Abstract
Coronary artery disease (CAD) is a multifactorial disease whose prevalence remains unabated especially in developing countries. Both lifestyle factors and genetic predisposition contribute to this disorder. Though notable achievements have been made in the medical, interventional and surgical management of CAD, the need for its prevention is more important. Among other modalities, this calls for defining evidence-based new biomarkers, which on their own or in combination with other known biomarkers may predict the risk of CAD to enable institution of appropriate preventive strategies. In the present communication, we have discussed the usefulness of shortening of telomeres as a potential biomarker of CAD. Clinical research evidence in favour of telomere shortening in CAD is well documented in different ethnic populations of the world. Establishing a well-standardized and accurate method of evaluating telomere length is essential before its routine use in preventive cardiology.
Keywords: Ageing, Biomarker, coronary artery disease, shortening, telomerase, telomere
Introduction
Cardiovascular disease (CVD) in general and coronary artery disease (CAD) in particular is a leading cause of death and disability1,2. According to the statistics released by the World Health Organization in early 2015, an estimated 17.5 million people died from CVD in 2012, representing 31 per cent of all global deaths2. Of these deaths, an estimated 7.4 million were due to coronary heart disease and 6.7 million were due to stroke. Over three-quarters of CVD deaths take place in low- and middle-income countries2. The largest increase in the number of deaths due to CAD and stroke is expected to occur in the south-East Asia region by 20301. Physical inactivity, unhealthy diet, tobacco use and lifestyle disorders such as type 2 diabetes mellitus (T2DM) and hypertension are known risk factors, raised levels of serum low-density lipoprotein (LDL) cholesterol, triglyceride (TG), lipoprotein(a), high sensitivity C-reactive protein (hs-CRP) and homocysteine are some of the recognized biomarkers of CAD3,4. However, none of these explain the total burden of CAD in a population. Therefore, search for potential biomarkers for early diagnosis and treatment of the disorder either independently or in combination with other markers continues to be an important goal of researches in preventive cardiology. With reference to a spate of research on telomere length (TL) in various disorders, recent population studies reported shortening of telomeres in patients affected with CAD5,6,7,8,9. This interesting finding prompted us to present the case of ‘telomere shortening’ as a potential biomarker of CAD in the present communication.
Telomere biology
Telomeres are terminal ends of linear chromosomes, which in all vertebrates consist of tandem repeats of ‘TTAGGG’ sequences and associated proteins. These form a cap and thereby protect chromosomes from double strand breaks, fusion, recombination and degradation5. Telomeres also play a role in the organization of the cellular nucleus. Their structure allows the end of linear DNA to be replicated completely. The length of telomeres varies among different species. Human telomeres are 8-14 kbp long. The telomeric DNA consists of noncoding tandemly repetitive sequences, with the exact replicate sequence varying from one species to the other. DNA polymerases replicate only in a 5’ 3’ direction by extending existing polynucleotide chain. The leading and the lagging DNA strands follow a different mechanism of DNA replication. DNA fragments, termed Okazaki fragments are formed to replicate the lagging strand by means of DNA polymerase elongating several RNA primers in succession. DNA sequences finally replace the RNA primers. The gap formed due to the removal of the terminal RNA primer on the lagging strand is filled in by extension of the next Okazaki fragment. Because there is no template for the ‘last’ Okazaki fragment beyond the 5’ end of the chromosome, one strand cannot be synthesized till the extreme end. This end replication problem causes a progressive decrease of chromosomal DNA at the 3’ ends as cell cycle progresses5. Although many questions still need answers, chromosomes must be processed and refolded into a stable structure before cells can proceed to the next step in the cell cycle. Free chromosome ends such as single-strand ends or blunt ends trigger a DNA damage response resolved by recruitment of DNA repair proteins. The need to remodel free ends into functional telomeres following replication requires efficient DNA damage responses and DNA repair reactions. Depending on the magnitude and the efficiency of DNA damage responses in a particular cell, these chromosome ends are at risk for other chromosomal abnormalities. Eukaryotic cells have a limited potential for growth in primary cultures and undergo senescence after a defined number of cell divisions which forms the basis for cellular ageing6,7. Tumour cells, however, exhibit unlimited growth and proliferation capability leading to cellular immortalization. There appears to be a relationship between telomere maintenance, telomerase expression and extension of cellular lifespan in mammalian cells8,9. Telomere shortening occurs with repeated cell divisions and, when the length is reduced to a critical point, the resulting genomic instability leads to further genetic abnormality that in most of the cells promotes cellular death or apoptosis, a hallmark of cellular ageing10. Telomere shortening is a mechanism that prevents replication error that would cause mutations in DNA. Once the telomeres are shortened, due to the multiple cell divisions, it will no longer divide. This replicative senescence is also termed as Hayflick limit or Mortality Stage I10. Telomerase, the TL modulator ribonucleoprotein, is ordinarily inactive in differentiated somatic cells, but its activity can be detected in tumour cells. The telomerase activation in malignant cancers is an imperative step in tumorigenesis. The cells gain the capability of indefinite propagation to become immortal8.
