Abstract
Background & objectives:
Type-1 diabetes mellitus (T1DM) and latent autoimmune diabetes in adults (LADA) share similar pathological features but differ in age of onset and progression. There is a scarcity of information on differences in CD4+ T-cell responses, particularly, cytokine secretion, between the two forms of autoimmune diabetes. Here proliferative potential and concentration of pro- and anti-inflammatory cytokines secreted by peripheral blood mononuclear cells (PBMCs) of T1DM and LADA patients were compared, after in vitro stimulation with β-cell autoantigens.
Methods:
A total of 19 patients with LADA, 37 with T1DM and 20 healthy controls were compared on the basis of lymphocyte proliferation and secretion of pro- and anti-inflammatory cytokines belonging to different T-helper types after in vitro stimulation of PBMCs with insulin and glutamic acid decarboxylase 65 (GAD65).
Results:
Following insulin stimulation, LADA group secreted higher concentration of interleukin-17 (IL-17) (P=0.02) and had higher proportion of interferon gamma (IFN-γ) secretors (P<0.001) than T1DM group. Post-GAD65 stimulation, higher proportion of LADA patients secreted IL-23 than T1DM group (P=0.02). Proportion of responders, as well as levels of secreted IL-10, were significantly higher in LADA than T1DM group, following stimulation with both insulin (P=0.01) and GAD65 (P=0.03). A significant positive correlation was observed between body mass index and IL-17 levels (r=0.41, P=0.04) and fasting plasma C-peptide with IL-10 levels (r=0.37, P=0.04).
Interpretation & conclusions:
There are differences in the portfolio of cytokine secretion in diabetic subjects with varying rates of β-cell destruction as LADA subjects secrete higher levels of both pro- and anti-inflammatory cytokines on exposure to β-cell autoantigens, thus highlighting another distinguishing feature in the pathophysiology of the two forms of autoimmune diabetes.
Keywords: CD4+ T-cells, cytokines, glutamic acid decarboxylase 65, insulin, latent autoimmune diabetes in adults, type-1 diabetes mellitus
Type-1 diabetes mellitus (T1DM) is an organ-specific autoimmune disease caused by selective destruction of insulin-producing β-cells in the pancreatic islets mainly by the autoreactive T-cells. A slower progressive form of this disease latent autoimmune diabetes in adults (LADA) has similar pathological features and accounts for 10 per cent of total diabetes cases but usually misdiagnosed as type-2 diabetes mellitus (T2DM)1,2. Insulin and glutamic acid decarboxylase 65 (GAD65) play an important role in disease pathogenesis as these are the earliest autoantigens recognized by autoreactive CD4+ T-cells3,4. Studies from India have also reported prevalence of islet-associated antibodies in patients with varying durations of T1DM5,6.
Cytokines are important inflammatory mediators and are implicated in the pathogenesis of autoimmune diabetes7. While the role of interferon gamma (IFN-γ) has been well documented as a major proinflammatory cytokine8, the role of interleukin-17 (IL-17) is increasingly being recognized in the pathogenesis of autoimmune diabetes9,10. It is suggested that T-helper 17 (Th17) cells may be actively involved in early stages of T1DM as higher levels of IL-17 coupled with increased IL-17 gene expression were detected in new-onset patients as compared to long-standing ones9,11. IL-6 has also been implicated in pathogenesis of both T1DM and T2DM as an important proinflammatory mediator12. To suppress autoreactive T-cell responses, regulatory T-cells (Tregs) produce anti-inflammatory cytokines, IL-10 and transforming growth factor beta13. In T1DM patients, Tregs are functionally defective with markedly reduced suppressive activity14,15. Another Th2 cytokine IL-4 counter-regulates the effects of IFN-γ16.
In humans, it is difficult to study local cytokine milieuduring disease progression due to inaccessibility of pancreas. LADA represents a unique subset of autoimmune diabetes with slow but ongoing β-cell destruction, and these individuals can provide valuable information on CD4+ T-cell function during disease progression. We observed decreased proliferative potential and pathogenicity of preproinsulin-specific CD8+ T-cells in individuals with LADA as compared to T1DM patients, suggestive of lower intensity of CD4+ T-cell pro-inflammatory responses in these individuals17. There are a few reports on comparison of cytokines in peripheral circulation in patients with T1DM and LADA, but they did not observe any distinction between the two groups18,19,20. One such study compared secretion of IFN-γ and IL-13 in patients with T1DM and LADA following stimulation with β-cell-associated peptides, and demonstrated no significant differences between the two groups in terms of number of cytokine-producing cells20. The present work was aimed to study differences in patients with T1DM and LADA in the proliferative potential of lymphocytes and secretion of cytokines belonging to different Th types, including IFN-γ, IL-17, IL-23, IL-6, IL-10 and IL-4 following in vitro stimulation of their peripheral blood mononuclear cells (PBMCs) with major β-cell autoantigens, insulin and GAD65.
