Abstract
Acyclovir, ganciclovir and (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine are active in vitro against the Epstein–Barr virus (EBV) but their in vivo anti-EBV activity is not well understood. We developed a novel, sensitive high-performance liquid chromatography assay with ultraviolet detection for measuring acyclovir, ganciclovir and (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine in human plasma to identify quantitative relationships between in vitro anti-EBV activity and therapeutic response. Characteristics of the assay include a low plasma volume (200 μL), perchloric acid protein precipitation, use of penciclovir as the internal standard, run times less than 8 min and a 50 ng/mL lower limit of quantification. The within- and between-assay variability is 0.7–4.8 and 1.0–7.9%, respectively. Accuracy for all three drugs ranges from 89.5 to 106.4% for four quality controls (50, 100, 1000 and 10,000 ng/mL). This assay supports pharmacokinetic and pharmacodynamic studies of candidate anti-EBV drugs in children and adults with EBV infections.
Keywords: acyclovir, ganciclovir, H2G, chromatography, Epstein–Barr virus
Introduction
Acyclovir, ganciclovir and (R)-9-[4-hydroxy-2-(hydroxymethyl) butyl]guanine (H2G) are guanosine nucleoside analogs with in vitro activity against several members of the herpesvirus group of DNA viruses, including the Epstein–Barr virus (EBV) (Colby et al., 1980; Lin et al., 1984; Lowe et al., 1995). Currently there are no antiviral drugs approved in the United States for the treatment or prevention of EBV infections. However, severe and occasionally life-threatening infections do occur in immunocompetent and immunocompromised individuals, many of whom are EBV-naive children and young adults (Smith et al., 2007; Tattevin et al., 2006). Infectious mononucleosis is a primary EBV infection of otherwise healthy teenagers and young adults that occasionally causes neurologic or hematologic complications, symptomatic hepatitis and rarely, death (Markin et al., 1987; Mroczek et al., 1987). Symptomatic infectious mononucleosis may also produce a permanent scarring of the immune system (Sauce et al., 2006). EBV infections in immunocompromised solid organ and bone marrow transplant recipients can lead to an uncommon but potentially fatal lymphoproliferative syndrome with tissue involvement known as post-transplant lymphoproliferative disorder (PTLD) (Katz et al., 2007; Newell et al., 1996). EBV has also been associated with chronic diseases including nasopharyngeal carcinoma, a subset of Hodgkin’s disease and multiple sclerosis (DeLorenze et al., 2006; Glaser et al., 1997; Pathmanathan et al., 1995). Given the spectrum of clinical syndromes related to EBV, an effective antiviral intervention would be significant.
Acyclovir, ganciclovir and the oral prodrugs valacyclovir and valganciclovir have been used in the treatment and prevention of EBV-related diseases including infectious mononucleosis and PTLD (Balfour et al., 2007; Funch et al., 2005; Humar et al., 2006; Torre and Tambini, 1999). In addition, EPB 348, an investigational oral prodrug of H2G, is being evaluated at our institution for the treatment of infectious mononucleosis. The success of antiviral interventions studied to date is highly variable. This is due in part to a poor understanding of the in vivo anti-EBV activity of candidate antiviral drugs. Knowledge of plasma drug exposure after the administration of a parent antiviral compound or an oral prodrug could help identify quantitative relationships between in vitro anti-EBV activity and therapeutic response. Such investigations first require a high-quality analytical method for measuring candidate anti-EBV drugs in plasma. Several methods for the simultaneous determination of acyclovir and ganciclovir have been developed (Kishino et al., 2002; McMullin et al., 1996; Perrottet et al., 2007; Teshima et al., 2003). One method also includes penciclovir, a guanosine nucleoside analog with in vitro anti-EBV activity comparable to that of acyclovir (Bacon and Boyd, 1995; Dao et al., 2008). However, there are no reports of a multi-drug assay that quantifies H2G in addition to acyclovir and ganciclovir.
We describe a novel high-performance liquid chromatography (HPLC) assay with ultraviolet (UV) detection for measuring acyclovir, ganciclovir and H2G in human plasma. The method involves a sample volume of 200 μL, making it suitable for pediatrics, sample preparation by perchloric acid protein precipitation, selection of penciclovir as the internal standard, run times less than 8 min, and a lower limit of quantification (LLOQ) of 50 ng/mL. It is more sensitive and has a shorter run time than other multi-drug HPLC-UV assays (McMullin et al., 1996; Teshima et al., 2003). Our method also has a unique application supporting pharmacokinetic and pharmacodynamic studies of valacyclovir, valganciclovir and EPB 348 in individuals with EBV infections.