The primary role of telomerase seems to be telomere lengthening. Alteration in telomerase expression is associated with many degenerative diseases, ageing and cancer-related functions. Other than telomere maintenance in the nucleus, several studies have shown that telomerase may also have extracurricular activities11,12, including sensitizing cells to oxidative damage leading to apoptosis in mitochondria, DNA repair, regulation of gene expression, chromatin organization and cell growth. It also has a role in stem cell maintenance and modulation of Wnt signaling11,12. These activities appear to require TERT (telomerase reverse transcriptase) instead of TERC (telomerase RNA component) and are thus free of typical telomerase activity. In the mitochondria, telomerase shows RNA-dependent DNA polymerase activity that is independently of TERC and uses tRNA as a template. Telomerase plays a role in the regulation of apoptosis12. It regulates gene expression by small interfering RNA (siRNA). Telomerase sensitizes the DNA of mitochondria to hydrogen peroxide (H2O2), which causes oxidative damage to mitochondrial (mt)-DNA12. Increased levels of oxidatively modified proteins and lipoproteins, e.g. oxidized LDL and lipid peroxidation products, have been seen in people with CAD13. Association of shorter telomeres with CVD could be a result of such an extracurricular activity of telomerase.
In addition to telomerase, a large number of factors help maintain telomere integrity. Shelterin proteins recruit a host of other factors to the telomere including PinX1. Overexpression of PinX1 results in decreased telomerase activity, telomere shortening and induction of crisis11. A strong association was found between PinX1 and coronary intima media thickness in a large genome wide study14.
Factors affecting telomere length in coronary artery disease
Telomeres shortening can often be caused by adopting an unhealthy lifestyle. Ageing, degenerative disease and stress are potential regulators of TL. As with the ageing process, cardiovascular disorders, most notably atherosclerosis, diabetes as well as insulin resistance, are found to be closely associated with telomere shortening. TL is also influenced by psychological stress (Table I). Patients with short telomeres show the lowest telomerase activity and equally high oxidative stress. Hypothalamus, pituitary, adrenal activation due to stress, elevates glucocorticoid production, which increases reactive oxygen species (ROS) level, resulting in shortened lifespan and increased risk for age-related disease, including CVD15,18. Animal models have shown that shortened telomeres with reduced telomerase activity are closely associated with an increase in inflammatory mediators, greater susceptibility to oxidative damage, stroke, blood-brain barrier permeability, as well as tight junction disturbances38,39. Cellular stress- or age-related disorder is linked to telomere shortening40. Mitochondrial dysfunction results in (ROS) production which initiates genomic instability and telomere shortening41,42. The persistent activation of p53 induces further mitochondrial damage, and a vicious cycle continues. Improving mitochondrial function may assist in healthy ageing41,42.
Table I.
Factors affecting telomere length
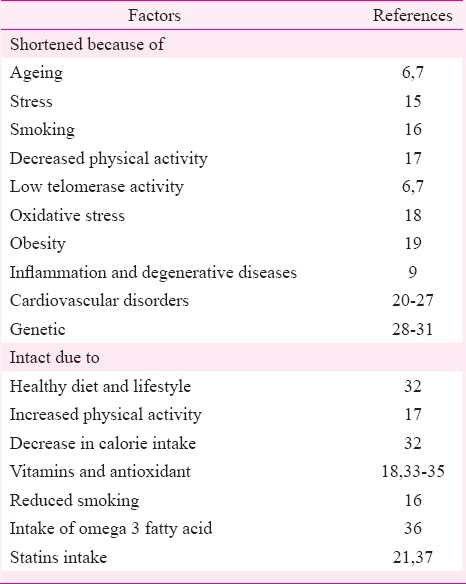
Shorter leucocyte telomeres in people prone to coronary heart disease could indicate the cumulative effect of other cardiovascular risk factors on TL. The association of shorter telomeres with an increased risk of coronary heart disease has a genetic basis that exacerbates or retards the TL28,29. In the genetic disorder dyskeratosis congenita, telomere shortening is accelerated30, and patients have premature onset of many age-related diseases and early death. It is also longer in African Americans than in whites of European descent. Although at the start of life, no sex difference in the length of telomeres is seen43, it is longer in women than in men. It is seen that men have shorter lifespan and greater telomere shortening44. This has led to speculation that sex-specific telomere shortening is one cause of sex-specific mortality. Survival advantage could be the result of variation in telomere maintenance alleles on the X-chromosome45.