Material & Methods
The study was conducted in the department of Endocrinology at the Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, from May 2012 to April 2015. A total of 76 individuals, consisting of 37 recently diagnosed T1DM patients and 19 with LADA, were selected from the departments of Paediatrics and Endocrinology, PGIMER, along with 20 age- and sex-matched non-diabetic healthy controls (HCs), majority of whom were siblings of T1DM and LADA patients. Diabetes was diagnosed as per the American Diabetes Association criteria21. Inclusion criteria for autoimmune diabetes were the presence of autoantibodies to GAD65 or islet antigen-2 or islet cell antigen or insulin. Inclusion criteria for LADA were age >25 yr, positive for at least one of the autoantibodies and insulin independence for at least six months after diagnosis of diabetes; in addition, none of the LADA patients had received exogenous insulin therapy at the time of recruitment. Exclusion criteria included anaemia (Hb <8.0 g/dl), any acute illness, other autoimmune diseases (including celiac disease), lymphomas, psychiatric illness and pregnancy.
The study protocol was approved by the Institutional Ethics Committee. After obtaining informed consent in writing, fasting peripheral blood samples were obtained from all participants in heparinized vacutainers. In addition to screening for autoantibodies, titres of anti-insulin (Orgentec Diagnostika, Mainz, Germany) and anti-GAD65 (Aesku Diagnostics, Wendelsheim, Germany) antibodies were analyzed by ELISA. The minimum detectable level (with intra-, inter-assay CV) for anti-insulin was 0.5 U/ml (3.1, 5.1%) and anti-GAD65 was 6 IU/ml (7.9, 7.3%). Fasting plasma C-peptide levels were determined by electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany), while glycated haemoglobin (HbA1c) was determined by spectrophotometry following cation-exchange chromatography (Bio-Rad, Hercules, USA).
Peripheral blood mononuclear cells (PBMCs) stimulation and proliferation: PBMCs were isolated by density-gradient centrifugation with Ficoll. For stimulation, 106 PBMCs per well were cultured in duplicate separately with optimized concentrations of insulin (10.0 μg/ml) and GAD65 (5.0 μg/ml) (Sigma-Aldrich, USA) in RPMI-1640 medium, supplemented with 0.1 per cent penicillin-streptomycin and 10 per cent foetal calf serum in 24-well tissue culture plates for 72 h at 37°C with 5 per cent CO217,22. After completion of incubation period, culture supernatants were collected by centrifugation and stored at −80°C in triplicates to avoid multiple freeze-thaw cycles until cytokine analysis. Lymphocyte proliferation was assessed by flow cytometry using carboxyfluorescein succinimidyl ester (CFSE) dye dilution assay23. Briefly, 2×106 PBMCs were stained with CFSE dye, of which 2×105 cells were acquired along with unstained cells and 2×105 cells/well were stimulated in triplicates. After incubation, cells were harvested, acquired and analyzed for proliferation on flow cytometer (FACS Canto II, BD Biosciences, USA). In all experiments, phytohaemagglutinin (PHA) (5.0 μg/ml) was used as positive control.
Extracellular cytokine analysis: ELISA was performed to determine the concentrations of cytokines in culture supernatants including, IFN-γ, IL-4 (Diaclone, Cedex, France), IL-10, IL-17 (subunits A-F) (Boster Biological Technology, Pleasanton, USA), IL-23 (Cusabio Biotech, Wuhan, China) and IL-6 (Mabtech, Nacka Strand, Sweden) as per manufacturer's instructions. The minimum detectable level (with intra-, inter-assay CV) for IL-4 was 0.31 pg/ml (9.2, 13.7%), IFN-γ, 0.69 pg/ml (3.9, 8.6%), IL-23, 6.25 pg/ml (8, 10%), IL-17, 1.0 pg/ml (5.9, 8.4%), IL-10, 0.5 pg/ml (5.7, 7.4%) and IL-6, 1.0 pg/ml (2.5, 3.8%), respectively. In case of GAD65 stimulation, culture supernatants of only T1DM and LADA groups were analyzed. IL-6 secretion was analyzed only in case of in vitro stimulation with insulin. Responders were defined as those secreting detectable amount of a cytokine at the end of the incubation period, as compared to baseline secretion at zero hour. Responders were compared between T1DM and LADA groups, as well as between HC and diabetic (T1DM+LADA) groups.
