesenteric and submucosal arterioles, epithelial necrosis, vacuolation and destruction of the epithelium of the crypts of Lieberkuhn are the prominent findings in the small intestine, cecum and colon. The affected crypts can be dilated and contained large amounts of cell debris with mixed neutrophils and macrophages (cryptitis) and herniated to submucosa (Fig. 2) (Khodakaram-Tafti and Miller, 2006 ▶; Liebler-Tenorio et al., 2006 ▶; Khodakaram-Tafti et al., 2015 ▶).
Fig. 2.
Ileum. Cryptitis and herniation of the crypts into the submucosa. The herniated crypts were dilated with mucus and cellular debris (bar = 150 μm
In respiratory tract, an acute catarrhal inflammation in nasal cavity and trachea can be observed. The lungs could present moderate congestion and lymphocytic interstitial reaction (Baule et al., 2001 ▶). In aborted fetus, histopathological changes are also noted in the cerebellum consisting of necrosis and depletion of the external germ layer (Swasdipan et al., 2002 ▶).
Diagnosis
There are different reliable methods for the detection of BVDV infected animals and, more importantly, differentiate acutely infected from PI animals because the identification and removal of PI animals that serve as the natural reservoirs is essential in preventing the spread of BVDV. Available methods to detect PI cattle include VI, RT-PCR, IHC, AC-ELISA which are some of the most commonly used tests to detect the presence of a PI animal (Table 1).
Table 1.
Suggested diagnostic laboratory tests for the detection of PI infected animals (Larson et al., 2004 ▶)
| Test | Cost | Advantages | Disvantages | Specimens/shipping |
|---|---|---|---|---|
|
Virus isolation
1-3 week turnaround |
Moderate to high cost |
-Gold standard for BVDV -High specificity -Virus is available for study at a later date |
-Slow procedure -Labor-intensive -Potential false negative due to interference by maternal Ab -Retest positive animals in 3-4 weeks to distinguish between PI and TI |
-Whole blood (10 ml) or serum (2-3 ml) and tissue samples -Send in container with cold packs -Do not freeze the samples |
|
Immunohistochemistry (IHC)
2-5 day turnaround |
Low cost | -High sensitivity -Usually identifies only PI -TI animals usually test negative |
-Labour-intensive -Formalin usage -Will not generally identify generally identify TI animals |
-Skin samples-ear notch and tissue samples -Send fresh on wet ice or stored in 1:10 volume of 10% neutral buffered formalin -Sample can be held in formalin for several weeks |
|
Antigen-Capture ELISA of serum
1-2 day turnaround |
Low cost | -High sensitivity -Easy to carry out |
-Potential false negative due to the interference by maternal antibodies -Variation of viremia -To distinguish between PI and TI animals, retest 3 weeks later |
-Serum (2 ml) -Send in insulated container with cold packs |
|
Antigen-Capture ELISA of skin
1-2 day turnaround |
Low cost | -High sensitivity -Usually identifies only PI animals -TI animals usually test negative |
-Will generally not identify TI animals | -Skin samples-ear notches -Send in insulated container with cold packs -Do not allow to dry out |
|
Antigen-Capture ELISA of tissue/leukocytes
1-3 day turnaround |
Low cost | -High sensitivity | -Labor-intensive to prepare buffy coat -Not used in a large screening |
-Whole blood (10 ml) using EDTA or heparin -Tissues -Send in insulated container with cold packs |
|
Polymerase chain reaction (PCR)
1-3 day turnaround |
Moderate to high cost (can be reduced pooling samples) | -High sensitivity -Can detect 1 ng/ml BVDV RNA |
-Potential of false positive due to laboratory contamination -Retest samples in 3 weeks to distinguish between PI and TI animals |
-Whole blood (10 ml) or serum (2-3 ml) -Ear notches in red top tubes -Milk, semen and tissues -Send in insulated container with cold packs |
Virus isolation (VI)
Culture and identification of BVDV from clinical specimens remains the “gold standard” diagnostic technique (Sandvik, 2005 ▶). Since BVDV appears to replicate best in lymphoid cells, samples that contain this cell type should be considered. The samples would include whole blood, buffy coat, lymphoid tissues such as Peyer’s patches, mesenteric lymph nodes, spleen and thymus from postmortem cattle or aborted fetuses. Buffy coat cells, whole blood, washed leukocytes or serum are suitable for isolation of the virus from live animals (Cornish et al., 2005 ▶).
Unfortunately, VI methods are labor intensive and take several days to be completed, and may not differentiate between TI and PI animals, unless positive cattle are re-tested and remain positive at a later date of 3 weeks (Cornish et al., 2005 ▶; Edmondson et al., 2007 ▶). In addition, colostral antibodies may temporarily reduce the amount of free virus in the serum of young calves and make the test less sensitive so that the virus from PI calves cannot be detected easily in serum by VI (Palfi et al., 1993 ▶). However, the virus can be isolated from mononuclear cells from calves receiving colostrum, but special procedures are needed.
Antigen-capture enzyme (ACE)-linked immunosorbent assay
Antigen-capture enzyme-linked immunosorbent assay has good sensitivity, specificity and repeatability for detecting antigen from BVDV; it is a robust, economical method of identifying PI cattle, easy to transfer and to perform (Bleak and Ballagi-Pordawy, 1993 ▶; Pacheco and Lager, 2003 ▶; Farjanikish et al., 2013 ▶).
The ACE, a relatively new assay available as a commercial test kit, uses MAb to capture viral antigen Erns glycoprotein (gp48). This structural protein is secreted from infected cells during virus replication and can be detected directly in blood, buffy coat cells, plasma, sera, ear notches or tissue extracts, producing reliable results (Brinkhof et al., 1996; ▶ Frey et al., 1996 ▶; Kuhne et al., 2005 ▶; Kennedy et al., 2006a ▶; Hill et al., 2007 ▶). For testing whole blood or peripheral blood leukocytes in the past often ACE was used (Saliki et al., 2000 ▶; Saliki and Dubovi, 2004 ▶).
