Abstract
People with epilepsy have increased risk of premature death, and their life expectancy may reduce by 2-10 yr. Population- and hospital-based studies have shown that the excess mortality in epilepsy is not entirely explained by deaths directly attributable to epilepsy such as accidents and drowning during a seizure. It is also significantly contributed by deaths from other causes such as cardiac deaths, deaths due to malignancies and other causes. It had recently been recognized that sudden unexpected deaths in epilepsy (SUDEP) contributed to a small yet important proportion of mortality in epilepsy. SUDEPs are deaths (witnessed or unwitnessed) unrelated to trauma, drowning or status epilepticus and not attributable to any specific medical conditions. Several factors related to epilepsy and drug therapy have been found to be associated with higher risk of SUDEP.
Keywords: Discussion with family members, epilepsy, premature death, risk assessment, sudden unexpected deaths, SUDEP
Introduction
Epilepsy is one of the most common neurological disorder that affect >50 million people across the world. It is now widely recognized that people with epilepsy are two to three times more likely to die early. It reduces life expectancy by 10 years for those with symptomatic epilepsy and by two years for those with idiopathic epilepsies1. Accidents, sudden unexpected deaths, status epilepticus and suicides constitute a vast majority of cause of death in epilepsy2. A population-based study in the UK showed that, during the period 1993-2005, the mortality from epilepsy increased by 31 per cent for males and 39 per cent for females, whereas the mortality from all causes declined by 16 per cent3. The standardized mortality ratio (SMR) for epilepsy in India varied from 2.58 to 7.64,5,6. The prevalence of epilepsy in low-income countries is comparable to that of high-income countries although the former has a higher incidence than the latter. This disparity between incidence and prevalence in low-income countries points towards higher mortality for epilepsy when compared to high-income countries7,8.
The excess mortality in epilepsy cannot be entirely explained by deaths directly related to epilepsy such as accidents and drowning due to seizures or status epilepticus. The proportion of deaths from suicide, malignancies and cardiac causes are also increased in people with epilepsy. Sudden unexpected death in epilepsy (SUDEP) has recently emerged as an important cause of death in people with epilepsy. Although the phenomenon of SUDEP was known as early as latter part of 19th century, it was rarely highlighted as important. Apparently, the stepdaughter of George Washington who was suffering from epilepsy had a sudden death9. SUDEP has considerable social significance as it is still not clear how and when the issue of SUDEP need to be presented to the patient or his or her caregivers10,11.
Definition
The first detailed definition of SUDEP was put forward by Nashef12 as sudden unexpected, witnessed or unwitnessed, non-traumatic and non-drowning death in patients with epilepsy with or without evidence for a seizure and excluding documented status epilepticus, in which post-mortem examination does not reveal a toxicologic or anatomic cause for death. A post-mortem examination is mandatory for the diagnosis according to this definition. Food and Drug Administration and Borroughs Welcome had set up a committee chaired by Leestma that had recommended a modified definition that included definite, probable and possible SUDEP13. This definition was easy to use in epidemiological studies and for classification of deaths where autopsies were not performed. However, it does not provide any operational cut-off values for ‘sudden death’ or have provisions to handle alternate or other causes of death. A new unified set of criteria for the diagnosis of SUDEP was proposed in 2012 (Table I)14.
Table I.
Unified criteria for diagnosis of sudden unexpected deaths in epilepsy (SUDEP)

Incidence
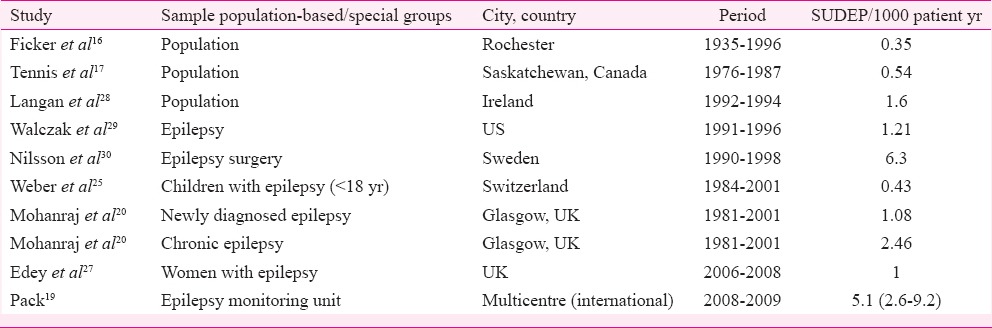
Incidence of SUDEP varies according to the study population. The SMR in patients with epilepsy is 2.55 i.e. 2-3 times more than that of general population15. In the population-based studies, SUDEP incidence rates have been found to vary between 0.35 and 1.35/1000 person-years16,17. The incidence reported was higher for people with chronic epilepsy (1.2-5.1/1000 patient years)18,19,20,21 and for those who had undergone surgery for refractory epilepsy (6.3-9.3/1000 person-years)22,23. The risk of SUDEP in children was lower (0.36-0.43/1000 person-years)24,25,26. Study on women in their reproductive age with epilepsy in the United Kingdom revealed a SUDEP rate of 11 per cent (1/1000 maternities) (Table II)27.