Statistical analysis: For comparison, the diabetic group comprised both T1DM and LADA patients taken together and results were presented as mean±standard error of mean (SEM). Unpaired t test was used to compare means between different groups, while Mann–Whitney U test was used when data were not normally distributed. Fisher's exact test was used to analyze differences in the proportions of patients secreting detectable amount of cytokines. Bivariate correlations between concentrations of individual cytokines with body mass index (BMI), fasting plasma C-peptide levels, anti-insulin and anti-GAD65 antibody titres were analyzed by Spearman's correlation test. Multivariate regression analysis was performed to investigate differences of cytokine concentrations (dependent variables) between different participant groups adjusted for BMI, age and C-peptide (independent variables). The data were log transformed to normalize the values of cytokines. Five models were used, which were adjusted for different combinations of variables: model 1, age; model 2, age and BMI; model 3, age, BMI and C-peptide; model 4, C-peptide; model 5, age and C-peptide; model-6, HbA1c. All the statistical analyses were performed using Graphpad Prism (v-4.0, La Jolla, CA, USA), SPSS (version 21.0, Armonk, NY, USA) and Microsoft Office Excel (2007).
Results
The mean (±SEM) age of LADA group individuals (28.8±1.6 yr) was significantly higher than T1DM patients (7.9±0.5 yr) (P<0.01) at the time of enrolment. Mean fasting plasma C-peptide levels were significantly higher in LADA (1.09±0.19 ng/ml) than T1DM patients (0.48±0.08 ng/ml) (P<0.01), whereas mean HbA1c (%, mmol/mol) was significantly higher in T1DM (11.65±0.44, 103±2.5) versus those with LADA (9.56±0.42, 81±2.2) (P<0.01). None of the participants had a history of any other autoimmune disease including celiac disease. The BMI of T1DM patients (16.34±0.69 kg/m2) was significantly lower than those with LADA (20.27±0.74 kg/m2) (P<0.01). Among those with diabetes, percentage of patients with T1DM and LADA demonstrating anti-insulin and anti-GAD65 antibodies had no significant difference (Table I). On comparison of antibody titres, no significant difference was observed between both the groups (data not shown).
Table I.
Clinical characteristics of patients and healthy controls and proliferation indices of their lymphocytes following in vitro stimulation with insulin, GAD65 and PHA
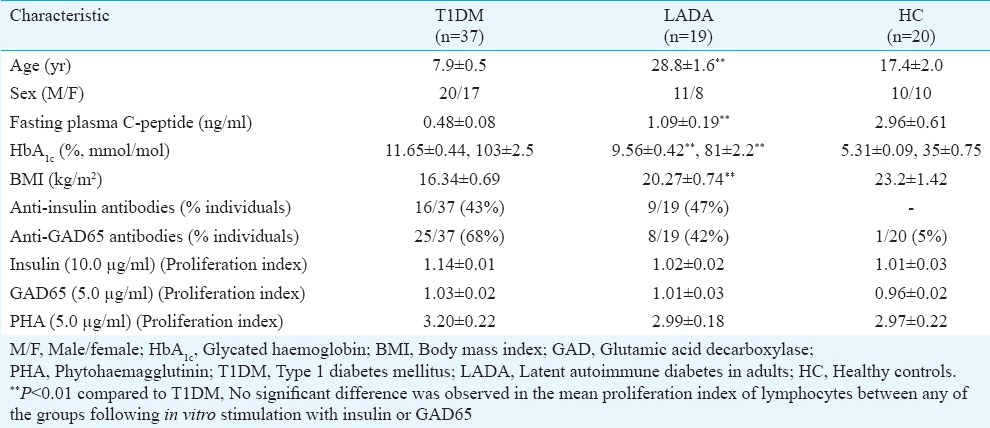
Lymphocytes proliferation following exposure to β-cell autoantigens: Following in vitro stimulation with insulin and GAD65, proliferation of lymphocytes was observed in all individuals (Fig. 1) and the mean proliferation index of lymphocytes was similar in patients with T1DM, LADA and HCs (Table I).
Fig. 1.
Representative images showing proliferation of lymphocytes stained with carboxyfluorescein succinimidyl ester (CFSE) following in vitro stimulation with autoantigens, insulin (10.0 μg/ml) and glutamic acid decarboxylase 65 (GAD65, 5.0 μg/ml), positive control, phytohaemagglutinin (PHA, 5.0 μg/ml) and negative control, media control (MC). (A) Lymphocytes were gated according to forward and side scatter. Histograms represent cells stimulated with (B) PHA, (C) media control, (D) insulin, (E) GAD65.
Cytokine analysis: Cytokine responses were clustered into four groups based on the type of cytokines secreted, as proinflammatory i.e. Th1 (IFN-γ) and Th17 (IL-17 and IL-23) and anti-inflammatory i.e. Treg (IL-10) and Th2 (IL-4, IL-6). In unstimulated cultures, the mean concentration of all cytokines, except IL-10 (3.6±0.25 pg/ml), was negligible compared to the insulin and GAD65 stimulated cultures.