Experimental
Chemicals and Reagents
Acyclovir, ganciclovir, perchloric acid, potassium phosphate monobasic, sodium carbonate and sodium hydroxide were purchased from Sigma-Aldrich (St Louis, MO, USA). H2G was a gift from Epiphany Biosciences Inc. (San Francisco, CA, USA). Penciclovir was selected as the internal standard and was kindly provided by Novartis International Pharmaceutical Ltd. (Ringaskiddy, Co. Cork, Ireland). Acetonitrile and methyl alcohol were HPLC-grade and, along with sodium phosphate, were obtained from Fisher Scientific (Fair Lawn, NJ, USA). EDTA-derived human plasma was purchased from Biological Specialty Corporation (Colmar, PA, USA).
Chromatography and Extraction Equipment and Supplies
The HPLC system was a series 1100 HP consisting of a G1322A quaternary pump, G1322A degasser, G1330A thermostatic control, G1316A column heater, G1329A autosampler and a G1314A UV detector (Agilent Technologies, Palo Alto, CA, USA). The HPLC column was an S-3, 3.0 × 150 mm YMC-Pack C8 column (Waters Corporation, Milford, MA, USA). Ultraviolet scanning was carried out using a Cary 50 Bio, UV–vis spectrophotometer (Varian Instruments, Walnut Creek, CA, USA). The sample preparation and extraction procedures included the use of Beckman model J-6B and Beckman model CS-15R centrifuges (Beckman Coulter Inc., Fullerton, CA, USA) and a Zymark model Turbo Vap LV nitrogen evaporator (Zymark Corp., Hopkinton, MA, USA). Data acquisition and analysis were accomplished with the software program Chrom Perfect® Spirit™, version 5.1.0 (Justice Innovations, Denville, NJ, USA).
Standard, Quality Control and Internal Standard Preparation
Individual 0.4 mg/mL stock solutions of acyclovir, ganciclovir and H2G were prepared separately in deionized water. Working standards for each drug were prepared directly in human plasma by dilution of the stock solutions. The high standard (20,000 ng/mL) was prepared by adding 1.25 mL of each of the stock solutions to a volumetric flask and diluting to 25 mL with plasma. Ten milliliter volumes of the 50, 200, 800, 2000 and 8000 ng/mL standards were prepared by adding 25 μl, 100 μl, 400 μl, 1 mL and 4 mL, respectively, of the 20,000 ng/mL standard to volumetric flasks and diluting to 10 mL with plasma.
Primary quality control stock solutions of each drug at 0.4 mg/mL in deionized water were prepared as described above by separate weighings. These solutions when diluted directly in human plasma to a concentration of 10,000 ng/mL resulted in the high quality control (QCH). The QCH was non-serially diluted in plasma to make concentrations equal to the quality control at the lowest standard (QCLLOQ), the low quality control (QCL), and the medium quality control (QCM), at final concentrations of 50, 100 and 1000 ng/mL, respectively. These four quality controls were assayed in triplicate on five occasions to determine within-and between-assay performance for accuracy and variability. The QCLLOQ was used to evaluate accuracy and variability at the concentration of the lowest standard, defining it as the LLOQ for this assay.
A primary stock solution of the internal standard, penciclovir, was prepared at 1 mg/mL in methanol. This stock solution was further diluted in deionized water to achieve a working concentration of 20,000 ng/mL.
Sample Preparation and Protein Precipitation Procedure
All standards, quality controls, plasma, and reagents were allowed to warm to room temperature (23°C) prior to extraction. Standards (200 μL in duplicate) and the quality controls (200 μL in triplicate) were added to 1.5 mL centrifuge tubes. A 100 μL volume of the internal standard working solution was added to all tubes except the plasma blanks, which received 100 μL of distilled water. A 50 μL volume of 22.4% perchloric acid solution was added to all tubes. All samples were capped and vortexed for 5 s. The samples were placed in the centrifuge at 4°C for 10 min then spun at 10,574 rpm for 5 min. Samples were removed from the centrifuge and a 170 μL volume of the supernatant was added to tubes containing 50 μL of 2 M sodium carbonate. These tubes were vortexed briefly and the contents transferred to an auto-sampler vial for analysis. The injection volume was 25 μL.
