Abstract
Background:
In considering the importance of postoperative pain management and its consequences on its related secondary outcomes including nausea, vomiting, and operation-related complications, we aimed to compare the effectiveness of the three analgesic agents including ketamine, paracetamol, and magnesium sulfate for postoperative pain relief and associated consequences in this trial.
Materials and Methods:
In this double-blinded randomized control clinical trial, patients scheduled for elective lower extremity orthopedic surgery under general anesthesia were enrolled and randomized into four groups for receiving intravenous ketamine (0.25 mg/kg), paracetamol (15 mg/kg), magnesium sulfate (7.5 mg/kg), and placebo (normal saline), immediately after the induction of anesthesia. Postoperative pain scores, analgesic, and metoclopramide use, and frequency of vomiting and satisfaction score of studied patients in the four studied groups during the 6 h, 6–12 h, and 12–24 h after recovery were recorded and compared.
Results:
In this trial, thirty patients randomized in each studied groups. Mean of postoperative pain score was significantly lower in ketamine group than others during 24 h after recovery (P < 0.001). Mean of additive analgesic use was significantly lower in ketamine group during 12 h after recovery (P < 0.001), but it was not significantly different during 12–24 h after recovery (P = 0.12). Mean of vomiting frequency and metoclopramide use was not different between groups (P > 0.05). Excellent and good satisfaction score were significantly higher in ketamine group than other groups (P = 0.04).
Conclusions:
Ketamine has more superior effect for during recovery and postoperative pain controlling and analgesic use than paracetamol and magnesium sulfate.
Keywords: Ketamine, paracetamol and magnesium sulfate, postoperative pain, preemptive analgesia
Introduction
Postoperative pain and its management are considered as a challenging issue for anesthesiologists. In spite of significant pharmacological advances in this field, evidence indicated that moderate and severe postoperative pain is reported in 80% and 31–37% of patients, respectively.[1,2,3]
In addition to physical, cognitive, and emotional discomfort for the patients, inappropriate postoperative pain management is associated with consequences such as impaired wound healing and rehabilitation, delayed gastrointestinal motility, pulmonary complication, higher risk of thromboembolism due to immobilization, and myocardial ischemia.[4,5,6,7,8] As a long-term effect, adequate and proper management of the pain could improve the quality of life of the patients.[9]
Although different treatment protocols have been introduced and evaluated in this regard, appropriate pain control still not achieved. One of the treatment modalities is preemptive analgesia which is defined an “antinociceptive treatment that prevents the establishment of altered central processing of afferent input, which amplifies postoperative pain.”[10] Various pharmacologic agents have been studied as preemptive analgesia, and different results have been reported regarding their effectiveness for postoperative pain management.[11]
Some of the agents including ketamine, paracetamol, and magnesium sulfate have received great attention due to their specific characteristics including utility, cost, effectiveness, and mechanism of action.[12,13,14] Ketamine is an N-methyl d-aspartate (NMDA) receptor antagonist which has a role in neural modulation. Results of different studies were controversial some did not support its effectiveness in this field, whereas others reported that it could have a significant effect on decreasing postoperative pain as well as analgesic and opioid use in different surgeries.[12,15]
Magnesium sulfate is another drug, which mechanism of action is not clearly determined, but it is suggested that it could suppress the neuropathic pain pathway through calcium regulation and as an antagonist of NMDA receptors in central nervous system. There are not conclusive results regarding the effectiveness of magnesium sulfate for its analgesic effects.[14,16]
Paracetamol is another agent which has indicated potential in preemptive analgesia. It has great popularity due to its higher safety profile, lack of allergic potential, and less contraindication for a different disease. The effectiveness of both oral and intravenous (IV) paracetamol has been reported in many studies and recently, the utility of its IV form has been evaluated in some studies.[13,17]
Considering the importance of postoperative pain management and its consequences on its related secondary outcomes including nausea, vomiting, and operation-related complications; we aimed to compare the effectiveness of the mentioned three analgesic agents for postoperative pain relief and associated operative and nonoperative consequences in this trial.