It was found in the elderly twins that the twin with shorter TL was more likely to die before the twin with longer TL46. TL is heritable and modified by paternal age at the time of conception31. Obesity, lack of exercise, smoking and psychological stress are some of the major factors that intensify the process (Table I).
Individual differences in biological ageing, as shown by shortening of TL, could affect susceptibility to coronary heart disease and might serve as a predictor of the disease6,7. Shorter TL may serve as a potential marker for the presence of atherothrombotic and haemorrhagic stroke and for the risk of post-stroke death47.
Oxidative stress could also be a possible contributor for the shorter TL in high-risk individuals for CAD. One of the many benefits of statins in CAD is that it may retard the shortening of telomeres. Short-telomere individuals might benefit most from statin treatment. The anti-ageing effects of statins are shown to be linked to their ability to inhibit telomere shortening by reducing either directly and indirectly oxidative telomeric DNA damage, as well as by a mechanism involving telomere capping proteins37.
Estimation of telomere length
TL measurement techniques used in epidemiological/clinical research include Southern blot analysis of the terminal restriction fragments (TRFs) length48; quantitative polymerase chain reaction (qPCR)49,50 and fluorescence in situ hybridization (FISH)51,52. Southern blot analysis to estimate mean TRF length generally involves digestion of genomic DNA with restriction enzymes. Determination of average TL by qPCR involves the comparison of copy number of telomere repeats with that of a single-copy gene (36b4) in a given sample (T/S ratio). The T/S ratio is proportional to the average TL49,50. The measurement of telomeres in vertebrate DNA by PCR amplification with oligonucleotide primers intended to hybridize to the TTAGGG and CCCTAA repeats was considered impossible until recently because only primer dimer-derived products were expected. However, a primer pair that removes this problem was found that allows easy measurement of telomeres in a closed tube, fluorescence-based assay49.
The FISH technique for determination of telomere repeats includes quantitative FISH (QFISH) using digital fluorescence microscopy or flow cytometry (flow-FISH). Flow-FISH is less time consuming than QFISH where TL measurements are performed on individual metaphase chromosomes. The flow-FISH technique can measure average TL in each cell and TL can be determined in specific cell populations. Southern blot and qPCR methods are more extensively used to measure leucocyte TL (LTL) than flow-FISH. The Southern blot method requires more DNA (μg quantities), is costly and more laborious. The qPCR method, on the other hand, requires less DNA. However, lack of good reference standards may make absolute TL measurement difficult. In the flow-FISH technique, a high variability in the length of alphoid centromere among individuals makes this method unreliable. Telomerase activity can, however, be tested by Telomeric Repeat Amplification Protocol (TRAP) assay53. The telomerase activity can be detected in extracts of cellular protein by TRAP assay. Thus, there remains the need for an easy, inexpensive and high throughput method to measure TL. Besides CAD and other clinical disorders, LTL is considered to be a biomarker of human ageing54. Short LTL was found to be associated with women19. Furthermore, investigations in twins suggested that short LTL was associated with their decreased survival46,55,56.