Agreement between ELISAs performed on serum or skin and PCR has been reported to be 100% (Hill et al., 2007 ▶). For ear notches samples, a sensitivity of 100% and specificity of 99.6% was reported using the commercially available kit on ear tagging obtained from PI animals (Kennedy et al., 2006a ▶, b ▶; Edmonson et al., 2007 ▶). Some researchers reported differences between the results obtained using sera and ear notches samples. Both samples from PI calves were tested using ACE, and while sera samples were negative after intake of colostrum, the ear tissue samples were positive for BVDV at all time points (Kuhne et al., 2005 ▶). The ACE test cannot be used reliably on pooled samples from any source.
Immunohistochemistry (IHC)
Because of reduced cost and ease of sample collection, IHC staining of formalin-fixed, paraffin-embedded skin biopsies is widely used for the detection of PI animals (Brodersen, 2004 ▶).
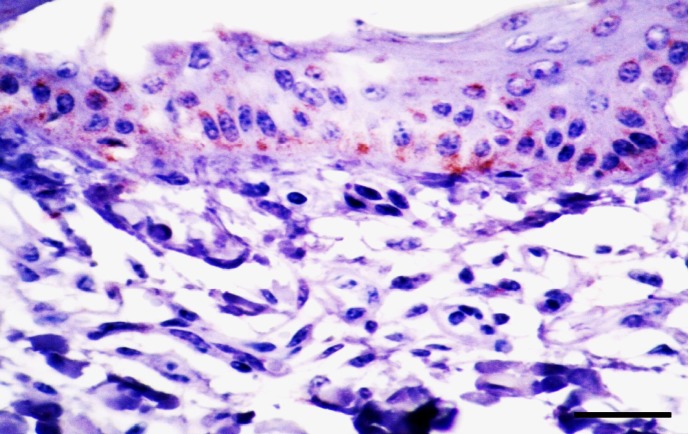
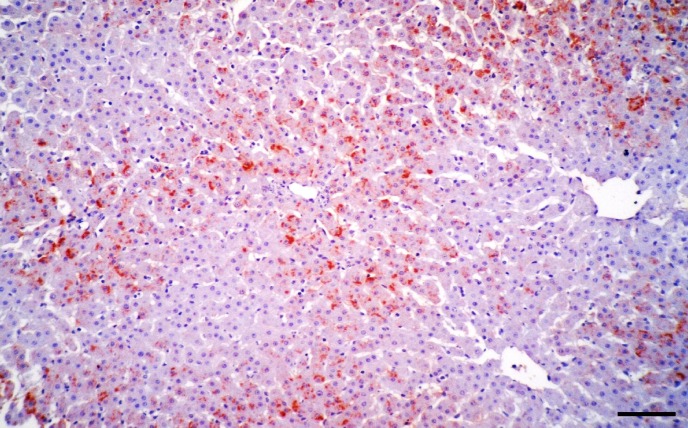
After the first report using skin biopsies as method of detection of PI cattle in 1996, where the agreement of IHC and VI in positive and negative animals was 100% (Thur et al., 1996 ▶), other studies have shown similar results (Njaa et al., 2000 ▶; Grooms et al., 2002 ▶). Previous studies described pronounced immunostaining in the basal epithelial cells of epidermis and hair follicles, subcutaneous stromal cells, endothelial cells of blood vessels and hepatocytes (Figs. 3, 4) (Thur et al., 1996 ▶; Njaa et al., 2000 ▶; Grooms and Keilen, 2002 ▶; Brodersen, 2004 ▶; Liebler-Tenorio et al., 2004 ▶; Saliki and Dubovi, 2004 ▶; Cornish et al., 2005 ▶; Loneragan et al., 2005 ▶; Khodakaram-Tafti and Miller, 2006 ▶; Luzzago et al., 2006 ▶; Hilbe et al., 2007a ▶, b ▶; Bedekovic et al., 2011 ▶; Khodakaram-Tafti et al., 2016). It should also be noted that IHC can detect virus in TI animals so care must be taken in interpreting positive results.
Fig. 3.
Ear-notch skin from a persistently infected calf. Immunopositivity for BVDV as finely brown cytoplasmic granules are observed in the cytoplasm of basal cells and other cells of epidermis, (IHC, bar = 40 μm
Fig. 4.
Liver. Positive immunostaining to BVDV antigen in hepatocytes and Kupffer’s cells (IHC, bar = 150 μm)
The advantages of the ear notch skin biopsy samples are include: they can be obtained easily and quickly, require less skill to obtain, provide a visible marker for the animal having been sampled, and these samples contain a high concentration of BVDV antigen. In addition, IHC to detect BVDV on skin biopsies is a good, fast and sensitive method, and the presence of maternal anti-BVDV antibodies therefore does not interfere with the detection of the BVDV antigen in the ear notch sample as it does with serum or plasma samples (Brodersen, 2004 ▶; Hilbe et al., 2007b ▶). This could potentially allow testing calves of any age, regardless of whether they received passive antibodies to BVDV via colostrum. Saliki and Dubovi (2004) ▶ recommended IHC or ACE from ear notch tissue samples to test cattle of all ages for BVDV. Two techniques performed on ear notches, IHC and ACE were compared for detection of BVDV PI animals. Both IHC and ACE detected 100% of PI calves (Cornish et al., 2005 ▶).
Recent studies support the conclusion that conventional tests may be replaced by the IHC on fixed tissues and ACE on unfixed tissues, and with these methods PI animals in a herd can be easily detected and eradicated (Hilbe et al., 2007b ▶).
