Abstract
Background & objectives:
Hospital-acquired infections (HAIs) are a major challenge to patient safety and have serious public health implications by changing the quality of life of patients and sometimes causing disability or even death. The true burden of HAI remains unknown, particularly in developing countries. The objective of this study was to estimate point prevalence of HAI and study the associated risk factors in a tertiary care hospital in Pune, India.
Methods:
A series of four cross-sectional point prevalence surveys were carried out between March and August 2014. Data of each patient admitted were collected using a structured data entry form. Centers for Disease Control and Prevention guidelines were used to identify and diagnose patients with HAI.
Results:
Overall prevalence of HAI was 3.76 per cent. Surgical Intensive Care Unit (ICU) (25%), medical ICU (20%), burns ward (20%) and paediatric ward (12.17%) were identified to have significant association with HAI. Prolonged hospital stay [odds ratio (OR=2.81), mechanical ventilation (OR=18.57), use of urinary catheter (OR=7.89) and exposure to central air-conditioning (OR=8.59) had higher odds of acquiring HAI (P<0.05).
Interpretation & conclusions:
HAI prevalence showed a progressive reduction over successive rounds of survey. Conscious effort needs to be taken by all concerned to reduce the duration of hospital stay. Use of medical devices should be minimized and used judiciously. Healthcare infection control should be a priority of every healthcare provider. Such surveys should be done in different healthcare settings to plan a response to reducing HAI.
Keywords: Developing country, hospital-acquired infections, nosocomial infections, risk factors, surveillance
Hospital-acquired infections (HAIs), also known as healthcare-associated infections (HCAI), are infections occurring in a patient in a hospital or other healthcare facility in whom the infection was not present or incubating at the time of admission1. HAI is a major problem for patient safety and has a high impact in terms of morbidity and mortality2,3.
Effective surveillance of HAIs helps in quality improvement by identifying a problem, suggesting an intervention and documenting the effectiveness of that intervention and thus directing future infection control practices. The risk to acquire HAI is universal and pervades every healthcare facility and health system worldwide with some high-income countries having a national surveillance system for HAIs. However, the true burden remains unknown in many nations, particularly in developing countries4. There are scant data available from India. Point prevalence survey of HAIs and use of indwelling devices and antimicrobials in a large tertiary care hospital in India reported overall prevalence of HAIs as seven per cent5. Another study carried out in north India in a burn unit of a tertiary care referral centre reported the infection density being 36.2 infections per 1000 patient-days6. The present study was an endeavour in this direction as a series of point prevalence surveys carried out in both acute and non-acute settings from paediatric to geriatric wards of a tertiary care hospital in Pune, India. The study was also aimed at identifying the common risk factors associated with the occurrence of HAI.
HAI Study Group (Names are arranged alphabetically, departmental affiliation mentioned in parenthesis)
Rohit Ambekar, Kuntal Bandyopadhyay, D R Basannar, Ayush Bhatnagar, MS Brar, Lee Budhathoki, Sunil Diwate, Mona Dubey, Manoj Gupta, Swatej Hanspal, Atul Kotwal, A Mahen, Manjunath SR, Kailas Methe, S Mukherji, Amol Nath, Seema Patrikar, Vivek Phutane, Naveen Phuyal, Sabreen B, Dinesh Sharma, Rekha Sharma, Gurpreet Singh, Neha Singh, Shruti Vashisht, Vikas Yadav (Community Medicine) Veena Kharayat, Biju Vasudevan, Rajesh Verma (Dermatology) Sunil Basukala, A Chakravarty, A Chatterjee, Saurabh Ghosh, Vikas H, Sania Shahbaz Hasnain, Ashish Jain, Hrishikesh Pisal, RK Ranyal, Anupam Sahu, Shashikant Sharma, Shailendra Singh, Swati Varshney (Hospital Administration) Anil Kumar Abbot, VA Arun, P Chauhan, Arnab Choudhury, Tashi Dema, Aditya Gupta, DK Jha, Khushboo, Velu Nair, N Naithani, AV Pachisiya, Ramakant, Makarand Randive, Bhupesh Saini, S Shankar, SK Singh, Dharmendra Singh, Smriti Sinha, Sambit Sundarey, Arun Valsan, Vasu Vardhan, RM Verghese, BK Rashmi Yadav (Internal Medicine) GS Bhalla, Vaibhav Dudhat, Nikunj Das, Naveen Grover, Santanu Hazra, DK Kalra, Mayuri Kulkarni, Mungunthan