Abstract
Diabetic cardiomyopathy is one of the major complications among patients with diabetes mellitus. Diabetic cardiomyopathy (DCM) is featured by left ventricular hypertrophy, myocardial fibrosis, and damaged left ventricular systolic and diastolic functions. The pathophysiological mechanisms include metabolic-altered substrate metabolism, dysfunction of microvascular, renin-angiotensin-aldosterone system (RAAS) activation, oxidative stress, cardiomyocyte apoptosis, mitochondrial dysfunction, and impaired Ca2+ handling. An array of molecules and signaling pathways such as p38 mitogen-activated protein kinase (p38 MAPK), c-Jun N-terminal kinase (JNK), and extracellular-regulated protein kinases (ERK) take roles in the pathogenesis of DCM. Currently, there was no remarkable effect in the treatment of DCM with application of single Western medicine. The myocardial protection actions of herbs have been gearing much attention. We present a review of the progress research of herbal medicine as a potential therapy for diabetic cardiomyopathy and the underlying mechanisms.
1. Introduction
Diabetic cardiomyopathy (DCM) resulted from diabetes mellitus ultimately leads to heart failure, increasing the mortality in diabetic patients. There was a 2.4-fold increase in the risk of heart failure in the male diabetic subjects and fivefold in the female diabetic subjects [1]. DCM is characterized by left ventricular hypertrophy, myocardial fibrosis, and compromised left ventricular systolic/diastolic function [2]. Currently, there is still lack of feasible therapeutic approach for DCM. The Chinese traditional medicine has a long history in the treatment of glucose metabolism disorder and cardiovascular disease. Most recently, Li et al. [3] uncovered the antidiabetes effect of artemisinins, and this finding has been published in the Journal of cell. Using herbs to treat many chronic diseases such as diabetes and its complication has been recognized by an increasing number of scientists and clinic physicians for their multitarget effect and comprehensive source. In this review, we discuss the molecular mechanisms for the pathogenesis of DCM and then the study progress of herbal medicines as potential therapeutic agents for DCM.
2. Pathophysiological Mechanisms of DCM
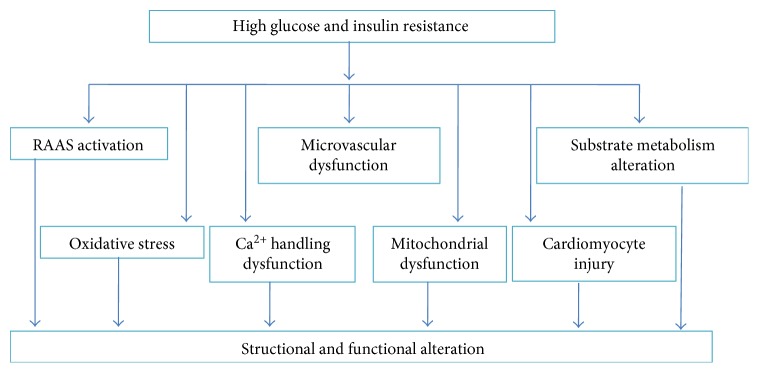
The function and structure of the heart are altered with the development of DCM. The left ventricular diastolic dysfunction is one of the pathological features of DCM, which occurs in isolation and precedes the development of systolic dysfunction [4]. The ventricular hypertrophy and myocardial fibrosis are the structural alteration of DCM. Hyperglycemia, insulin resistance, microcirculation dysfunction, and neurohormone activation are pathological triggers for DCM. The hyperglycemia and insulin resistance are responsible for altered substrate metabolisms including increased free fatty acid (FFA) oxidation, intramyocardial triglyceride accumulation, and reduced glucose utilization. The altered substrate metabolisms contribute to morphological changes of cardiomyocytes. Moreover, oxidative stress, the dysfunction of mitochondrion, abnormalities in Ca2+ homeostasis, and cardiomyocyte apoptosis all promote DCM development (Figure 1).
Figure 1.
Pathophysiological mechanisms for DCM. RASS: renin-angiotensin-aldosterone system; DCM: diabetic cardiomyopathy.
2.1. Neurohormone Activation and Microcirculation Dysfunction
Neurohormone activation is characterized by upregulation of sympathetic nervous system and renin-angiotensin-aldosterone system (RAAS). Circulating Ang II, the atrial natriuretic peptide (ANP), B-type natriuretic peptides (BNP), and catecholamines, as well as endothelin (ET-1) both in intramyocardium and circulating, are significantly increased in the context of hyperglycemia. Activation of RAAS and elevated ET-1 contribute to the myocardial fibrosis. ET-1 is responsible for vasoconstriction and myocardial ischemia. Endothelia-derived NO, an endogenous vasodilator, is remarkably decreased in the diabetic status. Impaired microvascular blood flow, sustained hyperglycemia, and excess generation of reactive oxygen species (ROS) are contributors for endothelial dysfunction.
2.2. Altered Substrate Metabolism
Substrate metabolism changes in diabetes are triggered by hyperglycemia and insulin resistance. In diabetes, the myocardial glucose utilization is significantly reduced for the depletion of glucose transporter proteins 1 (GLUT-1) and GLUT-4, resulting in the diabetic rely almost exclusively on FA as an energy production source [5]. Elevated circulating and cellular FFAs are attributable for increased adipose tissue lipolysis and hydrolysis of accumulated myocardial triglyceride. FFAs can inhibit glucose oxidation by activating peroxisome proliferator-activator receptor-α (PPAR-α), which increases the expression of pyruvate dehydrogenase kinase 4 (PDK4) involved in regulating enhanced mitochondrial FA uptake and reducing glucose oxidation. Moreover, elevated circulating and cellular FFAs can enhance peripheral insulin resistance. In the long term, increased myocardial FA utilization leads to lipotoxicity to the cardiomyocytes, characterized by myocyte lipid accumulation, mitochondrial dysfunction, increased oxygen demand, and excessive generation of ROS [6, 7]. Collectively, the enhanced peripheral insulin resistance, reduced cellular glucose utilization, and lipotoxicity are responsible for the cardiomyocyte injury and myocardial remodeling.
2.3. Oxidative Stress
In the physiological state, the ROS is eliminated by antioxidant system. However, in the diabetic settings, excessive production of ROS is responsible for oxidative stress and correlates with the development of DCM for its ability to damage proteins and DNA and lipid membranes. In the diabetic heart, ROS are derived from mitochondrial source, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and uncoupled NO synthases (NOS). The role of NADPH oxidase is the most important of the three sources in the development of DCM. Tumor necrosis factor-α (TNF-α) could induce cardiomyocyte hypertrophy by triggering the activity of NADPH oxidase [8]. Moreover, ROS derived from NADPH oxidase promotes the myocardial interstitial fibrosis and the mechanism involving increased activation of matrix metalloproteinases (MMP), expression of profibrotic genes, and activation of NF-κB [9]. As previously illustrated, mitochondrion is another major source for ROS production. The mitochondria themselves are susceptible to the ROS they produce, leading to local damage to mitochondrial DNA and membranes, generating more ROS as a result of a positive feedback mechanism [10].
2.4. Impaired Ca2+ Metabolism
Under the physiological state, Ca2+ influx induced by the activation of voltage-dependent L-type Ca2+ channels and then triggers the release of Ca2+ stored in the sarcoplasmic reticulum via ryanodine receptors (RyR) through a Ca2+-induced Ca2+-release mechanism. Free Ca2+ binds to troponin C and results in cardiomyocyte contraction. [Ca2+] is pumped out of cytosol and returns to a diastolic level mainly by the activation of the sarcolemmal Na+/Ca2+ exchanger, the sarcoplasmic reticulum Ca2+-ATPase2a (SERCA2a), and the sarcolemmal Ca2+-ATPase [11]. In the diabetic state, the decreased activity of SERCA results from the interaction of advanced glycation end products (AGE) with SERCA and the overexpression of SERCA2a inhibitor phospholamban (PLB) and could be reversed by insulin treatment [11]. Suppression of SERCA2a ultimately leads to Ca2+ overload in the cytosol and diastolic dysfunction [12]. The activity and expression of Na+/Ca2+ exchanger which contribute to the removal of [Ca2+] is also decreased in the diabetic state. The abnormal function of RyR induced by AGE/RAGE and oxidative stress contributes to sarcoplasmic reticulum Ca2+ leak, decreased sarcoplasmic reticulum-stored Ca2+, and systolic Ca2+ transient [13, 14]. Moreover, the decreased ATP synthesis rates which result from the reduced uptake of Ca2+ by mitochondria cause impaired contractility ability. Consequently, the disorder of Ca2+ handling contributes to diastolic/systolic dysfunction and left ventricular hypertrophy.
2.5. Cardiomyocyte Injury
Cardiomyocyte injury manners include apoptosis, necrosis, and autophagy. Apoptosis results from oxidative stress, mitochondrial dysfunction, and abnormalities in Ca2+ handling. Cardiomyocyte necrosis results in interstitial collagen deposition and ultimately leads to myocardial fibrosis [15].
Autophagy, a “housekeeping” subcellular process that maintains the cell nutrition homeostasis and self-renewal by degrading damaged proteins and organelles, is an alternate form of programmed cell death. Autophagy is also considered as a process that maintains cell survival in the condition of starvation and other cell stressors, for its regulation in the turnover of long-lived proteins, and protects cells. However, the dysregulated autophagy may result in excessive cell death. The role of autophagy in pathogenesis of DCM remains controversial. Xie et al. [16, 17] reported that a low constitutive autophagy is essential for protecting cardiomyocytes from hyperglycemic damage, whereas, the defect autophagy in diabetes contributes to the development of DCM. Coincidence with this finding, Zhao et al. showed that enhanced autophagy prevents DCM induced by STZ administration [18]. However, Hou et al. showed that AGE impairs the cell viability of rat neonate cardiomyocytes in a dose-dependent manner by inducing autophagy [19]. Collectively, well-regulated autophagy is essential for DCM attenuation.