Table II.
Sudden unexpected deaths in epilepsy (SUDEP) rates according to study characteristics
Predictors of sudden unexpected deaths in epilepsy (SUDEP)
Several studies have identified important predictors for SUDEP. The rate is marginally higher for males, particularly for those with generalized tonic-clonic seizures (GTCS)23. Studies have shown that onset of epilepsy before 16 yr of age carries higher risk of SUDEP31,32.
It is important to recognize that the presence of certain comorbidities increases the risk of SUDEP. Mental retardation, dementia, psychiatric illness, alcohol and other substance abuse are associated with increased risk of SUDEP, though none has been conclusively proven29,33. Jick et al34 have pointed towards the relationship between certain aspects of pharmacotherapy and SUDEP. There are conflicting reports on the association between polypharmacy and SUDEP. It has been shown that the addition of a second antiepileptic drug (AED) to the first one increases the risk of SUDEP and the presence of three AEDs increases risk 10 fold30. In contrast, Opeskin et al33 have failed to establish any association between SUDEP and polypharmacy.
A population-based study in Norway showed that of the 26 patients with SUDEP, 10 were on lamotrigine (9 females). There was a significant association between the use of lamotrigine and SUDEP in women. This observation is particularly significant as lamotrigine is increasingly recommended for the treatment of generalized epilepsy in women31. A secondary analysis of data from randomized controlled clinical trials involving lamotrigine showed that there was no significant difference in the rate of SUDEP between lamotrigine and comparator groups. The confidence intervals were wide and clinically significant effect could not be ruled out35. The possible association between lamotrigine and SUDEP may be mediated through cardiac autonomic dysfunction as in kainate model of epilepsy, lamotrigine was found to increase the cardiac autonomic instability36. Kloster and Engelskjon37 found that seizures were more common amongst patient taking carbamazepine and oxcarbazepine, but this was not proved in other studies. In SUDEP cases that had undergone autopsy studies, the blood level of AEDs were found to be in subtherapeutic range38.
The duration and severity of epilepsy seem to have an association with risk of SUDEP. People who suffered SUDEP had a longer duration of epilepsy than others in case-control studies39. The risk of SUDEP was 15-fold higher for people with >50 GTCS per year39. Nocturnal seizures were more common amongst the SUDEP group. Patients suffering from GTCS have been found to have increased risk of SUDEP39 when compared to patients with complex partial seizures and absences. Prolonged tonic phase of the seizure has been found to correlate with postictal immobility and has a role in mechanism of SUDEP40. Postictal generalized electroencephalogram (EEG) suppression following seizure has a predictive role in SUDEP but has not been proved in subsequent studies41.
Idiopathic/cryptogenic epilepsy was at a lower risk to SUDEP compared to symptomatic epilepsy and females with idiopathic generalized epilepsy were lesser prone for SUDEP compared to males with idiopathic generalized epilepsy. Patients with Dravet syndrome were at higher risk of sudden death and so were children younger than six years of age42,43.
Pathophysiology
SUDEP is a descriptive term that conveys little about the pathophysiology that leads to the sudden death. Various hypotheses involving respiratory, cardiac, cerebral and autonomic dysfunctions have been put forward to explain the phenomenon.
Cardiac hypothesis: One of the postulated mechanisms of SUDEP involves sudden cardiac arrest. Dysfunction of sodium channel has an important role in the electrophysiological basis of epilepsy and cardiac arrhythmias and altered cardiac electrical function may contribute to susceptibility for arrhythmogenesis and SUDEP. Ion channel abnormalities may be a potential mechanism for both epilepsy and cardiac dysfunction. The most common ion channel implicated is SCN gene mutation44,45 as in Dravet syndrome that is associated with higher risk of SUDEP. Channelopathies have been linked to long QT interval syndrome and familial epilepsies. There have been multiple case studies44,45 and animal experiments46 that have noted susceptibility of this gene in this condition. There is a possible pathogenic link between SUDEP, mutations in ion channel genes and familial Long QT syndrome (LQTS). Familial LQTS has been implicated in the sudden cardiac death. Ion channels such as KCNQ1, SCN1A, LQTS, KCNH2 and SCN5A regulating the QT interval are likely to play an important role in the predisposition of epilepsy patients to sudden death47.
