Abstract
Cases
Transarterial embolization of bilateral internal iliac arteries (TAE) is a useful hemostatic method for the management of pelvic fracture patients, but its effects on urinary functions remain unclear. In this study, we evaluated the impact of TAE on lower urinary tract symptoms (LUTS) in 10 pelvic fracture patients.
Outcomes
Lower urinary tract symptoms before and after hospitalization were evaluated by International Prostate Symptoms Score, Overactive Bladder Symptoms Score, and Quality Of Life score. All scores showed significant worsening. The changes did not correlate with sex, age, injury severity score, or durations of unstable hemodynamics or urethral catheterization. Changes of International Prostate Symptoms Score and Quality Of Life score showed significant positive correlations with intervals between the evaluations.
Conclusion
Pelvic fracture patients treated with TAE showed significant worsening of LUTS. Risk for exacerbation of LUTS should be taken into consideration when deciding to use TAE.
Keywords: Bilateral internal iliac arteries, bladder ischemia, lower urinary tract symptoms, pelvic fracture, transarterial embolization
Introduction
Transarterial embolization (TAE) is an effective method for control of arterial bleeding associated with pelvic fractures,1, 2 and its use is recommended in several guidelines as the primary method of hemostasis for hemodynamically unstable patients.3, 4 The standard embolization technique in this context is total embolization of the bilateral internal iliac arteries, even if the bleeding source is unilateral.5 Several studies investigated ischemic complications after TAE in pelvic fracture patients, but no study has examined its effect on lower urinary tract symptoms (LUTS), specifically impaired urinary voiding and storage functions.
Recent studies reported that obstruction of bilateral iliac arteries in an animal model leads to bladder ischemia and results in overactivity and impaired contractility of the detrusor.6, 7 As the standard TAE technique for hemostasis of pelvic fractures resembles temporary total occlusion of the bilateral iliac arteries in the animal model, we presumed a possible relation between TAE and exacerbation of LUTS in the clinical setting. The objective of the study was to clarify the impact of TAE on LUTS in patients with pelvic fractures.
The protocol for this research project was approved by a suitably constituted Ethics Committee of the institution and it conformed to the provisions of the Declaration of Helsinki (Committee of Saitama Medical Center, Approval No. 1015). Written informed consent was obtained from all patients who participated in the study.
Cases
Among 432 traumatic pelvic fracture patients who were admitted to Saitama Medical Center (Saitama, Japan) from January 2004 to January 2014, 97 underwent TAE to control bleeding associated with the fractures. Seventy‐one patients with brain, spinal cord, vesical, and urethral injuries, or TAE of lumber arteries, which may affect lower urinary tract function, were excluded. In all, 26 patients were included in the study. The complete obstruction of the bilateral internal iliac arteries was achieved by catheterization and infusion of narrow slips of gelatin (Sponzel; Astellas Pharma, Tokyo, Japan), and the presence of bladder ischemia was confirmed by angiography. Lower urinary tract symptoms before and after TAE were evaluated by questionnaires, namely, the International Prostate Symptoms Score (IPSS), Overactive Bladder Symptoms Score (OABSS), and Quality Of Life (QOL) score. We sent the questionnaires to the 26 patients asking for participation, and 10 patients gave consent.
Table 1A shows age, sex, durations of unstable hemodynamics and urethral catheterization, and intervals between the evaluations. Our institutional criteria for implementation of the standard TAE included not only unstable hemodynamics, but also extravasation of the contrast media confirmed by enhanced computed tomography scan. According to the criteria, TAE was carried out in the six patients (#2, 3, 4, 5, 8, and 10) who were hemodynamically stable.
Table 1.