Reverse transcriptase polymerase chain reaction (RT-PCR)
During the past 10 years, the RT-PCR has gained widespread use as a routine diagnostic method for BVDV (Smith et al., 2008 ▶). Recently, PCR has been used for BVDV detection in numerous clinical samples including serum, blood (buffy coats), tissues, fetal fluids, milk, nasal swab and soaked skin supernatant of PI animals (Drew et al., 1999 ▶; Renshaw et al., 2000 ▶; Stokstad et al., 2003 ▶; Kennedy, 2006 ▶; Young et al., 2006 ▶; Edmondson et al., 2007 ▶; Tajima et al., 2008 ▶; Khodakaram-Tafti et al., 2016).
In PI calves, RT-PCR test is a reliable diagnostic method at all ages and it has been demonstrated that RT-PCR is able to detect BVDV even in the presence of maternal antibodies to BVD virus that influence results obtained by VI, and ELISA (Bruschke et al., 1998 ▶; Letellier et al., 1999 ▶; Luzzago et al., 2001 ▶; Saliki et al., 2004 ▶; Goyal, 2005 ▶; Sandvik, 2005 ▶).
Some researchers tested pooled samples (pooled serum, milk and buffy coat samples or pooled ear notch phosphate buffered saline) by RT-PCR to screen many animals. If the pooled sample is positive by RT-PCR, samples are tested individually by PCR or ACE to identify the respective BVDV positive animal (Munoz-Zanzi et al., 2000 ▶; Kennedy et al., 2006 ▶; Khodakaram-Tafti et al., 2016 ▶). The pooling could provide an initial, rapid, cost-effective method of screening cattle herds for BVDV PI animals.
Complete agreement among IHC and ACE from skin samples, VI from white blood cells (WBC) lysates, and RT-PCR from WBC lysates from PI calves with BVDV was reported (Cornish et al., 2005 ▶). In the same way, using IHC as the relative gold standard, the RT-PCR has shown a sensitivity and specificity of 100% and 99%, respectively (Hilbe et al., 2007 ▶). As with IHC, RT-PCR can detect TI animals, so retesting of PCR positive animals may be necessary to establish PI status.
Because BVDV is an endemic disease in cattle populations in most parts of the world and due to its high prevalence and persistent economic losses in dairy and beef herds, it is considered one of the most significant infectious pathogens in the livestock industry. Due to the nature of infection, there is no treatment to fully cure an infected animal and the key lies in prevention of disease. Persistently infected animals in the domestic and wild populations are important reservoirs of the virus and shed large amounts of virus throughout their lives and it spreads among herds. All control programs which are in use in many countries, largely depend upon the detection and removal of PI animals, and preventing the introduction of PI animals in the herds. Detection of PI animals at early stage, particularly soon after birth is of significant benefit to implement BVDV control programs.
References
- Baker JC. The clinical manifestations of BVD infection. Vet. Clin. North Am.: Food Anim. Pract. 1987;11:425–445. doi: 10.1016/s0749-0720(15)30460-6. [DOI] [PubMed] [Google Scholar]
- Baule C, Kulcsar G, Belak K, Albert M, Mittelholzer C, Soos T, Kucsera L, Belak S. Pathogenesis of primary respiratory disease induced by isolates from a new genetic cluster of bovine viral diarrhea virus type I. J. Clin. Microbiol. 2001;39:146–153. doi: 10.1128/JCM.39.1.146-153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedekovic T, Lemo N, Lojki I, Beck A, Lojkić M, Madić J. Implementation of immuno-histochemistry on frozen ear notch tissue samples in diagnosis of bovine viral diarrhea virus in persistently infected cattle. Acta Vet. Scand. 2011;53:65–72. doi: 10.1186/1751-0147-53-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belak S, Ballagi-Pordany A. Experiences on the application of the polymerase chain reaction in a diagnostic laboratory. Mol. Cell. Prob. 1993;7:241–248. doi: 10.1006/mcpr.1993.1035. [DOI] [PubMed] [Google Scholar]
- Bezek DM, Grohn YT, Dubovi EJ. Effect of acute infection with noncytopathic or cytopathic bovine viral diarrhea virus isolates on bovine platelets. Am. J. Vet. Res. 1994;55:1115–1119. [PubMed] [Google Scholar]
- Bielanski A, Algire J, Lalonde A, Nadin-Davis S. Transmission of bovine viral diarrhea virus (BVDV) via in vitro-fertilized embryos to recipients, but not to their offspring. Theriogenology. 2009;71:499–508. doi: 10.1016/j.theriogenology.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Birk AV, Dubovi EJ, Cohen-Gould L, Donis R, Szeto HH. Cytoplasmic vacuolization responses to cytopathic bovine viral diarrhoea virus. Virus Res. 2008;132:76–85. doi: 10.1016/j.virusres.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin SR, Grooms DL. Origination and consequences of bovine viral diarrhea virus diversity. Vet. Clin. North Am.: Food Anim. Pract. 2004;20:51–68. doi: 10.1016/j.cvfa.2003.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin SR, McClurkin AW, Cutlip RC andCoria MF. Severe clinical disease induced in cattle persistently infected with noncytopathic bovine viral diarrhea virus by superinfection with cytopathic bovine viral diarrhea virus. Am. J. Vet. Res. 1985;46:573–576. [PubMed] [Google Scholar]