Materials and Methods
This double-blinded randomized control trial was conducted from October 2015 to February 2016, in Alzahra and Kashani hospitals, affiliated to Isfahan University of Medical Sciences, in Isfahan, Iran. During this study, 120 American Society of Anesthesiologists (ASA) I and II patients aged 15–60 years, scheduled for elective lower extremity orthopedic surgery under general anesthesia were enrolled.
Protocol of the trial was approved by the Regional Ethics Committee of Isfahan University of Medical Sciences with a research project number of 394605.
Patients without any history of chronic pain, analgesic use before surgery, psychological problem or disease, addiction to any drugs or alcohol, and allergy were included in the study. Those with allergy to the drugs assigned for preemptive analgesia, using higher doses of opioid during surgery, underwent changes in the anesthesia plan, and diagnoses of addiction were excluded from the study.
The aims of the study and details of the methods of the study were explained for all selected patients. Written informed consent was obtained for each patient.
Details and methods of visual analog scale (VAS) and satisfaction scale were explained for all patients during preoperative anesthesia consultation.
Selected patients randomized into four groups for receiving IV ketamine (0.25 mg/kg), paracetamol (15 mg/kg), magnesium sulfate (7.5 mg/kg), and placebo, immediately after the induction of anesthesia.
Induction of general anesthesia was performed in all four studied groups according to the protocol of our center.
The anesthesia was induced with IV fentanyl 2 μg/kg, thiopental sodium (5 mg/kg), and atracurium (0.6 mg/kg). After intubation, anesthesia was maintained with nitrous oxide 50% in oxygen and isoflurane 1–1.5%. As an analgesic, IV morphine (0.1 mg/kg) was also administered to all patients.
Residual neuromuscular blockade was terminated using IV neostigmine 0.04 mg/kg and atropine 0.02 mg/kg at the end of surgery. After neuromuscular blockade reversal, all patients were transferred to the recovery room.
Vital signs, systolic and diastolic blood pressure, mean arterial pressure, and heart rate.
During the postoperative period, if the patients had pain with VAS >3, pethidine (0.5 mg/kg) was administrated.
For patients with nausea or vomiting, metoclopramide (10 mg) was prescribed.
Duration of anesthesia, operation and recovery time (using modified Aldrete's scoring system), severity of pain (using VAS scoring system), time for the first request for analgesia at recovery, and opioid use at recovery were recorded for all patients.
Occurrence of any adverse effects including nausea, vomiting, and any other side effects associated with ketamine, magnesium sulfate, and paracetamol were recorded.
Postoperative pain scores (VAS), analgesic, and metoclopramide use and frequency of vomiting during the 6 h, 6–12 h, and 12–24 h after recovery in ketamine, paracetamol, magnesium sulfate, and control groups were recorded.
The frequency of different scores of satisfaction (excellent, good, moderate, and week) in the four studied groups was recorded over the 24 h postoperatively.
All mentioned recorded variables compared in the four studied groups.
In all steps of trial (preoperative, during operation, and postoperative time), the physicians and nursed were blinded. Allocation of the patients in the studied group was done by a physician who was not aware about the groups (labeled by A, B, C, and D). The nurse in recovery room who recorded the studied variables was not aware about the study and allocations.
Statistical analysis
Collected data were analyzed using SPSS version 20 (SPSS Inc., Chicago, IL, USA). To compare qualitative and nominal variables between four studied groups Chi-square test was used. Mann–Whitney test and repeated measure ANOVA test were used for comparing variables with ordinal scale and quantitative variables, respectively.
Results
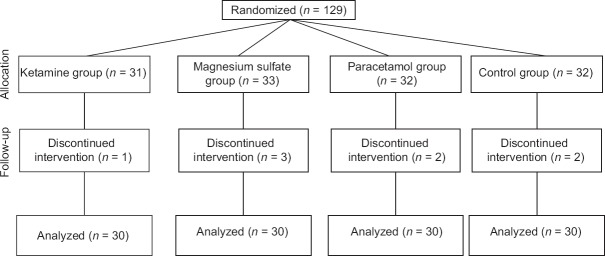
In this trial, from 129 initially enrolled patients, 120 patients (ASA I–II) randomized in four groups (thirty patients in each group) [Figure 1].