M, Priyanka Pandit, Anubha Patel, S Prasanna, G Gopal Rao, AK Sahni, Alina Singh (Microbiology) Vipin Kumar, Anupam Kapur, AK Srivastava, Vijaylakshmi, RD Wadhwa (Obstetrics & Gynaecology) Ashutosh Gupta (Ophthalnology) BK Chopra (Orthopaedics) Anvita Bhansali (Otorhinolaryngology) Anvita Bhansali (Otorhinolaryngology) SS Dalal, Aparajita Gupta, Gaurav Kulshrestha, I Lingamurthy, Sheila S Mathai, Kuldeep Mertiya (Paediatrics) Amit Kumar, Jyotiprakash, A Saha (Psychiatry) Manu Kumar Dhingra, SS Jaiswal, Imran Khan, Murali Krishna KG, Ankit Kumar, Dhinesh Kumar, Vipon Kumar, Satyaki Mukherjee, Vasu Nikunj, Rajat Prabhakar, AK Pujahari, PK Sharma, Ajit Singh, JK Singh, Tapan Sinha, Sivakumar, SP Tripathi, Venketesh, Dheeraj Yadav (Surgery) Deepak Batura, DP Joshi (Urology).
Material & Methods
A point prevalence cross-sectional study (which allows assessment of a disease or a health-related state in a population at a single point in time) was carried out by the Armed Forces Medical College at its affiliated and co-located Command Hospital (Southern Command), Pune. The study comprised a series of four rounds of point prevalence surveys carried out during March to August 2014. All hospital wards were included in this survey.
Data collection tool: The tool to collect data was designed by a multidisciplinary team comprising departments of Medicine, Community Medicine, Hospital Administration and Microbiology. This was prepared in-house by thorough, supportive literature search on various factors associated with HAIs. It was tested during pilot study and accordingly modified, validated and weighted by the concerned experts of various departments including Medicine, Community Medicine and statisticians of the institute. The comprehensive data collection tool included patient's admission details, demographic data, consultant speciality, ward-wise location, patient's diagnosis, use of indwelling devices, mechanical ventilation, surgical procedure carried out, antimicrobials used and presence of additional HAI risk factors. The survey team comprised of respective ward in-charge medical officer, resident from the concerned clinical speciality along with a resident each from the department of Community Medicine, Hospital Administration and Microbiology. The data were collected by the teams from all the eligible patients. Rigorous pre-survey training was imparted to 13 surveillance teams which covered the entire 1000-bedded Command Hospital, Pune, ranging from paediatric to all general and speciality wards on the male and female side.
Definition of hospital-acquired infections (HAIs): HAI patients were detected on the basis of HAI definition as per the Centers for Disease Control and Prevention (CDC) guidelines7. HAIs, also called ‘nosocomial infections’, are infections acquired during hospital care, which are not present, or incubating at the time of admission. Infections occurring more than 48 h after admission were considered nosocomial infections or HAIs.
Inclusion and exclusion criteria: All patients who were admitted for more than 48 h in the hospital and all transferred-in cases from other peripheral hospitals were included in the study. Hospitalization period till the dates of surveys during March 2014 to August 2014 was used for estimating patient-days. Ethical approval was obtained from the Institutional Ethics Committee. Written informed consent was obtained from each patient. Blood, pus, urine, tracheal aspirate, sputum, bronchoalveolar lavage and stool, as appropriate, were collected from HAI suspects for microbiological testing. Collection of samples for microbiology cultures was done as per the standard protocols and carried in person by the microbiology residents for further analysis in the microbiology laboratory for immediate processing. The antibacterial susceptibility testing was performed by Vitek 2 Compact (Biomerieux, France) automated system for the identification and antimicrobial susceptibility testing of microorganisms.
Statistical analysis: The questionnaire of the point prevalence survey was thoroughly scrutinized to ensure consistency of data in all the rounds. Statistical analysis was carried out using statistical software SPSS version 22.0 (IBM, USA) and Epi Info (CDC, Atlanta). Univariate analyses of the association between HAIs and the risk factors were performed using odds ratios (ORs). Maximum likelihood estimates of OR together with 95 per cent confidence intervals (CIs) were computed. Multivariate analysis was done by logistic regression.