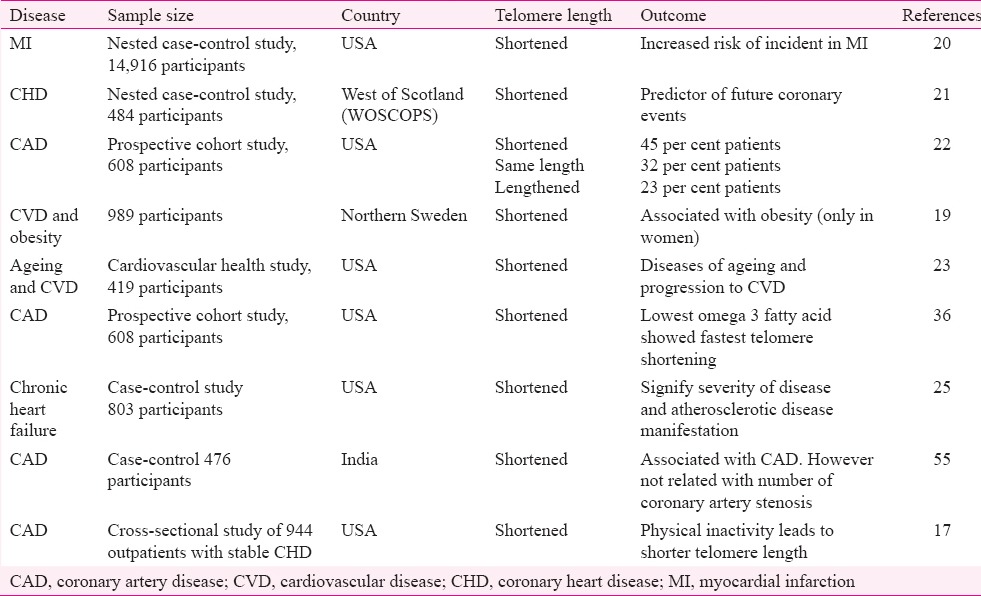
Shortening of telomeres in coronary artery disease
Research shows that telomere shortens in length over an individual's lifetime that may contribute to ageing and development of several diseases including CVD (Table II), diabetes, depression, pulmonary fibrosis, dementia and osteoarthritis.
Table II.
Studies showing telomere length is shorter in patients with coronary artery disease
Heart tissue is also affected by age. CAD is an age-related disorder57 where cellular senescence is a major feature for the development of atherosclerotic plaques58,59,60. In vitro studies have shown that induction of senescence causes expression of certain molecules which are entailed in atherogenesis60. As shown by previous studies, in many cell types, telomere shortening is the confounding factor for cellular ageing15. Almost all patients with CAD have shortening of telomeres. Endothelial injury occurring as a result of liberation of free radical produced by hypertension, diabetes mellitus, cigarette smoking, as well as increased serum levels of low-density lipoproteins may contribute to telomere shortening. Studies have also shown that suppression of the oxidative stress slows down this shortening process33,34. Telomere shortening was highly accelerated in the regions that were easily prone to atherosclerosis58,59,60. Increased cellular turnover may result in cellular senescence associated with telomere shortening and may participate in coronary atherogenesis.
A large body of evidence in ethnic populations across the world20,21,22,23,24,25,26,36,54,61,62,63,64,65,66 including Caucasian whites, Japanese and Asian Indians61 has demonstrated the association of shortened telomeres with CAD. One study showed LTL to be a valuable prognostic marker for carotid atherosclerosis54. This study provided preliminary evidence for a prospective association of LTL with risk assessment of carotid atherosclerosis. As atherosclerosis is a systemic disease, the prevalence of carotid atherosclerosis increases the prevalence of coronary atherosclerosis. Events originating from one artery have a strong prediction on the occurrence of events in the other artery65. Variation in the TERT gene also acts as a predictor of CAD in certain racial groups66. Middle-aged men with shortened telomeres appeared more prone to future coronary events64 including myocardial infarction (MI)20. Besides CAD and other clinical disorders, LTL is considered to be a biomarker of human ageing54.
It was demonstrated that the mean TL in leucocytes was shorter in patients with severe triple vessel CAD63 and patients with a history of premature MI64 as compared to corresponding age- and sex-matched controls. This was at variance to the finding that although telomere shortening was associated with CAD, it was not related to the number of coronary artery stenosis61. Similarly, patients who have suffered a premature MI also show shorter telomere compared with age- and sex-matched subjects without such a history21,64. Also future coronary events in middle-aged men can also be anticipated in individuals with shorter telomere21. A cohort study involving 603 participants found that lowest omega 3 fatty acid in the diet was associated with fastest telomere shortening36. Another study involving 803 participants, demonstrated significant shortening of the telomere in chronic heart failure patients25. Hence, all these studies suggest telomere shortening as a predictor of future coronary events. There are however, data disagreeing that there is a fundamental association between short telomeres and CVD27. Data suggest that changes in telomeres other than shortening can also trigger cell-ageing15. The TL varies throughout the life in an individual, and that is independent of the level of cardiovascular risk. In the only animal study, short telomeres seemed to protect apolipoprotein E deficient mice from diet-induced atherosclerosis67. When patients with shorter telomeres are treated with statins; their coronary heart disease risk becomes indistinguishable from that of patients with longer telomeres21. Diseases of ageing and progression to CVD23, and obesity particularly in women19, increase in coronary intima media thickness24 are also related to shortened telomere.