Figure 1.
Consort diagram of the study
Demographics characteristics and operative details of studied population in the four studied groups are presented in Table 1. All studied patients in the four studied groups were similar regarding their demographics characteristics and ASA score.
Table 1.
Demographics characteristics and operative details of studied population in ketamine, paracetamol, magnesium sulfate, and control groups
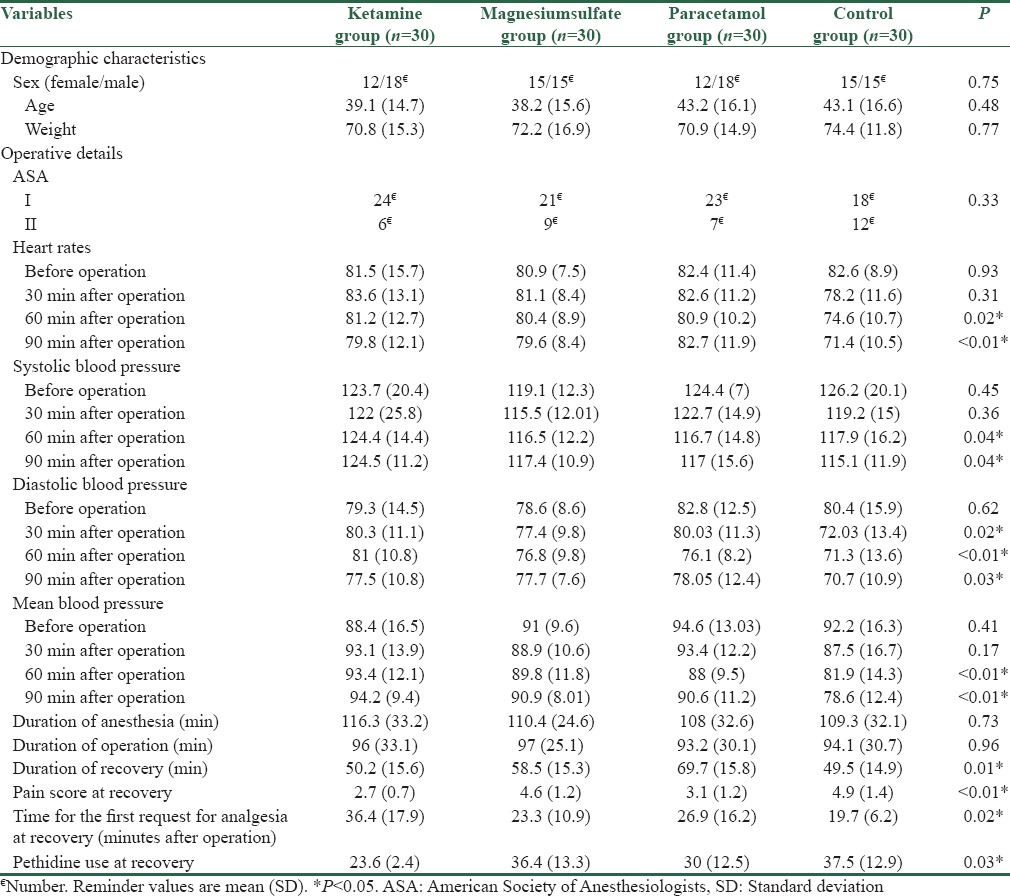
The mean heart rate of patients in control group was significantly lower than other groups during the 60 and 90 min after operation (P < 0.05) [Table 1].
Mean systolic blood pressure as significantly higher in ketamine group than other groups during the 60 and 90 min after operation (P < 0.05) [Table 1].
Mean diastolic blood pressure was significantly lower in control group than other groups during the 60 and 90 min after operation (P < 0.05) [Table 1].
Mean blood pressure was significantly lower in control group than other groups during the 60 and 90 min after operation (P < 0.05) [Table 1].
The mean duration of recovery was significantly lower in control and ketamine groups than other groups (P < 0.05) [Table 1].