- Brinkhof J, Zimmer G, Westenbrink F. Comparative study on four enzyme-linked immunosorbent assays and a cultivation assay for the detection of antigens associated with the bovine viral diarrhoea virus in persistently infected cattle. Vet. Microbiol. 1996;50:1–6. doi: 10.1016/0378-1135(95)00201-4. [DOI] [PubMed] [Google Scholar]
- Brock KV. The persistence of bovine viral diarrhea virus. Biologicals. 2003;31:133–135. doi: 10.1016/s1045-1056(03)00029-0. [DOI] [PubMed] [Google Scholar]
- Brock KV. The many faces of bovine viral diarrhoea virus. Vet. Clin. North Am.: Food Anim. Pract. 2004;20:1–3. doi: 10.1016/j.cvfa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Brodersen BW. Immunohistochemistry used as a screening method for persistent bovine viral diarrhea virus infection. Vet. Clin. North Am.: Food Anim. Pract. 2004;20:85–94. doi: 10.1016/j.cvfa.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Brownlie J. Pathogenesis of mucosal disease and molecular aspects of bovine virus diarrhea virus. Vet. Microbiol. 1990;23:371–382. doi: 10.1016/0378-1135(90)90169-v. [DOI] [PubMed] [Google Scholar]
- Bruschke CJM, Haghparast A, Hoek A, Rutten VP, Wentink GH, Van Rijn PA, Van Oirschot JT. The immune response of cattle persistently infected with non-cytopathic BVDV after superinfection with antigenetically semi-homologous cytopathic BVDV. Vet. Immunol. Immunopathol. 1998;62:37–50. doi: 10.1016/s0165-2427(97)00165-7. [DOI] [PubMed] [Google Scholar]
- Campbell JR. Effect of bovine viral diarrhea virus in the feedlot. Vet. Clin. North Am.: Food Anim. Pract. 2004;20:39–50. doi: 10.1016/j.cvfa.2003.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson U. Border disease in sheep caused by transmission of virus from cattle persistently infected with bovine virus diarrhoea virus. Vet. Rec. 1991;128:145–147. doi: 10.1136/vr.128.7.145. [DOI] [PubMed] [Google Scholar]
- Carlsson U, Belak K. Border disease virus transmitted to sheep and cattle by a persistently infected ewe: epidemiology and control. Acta Vet. Scand. 1994;35:79–88. doi: 10.1186/BF03548357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase CC, Elmowalid G, Yousif AA. The immune response to bovine viral diarrhea virus: a constantly changing picture. Vet. Clin. North Am.: Food Anim. Pract. 2004;20:95–114. doi: 10.1016/j.cvfa.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Cornish TE, Olphen AL, Cavender JL, Edwards JM, Jaeger PL, Vieyra LL, Woodard LF, Miller DR, O’Toole D. Comparison of ear notch immuno-histochemistry, ear notch antigen-capture ELISA, and buffy coat virus isolation for detection of calves persistently infected with bovine viral diarrhea virus. J.Vet. Diag. Investig. 2005;17:110–117. doi: 10.1177/104063870501700203. [DOI] [PubMed] [Google Scholar]
- Dabak M, Karapinar T, Gulacti I, Bulut H, Kizil O, Aydin S. Hemorrhagic syndrome-like disease in calves with bovine viral diarrhea and mucosal disease complex. J. Vet. Intern. Med. 2007;21:514–518. doi: 10.1892/0891-6640(2007)21[514:hsdicw]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Deregt D, Loewen KG. Bovine viral diarrhea virus: biotypes and disease. Can. Vet. J. 1995;36:371–378. [PMC free article] [PubMed] [Google Scholar]
- Deregt D, Tessaro SV, Baxi MK, Berezowski J, Ellis JA, Wu JT, Gilbert SA. Isolation of bovine viral diarrhoea viruses from bison. Vet. Rec. 2005;157:448–450. doi: 10.1136/vr.157.15.448. [DOI] [PubMed] [Google Scholar]
- Drew TW, Yapp F, Paton DJ. The detection of bovine viral diarrhoea virus in bulk milk samples by use of a singletube RT-PCR. Vet. Microbiol. 1999;64:145–154. doi: 10.1016/s0378-1135(98)00266-1. [DOI] [PubMed] [Google Scholar]
- Edmondson MA, Givens MD, Walz PH, Gard JA, Stringfellow DA, Carson RL. Comparison of tests for detection of bovine viral diarrhea virus in diagnostic samples. J.Vet. Diagn. Invest. 2007;19:376–381. doi: 10.1177/104063870701900406. [DOI] [PubMed] [Google Scholar]
- Evermann JF, Ridpath JF. Clinical and epidemiologic observations of bovine viral diarrhea virus in the northwestern United States. Vet. Microbiol. 2002;89:129–139. doi: 10.1016/s0378-1135(02)00178-5. [DOI] [PubMed] [Google Scholar]
- Ezanno P, Fourichon C, Seegers H. Influence of herd structure and type of virus introduction on the spread of bovine viral diarrhoea virus (BVDV) within a dairy herd. Vet. Res. 2008;39:39. doi: 10.1051/vetres:2008016. [DOI] [PubMed] [Google Scholar]
- Falcone E, Tollis M, Conti G. Bovine viral diarrhea disease associated with a contaminated vaccine. Vaccine. 1999;18:387–388. doi: 10.1016/s0264-410x(99)00244-3. [DOI] [PubMed] [Google Scholar]
- Farjanikish GH, Khodakaram-Tafti A, Mohammadi A. Serological survey of bovine viral diarrhea virus by antigen capture ELISA in dairy herds in Fars Province, Iran. Bulg. J. Vet. Med. 2013;16:217–222. [Google Scholar]
- Fenton A, Nettleton PF, Entrican G, Herring JA, Malloy C, Greig A, Low JC. Identification of cattle infected with bovine virus diarrhoea virus using a monoclonal antibody capture ELISA. Arch. Virol. 1991;3:169–174. doi: 10.1007/978-3-7091-9153-8_20. [DOI] [PubMed] [Google Scholar]