IFN-γ secretion in patients with LADA following insulin stimulation: After in vitro stimulation with insulin, IFN-γ secretion was observed from PBMCs of all of the 19 (100%) LADA patients, significantly higher than those with T1DM (22/37, 59.5%) (P<0.001) and HC subjects (9/20, 45%) (P=0.03) (Fig. 2A). However, comparison of mean concentration of secreted IFN-γ did not yield any significant difference between the T1DM (19.94±6.19 pg/ml) and LADA group (16.28±3.4 pg/ml). No significant difference was observed between the diabetic (18.35±3.74 pg/ml) and HC groups (9.20±2.70 pg/ml) (Fig. 3A) in terms of IFN-γ secretion. In response to in vitro stimulation with GAD65, the number of responders was similar in both T1DM (28/37, 76%) and LADA (13/19, 68%) groups (Fig. 2B). Likewise, both T1DM (9.89±3.43 pg/ml) and LADA patients (9.32±3.16 pg/ml) demonstrated similar levels of IFN-γ (Fig. 4A).
Fig. 2.

Percentage of individuals secreting various cytokines following in vitro stimulation of peripheral blood mononuclear cells. (A) Post-insulin stimulation, latent autoimmune diabetes in adults (LADA) group showed significantly higher number of responders secreting interferon gamma (IFN-γ) and interleukin-10 (IL-10). (B) Post-glutamic acid decarboxylase 65 (GAD-65) stimulation, LADA group showed significantly higher responders secreting IL-23 and IL-10. Interleukin-6 secretion was compared only in case of insulin stimulation. Responders were defined as those secreting detectable cytokine levels at the end of incubation period, as compared to zero hour.
Fig. 3.
Extracellular levels of cytokines, (A) IFN-γ, (B) IL-17, (C) IL-23, (D) IL-10, (E) IL-4 and (F) IL-6 in culture supernatants of peripheral blood mononuclear cell (PBMC) cultures in type-1 diabetes mellitus (T1DM), LADA and healthy control (HC) groups after in vitro stimulation with insulin. Values are shown as mean±standard error of mean. Levels of secreted interleukin-17 (P=0.02) and interleukin-10 (P=0.01) were significantly higher in LADA group than T1DM group post insulin stimulation. PBMCs of HC group secreted very low levels of IL-6 that could not be observed on the depicted scale.
Fig. 4.
Extracellular levels of cytokines, (A) IFN-γ, (B) IL-17, (C) IL-23, (D) IL-10 and (E) IL-4 in culture supernatants of peripheral blood mononuclear cells (PBMCs) isolated from T1DM and LADA group individuals after in vitro stimulation with GAD-65. Values are shown as mean±standard error of mean.
T-helper 17 (Th17) responses in LADA patients following stimulation with insulin and GAD65: To determine Th17 responses following in vitro stimulation of PBMCs with insulin and GAD65, concentrations of IL-17 and IL-23 were assessed. After stimulation with insulin, the number of patients secreting IL-17 was significantly higher in LADA (10/19, 53%) than T1DM groups (10/37, 27%) (P=0.08) (Fig. 2A) and mean concentration of secreted IL-17 was significantly higher in LADA (9.29±3.43 pg/ml) than T1DM group (1.18±0.56 pg/ml) (P=0.02) (Fig. 3B). Compared to the HC group (3/20, 15%), the proportion of responders were expectedly higher in diabetic group following insulin stimulation (P=0.09) (Fig. 2A). Following GAD65 stimulation, secretion of IL-17 was observed in only 9/37 (24%) T1DM and 8/19 (42%) LADA patients (P=0.22) (Fig. 2B), and the mean (±SEM) concentration of IL-17 was similar in both LADA (2.62±1.32 pg/ml) and T1DM subjects (2.29±1.75 pg/ml) (Fig. 4B).
IL-23 secretion by PBMCs in response to in vitro insulin stimulation was detected in most individuals, with all of the LADA, 32/37 (86%) T1DM patients and 13/20 HC (65%) demonstrating detectable IL-23 in culture supernatants (Fig. 2A). IL-23 secretion by PBMCs was similar in LADA (132.4±17.10 pg/ml) and T1DM patients (103.8±20.47 pg/ml) and no difference was observed between the diabetic (116.2±13.80 pg/ml) and HC group (81.01±15.82 pg/ml) (Fig. 3C). Following in vitro stimulation with GAD65, all of the LADA patients secreted detectable IL-23 which was significantly higher than T1DM patients (28/37, 76%) (P=0.02) (Fig. 2B). However, both the groups demonstrated similar levels of secreted IL-23 [T1DM (89.20±23.79 pg/ml) and LADA (129.9±13.01 pg/ml)] (Fig. 4C).