The mean of pain score at the end of recovery was significantly lower in ketamine and paracetamol groups than other groups (P < 0.05). Mean of pain score at the end of recovery was not different significantly in ketamine and paracetamol groups (P > 0.05) [Table 1].
Patients in ketamine group request opioid later than other groups (P < 0.05), and mean dose of opioid use at the end of recovery was also significantly lower than other groups (P < 0.05) [Table 1].
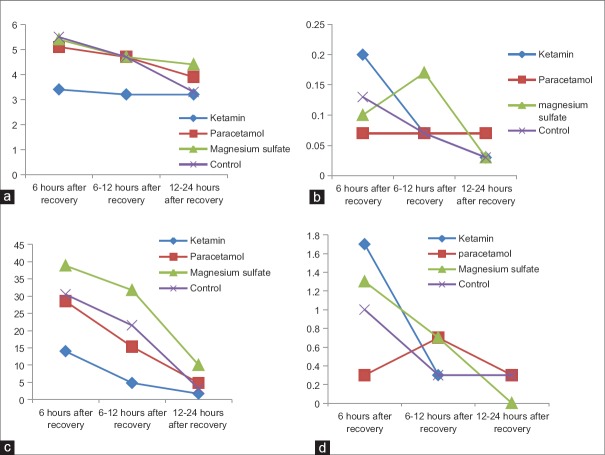
Mean of postoperative pain (VAS), analgesic, and metoclopramide use and vomiting during the 6 h, 6–12 h, and 12–24 h after recovery in ketamine, paracetamol, magnesium sulfate, and control groups are presented in Figure 2. Mean of vomiting frequency and metoclopramide use was not different between groups (P > 0.05). Mean of postoperative pain using VAS system was significantly lower in ketamine group than others during 24 h after recovery (P < 0.05). Mean of additive analgesic use (pethidine) was significantly lower in ketamine group during 12 h after recovery (P < 0.05), but it was not significantly different during 12–24 h after recovery (P > 0.05).
Figure 2.
Mean of postoperative pain (visual analog scale) (a), vomiting (b) analgesic (c) and metoclopramid use (d) during the 6 h, 6–12 h and 12–24 h after recovery in ketamine, paracetamol, magnesium sulfate and control groups
The frequency of different scores of patient's satisfaction (excellent, good, moderate, and week) in the four studied groups is presented in Figure 3. Excellent and good satisfaction score were significantly higher in ketamine group than other groups (P = 0.04), but it was not significantly different between the reminder groups.
Figure 3.
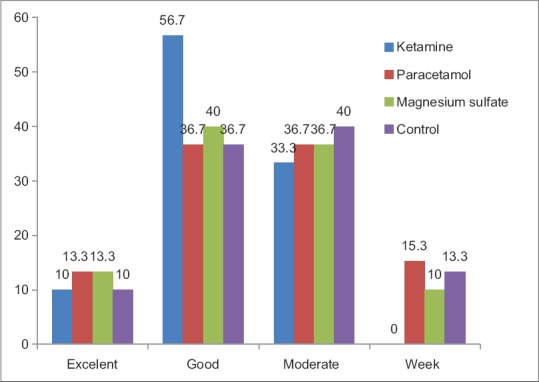
Frequency of different scores of patients satisfaction (excellent, good, moderate, week),24 hours after recovery, in ketamine, paracetamol, magnesium sulfate and control groups
Discussion
In this trial, we have evaluated the analgesic effect of three pharmacological agents including ketamine, paracetamol, and magnesium sulfate in comparison with control group in controlling postoperative pain, analgesic use, frequency of vomiting, and satisfaction score of patients underwent lower limb surgery using general anesthesia. Our results indicated that ketamine had a better analgesic effect than others both during and 24 h after surgery. In addition, the mean dose of administrated opioid and metoclopramide, mean of patients satisfaction score, frequency of vomiting, and duration of recovery were significantly lower in ketamine group.
Although several studies have been investigated the analgesic effect of the mentioned three agents in comparison with control group for postoperative pain relief,[12,13,14,15,16,17] there was not any similar study which compared the efficacy of the three agents for postoperative pain management.