3. Molecules and Signaling Pathways
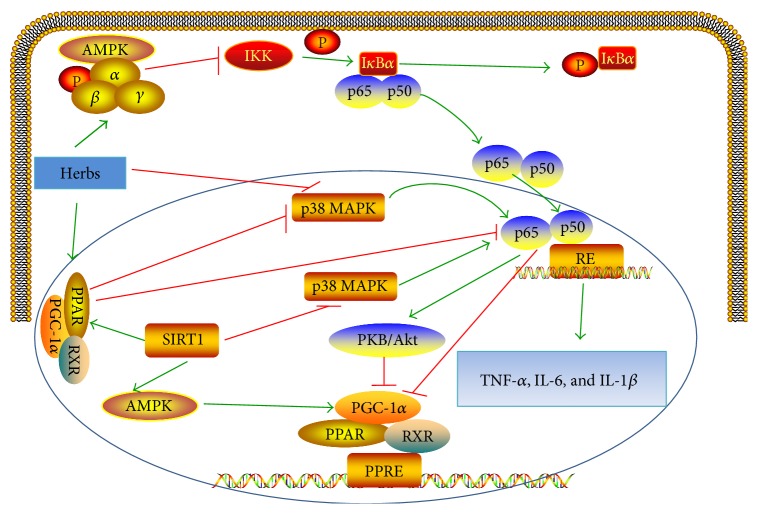
NF-κB is a key transcription factor that regulates inflammatory and cardiomyocyte injury processes. NF-κB consists of five members including p65 (RelA), RelB, c-Rel, NF-κB1 (p50 and its precursor p105), and NF-κB2 (p52 and its precursor p100). The most abundant form of the NF-κB family is the p65/p50 heterodimer. In resting cells, NF-κB is inactive by binding to IκBα in the cytoplasm. After high-glucose stimulation, the IκBα is phosphorylated by IκB kinase (IKK) complex, leading to the translocation of NF-κB to the nucleus and binding to NF-κB response element (RE) [20]. AMP-activated protein kinase (AMPK) suppresses NF-κB cascade through inhibition of IKK and decreased IκBα degradation. NF-κB cascade may be induced by phosphorylation of mitogen-activated protein kinase (MAPK). It has been also showed that signal transducer and activator of transcription (STAT) may contribute to activation of NF-κB and maintenance of NF-κB activity. PPAR has the ability to downregulate NF-κB activity by the interaction with the p65 subunit or inhibition of MAPK phosphorylation. Sirtuin 1 (SIRT1) inhibits NF-κB by inhibiting the MAPK or by increasing the interaction between PPAR and p65 subunit of NF-κB. SIRT1 activation leads to AMPK activation and deacetylation of PPAR-γ co-activator-1 (PGC1α). NF-κB decreases the activity of PGC-1α directly or indirectly by activating PKB/Akt. The activation of NF-κB leads to the increased proinflammatory cytokines, such as TNF-α, interleukin (IL)-6, and IL-1β, which contribute to the activation of profibrotic transforming growth factor beta (TGF-β) pathway. The NF-κB cascade is detailed in Figure 2. Herbs inhibit the activation of NF-κB by regulating AMPK, SIRT1, Akt, PPAR-γ, and MAPK cascades (Figure 2). Furthermore, herbs act on NFE2-related factor 2 (Nrf2), a master regulator of inflammation and oxidative status (Figure 3).
Figure 2.
Inflammation cascade in pathogenesis of DCM. AMPK: AMP activated-protein kinase; MAPK: mitogen-activated protein kinase; SIRT1: sirtuin 1; IKK: IκB kinase; RE: response element; PPAR: peroxisome proliferator-activated receptor; PGC1α: PPAR-γ co-activator-1; RXR: retinoid X receptor; PPRE: PPAR response elements; TNF-α: tumor necrosis factor-α; IL: interleukin.
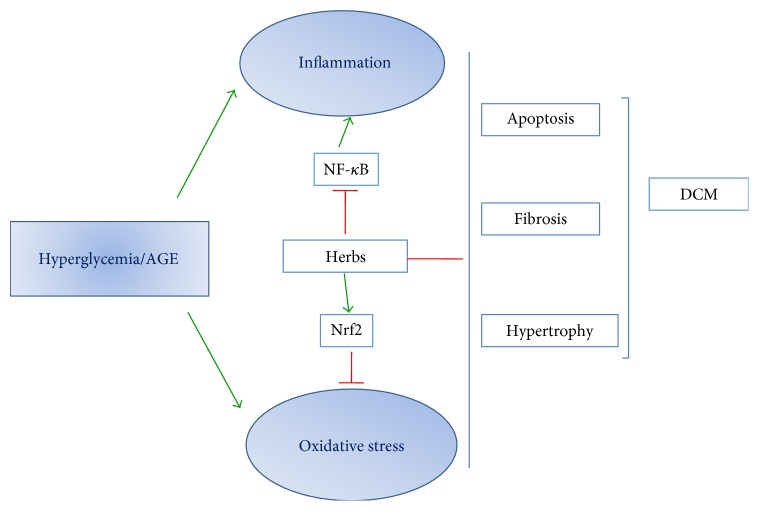
Figure 3.
Potential Mechanisms of herbal medicine protects against diabetic cardiomyopathy. Nrf2: transcription factor NFE2-related factor 2.
3.1. Mitogen-Activated Protein Kinase (MAPK) Cascade
Accumulated evidences have demonstrated that MAPK cascade including extracellular signal-regulated kinase 1/2 (ERK1/2), c-Jun N-terminal protein kinase (JNK), and p38 MAPK are involved in the diabetic complications [21]. p38 MAPK consists of four isoforms including p38α, p38β, p38γ, and p38δ. P38α is the major form expressed in a healthy heart, and p38β displays lower expression. p38 MAPK especially p38α MAPK contributes to the development of DCM owing to inflammation, oxidative stress, apoptosis, hypertrophy, metabolic abnormalities, and disordered Ca2+ handling. High glucose promotes the expression of protein kinase C (PKC) in the neonatal rat cardiomyocytes, leading to the upregulation of ROS, MAPK, and NF-κB [22]. ROS activates p38 MAPK, which in turn, promotes the production of ROS; alternatively, downregulation of p38 MAPK can inhibit ROS generation and oxidative stress [23]. p38α MAPK has been shown to promote the cardiomyocyte apoptosis by activating STAT1 and NF-κB and contribute to cardiomyocyte hypertrophy through activating the GATA4 transcription factor. MAPKPK-2 (MK2), a p38 MAPK downstream target, is responsible for downregulation of SERCA2a, FFA accumulation, and NF-κB activation in the development of DCM. On the contrary, the antiapoptotic function of p38β MAPK in DCM has been reported [21].
ERK1/2 signaling pathway is also known as the Ras-Raf-MEK-ERK cascade. ERK1/2 pathway is triggered by the activation of Ras at the myocyte membrane. The detrimental effects of ERK1/2 in a diabetic heart are manifested as oxidative stress, apoptosis, hypertrophy, and myocardial fibrosis. ERK1/2 activated by high glucose is associated with cardiac hypertrophy in DCM [24]. ROS and ET-1 stimulate the ERK1/2 cascade; hence, antioxidant agents block the ROS generation and ERK1/2 activation as well as cardiac hypertrophy [25]. The hyperglycemia-induced increased TGF-β is suppressed by ERK1/2 inhibitor U0126, suggesting that ERK1/2 mediates upregulation of TGF-β, closely related to cardiomyocyte fibrosis [26]. Interestingly, Zhang et al. showed that fibroblast growth factor 21 (FGF21) protects the diabetic heart from cardiac apoptosis, remodeling, and dysfunction by activating ERK1/2, and this protection effect could be abolished by ERK1/2 inhibitor PD98059 [27]. The antiapoptotic or proapoptotic effect of ERK1/2 is mainly dependent on the downstream effector activities. Taken together, ERK1/2 cascade is a two-edged sword in the development of DCM [28].
JNKs, members of the family of MAPKs, mediate inflammation and cell apoptosis. Tsai et al. [29] showed that hyperglycemia enhanced NADPH oxidase-derived superoxide generation, promoting activation of JNK and NF-κB activation as well as subsequent apoptosis of cardiomyocytes. JNK inhibitor and NF-ΚB siRNA abolished NF-ΚB-mediated inflammation and high-glucose-induced cardiomyocyte apoptosis.
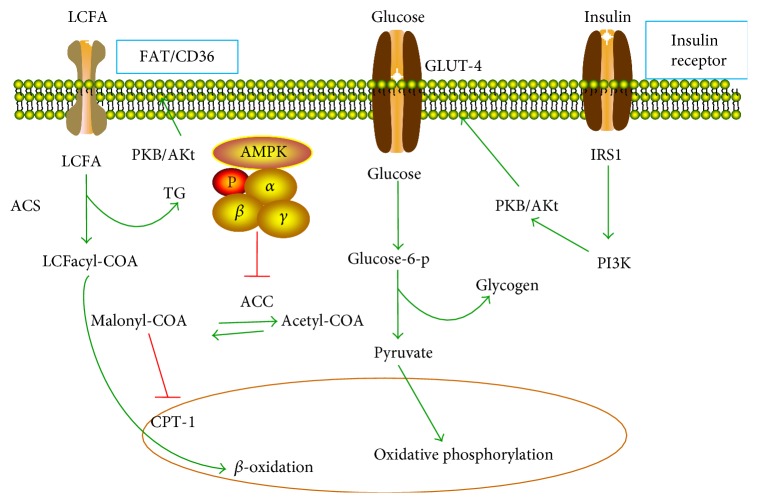
3.2. AMPK Cascade
AMPK, consists of α, β, and γ subunits, is an important regulator of insulin signaling, cardiac energy homeostasis, and oxidative stress. The phosphorylation at T172 of the α subunit is well-known mechanisms for AMPK activation [30]. AMPK is upregulated in response to an enhanced AMP/ATP ratio in a stressed cellular state [31]. Both liver kinase B1 (LKB1) and Ca2+/calmodulin-dependent protein kinase kinase (CAMMKK) are responsible for phosphorylation at T172 of AMPK α subunit in the cardiomyocytes under the condition of energy depletion [32, 33]. The activated AMPK facilitates the uptake of glucose by promoting myocardial GLUT-4 expression and translocation to the plasma membrane in a similar way to insulin [34]. Furthermore, AMPK increases FA uptake by cardiomyocytes via regulating the translocation of FA transporter (FAD/CD36) to the plasma membrane [35]. Carnitine palmitoyltransferase-1 (CPT-1) is responsible for the transportation of FAs into the mitochondria for β-oxidation. AMPK increases FA oxidation by inducing the phosphorylation-mediated inhibition of acetyl-COA carboxylase (ACC), leading to subsequent downregulation of malonyl-COA level, an inhibitor of CPT-1. Therefore, AMPK increases the energy production by increasing glucose uptake, FA oxidation, and glycolysis. However, AMPK inhibits lipolysis by inducing the phosphorylation of hormone-sensitive lipase [36]. The metabolic regulation by AMPK is illustrated in Figure 4.
Figure 4.