Chromatography Conditions
The mobile phase consisted of 97% 25 mM monobasic potassium phosphate (pH 6.0) and 3.0% acetonitrile, v/v. The stationary phase was an S-3, 3.0 mm × 150 mm YMC-Pack C8 column protected by the addition of a 0.5 μm pre-column filter apparatus. Column temperature was maintained at 35°C. The mobile phase flow rate was 0.4 mL/min. Ultraviolet scans were performed in mobile phase at a pH of 6.0 from 200 to 300 nm. The highest peak absorption for all three drugs and the internal standard was at 250 nm. Data were acquired at this wavelength over 8 min for each injection. The injection timing was set at 9 min intervals.
Assay Validation, Specificity and Stability
Assay validation was conducted in accordance with the United States Food and Drug Administration’s guidance for industry (2001). Standard curves for acyclovir, ganciclovir and H2G were calculated using peak height ratios with the internal standard and were linear fit with 1/x2 weighting. Variability and accuracy were calculated for the four quality controls (50, 100, 1000 and 10,000 ng/mL). These values were analyzed by a single factor analysis of variance (ANOVA) to determine accuracy as well as within- and between-assay variability. All variability was expressed as the coefficient of variation (CV%). The limit of detection (LOD) for each drug was calculated using a signal-to-noise ratio of 3:1. The value for background noise was measured in the plasma blank at the corresponding retention time window.
Extraction recovery was determined for acyclovir, ganciclovir, H2G and the internal standard at the four quality controls. Plasma and deionized water were spiked with 50, 100, 1000 and 10,000 ng/mL concentrations. The plasma samples were extracted in triplicate according to the protein precipitation procedure described previously. Unextracted water samples, also prepared in triplicate, were subjected to the same procedure however the extraction reagents were replaced with deionized water. Mean peak heights for each concentration were used to determine percent recovery, which was calculated as (mean extracted/mean unextracted) × 100.
Several medications commonly used in immunocompromised transplant patients and patients with malignancies were tested for co-elution with acyclovir, ganciclovir and H2G including cyclosporine, fluorouracil, fluconazole, gemcitabine, mycophenolic acid and sulfamethoxazole. Stock solutions of these drugs in methanol were diluted in mobile phase and injected directly onto the system.
The stability of acyclovir, ganciclovir and H2G in plasma was tested under three different laboratory conditions. Separate aliquots of the QCL (100 ng/mL) and the QCM (1000 ng/mL) were analyzed in triplicate to determine baseline concentrations. Additional aliquots of the QCL and the QCM were subjected to each laboratory condition and run in triplicate on a single assay. Under the first condition, aliquots were thawed, vortexed, stored at 4°C for 7 days, and refrozen. Under the second condition, aliquots were thawed, vortexed, and kept at room temperature (23°C) for 24 h prior to refreezing. Finally, to determine freeze–thaw stability, aliquots were removed from the freezer, thawed at room temperature (23°C) for no more than 2 h, vortexed briefly and refrozen. This process was repeated daily for 5 days. All of the samples exposed to the conditions described above were analyzed on the fifth validation assay. Stability was evaluated as a percentage of mean baseline QCL and QCM concentrations.
Clinical Application
This HPLC-UV assay is used to analyze samples collected from immunocompetent and immunocompromised individuals with EBV infections participating in pharmacokinetic and pharmacodynamic studies of acyclovir, ganciclovir or H2G, following the administration of the oral prodrugs valacyclovir, valganciclovir or EPB 348. Research protocols were approved by the Institutional Review Board at the University of Minnesota and written informed consent was obtained from all subjects or their guardians prior to enrollment. Timed post-dose blood samples were collected, centrifuged within 1 h of collection, and the resulting plasma separated and frozen at −80°C until analysis. Sample chromatograms from two representative subjects who completed pharmacokinetic studies of valacyclovir when used to treat EBV infectious mononucleosis and valganciclovir when used to prevent EBV-associated PTLD were available. A blinded study of EPB 348 for EBV infectious mononucleosis is ongoing.