- Fray MD, Paton DJ, Alenius S. The effects of bovine viral diarrhoea virus on cattle reproduction in relation to disease control. Anim. Reprod. Sci. 2000;2:615–627. doi: 10.1016/s0378-4320(00)00082-8. [DOI] [PubMed] [Google Scholar]
- Frey HR, Flebbe U, Liess B. Prevalence and clinical symptoms of persistent BVD-virus infection in cattle herds of Lower Saxony. Praktischer Tierarzt. 1996;14:14–18. [Google Scholar]
- Fulton RW, Johnson BJ, Briggs RE, Ridpath JF, Saliki JT, Confer AW, Burge LJ, Step DL, Walker DA, Payton ME. Challenge with bovine viral diarrhea virus by exposure to persistently infected calves: protection by vaccination and negative results of antigen testing in nonvaccinated acutely infected calves. Can. J.Vet. Res. 2006;70:121–127. [PMC free article] [PubMed] [Google Scholar]
- Givens MD, Heath AM, Brock KV, Brodersen BW, Carson RL, Stringfellow DA. Detection of bovine viral diarrhea virus in semen obtained after inoculation of seronegative postpubertal bulls. Am. J. Vet. Res. 2003;64:428–434. doi: 10.2460/ajvr.2003.64.428. [DOI] [PubMed] [Google Scholar]
- GoyalGoyal, SM. Diagnosis. In: Goyal, SM, Ridpath, JF, editors. Bovine viral diarrhea virus: diagnosis, management, and control. 1st Edn. Ames, IA: Blackwell Publishing; 2005. pp. 197–208. [Google Scholar]
- Grooms DL. Reproductive consequences of infection with bovine viral diarrhea virus. Vet. Clin. Food Anim. 2004;20:5–19. doi: 10.1016/j.cvfa.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Grooms DL, Keilen ED. Screening of neonatal calves for persistent infection with bovine viral diarrhea virus by immunohistochemistry on skin biopsy samples. Clin. Diagn. Lab Immunol. 2002;9:898–900. doi: 10.1128/CDLI.9.4.898-900.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn GJ, Sattkamp HW, Humphry RW, Stott AW. Assessing economic and social pressure for the control of bovine viral diarrhoea virus. Prev. Vet. Med. 2005;72:149–162. doi: 10.1016/j.prevetmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Hilbe M, Arquint A, Schaller P, Zlinszky K, Braun U, Peterhans E, Ehrensperger F. Immuno-histochemical diagnosis of persistent infection with Bovine Viral Diarrhea Virus (BVDV) on skin biopsies. Schw. Arch. Tierh. 2007b;149:337–344. doi: 10.1024/0036-7281.149.8.337. [DOI] [PubMed] [Google Scholar]
- Hilbe M, Stalder H, Peterhans E, Haessig M, Nussbaumer M, Egli C, Schelp C, Zlinszky K, Ehrensperger F. Comparison of five diagnostic methods for detecting bovine viral diarrhea virus infection in calves. J.Vet. Diagn. Invest. 2007a;19:28–34. doi: 10.1177/104063870701900105. [DOI] [PubMed] [Google Scholar]
- Hill FI, Reichel MP, McCoy RJ, Tisdall DJ. Evaluation of two commercial enzyme – linked immuno-sorbent assays for detection of bovine viral diarrhoea virus in serum and skin biopsies of cattle. New Zealand Vet. J. 2007;55:45–48. doi: 10.1080/00480169.2007.36734. [DOI] [PubMed] [Google Scholar]
- Houe H. Epidemiology of bovine viral diarrhea virus. Vet. Clin. North Am.: Food Anim. Pract. 1995;11:521–547. doi: 10.1016/s0749-0720(15)30465-5. [DOI] [PubMed] [Google Scholar]
- Houe H. Epidemiological features and economical importance of bovine virus diarrhoea virus (BVDV) infections. Vet. Microbiol. 1999;64:89–107. doi: 10.1016/s0378-1135(98)00262-4. [DOI] [PubMed] [Google Scholar]
- Kelling CL. Evolution of bovine viral diarrhea virus vaccines. Vet. Clin. Food Anim. Pract. 2004;20:115–129. doi: 10.1016/j.cvfa.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Kennedy JA. Diagnostic efficacy of a reverse transcriptase-polymerase chain reaction assay to screen cattle for persistent bovine viral diarrhea virus infection. J. Am. Vet. Med. Assoc. 2006;229:1472–1474. doi: 10.2460/javma.229.9.1472. [DOI] [PubMed] [Google Scholar]
- Kennedy JA, Mortimer RG, Powers B. Reverse transcription-polymerase chain reaction on pooled samples to detect bovine viral diarrhea virus by using fresh ear-notch-sample supernatants. J. Vet. Diagn. Invest. 2006;18:89–93. doi: 10.1177/104063870601800113. [DOI] [PubMed] [Google Scholar]
- Khodakaram-Tafti A, Ikede BO. A retrospective study of sporadic bovine abortions, stillbirths, and neonatal abnormalities in Atlantic Canada, from 1990 to 2001. Can. Vet. J. 2005;46:635–637. [PMC free article] [PubMed] [Google Scholar]
- Khodakaram-Tafti A, Miller L. The comparative evaluation of cellular localization of viral antigens with microscopic changes in the ileum of cattle infected with bovine viral diarrhea. Comp. Clin. Pathol. 2006;15:90–93. [Google Scholar]
- Khodakaram-Tafti A, Mohammadi A, Farjanikish GH. Histopathological and immuno-histochemical findings from bovine viral diarrhea virus infection in cattle. Onl. J. Vet. Res. 2015;19:317–321. [Google Scholar]
- Khodakaram-Tafti A, Mohammadi A, Farjanikish GH. Molecular characterization and phylogenetic analysis of bovine viral diarrhea virus in dairy herds of Fars province, Iran. Iran. J. Vet. Res. 2016;17:89–97. [PMC free article] [PubMed] [Google Scholar]