Metabolic regulation for normal heart. LCFA: long-chain fatty acids; FAT/CD36: translocation of FA transporter; GLUT: glucose transporter proteins; ACC: acetyl-COA carboxylase; ACS: acyl-CoA synthetase; CPT1: carnitine palmitoyltransferase-1; PI3K: phosphatidylinositol 3-kinase; IRS-1: insulin receptor substrate 1.
AMPK exerts an antioxidant effect by regulating the activation of Nrf2, another transcription factor that protects cardiomyocyte against oxidative stress. AMPK inhibits the NF-κB cascade by inhibiting IKK activity and IκBα degradation, or by activating downstream targets such as SIRT1, Forkhead box O (FOXO), and cardiac-enriched PGC1α [37, 38].
As previously illustrated, basic level of autophagy prevents against the apoptosis of cardiomyocytes. It has been demonstrated that AMPK directly activates ULK1, a homologue of yeast ATG1, through phosphorylation of Ser 317 and Ser 777 [39], or indirectly activates ULK1 by suppression of mammalian target of rapamycin complex 1 (mTORC1), resulting in enhanced autophagy [40]. Furthermore, AMPK regulates autophagy by activating FOXO, which upregulates expression of autophagy markers Bnip3, LC3, and ATG12 [30]. Recently, He et al. [41] reported that under starvation conditions, JNK1 activation leads to phosphorylation of BCL2 and dissociation of the Beclin1-BCL2 complex. However, diabetes inhibits the AMPK activity, suppresses its JNK1-BCL2 cascade, and promotes the interaction between Beclin1 and BCL2. Transfection of H9c2 cells with active JNK1 plasmid promotes BCL2 phosphorylation and disrupts the interaction between Beclin1 and BCL2, resulting in restoration of autophagy and reducing H9c2 cell apoptosis exposure to high glucose. Metformin, a well-known AMPK agonist, reduces apoptotic cell death and preserves the cardiac function by enhancing autophagy, and these effects were abolished by JNK1 inhibitor SP600125. These findings suggest that MAPK8/JNK1-BCL2 signaling is a new mechanism by which AMPK regulates autophagy [42].
3.3. PPAR Cascade
PPARs are responsible for the regulation of metabolism and inflammation. The subtypes of PPARs include PPAR α, PPAR β/δ, and PPAR-γ. Activation of PPARs is followed by the formation of heterodimers with the retinoid X receptor (RXR). Heterodimerization recruits PGC-1α and then binds to DNA-specific sequences called PPAR response elements (PPRE) and, consequently, allows the target gene transcription (CPT-1, FAT/CD36, PDK4, and GLUT-4 et al.). Of the three isoforms, PPAR α and PPAR β/δ express at high levels in the heart, while PPAR-γ enriched in adipose tissue shows a lower expression. According to Finck et al. [43] myocardial FA oxidation rates increased while glucose uptake and oxidation decreased in MHC-PPAR α mice, accompanied by the ventricular hypertrophy and systolic ventricular dysfunction. The MHC-PPAR α mice displayed metabolic phenotype and structure alteration similar to that of the diabetic heart. Burkart et al. [44] further showed that in contrast to MHC-PPAR α mice, MHC-PPAR β/δ mice did not develop cardiomyopathy, even in the context of a high-fat diet, owing to upregulation of GLUT-4 and enhanced rate of myocardial glucose uptake and utilization but without increased FA oxidation. Interestingly, in their study, both FAT/CD36 expression and GLUT-4 mRNA levels increased in MHC-PPAR-γ mice, suggesting that PPAR-γ shares the characteristic with both PPAR α and PPAR β/δ in regulation metabolism in diabetic hearts. Therefore, their findings provide the evidence that selective activation of PPAR β/δ is a promising therapeutic strategy for DCM. PGC-1α is the coactivator of PPARs enriched in the myocardium. PGC-1α regulates the mitochondrial biogenesis, FA oxidation, and glucose oxidative metabolism. PDK4 is a downstream molecule of PGC-1α. Selective overexpression of PDK4 in the heart of mice results in a remarkable decrease in glucose oxidation and an increase in FA oxidation. Overexpression of PGC-1α is associated with heart failure linked to increased FA oxidation. However, knockdown PGC-1α also leads to heart failure due to depletion of energy production in mitochondrion. According to Botta et al. [45] short-time exercise attenuated DCM in aged diabetic heart in db/db mice by activating PGC-1α. Wang et al. [46] showed that excise ameliorated DCM through activation of PGC-1α and Akt signaling.
All the three isoforms of PPARs exert anti-inflammation in the development of DCM for physical interaction with the p65 subunit of NF-κB and inhibit the activation of certain members of the MAPK signaling pathway [47–49]. Enhanced physical interaction between p65 and PGC-1α contributes to the decreased activation of PGC-1α.
3.4. Phosphatidylinositol 3-Kinase (PI3K)/PKB/Akt Cascade
PI3K takes a crucial role in insulin pathway and cardiac adaptation including protein synthesis, FA and glucose metabolisms, and cell survival regulation. Activation of PI3K subsequently targets to the upregulation of downstream effectors including PKB/Akt, glycogen synthase kinase (GSK)-3Β, and mTOR. The activation of PKB/Akt increases the uptake of glucose by inducing the translocation of the GLUT-4 protein to the cell membrane (Figure 4). However, PI3K can induce the cardiac glycogen synthesis by inhibition of PKB/Akt downstream effector, GSK-3Β. PI3K also has the ability to increase myocardial FA oxidation by promoting FAT/CD36 translocation to the sarcolemma in adult cardiomyocytes (Figure 4). PI3K stimulates autophagy by inhibiting mTORC1. NF-κB indirectly activates the PKB/Akt pathway, which phosphorylates PGC-1α and reduces its transcriptional activity.
3.5. SIRT1 Cascade
SIRT1, a class III (nicotinamide adenosine dinucleotide) NAD-dependent histone deacetylase, modulates AMPK activity by deacetylating LKB1 to induce its intracellular localization [50]. SIRT1 has the ability to promote the transcriptional activity of PGC-1α. SIRT1 inhibits NF-κB by enhancing the physical interaction between PPAR and p65 subunit or by inhibiting the phosphorylation of p38 MAPK [51]. Furthermore, Sulaiman et al. showed that upregulation of SIRT1 restores the SERCA2α gene expression in the context of hyperglycemia and improves the function left ventricular [52]. Taken together, SIRT1 prevents against the heart from diabetic injury by attenuating inflammation signaling cascade and improving Ca2+ handling [53].
3.6. Nrf2 Cascade
Under physiological conditions, Nrf2 locates in the cytoplasm and binds to its inhibitor kelch-like ECH-associated protein 1 (keap1). Under the condition of oxidative stress and high glucose, Nrf2 releases from keap1 and translocates into the nucleus to bind to antioxidant-responsive elements (AREs), leading to the expression of antioxidant enzymes such as NADPH quinone oxidoreductase (NQO1), heme oxygenase-1 (HO-1), superoxide dismutase (SOD), and catalase (CAT). He et al. [54] investigated the protection role of Nrf2 in the development of DCM using Nrf2-KO mice. There was an increased level of ROS in the cardiomyocytes of Nrf2-KO mice, and high glucose further increased the ROS generation in concentration and time-dependent manners.
Zhao et al. [18] have demonstrated that HO-1 prevents cardiac dysfunction by promoting the phosphorylation of AMPK and increasing the autophagy marker LC3II and Beclin1 expression in the STZ-induced diabetic heart. Activators target to Nrf2 are capable of protecting the heart from high-glucose injury.
3.7. MicroRNAs
miRNA is a class of conserved 19–25 nucleotide-noncoding RNAs that regulate gene expression posttranscriptionally. Recently, researchers have demonstrated that miRNAs play important roles in diabetes and related complications (Table 1). The miR-144 mimics enhance the generation of ROS and apoptosis in cardiomyocyte exposure to high glucose, which could be attenuated by an activator of Nrf2, Dh404. Inhibition of miR-144 results in suppressed ROS generation and cardiomyocyte apoptosis induced by high glucose, accompanied by the improved cardiac function in STZ-induced diabetic mice [55]. According to Jeyabal et al. [56], miR-9 expression was significantly reduced in high-glucose cultured cardiomyocytes and human diabetic hearts. miR-9 mimics attenuated hyperglycemia-induced ELAV-like protein 1 (ELAVL1) and inhibited cardiomyocyte apoptosis. Inhibition of miR-9 increased ELAVL1 and caspase-1 expression [56]. Zheng et al. showed that miR-195 expression was increased, and its target protein SIRT1 was decreased in STZ-induced type 1 and db/db type 2 diabetic mouse hearts. Anti-miR-195 in the heart improved myocardial function in STZ-induced mice by upregulating the activity of SIRT1 [57]. According to Liu et al., miR-21 promotes high-glucose-induced cardiac fibrosis though JAK/SAPK and p38 signaling pathway by suppression of dual specific phosphatase 8 (DUSP8) expression [58]. miR-200c expression is increased while its target molecule DUSP1 is decreased in DCM model and high-glucose-treated cardiomyocytes. Inhibition of miR-200c suppresses the expression of DUSP1, leading to decreased phosphorylation of ERK, p38, and JNK, as well as attenuating cardiomyocyte hypertrophy induced by high glucose [59]. Raut et al. showed that miR-30c overexpression attenuated high-glucose-induced cardiomyocyte hypertrophy by inhibiting the expression of cell division control protein 42 homolog (Cdc42) and p21-activated kinases (PAK1) [60]. Li et al. found that miR-30d promoted cardiomyocyte pyroptosis in DCM by direct repression of Foxo3a expression [61].
Table 1.
miRNA functions in DCM.
| miR type | Experimental model | Mechanism | Target gene | Reference |
|---|---|---|---|---|
| miR-144 | STZ-induced diabetic mice | Increased oxidative stress and cardiomyocyte apoptosis | Nrf2 | [55] |
| miR-9 | Human diabetic hearts, high-glucose cultured human | Prevented cardiomyocyte apoptosis | ELAVL1 | [56] |
| miR-195 | STZ-induced diabetic mice, db/db mice | Increased oxidative stress and apoptosis | SIRT1 | [57] |
| miR-21 | High-glucose cultured primary cardiac fibroblasts | Increased cardiac fibrosis | DUSP8 | [58] |
| miR-200c | High-fat diet plus STZ-induced diabetic rat, high-glucose cultured cardiomyocytes | Decreased cardiomyocyte hypertrophy | DUSP1 | [59] |
| miR-30c | STZ-induced diabetic rat, high-glucose cultured cardiomyocytes | Decreased cardiomyocyte hypertrophy | PAK1 and Cdc42 | [60] |
| miR-30d | STZ-induced diabetic rat | Increased cardiomyocyte pyroptosis | Foxo3a | [61] |
Nrf2: factor-erythroid 2-related factor 2; ELAVL1: ELAV-like protein 1; SIRT1: sirtuin 1; DUSP: dual specific phosphatase; PAK1: p21-activated kinases; Cdc42: cell division control protein 42 homolog; Foxo3a: Forkhead box O3.