IL-10 secretion in LADA patients following stimulation with insulin and GAD65: In response to in vitro stimulation of PBMCs with insulin, 11 of 19 (58%) LADA patients secreted IL-10, which was significantly higher than those with T1DM (9/37, 24%) (P=0.02). Compared to diabetic group, the IL-10 producers were significantly higher in the HC group (17/20, 85%) (P<0.01) (Fig. 2A). On comparison of mean concentration significantly higher levels of IL-10 were observed in LADA (57.12±23.70 pg/ml) than in T1DM group (1.48±1.05 pg/ml) (P=0.01) (Fig. 3D). Overall, the production of IL-10 by PBMCs obtained from the diabetic group (25.33±11.60 pg/ml) was significantly lower than the HC group (32.91±17.01 pg/ml) (P=0.08). Like in case of insulin, stimulation with GAD65 yielded similar results with significantly higher proportion of LADA patients (8/19, 42%) secreting detectable IL-10 compared to T1DM patients (5/37, 13%) (P=0.02) (Fig. 2B), with LADA group (2.82±1.82 pg/ml) demonstrating higher mean concentration of secreted IL-10 than T1DM group (0.08±0.08 pg/ml) (P=0.03) (Fig. 4D).
IL-4 levels in T1DM & LADA patients following insulin and GAD65 stimulation: Following in vitro stimulation with insulin, PBMCs of most of the individuals secreted very low levels of IL-4. The number of responders was similar in T1DM (31/37, 84%) and LADA groups (13/19, 68%) (Fig. 2A), and no significant difference was observed in mean concentrations of IL-4 in both the groups [T1DM (0.19±0.06 pg/ml) and LADA (0.08±0.03 pg/ml)] (Fig. 3E). The diabetic and the HC groups also did not demonstrate any differences in the number of responders (14/20, 70%) and levels of secreted IL-4 (0.13±0.04 pg/ml vs. 0.13±0.05 pg/ml). Response to GAD65 stimulation was also analogous, with number of patients secreting detectable IL-4 being comparable in T1DM (18/37, 49%) and LADA group (13/19, 68%) (Fig. 2B), with no significant difference in levels of secreted IL-4 [T1DM (0.14±0.08 pg/ml) and LADA (0.16±0.05 pg/ml)] (Fig. 4E).
IL-6 secretion in T1DM & LADA patients following stimulation with insulin: After in vitro stimulation with insulin, IL-6 secretion was observed from PBMCs of all T1DM and LADA patients, significantly higher than the HC (2/20, 10%) (P<0.01) (Fig. 2A). On comparing the mean concentration of cytokines, higher levels were observed in T1DM (102.5±27.65 pg/ml) than LADA group (40.88±23.12 pg/ml) though the difference was not significant. The HCs (0.35±0.09 pg/ml) secreted very low levels of IL-6 than the diabetic group (75.11±19.11 pg/ml (P=0.05) (Fig. 3F).
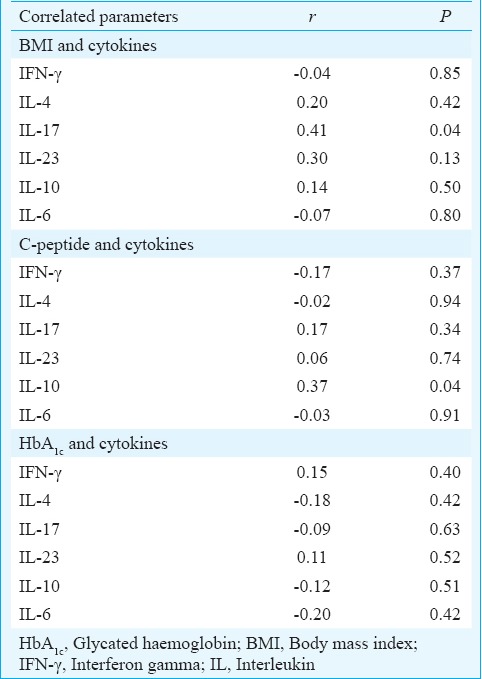
Positive association between IL-17 with BMI and IL-10 with C-peptide in autoimmune diabetes: All T1DM and LADA patients were pooled in one group, and correlation analysis was performed between concentration of individual cytokines secreted by PBMCs stimulated in vitro with insulin and BMI. Of all the cytokines, only IL-17 levels showed a significant positive correlation with BMI (r=0.41, P=0.04) (Table II). To observe the effect of remnant β-cell mass and hyperglycaemia on cytokine secretion pattern of PBMCs, fasting plasma C-peptide levels were correlated with HbA1c separately with concentrations of individual cytokines. A significant positive correlation was observed between IL-10 and C-peptide (r=0.37, P=0.04), whereas no correlation of fasting plasma C-peptide levels was observed with any of the other cytokines; further, no correlation was observed between HbA1c and any of the cytokine (Table II). Since insulin and GAD65 were used as major stimulants in cytokine analysis, correlation of anti-insulin and anti-GAD65 antibody titres with all cytokines was also assessed separately; however, no correlation was observed between antibody titres and any of the cytokines (data not shown).
Table II.
Correlation between levels of cytokines secreted by insulin stimulated peripheral blood mononuclear cells (PBMCs) with BMI, fasting plasma C-peptide and HbA1c in patients with autoimmune diabetes
Adjustment of confounding variables for analysis of cytokine data in relation to bivariate comparison of groups: The influence of potential confounding factors such as age, BMI, C-peptide and HbA1c was investigated on differences in levels of cytokines between the groups by employing various multiple regression models (Table III).