Results
Chromatography
An overlay of two chromatograms from injections of blank human plasma and a 2000 ng/mL standard is shown in Fig. 1. The retention times for the peak heights corresponding to ganciclovir, acyclovir, penciclovir and H2G were 3.91, 4.86, 6.48 and 7.32 min, respectively. No significant interfering plasma peaks were found in the blank extraction. The mean (CV%) retention times for ganciclovir, acyclovir, penciclovir and H2G at this standard for the five validation runs were 3.89 (0.55%), 4.83 (0.69%), 6.42 (0.91%) and 7.24 (1.03%) min, respectively. The retention times for the internal standard and for all drugs at the 50, 200, 800 and 20,000 ng/mL standards were also consistent for the five validations runs (data not shown).
Figure 1.
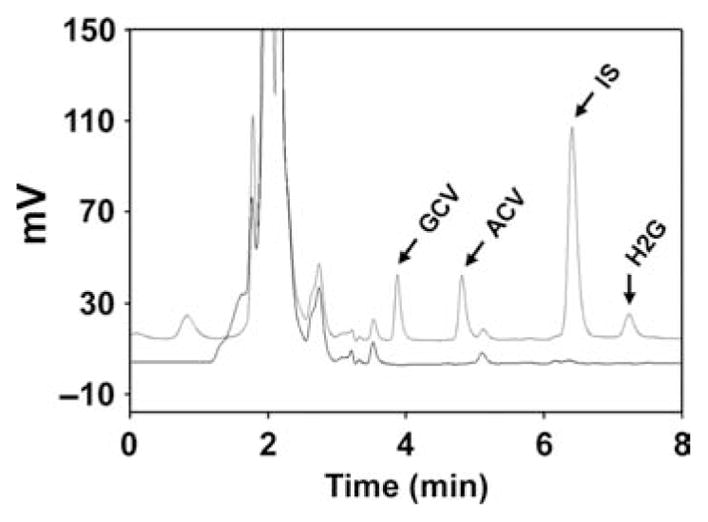
Representative chromatograms of blank human plasma (lower) and a 2000 ng/mL standard (upper). GCV, ganciclovir; ACV, acyclovir; IS, internal standard; H2G, (R)-9-[4-hydroxy-2- (hydroxymethyl)butyl]guanine.
Validation, Selectivity and Stability
The assay was linear with mean (±standard deviation) coefficients of determination for the acyclovir, ganciclovir and H2G standard curves of 0.9924 (±0.0007), 0.9927 (±0.0005) and 0.9936 (±0.0028), respectively. The mean (±standard deviation) slope and intercept from five validation runs were 0.1522 (±0.0044) and 0.7903 (±0.9112), respectively, for acyclovir, 0.1575 (±0.0068) and 0.8324 (±0.8361), respectively, for ganciclovir, and 0.5371 (±0.0075) and 0.6131 (±0.6612), respectively, for H2G. The ANOVA performed on the four quality controls from five validation runs resulted in ten and four degrees of freedom for within- and between-assay comparisons, respectively. Results for accuracy and assay variability are shown in Table 1. Within assay variability ranged from 0.7 to 4.8% and between assay variability ranged from 1.0 to 7.9%. Overall accuracy was determined by using the grand mean of each calculated concentration, and ranged from 89.5 to 106.4% for all target concentrations for all three drugs. The LOD was 4, 3 and 7 ng/mL for acyclovir, ganciclovir and H2G, respectively. The mean extraction recoveries of all three drugs and the internal standard ranged from 82.0 to 102.5% for the four quality controls.
Table 1.
Assay accuracy and variability results for acyclovir, ganciclovir, and H2G from five validation runs
| Quality control (ng/mL) | Acyclovir (%) | Ganciclovir (%) | H2G (%) |
|---|---|---|---|
| Accuracy | |||
| 50 | 106.4 | 99.0 | 102.0 |
| 100 | 100.5 | 92.1 | 94.1 |
| 1000 | 91.7 | 89.5 | 92.5 |
| 10,000 | 92.2 | 92.5 | 93.6 |
| Within assay variability | |||
| 50 | 4.8 | 1.6 | 4.8 |
| 100 | 2.6 | 1.9 | 2.6 |
| 1000 | 1.7 | 1.6 | 2.0 |
| 10,000 | 0.8 | 1.0 | 0.7 |
| Between assay variability | |||
| 50 | 7.9 | 4.5 | 3.7 |
| 100 | 4.7 | 2.8 | 1.9 |
| 1000 | 2.3 | 2.7 | 2.7 |
| 10,000 | 1.1 | 1.6 | 1.0 |
CV, coefficient of variation.