Autonomic dysfunction: Autonomic involvement in the form of excessive sympathetic and parasympathetic drive has been found to play a role in SUDEP. It is widely recognized that heart rate increases during seizures48. Two or more autonomic function tests have been found to be impaired in patients with refractory epilepsy49,50. Extensive derangement of autonomic functions such as higher sympathetic tone, higher vasomotor tone, lower parasympathetic reactivity, lower parasympathetic tone and severe dysautonomia has been demonstrated in patients with intractable epilepsy49,50. People with intractable epilepsy on treatment with antiepileptics have been shown to have decreased heart rate variability51. Decreased heart rate variability is an important risk factor of sudden cardiac death in patients with type II diabetes and myocardial infarction. The role of reduced heart rate variability in causation of SUDEP needs further validation. It is unclear whether the reduced variability in heart rate is a result of antiepileptic usage or a part of refractory epilepsy52,53.
Mortality in epilepsy monitoring unit study (MORTEMUS54) was an international initiative using in-hospital, presurgical data to assess SUDEP mechanisms and risk factors. Video EEG (VEEG) and electrocardiogram data at the time of cardiac arrest during epilepsy monitoring were retrospectively analyzed between January 2008 and December 2009. Of the 160 units enrolled in the study, 147 responded to the survey. A total of 16 SUDEP and nine near SUDEP were reported. The incidence of SUDEP was 5.1/1000 patient-years (95% confidence interval 2.6-9.2) and risk of 1.2/1000 was documented. This study proposed a centrally mediated alteration of respiration and cardiac function following GTCS. They found that following a GTCS, profound bradycardia and apnoea ensue after an initial variation in the cardiac and respiratory rates. This suppression of the cardiac and respiratory rhythm is associated with a postictal generalized suppression of the EEG. This study in epilepsy monitoring units showed altered cardiorespiratory function leading to terminal apnoea and cardiac arrest.
Peri-ictal hypoxaemia occurs in at least 25 per cent of patients with SUDEP55. The ictal phase of generalized seizures is more often associated with tachycardia than bradycardia. Both ictal hypoxaemia and ictal tachycardia were associated with risk factors implicated in SUDEP-refractory epilepsy, generalized seizures and normal neuroimaging55.
SUDEP often happens during sleep and nocturnal seizures were more frequent in patients who had SUDEP than survivors56. Almost all SUDEP deaths have occurred in prone position and during sleep. Excessive autonomic activity (ictal tachycardia), autonomic imbalance during seizure (switch from parasympathetic tone to sympathetic tone) and sympathetic overstimulation on AED withdrawal might be the probable precipitating factor for SUDEP.
Postictal generalized suppression of EEG (PGES) has been observed in some patients after GTCS. It has rarely been reported after other types of seizures. Lhatoo et al57 found that PGES of 50 sec or more in refractory epilepsy patients was associated with increased risk of SUDEP. However, this was not reconfirmed in later studies41,58. PGES is representative of the cortical electrical activity. It is unclear at present whether it is secondary to postictal depression of cortical activity or postictal hypoxia or due to derangement of the ascending arousal system.
Multiple neurotransmitters are released during a seizure, and their role in the pathogenesis of SUDEP needs further evaluation. The important neurotransmitter implicated in this is serotonin. Serotonin has been found low in patients with epilepsy and low serotonin levels have been found to be associated with SUDEP59,60. Serotonin levels are also found to be lower in sleep. Serotonin is a central neurotransmitter which stimulates thalamocortical circuits. Serotonin has been found to modulate respiratory activity in brainstem61. In an animal model (DBA/2 mouse model) treatment with serotonin antagonist fluoxetine reduced respiratory depression59. SUDEP may be related to sudden infant death syndrome where low medullary serotonin levels have been found62,63. Other substances released during seizures such as opioids and adenosine have also been found to have a role in SUDEP64,65. In a systematic review a significant association was found between prone position and SUDEP66, suggesting that prone position was a major risk factor.
Autopsy changes in SUDEP
In a study on SUDEP37 though pathological findings were found, there were no definitive changes noted in post-mortem findings of SUDEP and non-SUDEP patients. Most common findings in lung were pulmonary oedema, and this as a cause resulting in SUDEP was considered unlikely. Most common pathology in cardiac system was fibrosis of the conduction system. In brain, SUDEP patients had predominantly cerebral oedema, but there was no associated mass effect or hydrocephalus. Structural brain lesions were common amongst the SUDEP group, but this was a clue for aetiology rather than cause of death. Neuropathology showed evidence of acute neocortical and brainstem hypoxic neuronal changes67,68.