Table III.
Comparisons between LADA and T1DM groups following adjustment of confounding variables

After stepwise adjustment of confounding factors, the differences in the concentration of IL-17 between the T1DM and LADA patients were still observed. Although the concentration of IL-4 was low in both the groups as observed during bivariate comparison (Fig. 3), the mean levels became significantly lower in LADA group following adjustment analysis. For IL-10, the differences observed between the two groups were similar following stepwise adjustment of most models including HbA1c alone, except for model 1 and 5; for model 3, the difference was nearly significant (Table III).
Discussion
The underlying reasons for the slow progression of disease in LADA are not well understood. As indicated by plasma C-peptide levels, most of the LADA patients included in our study had appreciable residual β-cell mass at the time of diagnosis, suggestive of slower disease progression as compared to T1DM where β-cell mass was almost destroyed. This study was done to investigate cytokine profile of different Th types including proinflammatory (Th1, Th17) and anti-inflammatory (Treg, Th2) cytokines secreted by peripheral lymphocytes on exposure to β-cell autoantigens not only to understand differences if any, with the T1DM patients, but also to assess cytokines associated with disease progression, as patients with LADA represent those with ongoing pancreatic inflammation.
In our study it was possible to distinguish between T1DM and LADA patients on the basis of responders and concentration of secreted cytokines. This was in contrast to the previous reports advocating that T1DM and LADA are immunologically similar and cannot be differentiated on the basis of cytokine levels18,19. The LADA group in our study secreted higher levels of IL-17 after stimulation with insulin as compared to T1DM group. All patients were recently diagnosed, and hence, the duration of insulin therapy in T1DM patients was too less to have any impact on cytokine secretion following in vitro insulin stimulation. Similarly, more LADA patients secreted IFN-γ post-insulin stimulation and IL-23 post-GAD65 stimulation. In another previous ELISpot-based study20, IFN-γ secreting cells after GAD65 stimulation were similar in T1DM and LADA groups. Such discrepancies observed might be explained by differences in assay types, duration and concentration of stimuli and ethnicity of individuals. Increased secretion of proinflammatory cytokines by LADA patients could be attributed to the ongoing inflammation of β-cells, providing abundant antigenic target for the activated CD4+ T-cells, compared to T1DM patients, although it remains to be seen whether Th1 or Th17 responses prevail with the same intensity eventually when LADA patients loose β-cell mass and become insulin dependent. This finding was supported by the evidence of lower IL-4 production by the LADA group, following adjustment analysis, indicative of a poor Th2 response to counter the IFN-γ-mediated Th1 response. However, the IL-10 responses were higher in LADA group in terms of number of secretors and concentration, indicating that the immunosuppressive arm of the β-cell-specific Tregs was active and perhaps contributed to the slow rate of β-cell destruction. This was further confirmed when a positive correlation was observed between fasting plasma C-peptide levels and secreted IL-10 levels. It is known that IL-10 carries out immune regulation mainly by suppressing the release of inflammatory cytokines from other cell types, besides blocking B7/CD28 costimulatory pathway, inhibiting dendritic cell maturation and reducing major histocompatibility complex (MHC) class II expression24,25. Given the fact that β-cells die by multiple pathways, it must be noted that local cytokine milieu in the pancreas is a cumulative outcome of pro- and anti-inflammatory cytokines secreted by β-cell-specific CD4+ T-cells. Even though we did not investigate pancreatic IL-10 levels, it could be possible that there is local preponderance of IL-10 secreting CD4+ T-cells in the pancreatic region which is reflected in their measured peripheral T-cell responses. In addition, the concentration of IL-10 secreted by PBMCs of HCs was higher than diabetic patients, underlining the importance of functional Tregs14,15 and IL-10 in suppression of autoimmune diabetes24.
Most of our T1DM patients having negligible C-peptide levels demonstrated production of proinflammatory cytokines IL-17, IFN-γ, IL-6 and IL-23 while the levels of IL-10 and IL-4 were very low, indicating the dominance of proinflammatory over anti-inflammatory responses in T1DM. It confirms that memory CD4+ T-cells are still active in these patients even after absolute obliteration of β-cell mass and can mount a heightened pro-inflammatory response following re-exposure to β-cell autoantigens22,25. It has been suggested that initially there is inclination of autoreactive CD4+ T-cells towards a Th17 phenotype, which later transforms to Th1 type9. In this study, a significant difference was observed in the levels of secreted IL-17 between T1DM and LADA patients. Therefore, the role of local antigen presenting cells in priming β-cell-specific CD4+ T-cells towards a particular proinflammatory type may be relevant. Further, analysis of immunophenotype of antigen-specific CD4+ T-cells at initial stages of disease progression could provide more information about the cytokines involved and the environmental cues that favour a particular Th signature in pathogenesis of autoimmune diabetes. Differences in CD4+ T-cell immunophenotype are expected as observed in case of antigen-specific CD8+ T-cells reported by our group17.