Four of the six drugs tested for assay interference, cyclosporine, fluconazole, mycophenolic acid and sulfamethoxazole, did not elute during acquisition. Fluorouracil and gemcitabine had retention times of 3.31 and 5.53 min, respectively, and did not co-elute with assay components.
Mean and standard deviation results for the QCL and QCM at baseline and following stability testing are shown in Table 2. The percent of baseline ranged from 87.9 to 104.8%. This range was within ±15%, indicating that there were minimal changes in the quality controls under the three laboratory conditions tested.
Table 2.
Mean ± standard deviation (percentage of baseline) stability results for acyclovir, ganciclovir and H2G
| Acyclovir (ng/mL) | Ganciclovir (ng/mL) | H2G (ng/mL) | |
|---|---|---|---|
| Baseline QCL | 105.9 ± 3.5 | 94.5 ± 2.9 | 92.4 ± 7.8 |
| 4°C × 7 days | 99.3 ± 3.5 (93.8) | 94.7 ± 1.0 (100.2) | 97.2 ± 1.7 (105.2) |
| 23°C × 24 h | 96.2 ± 1.8 (90.8) | 93.1 ± 2.1 (98.5) | 96.6 ± 3.8 (104.6) |
| Five freeze–thaw cycles | 93.1 ± 5.6 (87.9) | 92.9 ± 2.0 (98.3) | 96.8 ± 7.4 (104.8) |
| Baseline QCM | 944 ± 25.4 | 932 ± 18.5 | 958 ± 14.2 |
| 4°C × 7 days | 969 ± 8.3 (102.6) | 949 ± 9.5 (101.8) | 999 ± 0.8 (104.3) |
| 23°C × 24 h | 975 ± 3.1 (103.3) | 947 ± 1.9 (101.6) | 984 ± 5.4 (102.7) |
| Five freeze–thaw cycles | 973 ± 2.4 (103.0) | 947 ± 4.1 (101.6) | 983 ± 3.7 (102.6) |
QCL, low quality control. QCM, medium quality control.
Clinical Application
A 12 h post-dose chromatogram for acyclovir from an otherwise healthy young adult receiving valacyclovir 1500 mg every 12 h for the treatment of antibody-confirmed EBV infectious mononucleosis is shown in Fig. 2. Similarly, a 12 h post-dose chromatogram for ganciclovir from a pediatric kidney transplant patient with stabile renal function taking 225 mg of valganciclovir suspension every 12 h for secondary prophylaxis against EBV-associated PTLD is shown in Fig. 3. The acyclovir and ganciclovir plasma trough concentrations associated with these chromatograms were 556 and 2206 ng/mL, respectively. No interfering peaks from either endogenous substances or, in the case of ganciclovir, concomitant medications (prednisolone, omeprazole and dapsone) were observed.
Figure 2.

A 12-hour post-dse chromatogram for acyclovir from a young adult receiving valacyclovir 1500 mg every 12 hour for EBV infectious mononucleosis. ACV, acyclovir; IS, internal standard.
Figure 3.
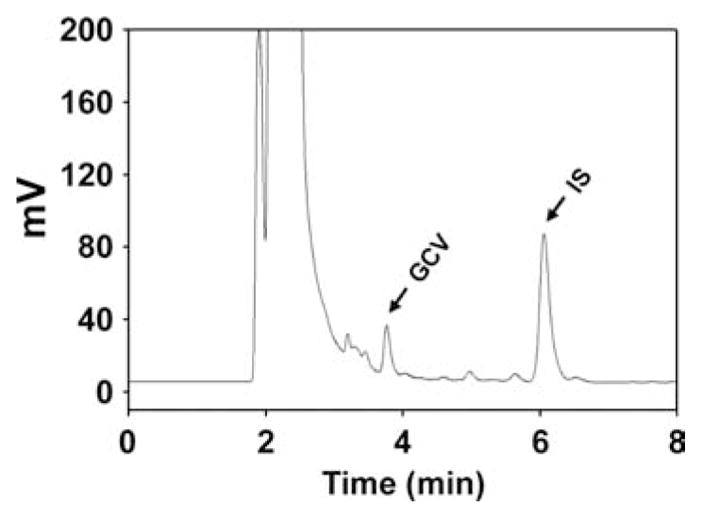
A 12-hour post-dse chromatogram for ganciclovir from a pediatric kidney transplant patient taking 225 mg of valganciclovir suspension every 12 hours for secondary prophylaxis of EBV-associated PTLD. GCV, ganciclovir; IS, internal standard.