Results
A total of 1886 patients, who had spent 40,610 patient-days in the hospital, were eligible for HAI surveys as per the inclusion criteria, of whom 77.3 per cent were males and 22.7 per cent were females with a male to female ratio of 1:0.29. The median age of all patients was 35 yr (interquartile range 26-51 yr). Table I shows the baseline data of the study population with respect to age, sex and admitting consultant speciality.
Table I.
Baseline characteristics of the study population
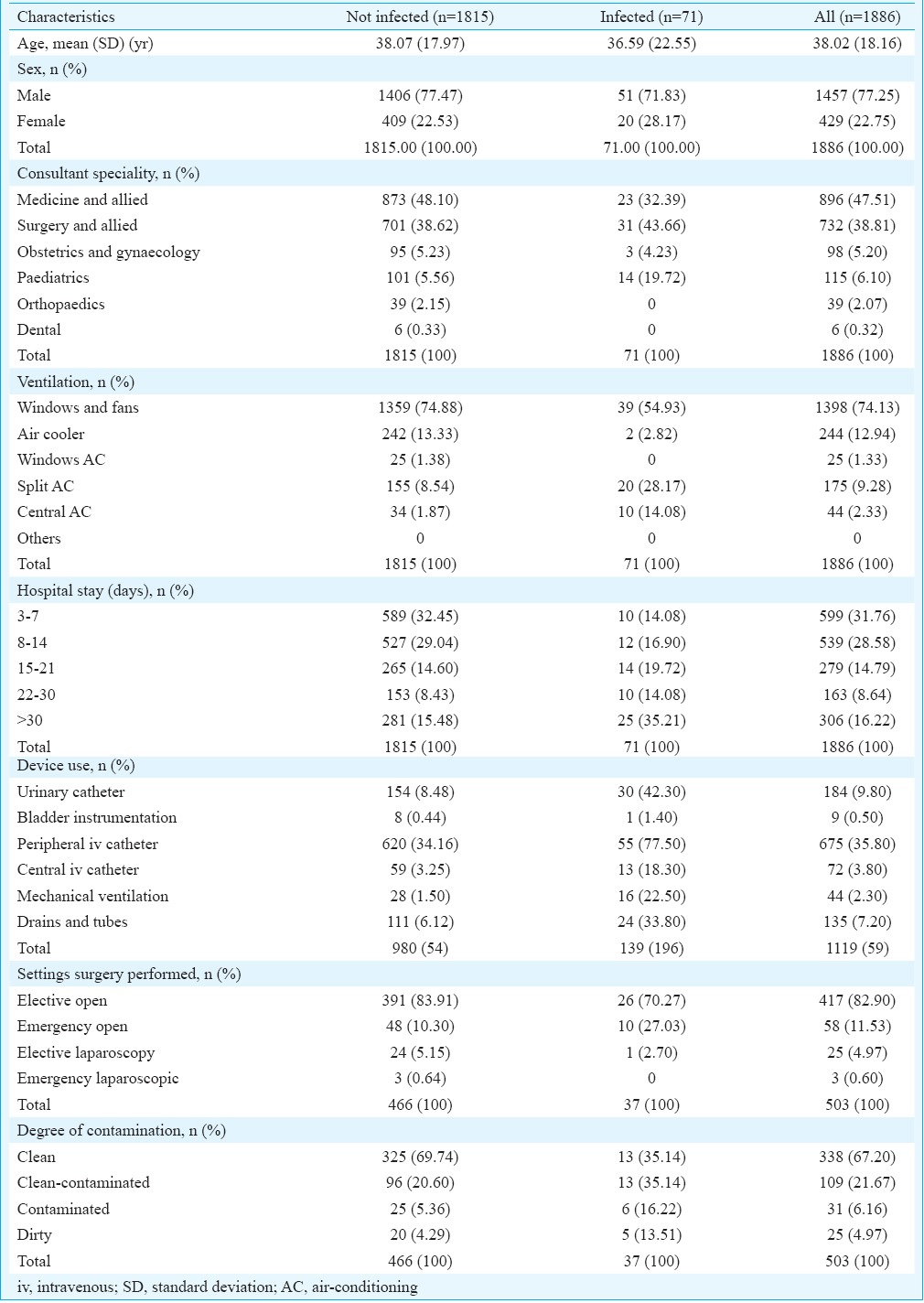
The survey was carried out as four rounds between March and August 2014. Seventy one cases of HAI were detected. Overall prevalence of HAI was 3.76 per cent (95% CI=2.97, 4.69). Prevalence of HAI cases detected went down with each successive round. It was 5.6 per cent in the first round, 4.9 per cent in the second round, 2.6 per cent in the third round and 1.6 per cent in the fourth round.