4. Herbal Medicines: Promising Therapeutic for DCM
Currently, a growing number of preclinical studies provide the evidences that herbal medicines are promising therapy for DCM (Tables 2 and 3). These herbs ameliorate cardiac injury of DCM owing to their antioxidant and anti-inflammation properties, via regulation of NF-κB and Nrf2 pathways (Figure 3).
Table 2.
In vitro studies of herbs in the application of DCM.
| Drug | Dosage | Experimental model | Reference |
|---|---|---|---|
| Triptolide | 20 ng/ml | High-glucose cultured H9c2 rat cardiac cells | [64] |
| C66 | 2.5, 5, or 10 μmol/L | High-glucose cultured H9c2 cells | [69] |
| C66 | 2.5, 5, or 10 μmol/L | High-glucose cultured neonatal rat cardiomyocytes | [69] |
| Resveratrol | 50 μM | High-glucose cultured neonatal rat cardiomyocytes | [79] |
| Astragalus polysaccharides | 0.8 mg/ml | High-glucose cultured H9c2 cardiomyocytes | [85] |
| Myricitrin | 25 μg/ml | AGE-induced H9c2 cells | [90] |
| Taxifolin | 20, 40 μg/ml | High-glucose cultured H9c2 cells | [92] |
| Naringin | 80 μM | High-glucose cultured H9c2 cells | [96] |
| Total saponins of Aralia taibaiensis | 25, 50, and 75 μg/ml | G/GO cultured H9c2 cardiomyocytes | [109] |
C66; Compound (2E, 6E)-2,6-bis (2-(trifluoromethyl)benzylidene) cyclohexanone; AGE: advanced glycation end products; G/GO: 33 mM glucose + 15 mU glucose oxidase.
Table 3.
In vivo studies of herbs in the application of DCM.
| Drug | Dosage | Administration | Experimental model | Reference |
|---|---|---|---|---|
| Triptolide | 100, 200, or 400 μg/kg/d | p.o. 6 weeks | STZ-induced diabetic rat | [64, 65] |
| Triptolide | 50, 100, or 200 μg/kg/d | p.o. 8 weeks | STZ-induced diabetic rat | [66] |
| Curcumin | 100 mg/kg/d | p.o. 8 weeks | STZ-induced diabetic rat | [68] |
| C66 | 5 mg/kg/d | p.o. every other day for 12 weeks | STZ-induced diabetic mice | [69, 70] |
| EGb761 | 100 mg/kg/d | p.o. 12 weeks | STZ-induced diabetic rat | [71, 72] |
| EGb761 | 50 mg/kg/d | p.o. 3 weeks | STZ-induced diabetic rat | [75] |
| Resveratrol | 2.5 mg/kg/d | p.o. 2 weeks | STZ-induced diabetic rat | [78] |
| Resveratrol | 10 mg/kg/d | i.p. 4 weeks | STZ-induced diabetic rat | [80] |
| Resveratrol | Diet enriched with resveratrol at 0.067% | p.o. 12 weeks | STZ-induced diabetic mice | [52] |
| Astragalus polysaccharides | 1-2 g/kg/d | p.o. 10 weeks | STZ-induced diabetic hamsters | [81–84] |
| Salvia miltiorrhiza | 100 mg/kg/d | i.p. 4 weeks | STZ-induced diabetic rat | [86] |
| Cryptotanshinone | 10 mg/kg/d | p.o. 28 days | STZ-induced diabetic rat | [87] |
| Myricitrin | 300 mg/kg/d | p.o. 8 weeks | STZ-induced diabetic mice | [90] |
| Taxifolin | 25, 50, 100 mg/kg/d | p.o. 4 weeks | STZ-induced diabetic mice | [92] |
| Troxerutin | 150 mg/kg/d | p.o. 4 weeks | STZ-induced diabetic rat | [93] |
| Nobiletin | 50 mg/kg/d | p.o. 11 weeks | STZ-induced diabetic mice | [95] |
| Liquirtin | 8, 16 mg/kg | p.o. 10 weeks | High fructose-induced diabetic mice | [98] |
| Shengmaisan | 4.5 g/kg/d | p.o. 24 weeks | db/db mice | [99] |
| Alcoholic ginseng root | 200 mg/kg/d | p.o. 2 or 4 months | STZ-induced diabetic mice and db/db mice | [101] |
| Total saponins of Panax ginseng | 30 mg/kg/d | p.o. 12 weeks | STZ-induced diabetic rat | [102] |
| Ginsenoside Rg1 | 10, 15, 20 mg/kg/d | i.p. 12 weeks | STZ-induced diabetic rat | [103] |
| Dendrobium officinale Kimura et Migo | 75, 150, 300 mg/kg/d | p.o. 8 weeks | STZ-induced diabetic mice | [105] |
| Flos Puerariae extract | 100, 200 mg/kg/d | p.o. 10 weeks | STZ-induced diabetic mice | [106] |
| Mangiferin | 20 mg/kg/d | p.o. 16 weeks | STZ and high-fat diet induced diabetic rat | [107] |
| TASAES | 4.9, 9.8, and 19.6 mg/kg/d | p.o. 8 weeks | STZ-induced diabetic rat | [108] |
| Berberine | 100 mg/kg/d | p.o. 16 weeks | High-fat diet and STZ-induced diabetic rat | [111] |
EGb761: Ginkgo biloba extract 761; TASAES: total aralosides of Aralia elata (Miq) seem.
4.1. Triptolide
Extracts of Tripterygium wilfordii Hook F are effective in traditional Chinese medicine for the treatment of immune inflammatory diseases including rheumatoid arthritis, systemic lupus erythematosus, and nephritis. Triptolide as the major active ingredient for Tripterygium wilfordii Hook F exerts immunosuppressive and anti-inflammatory functions [62] (Figure 5(a)). Li et al. [63] showed that triptolide at 20 μg/kg/d and 100 μg/kg/d attenuated the myocardial fibrosis, cardiomyocyte hypertrophy, and restored the impaired cardiac function in the rat that underwent transverse aortic constriction, associated with decreased production of profibrotic factors TNF-α and IL-1β. Wen et al. [64, 65] showed that triptolide (100, 200, or 400 μg/kg/d p.o) administration for 6 weeks improved the left ventricular function of STZ-induced diabetic heart by inhibiting the expression of cardiac p38 MAPK in the upstream of NF-κB activation; triptolide with dose of 200 μg/kg/d displayed the best improvement. Furthermore, triptolide (20 ng/ml) attenuated inflammation of H9c2 rat cardiac cell exposure to high glucose by inhibiting NF-κB activation. Guo et al. [66] showed that the left ventricle pathological structure and function of STZ-induced mice were significantly improved by triptolide (50, 100 or 200 μg/kg/d p.o.) treatment for 8 weeks. The mechanism through which triptolide protects against DCM is involving inhibition of NF-κB/IL-1β and NF-κB/TNF-α cascades.
Figure 5.
Molecular structure of the compounds described in this review.
4.2. Curcumin
Curcumin, a component of turmeric found in the Curcuma longa plant, has been used in treating inflammatory diseases for centuries due to its antioxidant property (Figure 5(b)). Soetikno et al. [67] showed that curcumin exerts antifibrotic effect in amelioration of diabetic nephropathy owing to inhibiting PKC-α and PKC-β2, as well as the downstream cascade ERK1/2. They also demonstrated that curcumin at dose of 100 mg/kg/d for an 8-week oral administration significantly improved the left ventricular function and attenuated the progression of cardiac remodeling of STZ-induced diabetic rats by downregulating PKC-α and PKC-β2 and subsequently inactivating p38 MAPK, ERK1/2, and NF-κB. Moreover, the effect of improved blood glucose of diabetic rat partly explained the decreased oxidative stress [68]. Compound (2E, 6E)-2,6-bis (2-(trifluoromethyl) benzylidene) cyclohexanone (C66) is a synthetic derivative of natural active curcumin. Pan et al. showed that pretreatment of H9c2 cells, and neonatal cardiomyocytes with C66, significantly reduced the high-glucose-induced inflammation cytokine overexpression by inhibiting NF-κB. Treatment of STZ-induced diabetic mice with C66 at a dose of 5 mg/kg every other day for 12 weeks decreased the levels of plasma and cardiac TNF-α, endoplasmic reticulum stress, and cardiomyocyte apoptosis, as well as improved the cardiac dysfunction by inhibiting JNK phosphorylation [69, 70].
4.3. Ginkgo biloba Extract (GBE)
Ginkgo biloba extract (GBE) contains terpenoids, flavonoids, alkylphenols, polyprenols, and organic acids. The standardized GBE, EGb761, is pharmacologically prepared containing ginkgo flavonoids (primarily quercetin, kaempferol, and isorhamnetin) comprising 22–24% of the GBE, 6% terpenoids (3.1% ginkgolides A, B, C, and J and 2.9% bilobalide), and <5 ppm ginkgolic acid (Figures 5(d), 5(e), and 5(f)). Fitzl et al. showed that 100 mg/kg/d EGb761 orally administered for 12 weeks significantly reduced the increase of interstitial volume and collagen fibers in a diabetic rat heart [71, 72]. Furthermore, EGb761 treatment improves the hypoxia tolerance of diabetic myocardium and myocardial microvessels [73, 74]. Saini et al. [75] demonstrated that EGb761 at a dose of 50 mg/kg/d for 3 weeks significantly attenuated the index of lipid peroxidation and oxidative stress in diabetic rats and inhibited the opening of mitochondrial permeability transition pore (mPTP), ultimately leading to improvement of cardiomyopathy.
4.4. Resveratrol
Resveratrol (3,5,4′-trihydroxylstilbene), a natural polyphenol present in red wine and grapes, is capable to reduce blood glucose level in STZ-induced diabetic rat [76, 77] (Figure 5(c)). GLUT-4 translocation and glucose uptake are increased in STZ-induced diabetic rat myocardium by orally administrated resveratrol at a dose of 2.5 mg/kg/d for 2 weeks, the mechanism involving activation of AMPK and AKt cascades by resveratrol [78]. Resveratrol prevents high-glucose cultured neonatal rat cardiomyocyte apoptosis by inhibiting NADPH-derived ROS production and by alleviating the reduction of cardiac antioxidant enzyme activities, possibly mediated by AMPK-related signaling pathway [79]. Yar et al. showed that 10 mg/kg/d resveratrol intraperitoneal injection for 4 weeks ameliorated diabetic heart failure by increasing the expression of SIRT1 [80]. A special diet enriched with resveratrol at 0.067% (the consumption of resveratrol is estimated to be less than 100 mg/kg/d) for 12 weeks effectively restores SERCA2α expression and cardiac function in diabetic mice, associated with STIR1 activation [52].