- Kuhne S, Schroeder C, Holmquist G, Wolf G, Horner S, Brem G, Ballagi A. Detection of bovine viral diarrhoea virus infected cattle testing tissue samples derived from ear tagging using an Erns capture ELISA. J. Vet. Med. B. Infect. Dis. Vet. Public Health. 2005;52:272–277. doi: 10.1111/j.1439-0450.2005.00861.x. [DOI] [PubMed] [Google Scholar]
- Kümmerer BM, Tautz N, Becher P, Thiel H, Meyers G. The genetic basis for cytopathogenicity of Pestiviruses. Vet. Microbiol. 2000;77:117–128. doi: 10.1016/s0378-1135(00)00268-6. [DOI] [PubMed] [Google Scholar]
- Lamm CG, Broaddus CC, Holyoak GR. Distribution of bovine viral diarrhea virus antigen in aborted fetal and neonatal goats by immunohistochemistry. Vet Pathol. 2009;46:54–58. doi: 10.1354/vp.46-1-54. [DOI] [PubMed] [Google Scholar]
- Larson RL, Groteloescher DM, Brock KV, Hunsaker BD, Smith RA, MacGregor DS, Daragatz DA. Bovine Viral Diarrhea (BVD): review for beef cattle veterinarians. Bov. Pract. 2004;38:93–102. [Google Scholar]
- Letellier C, Kerkhofs P, Wellemans G, Vanopdenbosch E. Detection and genotyping of bovine diarrhea virus by reverse transcription-polymerase chain amplification of the 5' untranslated region. Vet. Microbiol. 1999;64:155–167. doi: 10.1016/S0378-1135(98)00267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebler-Tenorio EM, Kenklies S, Greiser-Wilke I, Makoschey B, Pohlenz JF. Incidence of BVDV1 and BVDV2 infections in cattle submitted for necropsy in Northern Germany. 2006;J. Vet. Med. B. Infect. Dis. Vet. Public Health:363–369. doi: 10.1111/j.1439-0450.2006.00992.x. [DOI] [PubMed] [Google Scholar]
- Lindberg AL. Bovine viral diarrhea virus infections and its control. A review. Vet. Q. 2003;25:1–16. doi: 10.1080/01652176.2003.9695140. [DOI] [PubMed] [Google Scholar]
- Lindberg A, Brownlie J, Gunn GJ, Houe H, Moennig V, Saatkamp HW, Sandvik T, Valle PS. The control of bovine viral diarrhoea virus in Europe: today and in the future. Rev. Sci. Tech. 2006;25:961–979. [PubMed] [Google Scholar]
- Lindberg A, Houe H. Characteristics in the epidemiology of bovine viral diarrhea virus (BVDV) of relevance to control. Prev. Vet. Med. 2005;15:55–73. doi: 10.1016/j.prevetmed.2005.07.018. [DOI] [PubMed] [Google Scholar]
- Loneragan GH, Thomson DU, Montgomery DL, Mason GL, Larson RL. Prevalence, outcome, and health consequences associated with persistent infection with bovine viral diarrhea virus in feedlot cattle. J. Am. Vet. Med. Assoc. 2005;15:595–601. doi: 10.2460/javma.2005.226.595. [DOI] [PubMed] [Google Scholar]
- Lùken T. Ruminant Pestivirus infections in animals other than cattle and sheep. Vet. Clin. North Am.: Food Anim. Pract. 1995;11:597–614. doi: 10.1016/s0749-0720(15)30469-2. [DOI] [PubMed] [Google Scholar]
- Lunardi M, Headley SA, Lisboa JA, Amude AM, Alfieri AA. Outbreak of acute bovine viral diarrhea in Brazilian beef cattle: clinicopathological findings and molecular characterization of a wild-type BVDV strain subtype 1b. Res. Vet. Sci. 2008;85:599–604. doi: 10.1016/j.rvsc.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Luzzago C, Bandi C, Bronzo V, Ruffo G, Zecconi A. Distribution pattern of bovine viral diarrhoea virus strains in intensive cattle herds in Italy. Vet. Microbiol. 2001;26:265–274. doi: 10.1016/s0378-1135(01)00429-1. [DOI] [PubMed] [Google Scholar]
- Luzzago C, Frigerio M, Tolari F, Mazzei M, Salvadori C, Del Piero F, Arispici M. Indirect immuno-histochemistry on skin biopsy for the detection of persistently infected cattle with bovine viral diarrhoea virus in Italian dairy herds. New Microbiol. 2006;29:127–131. [PubMed] [Google Scholar]
- Moennig V, Houe H, Lindberg A. BVD control in Europe: current status and perspectives. Anim. Health Res. Rev. 2005;6:63–74. doi: 10.1079/ahr2005102. [DOI] [PubMed] [Google Scholar]
- Munoz-Zanzi CA, Johnson WO, Thurmond MC, Hietala SK. Pooled sample testing as a herd-screening tool for detection of bovine viral diarrhea virus persistently infected cattle. J. Vet. Diagn. Invest. 2000;12:195–203. doi: 10.1177/104063870001200301. [DOI] [PubMed] [Google Scholar]
- Neill JD, Ridpath JF, Lange A, Zuerner RL. Bovine viral diarrhoea virus infection alters global transcription profiles in bovine endothelial cells. Dev. Biol. 2008;132:93–98. doi: 10.1159/000317148. [DOI] [PubMed] [Google Scholar]
- Nelson DD, Duprau JL, Wolff PL, Evermann JF. Persistent bovine viral diarrhea virus infection in domestic and wild small ruminants and camelids including the mountain goat (Oreamnos americanus) Front Microbiol. 2015;6:1415–1422. doi: 10.3389/fmicb.2015.01415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niskanen R, Lindberg A. Transmission of bovine viral diarrhoea virus by unhygienic vaccination procedures, ambient air, and from contaminated pens. Vet. J. 2003;165:125–130. doi: 10.1016/s1090-0233(02)00161-2. [DOI] [PubMed] [Google Scholar]
- Niskanen, R, Lindberg, A, Larsson, B, Alenius, S. Lack of virus transmission from bovine viral diarrhoea virus infected calves to susceptible peers. Acta Vet. Scand. 2000;41:93–99. doi: 10.1186/BF03549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niskanen R, Lindberg A, Traven M. Failure to spread bovine virus diarrhea virus infection from primarily infected calves despite concurrent infection with bovine coronavirus. Vet. J. 2002;163:251–529. doi: 10.1053/tvjl.2001.0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njaa BL, Clark FG, Janzen E, Ellis JA, Haines DM. Diagnosis of persistent bovine viral diarrhea virus infection by immunohistochemical staining of formalin-fixed skin biopsy specimens. J. Vet. Diag. Invest. 2000;12:393–399. doi: 10.1177/104063870001200501. [DOI] [PubMed] [Google Scholar]