Overall rate of HAI was found to be 1.75 HAI cases per 1000 patient-days. It was 2.49 HAI cases per 1000 patient-days for the first round, 2.12 for the second round, 1.22 for the third round and 0.90 HAI cases per 1000 patient-days for the fourth round.
Surgical-site infections (SSIs) were identified to be the most common HAI (23.94%), followed by hospital-acquired pneumonia (HAP) (18.31%), urinary tract infection (UTI) (16.9%), catheter-related bloodstream infection (BSI) (16.9%), ventilator-associated pneumonia (VAP) (9.85%), septicaemia (8.45%) and others (5.65%).
Among consultant specialities, paediatric had the maximum odds of more than four times as compared to other specialities. The prevalence of HAI was highest in surgical Intensive Care Unit (ICU) ward (25%), followed by medical ICU (20%) and burns ward (20%). With regard to air-conditioning (AC) and ventilation in the wards, highest prevalence of HAI was seen in wards with central AC (22.72%), followed by split AC (11.43%). The odds of acquiring an HAI are 8.59 times more when a patient is exposed to central AC than other modalities of AC. Similarly, the odds were 4.20 times more when exposed to split AC (Table II)8,9,10,11. The association between AC and HAI was found significant (P<0.05) for all the modalities of ventilation.
Table II.
Prevalence of hospital-acquired infections according to patient characteristics (including risk factors) and specialties
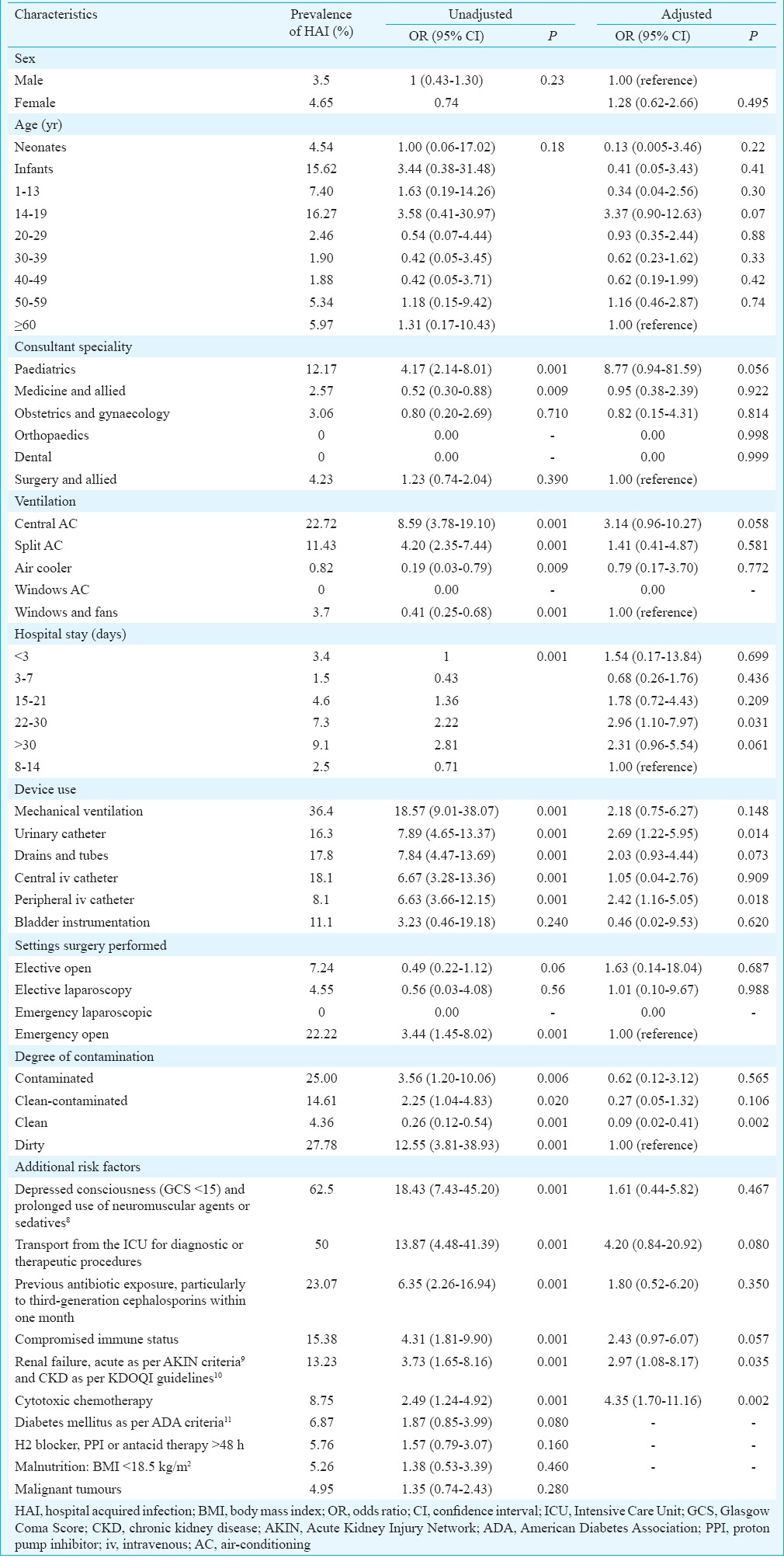
HAI prevalence was seen to rise with the increase in duration of hospital stay. The odds of acquiring an HAI were 3.11, 3.85 and 5.24 times more when the duration of hospital stay exceeded 15, 22-30 or more than 30 days respectively.
Peripheral intravenous (iv) line was the most commonly used device, but the highest HAI prevalence (36.4%) was found amongst patients on mechanical ventilation, followed by central iv line (18.1%). The odds of acquiring an HAI were highest (18.57 times more) when a patient is exposed to mechanical ventilation. Similarly, the odds are 7.89, 7.84, 6.67 and 6.63 times more when exposed to urinary catheter, drains and tubes, central iv catheter and peripheral iv catheter, respectively (Table II).