Prevention
Various mechanisms have been postulated for SUDEP. Understanding those mechanisms will help in prevention of SUDEP. SUDEP patients are mainly found in prone position, hence immediately post-seizure preventing the patient from compromised position may help in prevention of SUDEP. Prone position has been found to result in hypopnoea, leading to respiratory failure. Use of lattice pillows prevents suffocation and appears to reduce asphyxia risks69. The patients require nocturnal supervision. Post-seizure attention to recovery of patients after seizure and positioning after seizure or stimulating if necessary may be required to prevent SUDEP. MORTEMUS study54 suggested the routine use of pulse oximetry, EEG or pulse alarm system will help in improvement of nocturnal monitoring and supervision.
A reduction in frequency of GTCS can reduce the risk of SUDEP. Similarly, change adoption of monotherapy may also reduce the risk of SUDEP as polypharmacy has been found to have increased risk of seizures39. However, polytherapy and frequent GTCS may be indirect evidence of refractory epilepsy.
There have been reports of lamotrigine implicated in SUDEP; however, its role has not been conclusively proved. Lamotrigine and carbamazepine combination was found to have increased risk. A study by Hesdorffer et al39 suggested that prevention of SUDEP should include reduction of GTCS than AEDs alone. Some new detection devices are under study such as ‘wearable apnoea detection devices that could help prevent SUDEP’70.
Selective serotonin reuptake inhibitors on chronic administration have been found to prevent seizure-induced sudden cardiac death in chronic SUDEP model71. Furthermore, adenosine and opiod antagonists have also been under study for the prevention72.
Risk assessment
Studies have shown that neurophysicians fail to tell the risk of SUDEP amongst patients with epilepsy73. This may be due to non-availability of biomarkers or inventories. New SUDEP-7 inventory has been developed to help in risk assessment74. They correlated the risk of SUDEP with heart rate variability. Root mean square differences of successive R-R intervals (RMSSD), a measure of low-frequency heart rate variability (HRV), was significantly associated with SUDEP Risk Inventory (SUDEP-7) scores74. RMSSD is independent of respiratory effects and measure of vagal function. HRV is a measure of autonomic function, which can be assessed as a biomarker in SUDEP patients49,50. Low heart rate variability has been found in patients with SUDEP; however, these findings need further validation51. PGES duration has also been suggested to be useful as biomarkers75; however, its role in SUDEP is not proved.
Conclusion
SUDEP is an important yet under recognized aspect of epilepsy care. Several risk factors and possible predictors of SUDEP have been identified through epidemiological, clinical and experimental studies. Nevertheless, the precise mechanisms and possible preventive measures are yet to be found out. Health care professionals need to be aware of this condition and need to discuss regarding SUDEP with patients and their relatives for the better treatment and prevention of the same. Wider discussion and long term research programs are necessary in this direction.
Footnotes
Conflicts of Interest: None.
References
- 1.Gaitatzis A, Johnson AL, Chadwick DW, Shorvon SD, Sander JW. Life expectancy in people with newly diagnosed epilepsy. Brain. 2004;127(Pt 11):2427–32. doi: 10.1093/brain/awh267. [DOI] [PubMed] [Google Scholar]
- 2.Sander JW, Bell GS. Reducing mortality: An important aim of epilepsy management. J Neurol Neurosurg Psychiatry. 2004;75:349–51. doi: 10.1136/jnnp.2003.029223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ridsdale L, Charlton J, Ashworth M, Richardson MP, Gulliford MC. Epilepsy mortality and risk factors for death in epilepsy: A population-based study. Br J Gen Pract. 2011;61:e271–8. doi: 10.3399/bjgp11X572463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpio A, Bharucha NE, Jallon P, Beghi E, Campostrini R, Zorzetto S, et al. Mortality of epilepsy in developing countries. Epilepsia. 2005;46(Suppl 11):28–32. doi: 10.1111/j.1528-1167.2005.00404.x. [DOI] [PubMed] [Google Scholar]