- Odeon AC, Risatti G, Kaiser GG, Leunda MR, Odriozola E, Campero CM, Donis RO. Bovine viral diarrhea virus genomic associations in mucosal disease, enteritis and generalized dermatitis outbreaks in Argentina. Vet. Microbiol. 2003;17:133–144. doi: 10.1016/s0378-1135(03)00210-4. [DOI] [PubMed] [Google Scholar]
- Pacheco JM, Lager I. Indirect method ELISA for the detection of antibodies against bovine diarrhea virus in bovine serum. Rev. Argentin. Microbiol. 2003;35:19–23. [PubMed] [Google Scholar]
- Palfi V, Houe H, Philipsen J. Studies on the decline of bovine virus diarrhoea virus (BVDV) antibodies and detectability of BVDV in persistently infected calves. Acta Vet. Scand. 1993;34:105–107. doi: 10.1186/BF03548231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton DJ, Carlsson U, Lowings JP, Sands JJ, VilceÃk S, Alenius S. Identification of herdspecific bovine viral diarrhoea virus isolates from infected cattle and sheep. Vet. Microbiol. 1995;43:283–294. doi: 10.1016/0378-1135(94)00107-8. [DOI] [PubMed] [Google Scholar]
- Pellerin C, Vandenhurk J, Lecomte J, Tijssen P. Identification of a new group of bovine viral diarrhea virus strains associated with severe outbreaks and high mortalities. 1994;Virology:260–268. doi: 10.1006/viro.1994.1483. [DOI] [PubMed] [Google Scholar]
- Peterhans E, Jungi TW, Schweizer M. BVDV and innate immunity. 2003;Biologicals:107–112. doi: 10.1016/s1045-1056(03)00024-1. [DOI] [PubMed] [Google Scholar]
- Renshaw RW, Ray R, Dubovi EJ. Comparison of virus isolation and reverse transcription polymerase chain reaction assay for detection of bovine viral diarrhea virus in bulk milk tank samples. J. Vet. Diagn. Invest. 2000;12:184–186. doi: 10.1177/104063870001200219. [DOI] [PubMed] [Google Scholar]
- Ridpath, JF. Classification and molecular biology. In: Goyal, SM, Ridpath, JF, editors. Bovine viral diarrhea virus:diagnosis, management, and contro. 1st Edn. Ames, Iowa: Blackwell Publishing; 2005. pp. 65–80. [Google Scholar]
- Ridpath JF. Bovine viral diarrhea virus: global status. Vet. Clin. North Am.: Food Anim. Pract. 2010;26:105–121. doi: 10.1016/j.cvfa.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Ridpath JF, Fulton RW, Kirkland PD, Neill JD. Prevalence and antigenic differences observed between bovine viral diarrhea virus subgenotypes isolated from cattle in Australia and feedlots in the southwestern United States. J. Vet. Diagn. Invest. 2010;22:184–191. doi: 10.1177/104063871002200203. [DOI] [PubMed] [Google Scholar]
- Saliki JT, Dubovi EJ. Laboratory diagnosis of bovine viral diarrhea virus infections. Vet. Clin. North Am.: Food Anim. Pract. 2004;20:69–83. doi: 10.1016/j.cvfa.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Saliki JT, Huchzermeir R, Dubovi EJ. Evaluation of a new sandwich ELISA kit that uses serum for detection of cattle persistently infected with BVD virus. Ann. N. Y. Acad. Sci. 2000;916:358–363. doi: 10.1111/j.1749-6632.2000.tb05313.x. [DOI] [PubMed] [Google Scholar]
- Sandvik T. Selection and use of laboratory diagnostic assays in BVD control programmes. Preven. Vet. Med. 2005;72:3–16. doi: 10.1016/j.prevetmed.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Schirrmeier H, Strebelow G, Depner K, Hoffmann B, Beer M. Genetic and antigenic characterization of an atypical Pestivirus isolate, a putative member of a novel Pestivirus species. J. Gen. Virol. 2004;85:3647–3652. doi: 10.1099/vir.0.80238-0. [DOI] [PubMed] [Google Scholar]
- Schlafer DH, Gillespie JH, Foote RH, Quick S, Pennow NN, Dougherty EP, Schiff EI, Allen SE, Powers PA, Hall CE. Experimental transmission of bovine viral diseases by insemination with contaminated semen or during embryo transfer. Deutsche Tierärzt. Wochensch. 1990;97:68–72. [PubMed] [Google Scholar]
- Schweizer M, Matzener P, Pfaffen G, Stalder H, Peterhans E. "Self" and "Nonself" manipulation of interferon defense during persistent infection: bovine viral diarrhea virus resists alpha/beta interferon without blocking antiviral activity against unrelated viruses replicating in its host cells. J. Virol. 2006;80:6926–6935. doi: 10.1128/JVI.02443-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon AD, Richards SG, Kirkland PD, Moyle A. An antigen-capture ELISA detects Pestivirus antigens in blood and tissues of immunotolerant carrier cattle. J. Virol. Method. 1991;34:1–12. doi: 10.1016/0166-0934(91)90116-h. [DOI] [PubMed] [Google Scholar]