Of the 16 HAI patients on mechanical ventilation, seven (43.75%) had VAP. Similarly, in 30 HAI patients with urinary catheters, nine (30%) had UTI. In 13 HAI patients with central iv catheter, three (23.07%) had BSI in addition to other types of HAIs. Depending on the setting of surgery (elective/emergency and open/laparoscopic), HAI prevalence was highest (22.22%) in emergency open setting. The odds of acquiring an HAI are 3.44 times more when a patient is operated under the emergency open setting. With respect to degree of contamination at the time of surgery, most of the surgeries were performed under ‘clean’ conditions, while HAI prevalence (20%) was highest in the ‘dirty’category12. The odds of acquiring HAI are 3.48 times more for patient with ‘dirty’ category of contamination.
The presence of additional risk factors was also associated with prevalence of HAI. Odds of acquiring an HAI were 18.43 for a patient who had depressed consciousness (Glasgow Coma Score <15)13 and was on prolonged use of neuromuscular agents or sedatives. Similarly, the odds were 13.87 for a patient transported from ICU for diagnostic or therapeutic procedures; 6.35 for previous antibiotic exposure (particularly to third-generation cephalosporin within one month), 4.31 for compromised immune status, 3.73 for renal failure and 2.49 for cytotoxic chemotherapy. These results were found significant (P<0.05). Table II represents the OR along with 95 per cent CI and significance for various risk factors.
Logistic regression revealed that the association between HAI and use of urinary catheter and peripheral iv catheter was significant with increased odds of 2.69 and 2.42, respectively. Similarly, additional risk factors such as renal failure and cytotoxic chemotherapy were significantly associated with HAI, with increased odds of 2.97 and 4.35, respectively. Hospital stay of 22-30 days was significantly associated with HAI (OR=2.96), and surgeries performed under clean degree of contamination were found to be protective against HAI (OR=0.09).
Discussion
Most studies related to HAI conducted in developed countries demonstrate the efficacy of surveillance and its significant contribution to minimizing patient morbidity and mortality14,15,16,17. Conversely, in developing countries, only a few studies providing HAI using such standardized definitions are available. This study was carried out to establish HAI prevalence benchmarks. A highlight of this study was that it included all age groups including paediatrics, regarding which very few reports are available in India5.