Discussion and Conclusion
A high-quality analytical method for measuring candidate anti-EBV drugs in plasma is essential for conducting pharmacokinetic and pharmacodynamic studies that aim to identify quantitative relationships between in vitro anti-EBV activity and therapeutic response. The guanosine nucleoside analogs acyclovir, ganciclovir and H2G have in vitro activity against EBV with reported IC50 values of 0.3, 0.05 and 0.9 μM, respectively (Lin et al., 1984; Lowe et al., 1995). Several analytical methods for the simultaneous determination of acyclovir and ganciclovir in serum or plasma have been developed (Dao et al., 2008; Kishino et al., 2002; McMullin et al., 1996; Perrottet et al., 2007; Teshima et al., 2003). However, we report the first multi-drug plasma assay that measures H2G in addition to acyclovir and ganciclovir.
Our HPLC-UV assay compares favorably with other methods in the areas of accuracy and variability (Dao et al., 2008; Kishino et al., 2002; McMullin et al., 1996; Perrottet et al., 2007; Teshima et al., 2003). However, it is more sensitive and has a shorter run time than alternative HPLC-UV assays (McMullin et al., 1996; Teshima et al., 2003). We chose to use UV detection instead of fluorescence or pulsed amperometric detection in this assay for several reasons (Dao et al., 2008; Kishino et al., 2002; Perrottet et al., 2007). Ultraviolet scanning revealed good absorption at 250 nm for acyclovir, ganciclovir, H2G and the internal standard. This wavelength and our selection of a YMC C8 column (3 μm, 3.0 × 150 mm) minimized the number of pH and mobile phase adjustments needed to separate all three drugs and the internal standard, and to achieve good resolution in the presence of endogenous substances common in human plasma. Fluorescence detection has been used to measure acyclovir and ganciclovir because these compounds fluoresce at a pH less than 3.0 (Dao et al., 2008; Perrottet et al., 2007). However, a pH of 6.0 as used in our assay is less harsh compared with these acidic conditions and probably extends both column and equipment life. Furthermore, preliminary work in our laboratory with fluorescence scanning revealed no major advantage over UV detection for this plasma assay. Ultraviolet detection is also widely available and fairly inexpensive, making this an assay that can be easily adapted to other laboratory settings.
Sample preparation for the assay involves a perchloric acid protein precipitation. This procedure is simple, rapid, and less costly than liquid–liquid or solid-phase extractions (Kishino et al., 2002). Sodium carbonate is used to adjust the pH of the protein precipitate supernatant so that it more closely resembles mobile phase conditions. This salt was selected because of its high solubility, thereby maintaining assay sensitivity and sample integrity. Penciclovir was chosen as the internal standard because it is not an endogenous compound and it is structurally similar to acyclovir, ganciclovir and H2G. It also elutes before H2G, keeping run times under 8 min. Even though penciclovir is a guanosine nucleoside analog, it is not advantageous as a candidate anti-EBV drug because its in vitro anti-EBV activity is analogous to that of acyclovir (Bacon and Boyd, 1995).
Our analytical method performed well with representative clinical research samples collected from an otherwise healthy young adult taking valacyclovir for EBV infectious mononucleosis, and a pediatric kidney transplant recipient receiving valganciclovir for secondary prevention of EBV-PTLD. The dynamic range of the assay is from 50 to 20,000 ng/mL, making it well suited to evaluate both exposure-efficacy and exposure-toxicity relationships for candidate anti-EBV drugs. The assay uses 200 μL of plasma and an injection volume of 25 μL to achieve a LLOQ of 50 ng/mL, making it especially useful for pediatric patients or in other clinical situations where sample volume may be limited. Alternatively, the volume could be scaled down if the assay was modified for use in small animal research.
In summary, we describe a novel HPLC-UV assay for the simultaneous determination of acyclovir, ganciclovir and H2G in human plasma. The assay is simple, sensitive and specific, with high accuracy and low variability. This method supports pharmacokinetic and pharmacodynamic studies of valacyclovir, valganciclovir and EPB 348 in individuals with EBV infections.
Acknowledgments
Contract/grant sponsor: National Center for Research Resources, National Institutes of Health K12-RR023247.