Background characteristics and clinical courses related to lower urinary tract symptoms (A) and International Prostate Symptoms Score (IPSS), Overactive Bladder Symptoms Score (OABSS), and Quality of Life (QOL) score before and after transarterial embolization (TAE) (B) in pelvic fracture patients (n = 10)
| (A) | ||||||
|---|---|---|---|---|---|---|
| Case number | Age (years) | Sex | Injury severity score | Duration of unstable hemodynamics (min) | Duration of urethral catheterization (days) | Interval between evaluations (days) |
| 1 | 53 | M | 26 | 362 | 40 | 137 |
| 2 | 55 | M | 26 | 0 | 28 | 61 |
| 3 | 81 | F | 10 | 0 | 12 | 24 |
| 4 | 49 | M | 42 | 0 | 9 | 183 |
| 5 | 65 | F | 14 | 0 | 24 | 49 |
| 6 | 72 | M | 19 | 33 | 7 | 98 |
| 7 | 56 | F | 21 | 168 | 6 | 61 |
| 8 | 83 | M | 17 | 0 | 7 | 31 |
| 9 | 42 | M | 29 | 124 | 3 | 33 |
| 10 | 77 | M | 10 | 0 | 7 | 58 |
| Median (IQR) | 60 (54–74.5) | M : F 7:3 | 20 (15.5–26) | 0 (0–78.5) | 8 (7–8) | 59.3 (41–79.5) |
| (B) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Case number | IPSS before TAE | IPSS after TAE | ΔIPSS | OABSS before TAE | OABSS after TAE | ΔOABSS | QOL before TAE | QOL after TAE | ΔQOL |
| 1 | 0 | 14 | 14 | 0 | 1 | 1 | 0 | 6 | 6 |
| 2 | 2 | 2 | 0 | 3 | 6 | 3 | 3 | 3 | 0 |
| 3 | 2 | 5 | 3 | 1 | 1 | 0 | 1 | 3 | 2 |
| 4 | 9 | 20 | 11 | 0 | 1 | 1 | 2 | 5 | 3 |
| 5 | 6 | 7 | 1 | 4 | 4 | 0 | 2 | 2 | 0 |
| 6 | 10 | 10 | 0 | 3 | 3 | 0 | 3 | 3 | 0 |
| 7 | 3 | 3 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| 8 | 2 | 5 | 3 | 0 | 10 | 10 | 2 | 3 | 1 |
| 9 | 2 | 3 | 1 | 1 | 2 | 1 | 1 | 1 | 0 |
| 10 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| Median (IQR) | +1.0 (0 to +3)a | +0.5 (0 to +1)a | 0 (0 to +1)a | ||||||
∆IPSS, ∆OABSS, and ∆QOL indicate changes in scores between evaluations. M, Male; F, Female; IQR, interquartile range.
Significant change (P < 0.05) in score between evaluations.
Table 1B shows IPSS, OABSS, and QOL, before and after TAE, and their changes (∆IPSS, ∆OABSS, and ∆QOL) in each case. All scores showed a significant increase (P < 0.05) after TAE (Fig. 1).
Figure 1.
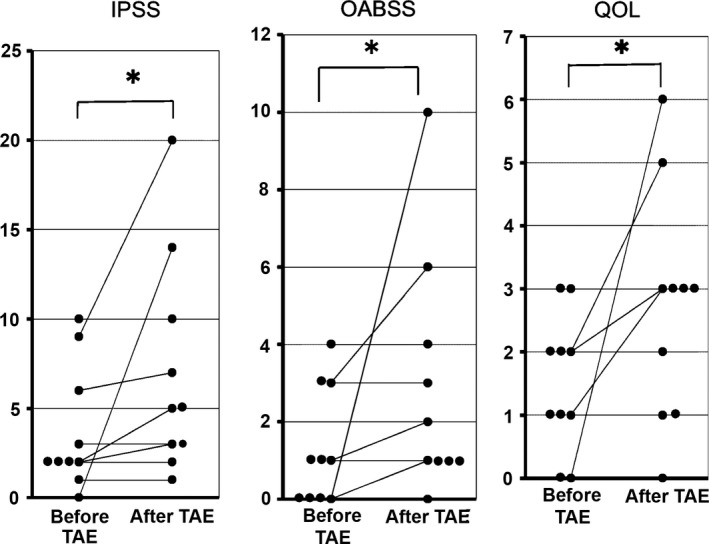
International Prostate Symptom Score (IPSS), Overactive Bladder Symptom Score (OABSS), and Quality Of Life (QOL) score before and after transarterial embolization (TAE) in 10 pelvic fracture patients, and their changes in each subject. *Statistically significant change (P < 0.05).