In our study, HAI prevalence decreased in successive rounds. The results of the different rounds were not shared with ward medical officers/treating consultants. Extensive training of the medical and nursing staff for carrying out this study, and doing the exercise multiple times, seemed to have sensitized them towards HAI control and encouraging them to follow measures to reduce its risk.
The present study has found point prevalence of HAI to be 3.76 per cent (95% CI=2.97, 4.69), which was lower than the rates reported by other hospitals in many developing5,6 and developed countries18 as well. A systematic review has estimated hospital-wide prevalence of HAIs in high-income countries at 7.6 per cent and in low- and middle-income countries at 10.1 per cent4.
The European Centre for Disease Prevention and Control has reported a mean HCAI prevalence of 7.1 per cent in Europe18, and public health reports from the USA estimate it to be 4.5 per cent in 200219. The English National Point Prevalence Survey on HCAI revealed that prevalence had reduced from 8.2 in 2006 to 6.4 per cent in 201120. Prospective surveillance of 12 ICUs of seven hospital members of the International Nosocomial Infection Control Consortium of seven Indian cities introduced from July 2004 to March 2007 reported an overall HCAI rate of 4.4 per cent and 9.06 HCAIs per 1000 ICU-days21. A study carried out at a tertiary hospital in north India has found point prevalence of HAI to be 7 per cent5.
Paediatrics had the highest odds of having HAI (>4 times) as compared to other specialities. A study from developing countries has reported that rates of neonatal infections were 3-20 times higher than those reported for hospital-born babies in industrialized countries22.
Our study showed that prevalence of HAI was highest in surgical ICU (25%), followed by medical ICU (20%) and burns ward (20%). Many studies found that the HAI burden was much more severe in patients admitted to ICUs, burn, transplant patients and neonates and might even go as high as 51 per cent23,24.
Our study results corroborated with another study which showed that the duration of hospital stay was significantly associated with higher HAI prevalence, suggesting that conscious efforts need to be taken by all concerned to reduce the duration of hospitalization stay, to the minimum25. High prevalence of HAI amongst patients on mechanical ventilation, central iv line and urinary catheter found in our study was similar to other studies26,27,28. In our study, SSI and HAP were the most common HAI types reported, which was similar to that reported in a systematic review and meta-analysis of HAI in developing countries3. As per other studies, the frequency of SSI varies between 1.2 and 5.2 per cent in high-income countries10,19,29. VAP was also reported by other authors with an alarming rate30,31. Our findings with regard to additional risk factors corroborated with European Prevalence of Infection in Intensive Care Study18.
Low prevalence of HAI in our study might be influenced by the significant number of chronic patients being treated in hospital wards such as psychiatry, dermatology and other lifestyle diseases and not exposed to other infectious patients. Second, many wards do not have AC and are dependent on traditional windows and fans for ventilation. These wards get more sunlight and fresh air as compared to wards with central AC/split AC which have recycled air and probably increased risk of HAI.
Single-patient rooms with independent window AC for acute care, rather than multiple-patients’ room with recycled central AC, and more exposure to fresh air and sunlight for non-acute wards, may sound a retrograde step, but, as per the study, it appears to be a healthier option to reduce the risk to acquire HAI.
Our study had several limitations. First, it was performed in only one of the hospitals in the selected area. The one-time prevalence study nature might have influenced the prevalence rate and not depict the true rate as also the inability to determine the causal factors. For calculating patient-days, the cut-off was taken as the date of survey and not the date of discharge. However, compared to time-consuming and costly resource intensive incidence studies, repeated prevalence surveys are practical and efficient method of measuring trends over time. Such surveys can be used to provide data on infected and non-infected patients and assess the impact of infection prevention and control programmes on HAI32.
In conclusion our study revealed that HAI prevalence was 5.6 per cent in the first round and it went down up to 1.6 per cent in the fourth round. Conscious effort needs to be taken by all concerned to reduce the duration of hospitalization stay, to the minimum. Use of devices such as urinary catheter and peripheral/central iv lines may be minimized. If these have to be used, these should be discontinued at the earliest. Role of type of AC and ventilation in effecting risk of HAI needs more study.
Acknowledgment
Authors acknowledge the sponsorship provided by Office of Director General of Armed Forces Medical Services, M-Block, Ministry of Defence, New Delhi-110 001.
Footnotes
Conflicts of Interest: None.