4.5. Astragalus Polysaccharides (APS)
APS is a main active extract from the traditional Chinese medicinal herb Astragalus membranaceus. Chen et al. demonstrated that APS improved cardiac function and myocardial collagen deposition by inhibiting the local chymase-Ang II system and Ang II-activated ERK1/2 in diabetic cardiomyopathy in hamsters [81–83]. They also showed that APS can ameliorate myocardial glucose metabolism disorders in diabetic hamster by promoting expression of myocardial GLUT-4 gene and inhibiting level of PPAR α [84]. Pretreatment of cells with 0.8 mg/ml APS could inhibit high-glucose-induced apoptosis of H9c2 cell by decreasing the expression of caspases and release of cytochrome C from mitochondria to cytoplasm and by modulating the ratio of BCL-2 to Bax in mitochondria [85].
4.6. Salvia Miltiorrhiza
Salvia miltiorrhiza (Danshen), a traditional Chinese herbal medicine, is commonly used for the prevention and treatment of cardiovascular disease. According to Yu et al. [86] intraperitoneal injection Salvia miltiorrhiza 100 mg/kg/d for 4 weeks improved the heart function of diabetic rats and protected against cardiomyopathy by downregulating thrombospondin-1 (TSP-1) and TGF-β1 in myocardial tissue. Cryptotanshinone is an active principal ingredient isolated from Salvia miltiorrhiza (Danshen). Oral administration of 10 mg/kg/d cryptotanshinone for 28 days attenuates the cardiac fibrosis in STZ-induced diabetic rats by inhibiting STAT3 pathway and MMP-9 expression [87].
4.7. Flavonoids
Chrysin, a PPAR-γ agonist, is a natural flavonoid present in honey, propolis, and various plant extracts. According to Rani et al. [88, 89], chrysin attenuated isoproterenol-induced myocardial injury in diabetic rats by activating PPAR-γ and inhibiting AGE-RAGE-mediated inflammation and oxidative stress signaling pathway. Myricitrin (Figure 5(g)) is a flavone exact from the root bark of Myrica cerifera, Myrica esculenta, Ampelopsis grossedentata, and other plants. Zhang et al. [90] reported that pretreated AGE-cultured H9c2 cells with 25 μg/ml Myricitrin for 12 h significantly decreased the AGE-induced inflammation cytokines and cell apoptosis by activating Nrf2 and inhibiting NF-κB. Oral administration of Myricitrin 300 mg/kg/d for 8 weeks attenuated the cardiomyocyte apoptosis and inflammation of diabetic mice heart via regulation of AKt- and ERK-mediated Nrf2 pathways. Apigenin, a flavonoid derived in fruits and vegetables, has been shown to protect against isoproterenol-challenged diabetic myocardial injury by activation of PPAR-γ pathway [91]. Taxifolin is a flavonoid abound in Pseudotsuga taxifolia, Dahurian larch, and syn Larix dahurica Turoz. According to Sun et al. [92], Taxifolin at concentration 20 and 40 μg/ml could decrease the apoptosis of high-glucose cultured H9c2 cells by inhibiting ROS generation. In vivo, Taxifolin attenuated the structure and function abnormalities by blocking NADPH oxidative activities. Troxerutin (Figure 5(h)), a bioflavonoid, protects against DCM through suppression of NF-κB and JNK in a diabetic rat [93]. Hesperidin, a flavonoid isolated from citrus, has been shown to reduce oxidative stress and apoptosis and attenuate myocardial injury in isoproterenol-STZ rat via activation of PPAR-γ [94]. Nobiletin treatment (50 mg/kg/d p.o. 11 weeks) attenuates diabetic heart injury by suppression of oxidative stress, JNK, p38 MAPK, and NF-κB pathways [95]. 80 μM Naringin (4,5,7-trihydroxyflavonone-7-rhamnoglucoside, Figure 5(i)) pretreated for 2 hours protects H9c2 cells from high-glucose injury by ROS scavenging and MAPK cascade inhibiting [96]. Liquirtin, a major constituent of Glycyrrhiza radix, exerts various pharmacological activities. Liquirtin prevents myocardial injury induced by high fructose feeding by inhibiting NF-κB and MAPK cascades [97, 98].
4.8. Ginseng
Shengmaisan, a traditional Chinese recipe, consists of Radix Ginseng, Radix Ophiopogonis, and Fructus Schisandrae. According to Zhao et al. [99], cardiac dysfunction, hypertrophy, and fibrosis in diabetic mice are improved by 4.5 g/kg daily Shengmaisan treatment for 24 weeks through suppression of TGF-β pathway. Ni et al. [100] showed that Shengmai powder and Danshen decoction (consists of Radix Ginseng 9 g, Radix Ophiopogonis 9 g, Fructus Schisandrae 6 g, Radix Salviae Miltiorrhizae 30 g, Lignum Santali 6 g, and Fructus Amomi 6 g) inhibited the myocardial fibrosis in the diabetic rat through inhibiting TGF-β and TSP-1. Sen et al. [101] showed that alcoholic ginseng root (200 mg/kg/d, daily oral gavage) for 2 or 4 months is effective in the protection of cardiomyopathy in both type 1 and type 2 diabetic mice attributed to its antioxidative and antihyperglycemia properties. According to Gu et al. [102], total saponins of Panax ginseng 30 mg/kg/d by gavage for 12 weeks attenuated myocardial ultrastructural injury in diabetic rat and improved the lipid profile, blood glucose, and myocardial oxidative stress level as well. The mechanism involves regulation of citric acid cycle, fatty acid metabolism, and oxidative stress. Yu et al. [103] showed that Ginsenoside Rg1 dose dependently reduced serum levels of creatinine kinase MB and cardiac troponin I and attenuated diabetic rat myocardial ultrastructural disorder. Myocardial apoptosis was reduced by Ginsenoside Rg1 associated with reduced levels of caspase-3 and increased levels of B-cell lymphoma-extra-large (Bcl-xL) in the diabetic rat myocardium.
4.9. Others
Broccoli sprout extract at high dose (estimate an Nrf2 activator- sulforaphane availability at 1.0 mg/kg) by gavage every other day for 3 months significantly prevents cardiac dysfunction of diabetic db/db mice by upregulating Nrf2 transcription [104]. Cardiac lipid accumulation and deposition of collagen are inhibited by 8-week oral treatment of Dendrobium officinale Kimura et Migo at dose of 75, 150, and 300 mg/kg/d [105]. Dendrobium officinale Kimura et Migo attenuates the diabetic heart injury by downregulating the NF-κB-mediated inflammation cascade [105]. Flos Puerariae extract at dose of 100 and 200 mg/kg/d for 10 weeks prevents myocardial apoptosis in STZ-induced diabetic heart through inhibiting oxidative stress, associated with suppression of JNK and p38 MAPK activation [106]. Mangiferin (20 mg/kg/d p.o. 16 weeks) inhibits ROS accumulation, AGEs/RAGE production, and NF-κB nuclear translocation, attenuating cardiac injury induced by STZ and high-fat diet [107]. Total aralosides of Aralia elata (Miq) seem (TASAES) from Chinese traditional herb Longya Aralia chinensis L was found to prevent diabetes-induced cardiac dysfunction and pathological damage through upregulating L-type Ca2+ channel current in cardiac cells and decreasing connective tissue growth factor expression at dose of 4.9, 9.8 mg/kg, and 19.6 mg/kg/d by gavage, respectively, for 8 weeks [108]. Duan et al. [109] showed that the total saponins of Aralia taibaiensis exerted cytoprotective effects against oxidative stress induced by hyperglycemia through the Nrf2/ARE pathway. Chang et al. showed that berberine, a plant alkaloid, improves insulin resistance in H9c2 cardiomyocytes partly due to stimulation of AMPK activity [110]. They further found that berberine improved cardiac function and attenuated cardiac hypertrophy and fibrosis in a high-fat diet and STZ-induced diabetic rats through activation of AMPK and Akt [111]. According to Shen et al. [112], Shensong Yangxin Capsule inhibits diabetic myocardial fibrosis via suppressing TGF-β pathway.
5. Conclusion and Future Perspectives
DCM, featured by structure and function alteration, is a multifactorial disease. Extensive preclinical studies investigated the molecular targets for pathogenesis of DCM, and identified herbs that act on these targets are potential therapeutic approaches for DCM. However, at present, most clinical studies have small sample sizes and are not performed using a randomized design, and thus hamper the application of herbal medicines in patients with DCM. The combination of the herbs with Western medicine and joint application of herbal medicine on diabetic cardiomyopathy are superior to individual applications and are still under exploring. Hence, clinical trials in high-quality are needed in the future. Furthermore, exploring potential therapeutic target will contribute to detect new herbs for the treatment of DCM. The safety and drug interaction should be paid attention to ensure the wild and effective application of herbs in the DCM treatment.
Acknowledgments
The authors gratefully acknowledge the financial support from Beijing NOVA Program (no. Z171100001117027), Key Projects in the National Science and Technology Pillar Program during the 12th Five-Year Plan Period (no. 2011BAI11B05), and Beijing Lab for cardiovascular Precision Medicine (PXM2017_014226_000037).
Contributor Information
Yue Liu, Email: liuyueheart@hotmail.com.
Shuzheng Lyu, Email: shuzheng@medmail.com.cn.
Conflicts of Interest
The authors have no conflict of interest to declare.
Authors' Contributions
Yue Liu and Shuzheng Lyu conceived the topic and drafted the paper together and were the cocorresponding authors; Jinfan Tian searched for the literature and wrote the manuscript together with Yingke Zhao and Yanfei Liu; Keji Chen helped in drafting the manuscript. All authors read and approved the final manuscript.