Conclusion
Besides lipoprotein and proinflammatory markers, TL appears to be a potential biomarker of CAD. Its evaluation may be introduced as a routine test in preventive cardiology practice, but it is essential to use a standard and an accurate method for the purpose. The TL of CAD patients may be compared with the normal range in samples of age, sex and ethnicity-matched asymptomatic people not affected by CAD, and its predictive power assessed over time.
Increased physical activity17,32, decrease calorie intake, resveratrol, antioxidant and mitochondrial support, avoidance of toxin exposure, reduced smoking16 and sufficient antioxidant intake18,35 are some of the factors which are associated with prevention of telomere shortening and maintaining telomere integrity. Drugs that target tumour cells by blocking their abnormally high levels of telomerase are already in different phases of clinical trials. It has been suggested that altered expression of miRNAs in the ageing heart contributes to the age-dependent turn down in cardiac function68. In vivo silencing or genetic deletion of miR-34a reduces age-associated death of cardiomyocytes68. The study documented PNUTS (also known as PPP1R10) as a novel direct miR-34a target, which reduces telomere shortening, DNA damage responses and cardiomyocyte apoptosis, and improves functional recovery after acute MI68.
Telomeres and telomerase are a focus of research aimed at understanding of ageing, stress and chronic diseases. Telomere shortening has been demonstrated in white blood cells from patients with coronary heart disease, premature MI, hypertension and diabetes mellitus69. Expression of 95 per cent telomerase activity has been detected in pancreatic adenocarcinoma. Thereby, the detection of telomerase activity has been proposed to be a useful tool in the diagnosis of pancreatic cancer70. To include telomere as CAD biomarkers, TL measurement variation needs to be reduced that presently restricts our means to perceive consistent result in TL assessment. Second, as TL in senescent lymphocytes is more informative than in leucocytes71, hence measuring the TL in specific cell populations may be more informative than measuring in mixed populations such as leucocytes. Merging conventional epidemiology with modern genomics at both bench and bedside will enhance our perceptive of CAD and switch discoveries into clinically useful application that can make a valuable impact in public health72.
Acknowledgment
Research at Cumballa Hill Hospital and Heart Institute is supported by the Department of Scientific and Industrial Research, Government of India.
Footnotes
Conflicts of Interest: None.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Cardiovascular Diseases (CVDs) [accessed on May 2, 2015]. Available from: http://www.who.int/mediacentre/factsheets/fs317/en/
- 3.Bogavac-Stanojević N, Jelić-Ivanović Z, Spasojević-Kalimanovska V, Spasić S, Kalimanovska-Ostrić D. Lipid and inflammatory markers for the prediction of coronary artery disease: A multi-marker approach. Clin Biochem. 2007;40:1000–6. doi: 10.1016/j.clinbiochem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Wierzbicki AS. Homocysteine and cardiovascular disease: A review of the evidence. Diab Vasc Dis Res. 2007;4:143–50. doi: 10.3132/dvdr.2007.033. [DOI] [PubMed] [Google Scholar]
- 5.Bailey SM, Meyne J, Chen DJ, Kurimasa A, Li GC, Lehnert BE, et al. DNA double-strand break repair proteins are required to cap the ends of mammalian chromosomes. Proc Natl Acad Sci U S A. 1999;96:14899–904. doi: 10.1073/pnas.96.26.14899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5:197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 7.Bekaert S, De Meyer T, van Oostveldt P. Telomere attrition as aging biomarker. Anticancer Res. 2005;25:3011–21. [PubMed] [Google Scholar]
- 8.Meyerson M. Telomerase enzyme activation and human cell immortalization. Toxicol Lett. 1998;102-103:41–5. doi: 10.1016/s0378-4274(98)00278-1. [DOI] [PubMed] [Google Scholar]
- 9.Meyerson M. Role of telomerase in normal and cancer cells. J Clin Oncol. 2000;18:2626–34. doi: 10.1200/JCO.2000.18.13.2626. [DOI] [PubMed] [Google Scholar]
- 10.Dahse R, Fiedler W, Ernst G. Telomeres and telomerase: Biological and clinical importance. Clin Chem. 1997;43:708–14. [PubMed] [Google Scholar]