- Smirnova NP, Bielefeldt-Ohmann H, Van Campen H, Austin KJ, Han H, Montgomery DL, Shoemaker ML, van Olphen AL, Hansen TR. Acute noncytopathic bovine viral diarrhea virus infection induces pronounced type I interferon response in pregnant cows and foetuses. Virus Res. 2008;132:49–58. doi: 10.1016/j.virusres.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Smith DR, Grotelueschen DM. Biosecurity and biocontainment of bovine viral diarrhea virus. Vet. Clin. North Am.: Food Anim. Pract. 2004;20:131–149. doi: 10.1016/j.cvfa.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Smith RL, Sanderson MW, Walz PH, Givens MD. Sensitivity of polymerase chain reaction for detection of bovine viral diarrhea virus in pooled serum samples and use of pooled polymerase chain reaction to determine prevalence of bovine viral diarrhea virus in auction market cattle. J. Vet. Diagn. Invest. 2008;20:75–78. doi: 10.1177/104063870802000115. [DOI] [PubMed] [Google Scholar]
- Stokstad M, Niskanen R, Lindberg A, Thoren P, Belak S, Alenius S, Loken T. Experimental infection of cows with bovine viral diarrhoea virus in early pregnancy – findings in serum and foetal fluids. J. Vet. Med. 2003;50:424–429. doi: 10.1046/j.0931-1793.2003.00699.x. [DOI] [PubMed] [Google Scholar]
- Stringfellow DA, Riddell KP, Givens MD, Galik PK, Sullivan E, Dykstra CC, Robl J, Kasinathan P. Bovine viral diarrhea virus (BVDV) in cell lines used for somatic cell cloning. Theriogenology. 2005;63:1004–1013. doi: 10.1016/j.theriogenology.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Swasdipan S, McGowan M, Phillips N, Bielefeldt-Ohmann H. Pathogenesis of transplacental virus infection: Pestivirus replication in the placenta and fetus following respiratory infection. Microb. Pathog. 2002;32:49–60. doi: 10.1006/mpat.2001.0480. [DOI] [PubMed] [Google Scholar]
- Taghipour Bazargani T, Khodakaram-Tafti A, Mousakhani F, Nekouie Jahromi OA. Occurrence of congenital tremor in Holstein calves due to infection with BVDV in two industrial dairies from Tehran and Kerman Provinces (case report) Sci. Res. Iranian Vet. J. 2011;7:92–96. [Google Scholar]
- Tajima M, Ohsaki T, Okazawa M, Yasutomi I. Availability of oral swab sample for the detection of bovine viral diarrhea virus (BVDV) gene from the cattle persistently infected with BVDV. Jpn. J. Vet. Res. 2008;56:3–8. [PubMed] [Google Scholar]
- Talafha AQ, Hirche SM, Ababneh MM, Al-Majali AM, Ababneh MM. Prevalence and risk factors associated with bovine viral diarrhea virus infection in dairy herds in Jordan. Trop. Anim. Health Prod. 2009;41:499–506. doi: 10.1007/s11250-008-9214-6. [DOI] [PubMed] [Google Scholar]
- Tautz N, Thiel HJ. Cytopathogenicity of Pestiviruses: cleavage of bovine viral diarrhoea virus NS2-3 has to occur at a defined position to allow viral replication. Arch. Virol. 2003;148:1405–1412. doi: 10.1007/s00705-003-0106-9. [DOI] [PubMed] [Google Scholar]
- Terpstra C, Wensvoort G. Natural infections of pigs with bovine viral diarrhoea virus associated with signs resembling swine fever. Res. Vet. Sci. 1988;45:137–142. [PubMed] [Google Scholar]
- Thur B, Zlinszky K, Ehrensperger F. Immuno-histochemical detection of bovine viral diarrhea virus in skin biopsies: a reliable and fast diagnostic tool. Zentral Veterin. 1996;43:163–166. doi: 10.1111/j.1439-0450.1996.tb00301.x. [DOI] [PubMed] [Google Scholar]
- Uzal, FA, Plattner, BL, Hostetter, JM. Alimentary system in pathology of domestic animals. In: Maxie, MG, editor. Jubb, Keneddy and Palmers pathology of domestic animals. 6th Edn. St. Louis, Missouri: Academic Press Inc; 2016. pp. 122–130. [Google Scholar]
- Van Campen H. Epidemiology and control of BVD in the U. S. Vet. Microbiol. 2010;14:94–98. doi: 10.1016/j.vetmic.2009.09.049. [DOI] [PubMed] [Google Scholar]
- Vilcek S, Nettleton PF. Pestiviruses in wild animals. Vet. Microbiol. 2006;116:1–12. doi: 10.1016/j.vetmic.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Vilcek S, Paton DJ, Rowe LW, Anderson EC. Typing of Pestiviruses from eland in Zimbabwe. J. Wildl. Dis. 2001;36:165–168. doi: 10.7589/0090-3558-36.1.165. [DOI] [PubMed] [Google Scholar]
- Voges H, Horner GW, Rowe S, Wellenberg GJ. Persistent bovine Pestivirus infection localized in the testes of an immuno-competent, non-viraemic bull. Vet. Microbiol. 1998;61:165–175. doi: 10.1016/s0378-1135(98)00177-1. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen CL, Bolin SR, Ridpath JF, Cheville NF, Kluge JP. Lesions and localization of viral antigen in tissues of cattle with experimentally induced or naturally acquired mucosal disease, or with naturally acquired chronic bovine viral diarrhea. Am. J. Vet. Res. 1991;52:269–275. [PubMed] [Google Scholar]
- Wittum TE, Grotelueschen DM, Brock KV. Persistent bovine viral diarrhoea virus infection in U. S. beef herds. Prev. Vet. Med. 2001;49:83–94. doi: 10.1016/s0167-5877(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Youngl NJ, Thomas CJ, Collins ME, Brownlie J. Real-time RT-PCR detection of bovine viral diarrhoea virus in whole blood using an external RNA reference. 2006;J. Virol. Methods:218–222. doi: 10.1016/j.jviromet.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer GM, Van Maanen C, De Goey I, Brinkhof J, Wentink GH. The effect of maternal antibodies on the detection of bovine virus diarrhoea virus in peripheral blood samples. Vet. Microbiol. 2004;100:145–149. doi: 10.1016/j.vetmic.2004.03.008. [DOI] [PubMed] [Google Scholar]