References
- 1.Benenson AS. Control of communicable diseases manual. 16th ed. Washington: American Public Health Association; 1995. [Google Scholar]
- 2.Burke JP. Infection control – A problem for patient safety. N Engl J Med. 2003;348:651–6. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 3.Allegranzi B, Bagheri Nejad S, Combescure C, Graafmans W, Attar H, Donaldson L, et al. Burden of endemic health-care-associated infection in developing countries: Systematic review and meta-analysis. Lancet. 2011;377:228–41. doi: 10.1016/S0140-6736(10)61458-4. [DOI] [PubMed] [Google Scholar]
- 4.Ogwang M, Paramatti D, Molteni T, Ochola E, Okello TR, Ortiz Salgado JC, et al. Prevalence of hospital-associated infections can be decreased effectively in developing countries. J Hosp Infect. 2013;84:138–42. doi: 10.1016/j.jhin.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Biswal M, Dhaliwal N, Mahesh R, Appannanavar SB, Gautam V, et al. Point prevalence surveys of healthcare-associated infections and use of indwelling devices and antimicrobials over three years in a tertiary care hospital in India. J Hosp Infect. 2014;86:272–4. doi: 10.1016/j.jhin.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Taneja N, Emmanuel R, Chari PS, Sharma M. A prospective study of hospital-acquired infections in burn patients at a tertiary care referral centre in North India. Burns. 2004;30:665–9. doi: 10.1016/j.burns.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC). Procedure-Associated Module, Surgical Site Infection (SSI) Event. 2015. Jan, [accessed on May 10, 2014]. Available from: http://www.cdc.gov/HAI/ssi/ssi.html .
- 8.Nseir S, Makris D, Mathieu D, Durocher A, Marquette CH. Intensive Care Unit-acquired infection as a side effect of sedation. Crit Care. 2010;14:R30. doi: 10.1186/cc8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao CT, Tsai HB, Wu CY, Lin YF, Hsu NC, Chen JS, et al. The severity of initial acute kidney injury at admission of geriatric patients significantly correlates with subsequent in-hospital complications. Sci Rep. 2015;5:13925. doi: 10.1038/srep13925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redmond A, Donlon S, Boyle G, McCann M, Einarsdottir H. Prevention of infection in patients with chronic kidney disease. Part II: Healthcare-associated infections. J Ren Care. 2011;37:52–62. doi: 10.1111/j.1755-6686.2011.00216.x. [DOI] [PubMed] [Google Scholar]
- 11.Datta P, Rani H, Chauhan R, Gombar S, Chander J. Health-care-associated infections: Risk factors and epidemiology from an intensive care unit in Northern India. Indian J Anaesth. 2014;58:30–5. doi: 10.4103/0019-5049.126785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surveillance Definitions. CDC/NHSN Surveillance Definitions for Specific Types of Infections. 2014. Jan, [accessed on June 10, 2014]. Available from: http://www.socinorte.com/wp-content/uploads/2014/06/17pscNosInfDef_current.pdf .
- 13.Gajović O, Tomović M, Stanarcić J, Canović P, Todorović Z, Lazić Z. Clinical characteristics of nosocomial infections of patients with acute central nervous system infections treated in ICU. Med Glas (Zenica) 2011;8:277–9. [PubMed] [Google Scholar]
- 14.Gastmeier P, Geffers C, Brandt C, Zuschneid I, Sohr D, Schwab F, et al. Effectiveness of a nationwide nosocomial infection surveillance system for reducing nosocomial infections. J Hosp Infect. 2006;64:16–22. doi: 10.1016/j.jhin.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 15.National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 16.Haley RW, Morgan WM, Culver DH, White JW, Emori TG, Mosser J, et al. Update from the SENIC project. Hospital infection control: Recent progress and opportunities under prospective payment. Am J Infect Control. 1985;13:97–108. doi: 10.1016/s0196-6553(85)80010-9. [DOI] [PubMed] [Google Scholar]
- 17.Gastmeier P, Sohr D, Geffers C, Behnke M, Daschner F, Rüden H. Mortality risk factors with nosocomial Staphylococcus aureus infections in Intensive Care Units: Results from the German Nosocomial Infection Surveillance System (KISS) Infection. 2005;33:50–5. doi: 10.1007/s15010-005-3186-5. [DOI] [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control. Annual Epidemiological Report 2011. Reporting on 2009 Surveillance Data and 2010 Epidemic Intelligence Data. 2011. [accessed on January 17, 2013]. p. 239. Available from: http://www.ecdc.europa.eu/en/publications/Publications/1111_SUR_Annual_Epidemiological_Report_on_Communicable_Diseases_in_Europe.pdf .