Association between concentration of proinflammatory cytokines and obesity has been shown26. In our patients with autoimmune diabetes, particularly LADA, we observed a positive correlation between BMI and IL-17 indicating that IL-17 might be part of proinflammatory cytokines leading to high risk of diabetes, including type-2 diabetes, where its role is suggested by several studies19,27. Increased Th17 cells have been observed in obese individuals causing metabolic dysfunction in muscle and liver cells in vitro28; in addition, high IL-17 levels were observed in obese than lean individuals29. Although most of the LADA patients in our study did not have obese phenotype, their BMI was significantly higher than T1DM patients. Therefore, our data merely indicate but not enough to provide evidence that increased activity of IL-17 secreting CD4+ T-cells is influenced by BMI and contributes to disease progression in patients with LADA.
There were some limitations in this study, such as the sample size; however, it must be considered that LADA patients were difficult to find. The minimum age of LADA patients included was chosen as 25 yr so as to include more individuals with late-onset diabetes and compared them with significantly younger individuals with early onset i.e. T1DM. Most of the patients in our study were either pre-pubertal (T1DM) or adults (LADA); therefore, any puberty-associated variations in immune responses were automatically eliminated. It must also be noted that the cytokine secretion profile of patients was compared following stimulation with β-cell autoantigens and not just cytokines in circulation. The study design could be further improved by conducting a longitudinal follow up study involving stimulation of PBMCs obtained at different time points to get a better insight of cytokine secretion pattern at different stages of progression of autoimmune diabetes. In addition, the phenotype of T-cells, including activated/memory/regulatory subsets, co-expression of cytokines can be assessed to have a better picture of T-cell responses involved in disease progression. In this context, the results obtained from stimulation assays in this study are preliminary.
In conclusion, our findings demonstrated that patients with LADA could be distinguished from those with T1DM on the basis of concentrations of cytokines secreted by their peripheral lymphocytes stimulated with β-cell autoantigens, and differences in the cytokines secreted between the two variants of autoimmune diabetes. With no definite guidelines for therapy of patients with LADA to prolong insulin dependence, we speculate that these patients might have better prospects for immune therapies involving modulation of proinflammatory cytokines, like IL-17, particularly during the initial stages of the disease.
Acknowledgment
The study was supported by Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Government of India, New Delhi. Authors thank the technical staff of Department of Endocrinology, PGIMER, Chandigarh, for performing routine diagnostic investigations.
Footnotes
Conflicts of Interest: None.
References
- 1.Zimmet P, Turner R, McCarty D, Rowley M, Mackay I. Crucial points at diagnosis. Type 2 diabetes or slow type 1 diabetes. Diabetes Care. 1999;22(Suppl 2):B59–64. [PubMed] [Google Scholar]
- 2.Leslie RD, Kolb H, Schloot NC, Buzzetti R, Mauricio D, De Leiva A, et al. Diabetes classification: Grey zones, sound and smoke: Action LADA 1. Diabetes Metab Res Rev. 2008;24:511–9. doi: 10.1002/dmrr.877. [DOI] [PubMed] [Google Scholar]
- 3.Tuomilehto J, Vidgren G, Toivanen L, Tuomilehto-Wolf E, Kohtamaki K, Stengård J, et al. Antibodies to glutamic acid decarboxylase as predictors of insulin-dependent diabetes mellitus before clinical onset of disease. Lancet. 1994;343:1383–5. doi: 10.1016/s0140-6736(94)92521-6. [DOI] [PubMed] [Google Scholar]
- 4.Nakayama M, Abiru N, Moriyama H, Babaya N, Liu E, Miao D, et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature. 2005;435:220–3. doi: 10.1038/nature03523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh AK, Bhatia E, Dabadghao P, Bhatia V, Gellert SA, Colman PG. Role of islet autoimmunity in the aetiology of different clinical subtypes of diabetes mellitus in young North Indians. Diabet Med. 2000;17:275–80. doi: 10.1046/j.1464-5491.2000.00267.x. [DOI] [PubMed] [Google Scholar]
- 6.Tandon N, Shtauvere-Brameus A, Hagopian WA, Sanjeevi CB. Prevalence of ICA-12 and other autoantibodies in North Indian patients with early-onset diabetes. Ann N Y Acad Sci. 2002;958:214–7. doi: 10.1111/j.1749-6632.2002.tb02972.x. [DOI] [PubMed] [Google Scholar]