- 5.Banerjee TK, Ray BK, Das SK, Hazra A, Ghosal MK, Chaudhuri A, et al. A longitudinal study of epilepsy in Kolkata, India. Epilepsia. 2010;51:2384–91. doi: 10.1111/j.1528-1167.2010.02740.x. [DOI] [PubMed] [Google Scholar]
- 6.Thomas SV, Reghunath B, Sankara Sarma P. Mortality among epilepsy patients attending a tertiary referral center in a developing country. Seizure. 2001;10:370–3. doi: 10.1053/seiz.2000.0514. [DOI] [PubMed] [Google Scholar]
- 7.Bell GS, Neligan A, Sander JW. An unknown quantity - The worldwide prevalence of epilepsy. Epilepsia. 2014;55:958–62. doi: 10.1111/epi.12605. [DOI] [PubMed] [Google Scholar]
- 8.Yemadje LP, Houinato D, Quet F, Druet-Cabanac M, Preux PM. Understanding the differences in prevalence of epilepsy in tropical regions. Epilepsia. 2011;52:1376–81. doi: 10.1111/j.1528-1167.2011.03099.x. [DOI] [PubMed] [Google Scholar]
- 9.DeToledo JC, DeToledo MB, Lowe MR. Epilepsy and sudden death: Notes from George Washington's diaries on the illness and death of Martha Parke-Custis (1756-1773) Epilepsia. 1999;40:1835–6. doi: 10.1111/j.1528-1157.1999.tb01608.x. [DOI] [PubMed] [Google Scholar]
- 10.Miller WR, Young N, Friedman D, Buelow JM, Devinsky O. Discussing sudden unexpected death in epilepsy (SUDEP) with patients: Practices of health-care providers. Epilepsy Behav. 2014;32:38–41. doi: 10.1016/j.yebeh.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramachandran Nair R, Jack SM, Strohm S. SUDEP: To discuss or not? Recommendations from bereaved relatives. Epilepsy Behav. 2016;56:20–5. doi: 10.1016/j.yebeh.2015.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Nashef L. Sudden unexpected death in epilepsy: Terminology and definitions. Epilepsia. 1997;38(11 Suppl):S6–8. doi: 10.1111/j.1528-1157.1997.tb06130.x. [DOI] [PubMed] [Google Scholar]
- 13.Leestma JE, Annegers JF, Brodie MJ, Brown S, Schraeder P, Siscovick D, et al. Sudden unexpected death in epilepsy. Observations from a large clinical developmental program. Epilepsia. 1997;38:47–55. doi: 10.1111/j.1528-1157.1997.tb01076.x. [DOI] [PubMed] [Google Scholar]
- 14.Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia. 2012;53:227–33. doi: 10.1111/j.1528-1167.2011.03358.x. [DOI] [PubMed] [Google Scholar]
- 15.Neligan A, Bell GS, Johnson AL, Goodridge DM, Shorvon SD, Sander JW. The long-term risk of premature mortality in people with epilepsy. Brain. 2011;134(Pt 2):388–95. doi: 10.1093/brain/awq378. [DOI] [PubMed] [Google Scholar]
- 16.Ficker DM, So EL, Shen WK, Annegers JF, O’Brien PC, Cascino GD, et al. Population-based study of the incidence of sudden unexplained death in epilepsy. Neurology. 1998;51:1270–4. doi: 10.1212/wnl.51.5.1270. [DOI] [PubMed] [Google Scholar]
- 17.Tennis P, Cole TB, Annegers JF, Leestma JE, McNutt M, Rajput A. Cohort study of incidence of sudden unexplained death in persons with seizure disorder treated with antiepileptic drugs in Saskatchewan, Canada. Epilepsia. 1995;36:29–36. doi: 10.1111/j.1528-1157.1995.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 18.Vlooswijk MC, Majoie HJ, De Krom MC, Tan IY, Aldenkamp AP. SUDEP in the Netherlands: A retrospective study in a tertiary referral center. Seizure. 2007;16:153–9. doi: 10.1016/j.seizure.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Pack AM. Sudden unexpected death in the epilepsy monitoring unit. Epilepsy Curr. 2014;14:78–80. doi: 10.5698/1535-7597-14.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohanraj R, Norrie J, Stephen LJ, Kelly K, Hitiris N, Brodie MJ. Mortality in adults with newly diagnosed and chronic epilepsy: A retrospective comparative study. Lancet Neurol. 2006;5:481–7. doi: 10.1016/S1474-4422(06)70448-3. [DOI] [PubMed] [Google Scholar]
- 21.Nashef L, Fish DR, Sander JW, Shorvon SD. Incidence of sudden unexpected death in an adult outpatient cohort with epilepsy at a tertiary referral centre. J Neurol Neurosurg Psychiatry. 1995;58:462–4. doi: 10.1136/jnnp.58.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dasheiff RM. Sudden unexpected death in epilepsy: A series from an epilepsy surgery program and speculation on the relationship to sudden cardiac death. J Clin Neurophysiol. 1991;8:216–22. [PubMed] [Google Scholar]