Score changes for ∆IPSS, ∆OABSS, and ∆QOL were rated as: +1 (0 to +7) (median [interquartile range]), +1 (+0.5 to +2), and 0 (0 to +2), respectively, for men; and +1 (+0.5 to +2), 0 (0 to 0), and 0 (0 to +1), respectively, for women. The change in scores showed no significant difference (P < 0.05) according to sex. Pearson's product‐moment correlation coefficients between ∆IPSS, ∆OABSS, and ∆QOL and age were −0.32, 0.32, and −0.19, respectively. Those between the scores and the injury severity score (ISS) were 0.55, 0.01, and 0.35, respectively. Those between the scores and the durations of unstable hemodynamics or urethral catheterization were 0.29, 0.18, and 0.19, or 0.40, 0.17, and 0.46, respectively. Those between the scores and the intervals were 0.75, −0.23, and 0.62, respectively. There was no significant correlation between changes in the scores and age, ISS, and durations. In contrast, there were significant positive correlations (P < 0.05) between ∆IPSS or ∆QOL and the intervals (Fig. 2).
Figure 2.
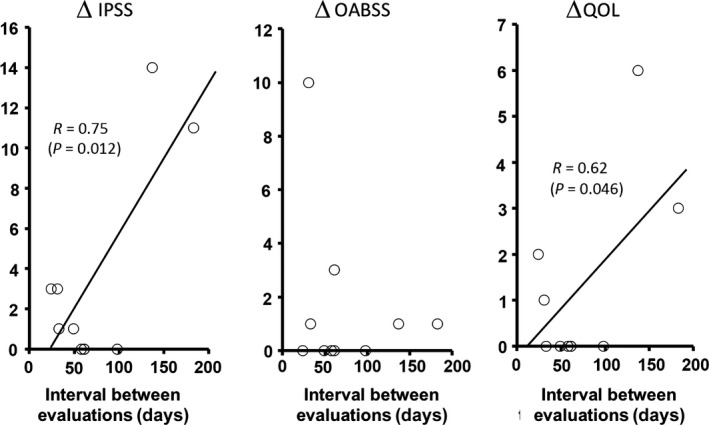
Changes in the International Prostate Symptom Score (∆IPSS), Overactive Bladder Symptom Score (∆OABSS), and Quality Of Life (∆QOL) score before and after transarterial embolization (TAE) in 10 pelvic fracture patients plotted against intervals between the evaluations. Solid lines represent linear regression lines. Formulas indicate Pearson's product‐moment correlation coefficients and their P‐values.
Discussion
The IPSS, OABSS, and QOL have verified validity in the evaluation of LUTS, and have been recommended for assessment of LUTS in clinical practice guidelines for both men and women.8, 9
Both IPSS and OABSS showed a statistically significant worsening after TAE and the adverse impact of TAE on voiding and storage functions. Quality of Life scores also showed a significant worsening after TAE and indicated that urinary tract dysfunction after TAE adversely affected patients’ quality of life. Exacerbation of IPSS, OABSS, and QOL showed no significant correlation with background characteristics and clinical courses such as sex, age, ISS, and durations of unstable hemodynamics and urethral catheterization. However, the exacerbation was significantly correlated with intervals between the evaluations. Given that the cases with prolonged intervals were relatively young (53 and 49 years old), the exacerbation was more attributable to TAE than aging. A study with an animal acute bladder ischemia and reperfusion model revealed the recovery of contractile function within 14 days.10 The intervals between evaluations were 24 days or longer in all patients who participated in this study. Taken together, the results may indicate that the exacerbation of LUTS and its impact on quality of life were persistent and possibly progressive.