In literature review, there was review studies and meta-analysis for each of them which discussed about their utilities and limitations.
Paracetamol is a safe analgesic with good tolerance which is used commonly for reducing postoperative pain. After the introduction of its IV form, its use has become wider for both peri-and post-operative periods. Results of review studies on the effectiveness of IV paracetamol have indicated that it could relief or prevent postoperative pain and improve satisfaction score. It could decrease opioid use and opioid-related nausea and vomiting during and after operation. The advantages of acetaminophen are its fewer adverse events which are more significant when it is used IV possibly due to the lack of the first-pass effect.[17,18]
Sinatra et al. have investigated the effectiveness of single dose of 1 g IV acetaminophen for postoperative management of major orthopedic surgery.[17]
Gregoire et al. have evaluated the efficacy of IV paracetamol with a 2-g starting dose and a 5 g dose during the first 24 h.[18]
The dose which is used in this trial was similar to that administrated in the study of Sinatra et al.[17]
Results of another study showed that paracetamol could be used mainly for surgeries with mild to moderate postoperative pain.[19]
Many studies have investigated the effect of periopretive/preemptive administration of magnesium sulfate on postoperative pain of different surgeries. The results were different and inconsistent. Two review studies have systematically reviewed the studies and clinical trial which have investigated the outcome of magnesium sulfate use during different operations.[16,20] Albrecht et al. showed that irrespective to the mode of magnesium sulfate administration, it could decrease the use of opioid use, postoperative pain score during 24 h after various surgeries. They did not find any report regarding serious adverse effects of the agent.[16] Results of a meta-analysis from different clinical trial of magnesium sulfate use for postoperative pain by De Oliveira et al. have also confirmed that it reduces both postoperative pain and opioid use in different surgical procedures using general anesthesia without any toxic effects.[20]
There are also some recent regional studies which support the findings of the previous studies regrinding the effectiveness of magnesium sulfate for postoperative pain in different surgeries.[21,22,23]
There are some systematic review, narrative review, and meta-analysis regarding the preemptive analgesic effect of ketamine. Almost all of the studies demonstrated that ketamine in a dosage ranging from 0.15 to 1 mg/kg, have positive effect on the reduction of postoperative pain, opioid consumption, and prolonging the time to first analgesic request.[15,24,25,26] The results of a meta-analysis performed by Yang et al. indicated that ketamine could decrease the postoperative use and delay the time of first analgesic use, but it had not significant effect on nausea and vomiting, operation, and anesthetic time.[26] The results of another narrative review study confirmed the findings of the previous studies. In addition, they recommended that more randomized clinical trials is needed for the determination of the proper administration method of ketamine and type of surgeries which are best suited for ketamine analgesic effects. They also reported that ketamine could have some adverse effects including neurotoxicity and memory impairment.[25]
There are also regional studies in Isfahan and Kashan in this field which showed different results.[27,28]
There was only one study which compared the effectiveness of ketamine and magnesium sulfate. Helmy et al. in Egypt have evaluated the preemptive analgesic effect of low-dose ketamine (0.3 mg/kg) and magnesium sulfate (30 mg/kg) on postoperative pain of patients undergoing cesarean section under general anesthesia in comparison with placebo group. They result indicated that ketamine had more favorable effects than magnesium sulfate. They showed that total opioid use was lower, and the time for the first request for postoperative analgesia was longer for ketamine group. Postoperative pain score by VAS was significantly lower in ketamine group than others at 2 and 6 h postoperatively. They concluded that preemptive analgesic effect of ketamine is more significant than magnesium sulfate.[29]
In this study, ketamine has better analgesic effects at the end of recovery and 24 h after recovery and consequently, lower analgesic use than magnesium sulfate and paracetamol. Ketamine has increased patients’ satisfaction score and prolonged the time for the first analgesic request.
There was not any significant different in mean of vomiting frequency and metoclopramide use between groups.
We have not any reports regarding side effects of the three analgesics during and postoperation.
The strength of this study was that we compare the effectiveness of the three most commonly used preemptive analgesics in our center. There was not any similar study. Our findings could help us for better management of postoperative pain and selection of the most proper analgesic in different surgical procedures.