- 23.Nilsson L, Ahlbom A, Farahmand BY, Tomson T. Mortality in a population-based cohort of epilepsy surgery patients. Epilepsia. 2003;44:575–81. doi: 10.1046/j.1528-1157.2003.03302.x. [DOI] [PubMed] [Google Scholar]
- 24.Sillanpää M, Shinnar S. Long-term mortality in childhood-onset epilepsy. N Engl J Med. 2010;363:2522–9. doi: 10.1056/NEJMoa0911610. [DOI] [PubMed] [Google Scholar]
- 25.Weber P, Bubl R, Blauenstein U, Tillmann BU, Lütschg J. Sudden unexplained death in children with epilepsy: A cohort study with an eighteen-year follow-up. Acta Paediatr. 2005;94:564–7. doi: 10.1111/j.1651-2227.2005.tb01940.x. [DOI] [PubMed] [Google Scholar]
- 26.Sillanpää M, Shinnar S. SUDEP and other causes of mortality in childhood-onset epilepsy. Epilepsy Behav. 2013;28:249–55. doi: 10.1016/j.yebeh.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Edey S, Moran N, Nashef L. SUDEP and epilepsy-related mortality in pregnancy. Epilepsia. 2014;55:e72–4. doi: 10.1111/epi.12621. [DOI] [PubMed] [Google Scholar]
- 28.Langan Y, Nolan N, Hutchinson M. The incidence of sudden unexpected death in epilepsy (SUDEP) in South Dublin and Wicklow. Seizure. 1998;7:355–8. doi: 10.1016/s1059-1311(05)80002-0. [DOI] [PubMed] [Google Scholar]
- 29.Walczak TS, Leppik IE, D’Amelio M, Rarick J, So E, Ahman P, et al. Incidence and risk factors in sudden unexpected death in epilepsy: A prospective cohort study. Neurology. 2001;56:519–25. doi: 10.1212/wnl.56.4.519. [DOI] [PubMed] [Google Scholar]
- 30.Nilsson L, Farahmand BY, Persson PG, Thiblin I, Tomson T. Risk factors for sudden unexpected death in epilepsy: A case-control study. Lancet. 1999;353:888–93. doi: 10.1016/s0140-6736(98)05114-9. [DOI] [PubMed] [Google Scholar]
- 31.Aurlien D, Larsen JP, Gjerstad L, Taubøll E. Increased risk of sudden unexpected death in epilepsy in females using lamotrigine: A nested, case-control study. Epilepsia. 2012;53:258–66. doi: 10.1111/j.1528-1167.2011.03334.x. [DOI] [PubMed] [Google Scholar]
- 32.Berg AT, Nickels K, Wirrell EC, Geerts AT, Callenbach PM, Arts WF, et al. Mortality risks in new-onset childhood epilepsy. Pediatrics. 2013;132:124–31. doi: 10.1542/peds.2012-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Opeskin K, Berkovic SF. Risk factors for sudden unexpected death in epilepsy: A controlled prospective study based on coroners cases. Seizure. 2003;12:456–64. doi: 10.1016/s1059-1311(02)00352-7. [DOI] [PubMed] [Google Scholar]
- 34.Jick SS, Cole TB, Mesher RA, Tennis P, Jick H. Idiopathic epilepsy and sudden unexplained death. Pharmacoepidemiol Drug Saf. 1992;1:59–64. [Google Scholar]
- 35.Tomson T, Hirsch LJ, Friedman D, Bester N, Hammer A, Irizarry M, et al. Sudden unexpected death in epilepsy in lamotrigine randomized-controlled trials. Epilepsia. 2013;54:135–40. doi: 10.1111/j.1528-1167.2012.03689.x. [DOI] [PubMed] [Google Scholar]
- 36.Dibué M, Kamp MA, Neumaier F, Steiger HJ, Hänggi D, Hescheler J, et al. Cardiac phenomena during kainic-acid induced epilepsy and lamotrigine antiepileptic therapy. Epilepsy Res. 2014;108:666–74. doi: 10.1016/j.eplepsyres.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 37.Kloster R, Engelskjøn T. Sudden unexpected death in epilepsy (SUDEP): A clinical perspective and a search for risk factors. J Neurol Neurosurg Psychiatry. 1999;67:439–44. doi: 10.1136/jnnp.67.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhuo L, Zhang Y, Zielke HR, Levine B, Zhang X, Chang L, et al. Sudden unexpected death in epilepsy: Evaluation of forensic autopsy cases. Forensic Sci Int. 2012;223:171–5. doi: 10.1016/j.forsciint.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 39.Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, et al. Combined analysis of risk factors for SUDEP. Epilepsia. 2011;52:1150–9. doi: 10.1111/j.1528-1167.2010.02952.x. [DOI] [PubMed] [Google Scholar]