References
- 1.Falcão-Pires I., Leite-Moreira A. F. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Failure Reviews. 2012;17(3):325–344. doi: 10.1007/s10741-011-9257-z. [DOI] [PubMed] [Google Scholar]
- 2.Miki T., Yuda S., Kouzu H., Miura T. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Failure Reviews. 2013;18(2):149–166. doi: 10.1007/s10741-012-9313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J., Casteels T., Frogne T., et al. Artemisinins target GABAA receptor signaling and impair α cell identity. Cell. 2017;168(1-2):86–100.e15. doi: 10.1016/j.cell.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cosson S., Kevorkian J. P. Left ventricular diastolic dysfunction: an early sign of diabetic cardiomyopathy? Diabetes & Metabolism. 2003;29(5):455–466. doi: 10.1016/s1262-3636(07)70059-9. [DOI] [PubMed] [Google Scholar]
- 5.Amaral N., Okonko D. O. Metabolic abnormalities of the heart in type II diabetes. Diabetes and Vascular Disease Research. 2015;12(4):239–248. doi: 10.1177/1479164115580936. [DOI] [PubMed] [Google Scholar]
- 6.Taegtmeyer H., McNulty P., Young M. E. Adaptation and maladaptation of the heart in diabetes: Part I: general concepts. Circulation. 2002;105(14):1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 7.Young M. E., McNulty P., Taegtmeyer H. Adaptation and maladaptation of the heart in diabetes: Part II: potential mechanisms. Circulation. 2002;105(15):1861–1870. doi: 10.1161/01.cir.0000012467.61045.87. [DOI] [PubMed] [Google Scholar]
- 8.Zeng S. Y., Chen X., Chen S. R., et al. Upregulation of Nox4 promotes angiotensin II-induced epidermal growth factor receptor activation and subsequent cardiac hypertrophy by increasing ADAM17 expression. Canadian Journal of Cardiology. 2013;29(10):1310–1319. doi: 10.1016/j.cjca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Sirker A., Zhang M., Murdoch C., Shah A. M. Involvement of NADPH oxidases in cardiac remodelling and heart failure. American Journal of Nephrology. 2007;27(6):649–660. doi: 10.1159/000109148. [DOI] [PubMed] [Google Scholar]
- 10.Duncan J. G. Mitochondrial dysfunction in diabetic cardiomyopathy. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2011;1813(7):1351–1359. doi: 10.1016/j.bbamcr.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lebeche D., Davidoff A. J., Hajjar R. J. Interplay between impaired calcium regulation and insulin signaling abnormalities in diabetic cardiomyopathy. Nature Clinical Practice Cardiovascular Medicine. 2008;5(11):715–724. doi: 10.1038/ncpcardio1347. [DOI] [PubMed] [Google Scholar]
- 12.Zhao X. Y., Hu S. J., Li J., Mou Y., Chen B. P., Xia Q. Decreased cardiac sarcoplasmic reticulum Ca2+ −ATPase activity contributes to cardiac dysfunction in streptozotocin-induced diabetic rats. Journal of Physiology and Biochemistry. 2006;62(1):1–8. doi: 10.1007/bf03165800. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X., Liu W., Deng J., et al. Polydatin protects cardiac function against burn injury by inhibiting sarcoplasmic reticulum Ca2+ leak by reducing oxidative modification of ryanodine receptors. Free Radical Biology & Medicine. 2013;60:292–299. doi: 10.1016/j.freeradbiomed.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Yan D., Luo X., Li Y., et al. Effects of advanced glycation end products on calcium handling in cardiomyocytes. Cardiology. 2014;129(2):75–83. doi: 10.1159/000364779. [DOI] [PubMed] [Google Scholar]
- 15.Buja L. M., Vela D. Cardiomyocyte death and renewal in the normal and diseased heart. Cardiovascular Pathology. 2008;17(6):349–374. doi: 10.1016/j.carpath.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Xie Z., He C., Zou M. H. AMP-activated protein kinase modulates cardiac autophagy in diabetic cardiomyopathy. Autophagy. 2011;7(10):1254–1255. doi: 10.4161/auto.7.10.16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie Z., Lau K., Eby B., et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60(6):1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y., Zhang L., Qiao Y., et al. Heme oxygenase-1 prevents cardiac dysfunction in streptozotocin-diabetic mice by reducing inflammation, oxidative stress, apoptosis and enhancing autophagy. PLoS One. 2013;8(9, article e75927) doi: 10.1371/journal.pone.0075927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou X., Hu Z., Xu H., et al. Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovascular Diabetology. 2014;13(1):p. 78. doi: 10.1186/1475-2840-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palomer X., Salvadó L., Barroso E., Vázquez-Carrera M. An overview of the crosstalk between inflammatory processes and metabolic dysregulation during diabetic cardiomyopathy. International Journal of Cardiology. 2013;168(4):3160–3172. doi: 10.1016/j.ijcard.2013.07.150. [DOI] [PubMed] [Google Scholar]
- 21.Wang S., Ding L., Ji H., Xu Z., Liu Q., Zheng Y. The role of p38 MAPK in the development of diabetic cardiomyopathy. International Journal of Molecular Sciences. 2016;17(7) doi: 10.3390/ijms17071037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min W., Bin Z. W., Quan Z. B., Hui Z. J., Sheng F. G. The signal transduction pathway of PKC/NF-κB/c-fos may be involved in the influence of high glucose on the cardiomyocytes of neonatal rats. Cardiovascular Diabetology. 2009;8(1):p. 8. doi: 10.1186/1475-2840-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Linthout S., Riad A., Dhayat N., et al. Anti-inflammatory effects of atorvastatin improve left ventricular function in experimental diabetic cardiomyopathy. Diabetologia. 2007;50(9):1977–1986. doi: 10.1007/s00125-007-0719-8. [DOI] [PubMed] [Google Scholar]
- 24.Ko S. Y., Lin I. H., Shieh T. M., et al. Cell hypertrophy and MEK/ERK phosphorylation are regulated by glyceraldehyde-derived AGEs in cardiomyocyte H9c2 cells. Cell Biochemistry and Biophysics. 2013;66(3):537–544. doi: 10.1007/s12013-012-9501-8. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka K., Honda M., Takabatake T. Redox regulation of MAPK pathways and cardiac hypertrophy in adult rat cardiac myocyte. Journal of the American College of Cardiology. 2001;37(2):676–685. doi: 10.1016/s0735-1097(00)01123-2. [DOI] [PubMed] [Google Scholar]
- 26.Tang M., Zhang W., Lin H., Jiang H., Dai H., Zhang Y. High glucose promotes the production of collagen types I and III by cardiac fibroblasts through a pathway dependent on extracellular-signal-regulated kinase 1/2. Molecular and Cellular Biochemistry. 2007;301(1-2):109–114. doi: 10.1007/s11010-006-9401-6. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C., Huang Z., Gu J., et al. Fibroblast growth factor 21 protects the heart from apoptosis in a diabetic mouse model via extracellular signal-regulated kinase 1/2-dependent signalling pathway. Diabetologia. 2015;58(8):1937–1948. doi: 10.1007/s00125-015-3630-8. [DOI] [PubMed] [Google Scholar]
- 28.Xu Z., Sun J., Tong Q., et al. The role of ERK1/2 in the development of diabetic cardiomyopathy. International Journal of Molecular Sciences. 2016;17(12) doi: 10.3390/ijms17122001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai K. H., Wang W. J., Lin C. W., et al. NADPH oxidase-derived superoxide anion-induced apoptosis is mediated via the JNK-dependent activation of NF-κB in cardiomyocytes exposed to high glucose. Journal of Cellular Physiology. 2012;227(4):1347–1357. doi: 10.1002/jcp.22847. [DOI] [PubMed] [Google Scholar]
- 30.Jeon S. M. Regulation and function of AMPK in physiology and diseases. Experimental & Molecular Medicine. 2016;48(7, article e245) doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cabarcas S. M., Hurt E. M., Farrar W. L. Defining the molecular nexus of cancer, type 2 diabetes and cardiovascular disease. Current Molecular Medicine. 2010;10(8):744–755. doi: 10.2174/156652410793384187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods A., Johnstone S. R., Dickerson K., et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Current Biology. 2003;13(22):2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 33.Woods A., Dickerson K., Heath R., et al. Ca2+/calmodulin-dependent protein kinase kinase-β acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metabolism. 2005;2(1):21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Holmes B. F., Sparling D. P., Olson A. L., Winder W. W., Dohm G. L. Regulation of muscle GLUT4 enhancer factor and myocyte enhancer factor 2 by AMP-activated protein kinase. American Journal of Physiology - Endocrinology and Metabolism. 2005;289(6):E1071–E1076. doi: 10.1152/ajpendo.00606.2004. [DOI] [PubMed] [Google Scholar]
- 35.Habets D. D., Coumans W. A., El Hasnaoui M., et al. Crucial role for LKB1 to AMPKα2 axis in the regulation of CD36-mediated long-chain fatty acid uptake into cardiomyocytes. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2009;1791(3):212–219. doi: 10.1016/j.bbalip.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Garton A. J., Campbell D. G., Carling D., Hardie D. G., Colbran R. J., Yeaman S. J. Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. European Journal of Biochemistry. 1989;179(1):249–254. doi: 10.1111/j.1432-1033.1989.tb14548.x. [DOI] [PubMed] [Google Scholar]