Contract/grant sponsor: National Center for Research Resources, National Institutes of Health M01-RR00400.
Contract/grant sponsor: University of Minnesota International Center for Antiviral Research and Epidemiology (I CARE).
We thank Brent Williams and Mark N. Kirstein, Pharm.D. for their assistance with graphics.
Abbreviations used
- EBV
Epstein–Barr virus
- H2G
(R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine
- PTLD
post-transplant lymphoproliferative disorder
References
- Bacon TH, Boyd MR. Activity of penciclovir against Epstein-Barr virus. Antimicrobial Agents and Chemotherapy. 1995;39:1599–1602. doi: 10.1128/aac.39.7.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour HH, Jr, Hokanson KM, Schacherer RM, Fietzer CM, Schmeling DO, Holman CJ, Vezina HE, Brundage RC. A virologic pilot study of valacyclovir in infectious mononucleosis. Journal of Clinical Virology. 2007;39:16–21. doi: 10.1016/j.jcv.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Colby BM, Shaw JE, Elion GB, Pagano JS. Effect of acyclovir [9-(2-hydroxyethoxymethyl)guanine] on Epstein–Barr virus DNA replication. Journal of Virology. 1980;34:560–568. doi: 10.1128/jvi.34.2.560-568.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao YJ, Jiao Z, Zhong MK. Simultaneous determination of acyclovir, ganciclovir, and penciclovir in human plasma by high-performance liquid chromatography with fluorescence detection. Journal of Chromatography B. 2008;867:270–276. doi: 10.1016/j.jchromb.2008.04.022. [DOI] [PubMed] [Google Scholar]
- DeLorenze GN, Munger KL, Lennette ET, Orentreich N, Vogelman JH, Ascherio A. Epstein-Barr virus and multiple sclerosis: evidence of association from a prospective study with long-term follow-up. Archives of Neurology. 2006;63:839–844. doi: 10.1001/archneur.63.6.noc50328. [DOI] [PubMed] [Google Scholar]
- Funch DP, Walker AM, Schneider G, Ziyadeh NJ, Pescovitz MD. Ganciclovir and acyclovir reduce the risk of post-transplant lymphoproliferative disorder in renal transplant recipients. American Journal of Transplantation. 2005;5:2894–2900. doi: 10.1111/j.1600-6143.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, Pallesen G, Gulley ML, Khan G, O’Grady J, Hummel M, Preciado MV, Knecht H, Chan JK, Claviez A. Epstein-Barr virus-associated Hodgkin’s disease: epidemiologic characteristics in international data. International Journal of Cancer. 1997;70:375–382. doi: 10.1002/(sici)1097-0215(19970207)70:4<375::aid-ijc1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Humar A, Hebert D, Davies HD, Humar A, Stephens D, O’Doherty B, Allen U. A randomized trial of ganciclovir versus ganciclovir plus immune globulin for prophylaxis against Epstein–Barr virus related postransplant lymphoproliferative disorder. Transplantation. 2006;81:856–861. doi: 10.1097/01.tp.0000202724.07714.a2. [DOI] [PubMed] [Google Scholar]
- Katz BZ, Pahl E, Crawford SE, Kostyk MC, Rodgers S, Seshadri R, Proytcheva M, Pophal S. Case–control study of risk factors for the development of post-transplant lymphoproliferative disease in a pediatyric heart transplant cohort. Pediatric Transplantation. 2007;11:58–65. doi: 10.1111/j.1399-3046.2006.00609.x. [DOI] [PubMed] [Google Scholar]
- Kishino S, Takekuma Y, Sugawara M, Shimamura T, Furukawa H, Todo S, Miyazaki K. Liquid chromatographic method for the determination of ganciclovir and/or acyclovir in human plasma using pulsed amperometric detection. Journal of Chromatography B. 2002;780:289–294. doi: 10.1016/s1570-0232(02)00538-x. [DOI] [PubMed] [Google Scholar]