- 11.Johnson FB. PinX1 the tail on the chromosome. J Clin Invest. 2011;121:1242–4. doi: 10.1172/JCI57024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaiswal RK, Kumar P, Yadava PK. Telomerase and its extracurricular activities. Cell Mol Biol Lett. 2013;18:538–54. doi: 10.2478/s11658-013-0105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, et al. Hydrogen peroxide- and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 2000;86:960–6. doi: 10.1161/01.res.86.9.960. [DOI] [PubMed] [Google Scholar]
- 14.Bis JC, Kavousi M, Franceschini N, Isaacs A, Abecasis GR, Schminke U, et al. Meta-analysis of genome-wide association studies from the CHARGE consortium identifies common variants associated with carotid intima media thickness and plaque. Nat Genet. 2011;43:940–7. doi: 10.1038/ng.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hewitt G, Jurk D, Marques FD, Correia-Melo C, Hardy T, Gackowska A, et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babizhayev MA, Yegorov YE. Smoking and health: Association between telomere length and factors impacting on human disease, quality of life and life span in a large population-based cohort under the effect of smoking duration. Fundam Clin Pharmacol. 2011;25:425–42. doi: 10.1111/j.1472-8206.2010.00866.x. [DOI] [PubMed] [Google Scholar]
- 17.Krauss J, Farzaneh-Far R, Puterman E, Na B, Lin J, Epel E, et al. Physical fitness and telomere length in patients with coronary heart disease: Findings from the Heart and Soul Study. PLoS One. 2011;6:e26983. doi: 10.1371/journal.pone.0026983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi N, Machida T, Takahashi T, Takatsu H, Shinkai T, Abe K, et al. Elevation by oxidative stress and aging of hypothalamic-pituitary-adrenal activity in rats and its prevention by vitamin E. J Clin Biochem Nutr. 2009;45:207–13. doi: 10.3164/jcbn.09-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordfjäll K, Eliasson M, Stegmayr B, Melander O, Nilsson P, Roos G. Telomere length is associated with obesity parameters but with a gender difference. Obesity (Silver Spring) 2008;16:2682–9. doi: 10.1038/oby.2008.413. [DOI] [PubMed] [Google Scholar]
- 20.Zee RY, Michaud SE, Germer S, Ridker PM. Association of shorter mean telomere length with risk of incident myocardial infarction: A prospective, nested case-control approach. Clin Chim Acta. 2009;403:139–41. doi: 10.1016/j.cca.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: A nested case-control study. Lancet. 2007;369:107–14. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 22.Farzaneh-Far R, Lin J, Epel E, Lapham K, Blackburn E, Whooley MA. Telomere length trajectory and its determinants in persons with coronary artery disease: Longitudinal findings from the heart and soul study. PLoS One. 2010;5:e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 24.O’Donnell CJ, Demissie S, Kimura M, Levy D, Gardner JP, White C, et al. Leukocyte telomere length and carotid artery intimal medial thickness: The Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2008;28:1165–71. doi: 10.1161/ATVBAHA.107.154849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, Mulder MJ, et al. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–64. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, Yoshimi N, et al. Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol. 2004;24:546–50. doi: 10.1161/01.ATV.0000117200.46938.e7. [DOI] [PubMed] [Google Scholar]
- 27.Spyridopoulos I, von Zglinicki T. Telomere length predicts cardiovascular disease. BMJ. 2014;349:g4373. doi: 10.1136/bmj.g4373. [DOI] [PubMed] [Google Scholar]
- 28.Ning XH, Zhang N, Li T, Wu PJ, Wang X, Li XY, et al. Telomere shortening is associated with genetic anticipation in Chinese Von Hippel-Lindau disease families. Cancer Res. 2014;74:3802–9. doi: 10.1158/0008-5472.CAN-14-0024. [DOI] [PubMed] [Google Scholar]
- 29.Zhu Y, Voruganti VS, Lin J, Matsuguchi T, Blackburn E, Best LG, et al. QTL mapping of leukocyte telomere length in American Indians: The Strong Heart Family Study. Aging (Albany NY) 2013;5:704–16. doi: 10.18632/aging.100600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shay JW, Wright WE. Telomeres in dyskeratosis congenita. Nat Genet. 2004;36:437–8. doi: 10.1038/ng0504-437. [DOI] [PubMed] [Google Scholar]