- 37.Salminen A., Ojala J., Huuskonen J., Kauppinen A., Suuronen T., Kaarniranta K. Interaction of aging-associated signaling cascades: Inhibition of NF-κB signaling by longevity factors FoxOs and SIRT1. Cellular and Molecular Life Sciences. 2008;65(7-8):1049–1058. doi: 10.1007/s00018-008-7461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salminen A., Hyttinen J. M., Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on healthspan and lifespan. Journal of Molecular Medicine. 2011;89(7):667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egan D., Kim J., Shaw R. J., Guan K. L. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy. 2011;7(6):643–644. doi: 10.4161/auto.7.6.15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw R. J. LKB1 and AMP-activated protein kinase control of mTOR signalling and growth. Acta Physiologica. 2009;196(1):65–80. doi: 10.1111/j.1748-1716.2009.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He C., Zhu H., Li H., Zou M. H., Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes. 2013;62(4):1270–1281. doi: 10.2337/db12-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou M. H., Xie Z. Regulation of interplay between autophagy and apoptosis in the diabetic heart: new role of AMPK. Autophagy. 2013;9(4):624–625. doi: 10.4161/auto.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Finck B. N., Lehman J. J., Leone T. C., et al. The cardiac phenotype induced by PPARα overexpression mimics that caused by diabetes mellitus. Journal of Clinical Investigation. 2002;109(1):121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burkart E. M., Sambandam N., Han X., et al. Nuclear receptors PPARβ/δ and PPARα direct distinct metabolic regulatory programs in the mouse heart. Journal of Clinical Investigation. 2007;117(12):3930–3939. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Botta A., Laher I., Beam J., et al. Short term exercise induces PGC-1α, ameliorates inflammation and increases mitochondrial membrane proteins but fails to increase respiratory enzymes in aging diabetic hearts. PLoS One. 2013;8(8, article e70248) doi: 10.1371/journal.pone.0070248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang H., Bei Y., Lu Y., et al. Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1a and Akt activation. Cellular Physiology and Biochemistry. 2015;35(6):2159–2168. doi: 10.1159/000374021. [DOI] [PubMed] [Google Scholar]
- 47.Buroker N. E., Barboza J., Huang J. Y. The IκBα gene is a peroxisome proliferator-activated receptor cardiac target gene. FEBS Journal. 2009;276(12):3247–3255. doi: 10.1111/j.1742-4658.2009.07039.x. [DOI] [PubMed] [Google Scholar]
- 48.Planavila A., Rodríguez-Calvo R., Jové M., et al. Peroxisome proliferator-activated receptor β/δ activation inhibits hypertrophy in neonatal rat cardiomyocytes. Cardiovascular Research. 2005;65(4):832–841. doi: 10.1016/j.cardiores.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 49.Asakawa M., Takano H., Nagai T., et al. Peroxisome proliferator-activated receptor γ plays a critical role in inhibition of cardiac hypertrophy in vitro and in vivo. Circulation. 2002;105(10):1240–1246. doi: 10.1161/hc1002.105225. [DOI] [PubMed] [Google Scholar]
- 50.Lan F., Cacicedo J. M., Ruderman N., Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. Journal of Biological Chemistry. 2008;283(41):27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan W., Yu H., Huang S., Zhu P. Resveratrol protects against TNF-α-induced injury in human umbilical endothelial cells through promoting sirtuin-1-induced repression of NF-KB and p38 MAPK. PLoS One. 2016;11(1, article e0147034) doi: 10.1371/journal.pone.0147034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sulaiman M., Matta M. J., Sunderesan N. R., Gupta M. P., Periasamy M., Gupta M. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. American Journal of Physiology - Heart and Circulatory Physiology. 2010;298(3):H833–H843. doi: 10.1152/ajpheart.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karbasforooshan H., Karimi G. The role of SIRT1 in diabetic cardiomyopathy. Biomedicine & Pharmacotherapy. 2017;90:386–392. doi: 10.1016/j.biopha.2017.03.056. [DOI] [PubMed] [Google Scholar]
- 54.He X., Kan H., Cai L., Ma Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. Journal of Molecular and Cellular Cardiology. 2009;46(1):47–58. doi: 10.1016/j.yjmcc.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Yu M., Liu Y., Zhang B., Shi Y., Cui L., Zhao X. Inhibiting microRNA-144 abates oxidative stress and reduces apoptosis in hearts of streptozotocin-induced diabetic mice. Cardiovascular Pathology. 2015;24(6):375–381. doi: 10.1016/j.carpath.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Jeyabal P., Thandavarayan R. A., Joladarashi D., et al. MicroRNA-9 inhibits hyperglycemia-induced pyroptosis in human ventricular cardiomyocytes by targeting ELAVL1. Biochemical and Biophysical Research Communications. 2016;471(4):423–429. doi: 10.1016/j.bbrc.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng D., Ma J., Yu Y., et al. Silencing of miR-195 reduces diabetic cardiomyopathy in C57BL/6 mice. Diabetologia. 2015;58(8):1949–1958. doi: 10.1007/s00125-015-3622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu S., Li W., Xu M., Huang H., Wang J., Chen X. Micro-RNA 21 targets dual specific phosphatase 8 to promote collagen synthesis in high glucose-treated primary cardiac fibroblasts. Canadian Journal of Cardiology. 2014;30(12):1689–1699. doi: 10.1016/j.cjca.2014.07.747. [DOI] [PubMed] [Google Scholar]
- 59.Singh G. B., Raut S. K., Khanna S., et al. MicroRNA-200c modulates DUSP-1 expression in diabetes-induced cardiac hypertrophy. Molecular and Cellular Biochemistry. 2017;424(1-2):1–11. doi: 10.1007/s11010-016-2838-3. [DOI] [PubMed] [Google Scholar]
- 60.Raut S. K., Kumar A., Singh G. B., et al. miR-30c mediates upregulation of Cdc42 and Pak1 in diabetic cardiomyopathy. Cardiovascular Therapeutics. 2015;33(3):89–97. doi: 10.1111/1755-5922.12113. [DOI] [PubMed] [Google Scholar]
- 61.Li X., Du N., Zhang Q., et al. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death and Disease. 2014;5, article e1479 doi: 10.1038/cddis.2014.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qiu D., Kao P. N. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medicinal herb Tripterygium wilfordii Hook. f. Drugs in R&D. 2003;4(1):1–18. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 63.Li R., Lu K., Wang Y., et al. Triptolide attenuates pressure overload-induced myocardial remodeling in mice via the inhibition of NLRP3 inflammasome expression. Biochemical and Biophysical Research Communications. 2017;485(1):69–75. doi: 10.1016/j.bbrc.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 64.Wen H. L., Liang Z. S., Zhang R., Yang K. Anti-inflammatory effects of triptolide improve left ventricular function in a rat model of diabetic cardiomyopathy. Cardiovascular Diabetology. 2013;12(1):p. 50. doi: 10.1186/1475-2840-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liang Z., Leo S., Wen H., Ouyang M., Jiang W., Yang K. Triptolide improves systolic function and myocardial energy metabolism of diabetic cardiomyopathy in streptozotocin-induced diabetic rats. BMC Cardiovascular Disorders. 2015;15:p. 42. doi: 10.1186/s12872-015-0030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo X., Xue M., Li C. J., et al. Protective effects of triptolide on TLR4 mediated autoimmune and inflammatory response induced myocardial fibrosis in diabetic cardiomyopathy. Journal of Ethnopharmacology. 2016;193:333–344. doi: 10.1016/j.jep.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 67.Soetikno V., Watanabe K., Sari F. R., et al. Curcumin attenuates diabetic nephropathy by inhibiting PKC-α and PKC-β1 activity in streptozotocin-induced type I diabetic rats. Molecular Nutrition & Food Research. 2011;55(11):1655–1665. doi: 10.1002/mnfr.201100080. [DOI] [PubMed] [Google Scholar]
- 68.Soetikno V., Sari F. R., Sukumaran V., et al. Curcumin prevents diabetic cardiomyopathy in streptozotocin-induced diabetic rats: possible involvement of PKC-MAPK signaling pathway. European Journal of Pharmaceutical Sciences. 2012;47(3):604–614. doi: 10.1016/j.ejps.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 69.Pan Y., Wang Y., Zhao Y., et al. Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes. 2014;63(10):3497–3511. doi: 10.2337/db13-1577. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y., Zhou S., Sun W., et al. Inhibition of JNK by novel curcumin analog C66 prevents diabetic cardiomyopathy with a preservation of cardiac metallothionein expression. American Journal of Physiology - Endocrinology and Metabolism. 2014;306(11):E1239–E1247. doi: 10.1152/ajpendo.00629.2013. [DOI] [PubMed] [Google Scholar]
- 71.Fitzl G., Martin R., Dettmer D., Hermsdorf V., Drews H., Welt K. Protective effects of Gingko biloba extract EGb 761 on myocardium of experimentally diabetic rats. I: ultrastructural and biochemical investigation on cardiomyocytes. Experimental and Toxicologic Pathology. 1999;51(3):189–198. doi: 10.1016/S0940-2993(99)80093-1. [DOI] [PubMed] [Google Scholar]
- 72.Welt K., Weiss J., Koch S., Fitzl G. Protective effects of Ginkgo biloba extract EGb 761 on the myocardium of experimentally diabetic rats. II. Ultrastructural and immunohistochemical investigation on microvessels and interstitium. Experimental and Toxicologic Pathology. 1999;51(3):213–222. doi: 10.1016/S0940-2993(99)80099-2. [DOI] [PubMed] [Google Scholar]
- 73.Fitzl G., Welt K., Martin R., et al. The influence of hypoxia on the myocardium of experimentally diabetic rats with and without protection by Ginkgo biloba extract. I. Ultrastructural and biochemical investigations on cardiomyocytes. Experimental and Toxicologic Pathology. 2000;52(5):419–430. doi: 10.1016/S0940-2993(00)80075-5. [DOI] [PubMed] [Google Scholar]
- 74.Welt K., Fitzl G., Schepper A. Experimental hypoxia of STZ-diabetic rat myocardium and protective effects of Ginkgo biloba extract. II. Ultrastructural investigation of microvascular endothelium. Experimental and Toxicologic Pathology. 2001;52(6):503–512. doi: 10.1016/S0940-2993(01)80006-3. [DOI] [PubMed] [Google Scholar]
- 75.Saini A. S., Taliyan R., Sharma P. L. Protective effect and mechanism of Ginkgo biloba extract-EGb 761 on STZ-induced diabetic cardiomyopathy in rats. Pharmacognosy Magazine. 2014;10(38):172–178. doi: 10.4103/0973-1296.131031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su H. C., Hung L. M., Chen J. K. Resveratrol, a red wine antioxidant, possesses an insulin-like effect in streptozotocin-induced diabetic rats. American Journal of Physiology - Endocrinology and Metabolism. 2006;290(6):E1339–E1346. doi: 10.1152/ajpendo.00487.2005. [DOI] [PubMed] [Google Scholar]
- 77.Turan B., Tuncay E., Vassort G. Resveratrol and diabetic cardiac function: focus on recent in vitro and in vivo studies. Journal of Bioenergetics and Biomembranes. 2012;44(2):281–296. doi: 10.1007/s10863-012-9429-0. [DOI] [PubMed] [Google Scholar]