- 7.Nepom GT, Ehlers M, Mandrup-Poulsen T. Anti-cytokine therapies in T1D: Concepts and strategies. Clin Immunol. 2013;149:279–85. doi: 10.1016/j.clim.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldeón ME, Chun T, Gaskins HR. Interferon-alpha and interferon-gamma differentially affect pancreatic beta-cell phenotype and function. Am J Physiol. 1998;275(1 Pt 1):C25–32. doi: 10.1152/ajpcell.1998.275.1.C25. [DOI] [PubMed] [Google Scholar]
- 9.Arif S, Moore F, Marks K, Bouckenooghe T, Dayan CM, Planas R, et al. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated ß-cell death. Diabetes. 2011;60:2112–9. doi: 10.2337/db10-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grieco FA, Moore F, Vigneron F, Santin I, Villate O, Marselli L, et al. IL-17A increases the expression of proinflammatory chemokines in human pancreatic islets. Diabetologia. 2014;57:502–11. doi: 10.1007/s00125-013-3135-2. [DOI] [PubMed] [Google Scholar]
- 11.Han D, Leyva CA, Matheson D, Mineo D, Messinger S, Blomberg BB, et al. Immune profiling by multiple gene expression analysis in patients at-risk and with type 1 diabetes. Clin Immunol. 2011;139:290–301. doi: 10.1016/j.clim.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Targher G, Zenari L, Bertolini L, Muggeo M, Zoppini G. Elevated levels of interleukin-6 in young adults with type 1 diabetes without clinical evidence of microvascular and macrovascular complications. Diabetes Care. 2001;24:956–7. doi: 10.2337/diacare.24.5.956. [DOI] [PubMed] [Google Scholar]
- 13.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: A jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 15.Haseda F, Imagawa A, Murase-Mishiba Y, Terasaki J, Hanafusa T. CD4+ CD45RA- FoxP3high activated regulatory T cells are functionally impaired and related to residual insulin-secreting capacity in patients with type 1 diabetes. Clin Exp Immunol. 2013;173:207–16. doi: 10.1111/cei.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron MJ, Arreaza GA, Waldhauser L, Gauldie J, Delovitch TL. Immunotherapy of spontaneous type 1 diabetes in nonobese diabetic mice by systemic interleukin-4 treatment employing adenovirus vector-mediated gene transfer. Gene Ther. 2000;7:1840–6. doi: 10.1038/sj.gt.3301309. [DOI] [PubMed] [Google Scholar]
- 17.Sachdeva N, Paul M, Badal D, Kumar R, Jacob N, Dayal D, et al. Preproinsulin specific CD8+ T cells in subjects with latent autoimmune diabetes show lower frequency and different pathophysiological characteristics than those with type 1 diabetes. Clin Immunol. 2015;157:78–90. doi: 10.1016/j.clim.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Pham MN, Hawa MI, Pfleger C, Roden M, Schernthaner G, Pozzilli P, et al. Pro- and anti-inflammatory cytokines in latent autoimmune diabetes in adults, type 1 and type 2 diabetes patients: Action LADA 4. Diabetologia. 2011;54:1630–8. doi: 10.1007/s00125-011-2088-6. [DOI] [PubMed] [Google Scholar]
- 19.Xiang Y, Zhou P, Li X, Huang G, Liu Z, Xu A, et al. Heterogeneity of altered cytokine levels across the clinical spectrum of diabetes in China. Diabetes Care. 2011;34:1639–41. doi: 10.2337/dc11-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strom A, Menart B, Simon MC, Pham MN, Kolb H, Roden M, et al. Cellular interferon-γ and interleukin-13 immune reactivity in type 1, type 2 and latent autoimmune diabetes: Action LADA 6. Cytokine. 2012;58:148–51. doi: 10.1016/j.cyto.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–9. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monti P, Scirpoli M, Rigamonti A, Mayr A, Jaeger A, Bonfanti R, et al. Evidence for in vivo primed and expanded autoreactive T cells as a specific feature of patients with type 1 diabetes. J Immunol. 2007;179:5785–92. doi: 10.4049/jimmunol.179.9.5785. [DOI] [PubMed] [Google Scholar]
- 23.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–7. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 24.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: The role of T regulatory cells. Immunology. 2006;117:433–42. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chee J, Ko HJ, Skowera A, Jhala G, Catterall T, Graham KL, et al. Effector-memory T cells develop in islets and report islet pathology in type 1 diabetes. J Immunol. 2014;192:572–80. doi: 10.4049/jimmunol.1302100. [DOI] [PubMed] [Google Scholar]
- 26.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: The link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Jagannathan-Bogdan M, McDonnell ME, Shin H, Rehman Q, Hasturk H, Apovian CM, et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol. 2011;186:1162–72. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fabbrini E, Cella M, McCartney SA, Fuchs A, Abumrad NA, Pietka TA, et al. Association between specific adipose tissue CD4+ T-cell populations and insulin resistance in obese individuals. Gastroenterology. 2013;145:366–74.e1-3. doi: 10.1053/j.gastro.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond) 2009;33:151–6. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]