None of the patients underwent magnetic resonance imaging to examine direct nerve injuries associated with pelvic fracture, and injuries could not be excluded as a cause for exacerbation of LUTS. Furthermore, the study did not compare the exacerbation in the TAE group with that in a non‐TAE or more selective TAE group. Therefore, the results only showed exacerbation of LUTS among the severe pelvic fracture patients treated with the standard TAE. However, taken together with the studies that reported lower urinary tract dysfunctions in animal models with obstruction of bilateral iliac arteries,6, 7 the results suggested that TAE might be one of the causes of exacerbation of LUTS in the patients.
The case series included six hemodynamically stable patients (#2, 3, 4, 5, 8, and 10), who underwent standard TAE according to the institutional criteria and the Eastern Association for the Surgery of Trauma guidelines. The guidelines recommend TAE in patients with evidence of arterial intravenous contrast extravasation in the pelvis by computed tomography, regardless of their hemodynamic status.3 However, the guidelines do not refer to LUTS as a complication of TAE.
In these contexts, the development of LUTS should be noted as a complication of TAE in pelvic fracture patients, and the risk for developing LUTS has to be taken into consideration when deciding TAE in this patient population, as LUTS may have long‐term adverse impacts on function and quality of life. The decision to avoid standard TAE in hemodynamically unstable patients is difficult as it may risk their lives. However, when deciding TAE in hemodynamically stable patients, more selective or unilateral embolization may be considered to reduce the risk.
Disclosure
Conflict of Interest: None declared.
Funding Information
No funding information provided.
References
- 1. Cook RE, Keating JF, Gillespie I. The role of angiography in the management of haemorrhage from major fractures of the pelvis. J. Bone Joint Surg. Br. 2002; 84: 178–82. [DOI] [PubMed] [Google Scholar]
- 2. Fangio P, Asehnoune K, Edouard A, Smail N, Benhamou D. Early embolization and vasopressor administration for management of life‐threatening hemorrhage from pelvic fracture. J. Trauma 2005; 58: 978–84. discussion 984. [DOI] [PubMed] [Google Scholar]
- 3. Cullinane DC, Schiller HJ, Zielinski MD et al Eastern Association for the Surgery of Trauma practice management guidelines for hemorrhage in pelvic fracture–update and systematic review. J. Trauma 2011; 71: 1850–68. [DOI] [PubMed] [Google Scholar]
- 4. Heetveld MJ, Harris I, Schlaphoff G, Sugrue M. Guidelines for the management of haemodynamically unstable pelvic fracture patients. ANZ J. Surg. 2004; 74: 520–9. [DOI] [PubMed] [Google Scholar]
- 5. Fang JF, Shih LY, Wong YC, Lin BC, Hsu YP. Repeat transcatheter arterial embolization for the management of pelvic arterial hemorrhage. J. Trauma 2009; 66: 429–35. [DOI] [PubMed] [Google Scholar]
- 6. Sagawa K, Aikawa K, Nomiya M et al Impaired detrusor contractility in a rat model of chronic bladder ischemia. Urology 2013; 81: e9–14. [DOI] [PubMed] [Google Scholar]
- 7. Nomiya M, Yamaguchi O, Andersson KE et al The effect of atherosclerosis‐induced chronic bladder ischemia on bladder function in the rat. Neurourol. Urodyn. 2012; 31: 195–200. [DOI] [PubMed] [Google Scholar]
- 8. Okamura K, Usami T, Nagahama K, Maruyama S, Mizuta E. The relationship among filling, voiding subscores from International Prostate Symptom Score and quality of life in Japanese elderly men and women. Eur. Urol. 2002; 42: 498–505. [DOI] [PubMed] [Google Scholar]
- 9. Homma Y, Yoshida M, Seki N et al Symptom assessment tool for overactive bladder syndrome‐ overactive bladder symptom score. Urology 2006; 68: 318–23. [DOI] [PubMed] [Google Scholar]
- 10. Juan YS, Chuang SM, Kogan BA et al Effect of ischemia/reperfusion on bladder nerve and detrusor cell damage. Int. Urol. Nephrol. 2009; 41: 513–21. [DOI] [PubMed] [Google Scholar]