- 40.Tao JX, Yung I, Lee A, Rose S, Jacobsen J, Ebersole JS. Tonic phase of a generalized convulsive seizure is an independent predictor of postictal generalized EEG suppression. Epilepsia. 2013;54:858–65. doi: 10.1111/epi.12094. [DOI] [PubMed] [Google Scholar]
- 41.Lamberts RJ, Gaitatzis A, Sander JW, Elger CE, Surges R, Thijs RD. Postictal generalized EEG suppression: An inconsistent finding in people with multiple seizures. Neurology. 2013;81:1252–6. doi: 10.1212/WNL.0b013e3182a6cbeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Skluzacek JV, Watts KP, Parsy O, Wical B, Camfield P. Dravet syndrome and parent associations: The IDEA League experience with comorbid conditions, mortality, management, adaptation, and grief. Epilepsia. 2011;52(Suppl 2):95–101. doi: 10.1111/j.1528-1167.2011.03012.x. [DOI] [PubMed] [Google Scholar]
- 43.Sakauchi M, Oguni H, Kato I, Osawa M, Hirose S, Kaneko S, et al. Mortality in Dravet syndrome: Search for risk factors in Japanese patients. Epilepsia. 2011;52(Suppl 2):50–4. doi: 10.1111/j.1528-1167.2011.03002.x. [DOI] [PubMed] [Google Scholar]
- 44.Le Gal F, Korff CM, Monso-Hinard C, Mund MT, Morris M, Malafosse A, et al. A case of SUDEP in a patient with Dravet syndrome with SCN1A mutation. Epilepsia. 2010;51:1915–8. doi: 10.1111/j.1528-1167.2010.02691.x. [DOI] [PubMed] [Google Scholar]
- 45.Aurlien D, Leren TP, Taubøll E, Gjerstad L. New SCN5A mutation in a SUDEP victim with idiopathic epilepsy. Seizure. 2009;18:158–60. doi: 10.1016/j.seizure.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 46.Auerbach DS, Jones J, Clawson BC, Offord J, Lenk GM, Ogiwara I, et al. Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome. PLoS One. 2013;8:e77843. doi: 10.1371/journal.pone.0077843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glasscock E. Genomic biomarkers of SUDEP in brain and heart. Epilepsy Behav. 2014;38:172–9. doi: 10.1016/j.yebeh.2013.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zijlmans M, Flanagan D, Gotman J. Heart rate changes and ECG abnormalities during epileptic seizures: Prevalence and definition of an objective clinical sign. Epilepsia. 2002;43:847–54. doi: 10.1046/j.1528-1157.2002.37801.x. [DOI] [PubMed] [Google Scholar]
- 49.Mukherjee S, Tripathi M, Chandra PS, Yadav R, Choudhary N, Sagar R, et al. Cardiovascular autonomic functions in well-controlled and intractable partial epilepsies. Epilepsy Res. 2009;85:261–9. doi: 10.1016/j.eplepsyres.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 50.Sathyaprabha TN, Satishchandra P, Netravathi K, Sinha S, Thennarasu K, Raju TR. Cardiac autonomic dysfunctions in chronic refractory epilepsy. Epilepsy Res. 2006;72:49–56. doi: 10.1016/j.eplepsyres.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Lotufo PA, Valiengo L, Benseñor IM, Brunoni AR. A systematic review and meta-analysis of heart rate variability in epilepsy and antiepileptic drugs. Epilepsia. 2012;53:272–82. doi: 10.1111/j.1528-1167.2011.03361.x. [DOI] [PubMed] [Google Scholar]
- 52.Kataoka M, Ito C, Sasaki H, Yamane K, Kohno N. Low heart rate variability is a risk factor for sudden cardiac death in type 2 diabetes. Diabetes Res Clin Pract. 2004;64:51–8. doi: 10.1016/j.diabres.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Kiviniemi AM, Tulppo MP, Wichterle D, Hautala AJ, Tiinanen S, Seppänen T, et al. Novel spectral indexes of heart rate variability as predictors of sudden and non-sudden cardiac death after an acute myocardial infarction. Ann Med. 2007;39:54–62. doi: 10.1080/07853890600990375. [DOI] [PubMed] [Google Scholar]
- 54.Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, et al. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): A retrospective study. Lancet Neurol. 2013;12:966–77. doi: 10.1016/S1474-4422(13)70214-X. [DOI] [PubMed] [Google Scholar]
- 55.Moseley BD, Wirrell EC, Nickels K, Johnson JN, Ackerman MJ, Britton J. Electrocardiographic and oximetric changes during partial complex and generalized seizures. Epilepsy Res. 2011;95:237–45. doi: 10.1016/j.eplepsyres.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Lamberts RJ, Thijs RD, Laffan A, Langan Y, Sander JW. Sudden unexpected death in epilepsy: People with nocturnal seizures may be at highest risk. Epilepsia. 2012;53:253–7. doi: 10.1111/j.1528-1167.2011.03360.x. [DOI] [PubMed] [Google Scholar]