The limitation of this trial was shorter duration of postoperative follow-up. It is suggested that evaluation of different long-term complications specially those related to postoperative pain would be more helpful for providing more effective postoperative pain control protocol.
Conclusions
The findings of this trial indicated that ketamine has more superior effect for during recovery and postoperative pain controlling and analgesic use than paracetamol and magnesium sulfate. Patients were more satisfied in ketamine group, and the duration of recovery was shorter. It could also delay the time for the first analgesic request. It is recommended to design more trials for evaluation of different doses of ketamine as well as its long-term postoperative consequences and adverse effects for achieving more conclusive results in this field.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vadivelu N, Mitra S, Schermer E, Kodumudi V, Kaye AD, Urman RD. Preventive analgesia for postoperative pain control: A broader concept. Local Reg Anesth. 2014;7:17–22. doi: 10.2147/LRA.S62160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carr DB, Goudas LC. Acute pain. Lancet. 1999;353:2051–8. doi: 10.1016/S0140-6736(99)03313-9. [DOI] [PubMed] [Google Scholar]
- 3.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–40. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 4.Kehlet H, Dahl JB. Anaesthesia, surgery, and challenges in postoperative recovery. Lancet. 2003;362:1921–8. doi: 10.1016/S0140-6736(03)14966-5. [DOI] [PubMed] [Google Scholar]
- 5.Holte K, Kehlet H. Postoperative ileus: A preventable event. Br J Surg. 2000;87:1480–93. doi: 10.1046/j.1365-2168.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 6.Gandhi K, Heitz JW, Viscusi ER. Challenges in acute pain management. Anesthesiol Clin. 2011;29:291–309. doi: 10.1016/j.anclin.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 7.American Society of Anesthesiologists Task Force on Acute Pain Management. Practice guidelines for acute pain management in the perioperative setting: An updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–73. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 8.Lucas CE, Vlahos AL, Ledgerwood AM. Kindness kills: The negative impact of pain as the fifth vital sign. J Am Coll Surg. 2007;205:101–7. doi: 10.1016/j.jamcollsurg.2007.01.062. [DOI] [PubMed] [Google Scholar]
- 9.Taylor RS, Ullrich K, Regan S, Broussard C, Schwenkglenks M, Taylor RJ, et al. The impact of early postoperative pain on health-related quality of life. Pain Pract. 2013;13:515–23. doi: 10.1111/papr.12026. [DOI] [PubMed] [Google Scholar]
- 10.Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: A meta-analysis. Anesth Analg. 2005;100:757–73. doi: 10.1213/01.ANE.0000144428.98767.0E. [DOI] [PubMed] [Google Scholar]
- 11.Hariharan S, Moseley H, Kumar A, Raju S. The effect of preemptive analgesia in postoperative pain relief – A prospective double-blind randomized study. Pain Med. 2009;10:49–53. doi: 10.1111/j.1526-4637.2008.00547.x. [DOI] [PubMed] [Google Scholar]
- 12.Dullenkopf A, Müller R, Dillmann F, Wiedemeier P, Hegi TR, Gautschi S. An intraoperative pre-incision single dose of intravenous ketamine does not have an effect on postoperative analgesic requirements under clinical conditions. Anaesth Intensive Care. 2009;37:753–7. doi: 10.1177/0310057X0903700519. [DOI] [PubMed] [Google Scholar]
- 13.Koh W, Nguyen KP, Jahr JS. Intravenous non-opioid analgesia for peri- and postoperative pain management: A scientific review of intravenous acetaminophen and ibuprofen. Korean J Anesthesiol. 2015;68:3–12. doi: 10.4097/kjae.2015.68.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han JU. About uses of magnesium during perioperative period. Korean J Anesthesiol. 2012;62:509–11. doi: 10.4097/kjae.2012.62.6.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jouguelet-Lacoste J, La Colla L, Schilling D, Chelly JE. The use of intravenous infusion or single dose of low-dose ketamine for postoperative analgesia: A review of the current literature. Pain Med. 2015;16:383–403. doi: 10.1111/pme.12619. [DOI] [PubMed] [Google Scholar]