- Lin JC, Smith MC, Pagano JS. Prolonged inhibitory effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine against replication of Epstein–Barr virus. Journal of Virology. 1984;50:50–55. doi: 10.1128/jvi.50.1.50-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe DM, Alderton WK, Ellis MR, Parmar V, Miller WH, Roberts GB, Fyfe JA, Gaillard R, Ertl P, Snowden W, Littler E. Mode of action of (R)-9-[4-hydroxy-2-(hydroxymethyl)butyl]guanine against herpesviruses. Antimicrobial Agents and Chemotherapy. 1995;39:1802–1808. doi: 10.1128/aac.39.8.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markin RS, Linder J, Zuerlein K, Mroczek E, Grierson HL, Brichacek B, Purtilo DT. Hepatitis in fatal infectious mononucleosis. Gastroenterology. 1987;93:1210–1217. doi: 10.1016/0016-5085(87)90246-0. [DOI] [PubMed] [Google Scholar]
- McMullin CM, Kirk B, Sunderland J, White LO, Reeves DS, MacGowan AP. A simple high performance liquid chromatography (HPLC) assay for aciclovir and ganciclovir in serum. Journal of Antimicrobial Chemotherapy. 1996;38:739–740. doi: 10.1093/jac/38.4.739. [DOI] [PubMed] [Google Scholar]
- Mroczek EC, Weisenburger DD, Grierosn HL, Markin R, Purtilo DT. Fatal infectious mononucleosis and virus-associated hemophagocytic syndrome. Archives of Pathology and Laboratory Medicine. 1987;111:530–535. [PubMed] [Google Scholar]
- Newell KA, Alonso EM, Whitington PF, Bruce DS, Millis JM, Piper JB, Woodle ES, Kelly SM, Koeppen H, Hart J, Rubin CM, Thistlethwaite JR., Jr Posttransplant lymphoproliferative disease in pediatric liver transplantation. Interplay between primary Epstein-Barr virus infection and immunosuppression. Transplantation. 1996;62:370–375. doi: 10.1097/00007890-199608150-00012. [DOI] [PubMed] [Google Scholar]
- Pathmanathan R, Prasad U, Chandrika G, Sadler R, Flynn K, Raab-Traub N. Undifferentiated, nonkeratinizing, and squamous cell carcinoma of the nasopharynx. Variants of Epstein–Barr virus-infected neoplasia. American Journal of Pathology. 1995;146:1355–1367. [PMC free article] [PubMed] [Google Scholar]
- Perrottet N, Beguin A, Meylan P, Pascual M, Manuel O, Buclin T, Biollaz J, Decosterd LA. Determination of aciclovir and ganciclovir in human plasma by liquid chromatography–spectrofluorimetric detection and stability studies in blood samples. Journal of Chromatography B. 2007;852:420–429. doi: 10.1016/j.jchromb.2007.01.045. [DOI] [PubMed] [Google Scholar]
- Sauce D, Larsen M, Curnow SJ, Leese AM, Moss PAH, Hislop AD, Salmon M, Rickinson AB. EBV-associated mononucleosis leads to a long-term global deficit in T-cell responsiveness to IL-15. Blood. 2006;108:11–18. doi: 10.1182/blood-2006-01-0144. [DOI] [PubMed] [Google Scholar]
- Smith JM, Corey L, Healey PJ, Davis CL, McDonald RA. Adolescents are more likely to develop posttransplant lymphoproliferative disorder after primary Epstein–Barr virus infection than younger renal transplant recipients. Transplantation. 2007;83:1423–1428. doi: 10.1097/01.tp.0000265914.16491.7d. [DOI] [PubMed] [Google Scholar]
- Tattevin P, Le Tulzo Y, Minjolle S, Person A, Chapplain JM, Arvieux C, Thomas R, Michelet C. Increasing incidence of severe Epstein–Barr virus-related infectious mononucleosis: surveillance study. Journal of Clinical Microbiology. 2006;44:1873–1874. doi: 10.1128/JCM.44.5.1873-1874.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima D, Otsubo K, Yoshida T, Itoh Y, Oishi R. A simple and simultaneous determination of acyclovir and ganciclovir in human plasma by high-performance liquid chromatography. Biomedical Chromatography. 2003;17:500–503. doi: 10.1002/bmc.258. [DOI] [PubMed] [Google Scholar]
- Torre D, Tambini R. Acyclovir for treatment of infectious mononucleosis: a meta-analysis. Scandinavian Journal of Infectious Diseases. 1999;31:543–547. doi: 10.1080/00365549950164409. [DOI] [PubMed] [Google Scholar]
- United States Department of Health and Human Services Food and Drug Administration. [accessed 6 October 2008];Guidance for Industry: Bioanalytical Method Validation. 2001 May; http://www.fda.gov/cder/guidance/4252fnl.htm.