- 31.Njajou OT, Cawthon RM, Damcott CM, Wu SH, Ott S, Garant MJ, et al. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci U S A. 2007;104:12135–9. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bishop NA, Guarente L. Genetic links between diet and lifespan: Shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–44. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- 33.Furumoto K, Inoue E, Nagao N, Hiyama E, Miwa N. Age-dependent telomere shortening is slowed down by enrichment of intracellular Vitamin C via suppression of oxidative stress. Life Sci. 1998;63:935–48. doi: 10.1016/s0024-3205(98)00351-8. [DOI] [PubMed] [Google Scholar]
- 34.Xu D, Neville R, Finkel T. Homocysteine accelerates endothelial cell senescence. FEBS Lett. 2000;470:20–4. doi: 10.1016/s0014-5793(00)01278-3. [DOI] [PubMed] [Google Scholar]
- 35.Xu Q, Parks CG, DeRoo LA, Cawthon RM, Sandler DP, Chen H. Multivitamin use and telomere length in women. Am J Clin Nutr. 2009;89:1857–63. doi: 10.3945/ajcn.2008.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–7. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olivieri F, Mazzanti I, Abbatecola AM, Recchioni R, Marcheselli F, Procopio AD, et al. Telomere/Telomerase system: A new target of statins pleiotropic effect? Curr Vasc Pharmacol. 2012;10:216–24. doi: 10.2174/157016112799305076. [DOI] [PubMed] [Google Scholar]
- 38.Lee J, Jo YS, Sung YH, Hwang IK, Kim H, Kim SY, et al. Telomerase deficiency affects normal brain functions in mice. Neurochem Res. 2010;35:211–8. doi: 10.1007/s11064-009-0044-3. [DOI] [PubMed] [Google Scholar]
- 39.Flanary BE, Streit WJ. Telomeres shorten with age in rat cerebellum and cortex in vivo. J Anti Aging Med. 2003;6:299–308. doi: 10.1089/109454503323028894. [DOI] [PubMed] [Google Scholar]
- 40.Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bratic A, Larsson NG. The role of mitochondria in aging. J Clin Invest. 2013;123:951–7. doi: 10.1172/JCI64125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longev Healthspan. 2014;3:6. doi: 10.1186/2046-2395-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youngren K, Jeanclos E, Aviv H, Kimura M, Stock J, Hanna M, et al. Synchrony in telomere length of the human fetus. Hum Genet. 1998;102:640–3. doi: 10.1007/s004390050755. [DOI] [PubMed] [Google Scholar]
- 44.Stindl R. Tying it all together: Telomeres, sexual size dimorphism and the gender gap in life expectancy. Med Hypotheses. 2004;62:151–4. doi: 10.1016/s0306-9877(03)00316-5. [DOI] [PubMed] [Google Scholar]
- 45.Chuaire-Noack L, Sánchez-Corredor MC, Martínez-Agüero M. Revisiting the X-chromosome inactivation and its impact on female longevity. Adv Biosci Biotechnol. 2014;5:572–83. [Google Scholar]
- 46.Kimura M, Hjelmborg JV, Gardner JP, Bathum L, Brimacombe M, Lu X, et al. Telomere length and mortality: A study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167:799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang W, Chen Y, Wang Y, Liu P, Zhang M, Zhang C, et al. Short telomere length in blood leucocytes contributes to the presence of atherothrombotic stroke and haemorrhagic stroke and risk of post-stroke death. Clin Sci (Lond) 2013;125:27–36. doi: 10.1042/CS20120691. [DOI] [PubMed] [Google Scholar]
- 48.Kimura M, Stone RC, Hunt SC, Skurnick J, Lu X, Cao X, et al. Measurement of telomere length by the Southern blot analysis of terminal restriction fragment lengths. Nat Protoc. 2010;5:1596–607. doi: 10.1038/nprot.2010.124. [DOI] [PubMed] [Google Scholar]
- 49.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Callaghan N, Dhillon V, Thomas P, Fenech M. A quantitative real-time PCR method for absolute telomere length. Biotechniques. 2008;44:807–9. doi: 10.2144/000112761. [DOI] [PubMed] [Google Scholar]