- 57.Lhatoo SD, Faulkner HJ, Dembny K, Trippick K, Johnson C, Bird JM. An electroclinical case-control study of sudden unexpected death in epilepsy. Ann Neurol. 2010;68:787–96. doi: 10.1002/ana.22101. [DOI] [PubMed] [Google Scholar]
- 58.Surges R, Strzelczyk A, Scott CA, Walker MC, Sander JW. Postictal generalized electroencephalographic suppression is associated with generalized seizures. Epilepsy Behav. 2011;21:271–4. doi: 10.1016/j.yebeh.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 59.Tupal S, Faingold CL. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia. 2006;47:21–6. doi: 10.1111/j.1528-1167.2006.00365.x. [DOI] [PubMed] [Google Scholar]
- 60.Toczek MT, Carson RE, Lang L, Ma Y, Spanaki MV, Der MG, et al. PET imaging of 5HT1A receptor binding in patients with temporal lobe epilepsy. Neurology. 2003;60:749–56. doi: 10.1212/01.wnl.0000049930.93113.20. [DOI] [PubMed] [Google Scholar]
- 61.Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: Contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J Appl Physiol (1985) 2010;108:1425–32. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–50. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, et al. Multiple serotonergic abnormalities in sudden infant death syndrome. JAMA. 2006;296:21254–32. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 64.Shen HY, Li T, Boison D. A novel mouse model for sudden unexpected death in epilepsy (SUDEP): Role of impaired adenosine clearance. Epilepsia. 2010;51:465–8. doi: 10.1111/j.1528-1167.2009.02248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tortella FC, Long JB, Holaday JW. Endogenous opiate systems: Physiological role in the self limitation of seizures. Brain Res. 1985;332:174–8. doi: 10.1016/0006-8993(85)90403-2. [DOI] [PubMed] [Google Scholar]
- 66.Liebenthal JA, Wu S, Rose S, Ebersole JS, Tao JX. Association of prone position with sudden unexpected death in epilepsy. Neurology. 2015;84:703–9. doi: 10.1212/WNL.0000000000001260. [DOI] [PubMed] [Google Scholar]
- 67.Thorn M. Neuropathologic findings in postmortem studies of sudden death in epilepsy. Epilepsia. 1997;38(11 Suppl):S32–4. doi: 10.1111/j.1528-1157.1997.tb06123.x. [DOI] [PubMed] [Google Scholar]
- 68.Tu E, Bagnall RD, Duflou J, Semsarian C. Post-mortem review and genetic analysis of sudden unexpected death in epilepsy (SUDEP) cases. Brain Pathol. 2011;21:201–8. doi: 10.1111/j.1750-3639.2010.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Catcheside PG, Mohtar AA, Reynolds KJ. Airflow resistance and CO2 rebreathing properties of antiasphyxia pillows designed for epilepsy. Seizure. 2014;23:462–7. doi: 10.1016/j.seizure.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 70.Rodrigues-Villegas E, Chen G, Radcliffe J, Duncan J. A pilot study of wearable apnoea detection device. BMJ Open. 2014;4:e005299. doi: 10.1136/bmjopen-2014-005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Faingold CL, Tupal S, Randall M. Prevention of seizure-induced sudden death in a chronic SUDEP model by semichronic administration of a selective serotonin reuptake inhibitor. Epilepsy Behav. 2011;22:186–90. doi: 10.1016/j.yebeh.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 72.Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–80. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 73.Waddell B, McColl K, Turner C, Norman A, Coker A, White K. Are we discussing SUDEP?-A retrospective case note analysis. Seizure. 2013;22:74–6. doi: 10.1016/j.seizure.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 74.Novak JL, Miller PR, Markovic D, Meymandi SK, DeGiorgio CM. Risk assessment for Sudden Death in Epilepsy. The SUDEP-7 Inventory. Front Neurol. 2015;6:252. doi: 10.3389/fneur.2015.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moseley BD, So E, Wirrell EC, Nelson C, Lee RW, Mandrekar J, et al. Characteristics of postictal generalized EEG suppression in children. Epilepsy Res. 2013;106:123–7. doi: 10.1016/j.eplepsyres.2013.05.007. [DOI] [PubMed] [Google Scholar]