- 78.Penumathsa S. V., Thirunavukkarasu M., Zhan L., et al. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. Journal of Cellular and Molecular Medicine. 2008;12(6A):2350–2361. doi: 10.1111/j.1582-4934.2008.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo S., Yao Q., Ke Z., Chen H., Wu J., Liu C. Resveratrol attenuates high glucose-induced oxidative stress and cardiomyocyte apoptosis through AMPK. Molecular and Cellular Endocrinology. 2015;412:85–94. doi: 10.1016/j.mce.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 80.Yar A. S., Menevse S., Alp E. The effects of resveratrol on cyclooxygenase-1 and -2, nuclear factor kappa beta, matrix metalloproteinase-9, and sirtuin 1 mRNA expression in hearts of streptozotocin-induced diabetic rats. Genetics and Molecular Research. 2011;10(4):2962–2975. doi: 10.4238/2011.November.29.7. [DOI] [PubMed] [Google Scholar]
- 81.Chen W., Li Y. M., Yu M. H. Effects of astragalus polysaccharides on chymase, angiotensin-converting enzyme and angiotensin II in diabetic cardiomyopathy in hamsters. Journal of International Medical Research. 2007;35(6):873–877. doi: 10.1177/147323000703500615. [DOI] [PubMed] [Google Scholar]
- 82.Chen W., Yu M. H., Li Y. M., Chen W. J., Xia Y. P. Beneficial effects of astragalus polysaccharides treatment on cardiac chymase activities and cardiomyopathy in diabetic hamsters. Acta Diabetologica. 2010;47(Supplement 1):35–46. doi: 10.1007/s00592-009-0116-5. [DOI] [PubMed] [Google Scholar]
- 83.Chen W., Li Y. M., Yu M. H. Astragalus polysaccharides inhibited diabetic cardiomyopathy in hamsters depending on suppression of heart chymase activation. Journal of Diabetes and its Complications. 2010;24(3):199–208. doi: 10.1016/j.jdiacomp.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 84.Chen W., Xia Y. P., Chen W. J., Yu M. H., Li Y. M., Ye H. Y. Improvement of myocardial glycolipid metabolic disorder in diabetic hamster with astragalus polysaccharides treatment. Molecular Biology Reports. 2012;39(7):7609–7615. doi: 10.1007/s11033-012-1595-y. [DOI] [PubMed] [Google Scholar]
- 85.Sun S., Yang S., Dai M., et al. The effect of astragalus polysaccharides on attenuation of diabetic cardiomyopathy through inhibiting the extrinsic and intrinsic apoptotic pathways in high glucose -stimulated H9C2 cells. BMC Complementary and Alternative Medicine. 2017;17(1):p. 310. doi: 10.1186/s12906-017-1828-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu J., Fei J., Azad J., Gong M., Lan Y., Chen G. Myocardial protection by Salvia miltiorrhiza injection in streptozotocin-induced diabetic rats through attenuation of expression of thrombospondin-1 and transforming growth factor-β1. Journal of International Medical Research. 2012;40(3):1016–1024. doi: 10.1177/147323001204000320. [DOI] [PubMed] [Google Scholar]
- 87.Lo S. H., Hsu C. T., Niu H. S., Niu C. S., Cheng J. T., Chen Z. C. Cryptotanshinone inhibits STAT3 signaling to alleviate cardiac fibrosis in type 1-like diabetic rats. Phytotherapy Research. 2017;31(4):638–646. doi: 10.1002/ptr.5777. [DOI] [PubMed] [Google Scholar]
- 88.Rani N., Bharti S., Bhatia J., Nag T. C., Ray R., Arya D. S. Chrysin, a PPAR-γ agonist improves myocardial injury in diabetic rats through inhibiting AGE-RAGE mediated oxidative stress and inflammation. Chemico-Biological Interactions. 2016;250:59–67. doi: 10.1016/j.cbi.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 89.Rani N., Bharti S., Bhatia J., et al. Inhibition of TGF-β by a novel PPAR-γ agonist, chrysin, salvages β-receptor stimulated myocardial injury in rats through MAPKs-dependent mechanism. Nutrition & Metabolism. 2015;12:p. 11. doi: 10.1186/s12986-015-0004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang B., Shen Q., Chen Y., et al. Myricitrin alleviates oxidative stress-induced inflammation and apoptosis and protects mice against diabetic cardiomyopathy. Scientific Reports. 2017;7, article 44239 doi: 10.1038/srep44239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mahajan U. B., Chandrayan G., Patil C. R., et al. The protective effect of apigenin on myocardial injury in diabetic rats mediating activation of the PPAR-γ pathway. International Journal of Molecular Sciences. 2017;18(4) doi: 10.3390/ijms18040756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sun X., Chen R. C., Yang Z. H., et al. Taxifolin prevents diabetic cardiomyopathy in vivo and in vitro by inhibition of oxidative stress and cell apoptosis. Food and Chemical Toxicology. 2014;63:221–232. doi: 10.1016/j.fct.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 93.Yu Y., Zheng G. Troxerutin protects against diabetic cardiomyopathy through NF-κB/AKT/IRS1 in a rat model of type 2 diabetes. Molecular Medicine Reports. 2017;15(6):3473–3478. doi: 10.3892/mmr.2017.6456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Agrawal Y. O., Sharma P. K., Shrivastava B., Arya D. S., Goyal S. N. Hesperidin blunts streptozotocin-isoproternol induced myocardial toxicity in rats by altering of PPAR-γ receptor. Chemico-Biological Interactions. 2014;219:211–220. doi: 10.1016/j.cbi.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 95.Zhang N., Yang Z., Xiang S. Z., et al. Nobiletin attenuates cardiac dysfunction, oxidative stress, and inflammatory in streptozotocin: induced diabetic cardiomyopathy. Molecular and Cellular Biochemistry. 2016;417(1-2):87–96. doi: 10.1007/s11010-016-2716-z. [DOI] [PubMed] [Google Scholar]
- 96.Chen J., Guo R., Yan H., et al. Naringin inhibits ROS-activated MAPK pathway in high glucose-induced injuries in H9c2 cardiac cells. Basic & Clinical Pharmacology & Toxicology. 2014;114(4):293–304. doi: 10.1111/bcpt.12153. [DOI] [PubMed] [Google Scholar]
- 97.Zhang Y., Zhang L., Zhang Y., Xu J. J., Sun L. L., Li S. Z. The protective role of liquiritin in high fructose-induced myocardial fibrosis via inhibiting NF-κB and MAPK signaling pathway. Biomedicine & Pharmacotherapy. 2016;84:1337–1349. doi: 10.1016/j.biopha.2016.10.036. [DOI] [PubMed] [Google Scholar]
- 98.Xie X. W. Liquiritigenin attenuates cardiac injury induced by high fructose-feeding through fibrosis and inflammation suppression. Biomedicine & Pharmacotherapy. 2017;86:694–704. doi: 10.1016/j.biopha.2016.12.066. [DOI] [PubMed] [Google Scholar]
- 99.Zhao J., Cao T. T., Tian J., et al. Shengmai san ameliorates myocardial dysfunction and fibrosis in diabetic db/db mice. Evidence-Based Complementary and Alternative Medicine. 2016;2016:9. doi: 10.1155/2016/4621235.4621235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ni Q., Wang J., Li E. Q., et al. Study on the protective effect of the mixture of Shengmai powder and Danshen decoction on the myocardium of diabetic cardiomyopathy in the rat model. Chinese Journal of Integrative Medicine. 2011;17(2):116–125. doi: 10.1007/s11655-011-0639-9. [DOI] [PubMed] [Google Scholar]
- 101.Sen S., Chen S., Wu Y., Feng B., Lui E. K., Chakrabarti S. Preventive effects of North American ginseng (Panax quinquefolius) on diabetic retinopathy and cardiomyopathy. Phytotherapy Research. 2013;27(2):290–298. doi: 10.1002/ptr.4719. [DOI] [PubMed] [Google Scholar]
- 102.Gu J. N., Niu J., Pi Z. F., Yue H., Wu S. S., Liu S. Y. Study on the mechanism of total saponins of panax ginseng in treatment of diabetic cardiomyopathy in rat assessed by urine metabonomics. Chinese Journal of Analytical Chemistry. 2013;41(3):371–376. [Article in Chinese] [Google Scholar]
- 103.Yu H. T., Zhen J., Pang B., Gu J. N., Wu S. S. Ginsenoside Rg1 ameliorates oxidative stress and myocardial apoptosis in streptozotocin-induced diabetic rats. Journal of Zhejiang University-Science B. 2015;16(5):344–354. doi: 10.1631/jzus.B1400204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xu Z., Wang S., Ji H., et al. Broccoli sprout extract prevents diabetic cardiomyopathy via Nrf2 activation in db/db T2DM mice. Science Reports. 2016;6, article 30252 doi: 10.1038/srep30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Z., Zhang D., Dou M., Li Z., Zhang J., Zhao X. Dendrobium officinale Kimura et Migo attenuates diabetic cardiomyopathy through inhibiting oxidative stress, inflammation and fibrosis in streptozotocin-induced mice. Biomedicine & Pharmacotherapy. 2016;84:1350–1358. doi: 10.1016/j.biopha.2016.10.074. [DOI] [PubMed] [Google Scholar]
- 106.Yu W., Zha W., Guo S., Cheng H., Wu J., Liu C. Flos Puerariae extract prevents myocardial apoptosis via attenuation oxidative stress in streptozotocin-induced diabetic mice. PLoS One. 2014;9(5, article e98044) doi: 10.1371/journal.pone.0098044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hou J., Zheng D., Fung G., et al. Mangiferin suppressed advanced glycation end products (AGEs) through NF-κB deactivation and displayed anti-inflammatory effects in streptozotocin and high fat diet-diabetic cardiomyopathy rats. Canadian Journal of Physiology and Pharmacology. 2016;94(3):332–340. doi: 10.1139/cjpp-2015-0073. [DOI] [PubMed] [Google Scholar]
- 108.Xi S., Zhou G., Zhang X., Zhang W., Cai L., Zhao C. Protective effect of total aralosides of Aralia elata (Miq) Seem (TASAES) against diabetic cardiomyopathy in rats during the early stage, and possible mechanisms. Experimental and Molecular Medicine. 2009;41(8):538–547. doi: 10.3858/emm.2009.41.8.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Duan J., Wei G., Guo C., et al. Aralia taibaiensis protects cardiac myocytes against high glucose-induced oxidative stress and apoptosis. The American Journal of Chinese Medicine. 2015;43(6):1159–1175. doi: 10.1142/S0192415X15500664. [DOI] [PubMed] [Google Scholar]
- 110.Chang W., Zhang M., Li J., et al. Berberine improves insulin resistance in cardiomyocytes via activation of 5′-adenosine monophosphate-activated protein kinase. Metabolism. 2013;62(8):1159–1167. doi: 10.1016/j.metabol.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 111.Chang W., Zhang M., Meng Z., et al. Berberine treatment prevents cardiac dysfunction and remodeling through activation of 5′-adenosine monophosphate-activated protein kinase in type 2 diabetic rats and in palmitate-induced hypertrophic H9c2 cells. European Journal of Pharmacology. 2015;769:55–63. doi: 10.1016/j.ejphar.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 112.Shen N., Li X., Zhou T., et al. Shensong Yangxin capsule prevents diabetic myocardial fibrosis by inhibiting TGF-β1/Smad signaling. Journal of Ethnopharmacology. 2014;157:161–170. doi: 10.1016/j.jep.2014.09.035. [DOI] [PubMed] [Google Scholar]