- 51.Lauzon W, Sanchez Dardon J, Cameron DW, Badley AD. Flow cytometric measurement of telomere length. Cytometry. 2000;42:159–64. doi: 10.1002/1097-0320(20000615)42:3<159::aid-cyto1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 52.Hultdin M, Grönlund E, Norrback K, Eriksson-Lindström E, Just T, Roos G. Telomere analysis by fluorescence in situ hybridization and flow cytometry. Nucleic Acids Res. 1998;26:3651–6. doi: 10.1093/nar/26.16.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mender I, Shay JW. Telomerase repeated amplification protocol (TRAP) Bio Protoc. 2015;5:pii: e1657. doi: 10.21769/bioprotoc.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen S, Lin J, Matsuguchi T, Blackburn E, Yeh F, Best LG, et al. Short leukocyte telomere length predicts incidence and progression of carotid atherosclerosis in American Indians: The Strong Heart Family Study. Aging (Albany NY) 2014;6:414–27. doi: 10.18632/aging.100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hjelmborg JB, Dalgård C, Mangino M, Spector TD, Halekoh U, Kimura M, et al. Paternal age and telomere length in twins: The germ stem cell selection paradigm. Aging Cell. 2015;14:701–3. doi: 10.1111/acel.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, et al. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6:769–74. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- 57.Lloyd-Jones DM, Larson MG, Beiser A, Levy D. Lifetime risk of developing coronary heart disease. Lancet. 1999;353:89–92. doi: 10.1016/S0140-6736(98)10279-9. [DOI] [PubMed] [Google Scholar]
- 58.Davies MJ, Woolf N, Rowles PM, Pepper J. Morphology of the endothelium over atherosclerotic plaques in human coronary arteries. Br Heart J. 1988;60:459–64. doi: 10.1136/hrt.60.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bürrig KF. The endothelium of advanced arteriosclerotic plaques in humans. Arterioscler Thromb. 1991;11:1678–89. [PubMed] [Google Scholar]
- 60.Minamino T, Miyauchi H, Yoshida T, Ishida Y, Yoshida H, Komuro I. Endothelial cell senescence in human atherosclerosis: Role of telomere in endothelial dysfunction. Circulation. 2002;105:1541–4. doi: 10.1161/01.cir.0000013836.85741.17. [DOI] [PubMed] [Google Scholar]
- 61.Mukherjee M, Brouilette S, Stevens S, Shetty KR, Samani NJ. Association of shorter telomeres with coronary artery disease in Indian subjects. Heart. 2009;95:669–73. doi: 10.1136/hrt.2008.150250. [DOI] [PubMed] [Google Scholar]
- 62.Batty GD, Wang Y, Brouilette SW, Shiels P, Packard C, Moore J, et al. Socioeconomic status and telomere length: The West of Scotland Coronary Prevention Study. J Epidemiol Community Health. 2009;63:839–41. doi: 10.1136/jech.2009.088427. [DOI] [PubMed] [Google Scholar]
- 63.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–3. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
- 64.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–6. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 65.Jashari F, Ibrahimi P, Nicoll R, Bajraktari G, Wester P, Henein MY. Coronary and carotid atherosclerosis: Similarities and differences. Atherosclerosis. 2013;227:193–200. doi: 10.1016/j.atherosclerosis.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 66.Bressler J, Franceschini N, Demerath EW, Mosley TH, Folsom AR, Boerwinkle E, et al. Sequence variation in telomerase reverse transcriptase (TERT) as a determinant of risk of cardiovascular disease: The Atherosclerosis Risk in Communities (ARIC) study. BMC Med Genet. 2015;16:52. doi: 10.1186/s12881-015-0194-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Poch E, Carbonell P, Franco S, Díez-Juan A, Blasco MA, Andrés V. Short telomeres protect from diet-induced atherosclerosis in apolipoprotein E-null mice. FASEB J. 2004;18:418–20. doi: 10.1096/fj.03-0710fje. [DOI] [PubMed] [Google Scholar]
- 68.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–10. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 69.Balasubramanyam M, Adaikalakoteswari A, Monickaraj SF, Mohan V. Telomere shortening & metabolic/vascular diseases. Indian J Med Res. 2007;125:441–50. [PubMed] [Google Scholar]
- 70.Zisuh AV, Han TQ, Zhan SD. Expression of telomerase & its significance in the diagnosis of pancreatic cancer. Indian J Med Res. 2012;135:26–30. doi: 10.4103/0971-5916.93420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spyridopoulos I, Hoffmann J, Aicher A, Brümmendorf TH, Doerr HW, Zeiher AM, et al. Accelerated telomere shortening in leukocyte subpopulations of patients with coronary heart disease: Role of cytomegalovirus seropositivity. Circulation. 2009;120:1364–72. doi: 10.1161/CIRCULATIONAHA.109.854299. [DOI] [PubMed] [Google Scholar]
- 72.Padmanabhan S, Hastie C, Prabhakaran D, Dominczak AF. Genomic approaches to coronary artery disease. Indian J Med Res. 2010;132:567–78. [PMC free article] [PubMed] [Google Scholar]