- 19.Klevens RM, Edwards JR, Richards CL, Jr, Horan TC, Gaynes RP, Pollock DA, et al. Estimating health care-associated infections and deaths in U. S hospitals, 2002. Public Health Rep. 2007;122:160–6. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins S, Karen S, Lisa S. for Healthcare Protection Agency. English National Point Prevalence Survey on Healthcare-Associated Infections and Antimicrobial Use, 2011: Preliminary Data. London: Health Protection Agency; 2012. [Google Scholar]
- 21.Mehta A, Rosenthal VD, Mehta Y, Chakravarthy M, Todi SK, Sen N, et al. Device-associated nosocomial infection rates in Intensive Care Units of seven Indian cities. Findings of the International Nosocomial Infection Control Consortium (INICC) J Hosp Infect. 2007;67:168–74. doi: 10.1016/j.jhin.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Zaidi AKM, Huskins WC, Thaver D, Bhutta ZA, Abbas Z, Goldmann DA. Hospital-acquired neonatal infections in developing countries. Lancet. 2005;36 5:1175–88. doi: 10.1016/S0140-6736(05)71881-X. [DOI] [PubMed] [Google Scholar]
- 23.Burns K, Foley M, Donlon S. Point Prevalence Survey of Hospital – Acquired Infections & Antimicrobial Use in European Acute Care Hospitals: May, 2012. Republic of Ireland: National Report-November; 2012. [Google Scholar]
- 24.Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, et al. International study of the prevalence and outcomes of infection in Intensive Care Units. JAMA. 2009;302:2323–9. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 25.Balkhy HH, Cunningham G, Chew FK, Francis C, Al Nakhli DJ, Almuneef MA, et al. Hospital- and community-acquired infections: A point prevalence and risk factors survey in a tertiary care center in Saudi Arabia. Int J Infect Dis. 2006;10:326–33. doi: 10.1016/j.ijid.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Klavs I, Bufon Luznik T, Skerl M, Grgic-Vitek M, Lejko Zupanc T, Dolinsek M, et al. Prevalance of and risk factors for hospital-acquired infections in Slovenia-results of the first national survey, 2001. J Hosp Infect. 2003;54:149–57. doi: 10.1016/s0195-6701(03)00112-9. [DOI] [PubMed] [Google Scholar]
- 27.Sohn AH, Garrett DO, Sinkowitz-Cochran RL, Grohskopf LA, Levine GL, Stover BH, et al. Prevalence of nosocomial infections in neonatal Intensive Care Unit patients: Results from the first national point-prevalence survey. J Pediatr. 2001;139:821–7. doi: 10.1067/mpd.2001.119442. [DOI] [PubMed] [Google Scholar]
- 28.Richards MJ, Edwards JR, Culver DH, Gaynes RP. Nosocomial infections in combined medical-surgical Intensive Care Units in the United States. Infect Control Hosp Epidemiol. 2000;21:510–5. doi: 10.1086/501795. [DOI] [PubMed] [Google Scholar]
- 29.Moro ML, Morsillo F, Tangenti M, Mongardi M, Pirazzini MC, Ragni P, et al. Rates of surgical-site infection: An international comparison. Infect Control Hosp Epidemiol. 2005;26:442–8. doi: 10.1086/502565. [DOI] [PubMed] [Google Scholar]
- 30.Stevens RM, Teres D, Skillman JJ, Feingold DS. Pneumonia in an Intensive Care Unit. A 30-month experience. Arch Intern Med. 1974;134:106–11. [PubMed] [Google Scholar]
- 31.Mukhopadhyay C, Bhargava A, Ayyagari A. Role of mechanical ventilation & development of multidrug resistant organisms in hospital acquired pneumonia. Indian J Med Res. 2003;118:229–35. [PubMed] [Google Scholar]
- 32.Gravel D, Taylor G, Ofner M, Johnston L, Loeb M, Roth VR, et al. Point prevalence survey for healthcare-associated infections within Canadian adult acute-care hospitals. J Hosp Infect. 2007;66:243–8. doi: 10.1016/j.jhin.2007.04.008. [DOI] [PubMed] [Google Scholar]