- 16.Albrecht E, Kirkham KR, Liu SS, Brull R. Peri-operative intravenous administration of magnesium sulphate and postoperative pain: A meta-analysis. Anaesthesia 2013. 2013;68:79–90. doi: 10.1111/j.1365-2044.2012.07335.x. [DOI] [PubMed] [Google Scholar]
- 17.Sinatra RS, Jahr JS, Reynolds LW, Viscusi ER, Groudine SB, Payen-Champenois C. Efficacy and safety of single and repeated administration of 1 gram intravenous acetaminophen injection (paracetamol) for pain management after major orthopedic surgery. Anesthesiology. 2005;102:822–31. doi: 10.1097/00000542-200504000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Gregoire N, Hovsepian L, Gualano V, Evene E, Dufour G, Gendron A. Safety and pharmacokinetics of paracetamol following intravenous administration of 5 g during the first 24 h with a 2-g starting dose. Clin Pharmacol Ther. 2007;81:401–5. doi: 10.1038/sj.clpt.6100064. [DOI] [PubMed] [Google Scholar]
- 19.Brodner G, Gogarten W, Van Aken H, Hahnenkamp K, Wempe C, Freise H, et al. Efficacy of intravenous paracetamol compared to dipyrone and parecoxib for postoperative pain management after minor-to-intermediate surgery: A randomised, double-blind trial. Eur J Anaesthesiol. 2011;28:125–32. doi: 10.1097/EJA.0b013e32833fedfa. [DOI] [PubMed] [Google Scholar]
- 20.De Oliveira GS, Jr, Castro-Alves LJ, Khan JH, McCarthy RJ. Perioperative systemic magnesium to minimize postoperative pain: A meta-analysis of randomized controlled trials. Anesthesiology. 2013;119:178–90. doi: 10.1097/ALN.0b013e318297630d. [DOI] [PubMed] [Google Scholar]
- 21.Rezae M, Naghibi K, Taefnia AM. Effect of pre-emptive magnesium sulfate infusion on the post-operative pain relief after elective cesarean section. Adv Biomed Res. 2014;3:164. doi: 10.4103/2277-9175.139127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabbagh A, Elyasi H, Razavi SS, Fathi M, Rajaei S. Intravenous magnesium sulfate for post-operative pain in patients undergoing lower limb orthopedic surgery. Acta Anaesthesiol Scand. 2009;53:1088–91. doi: 10.1111/j.1399-6576.2009.02025.x. [DOI] [PubMed] [Google Scholar]
- 23.Shariat Moharari R, Motalebi M, Najafi A, Zamani MM, Imani F, Etezadi F, et al. Magnesium can decrease postoperative physiological ileus and postoperative pain in major non laparoscopic gastrointestinal surgeries: A randomized controlled trial. Anesth Pain Med. 2013;4:e12750. doi: 10.5812/aapm.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radvansky BM, Shah K, Parikh A, Sifonios AN, Le V, Eloy JD. Role of ketamine in acute postoperative pain management: A narrative review. Biomed Res Int. 2015;2015:749837. doi: 10.1155/2015/749837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh H, Kundra S, Singh RM, Grewal A, Kaul TK, Sood D. Preemptive analgesia with Ketamine for Laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol. 2013;29:478–84. doi: 10.4103/0970-9185.119141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang L, Zhang J, Zhang Z, Zhang C, Zhao D, Li J. Preemptive analgesia effects of ketamine in patients undergoing surgery. A meta-analysis. Acta Cir Bras. 2014;29:819–25. doi: 10.1590/S0102-86502014001900009. [DOI] [PubMed] [Google Scholar]
- 27.Safavi M, Honarmand A, Nematollahy Z. Pre-incisional analgesia with intravenous or subcutaneous infiltration of ketamine reduces postoperative pain in patients after open cholecystectomy: A randomized, double-blind, placebo-controlled study. Pain Med. 2011;12:1418–26. doi: 10.1111/j.1526-4637.2011.01205.x. [DOI] [PubMed] [Google Scholar]
- 28.Reza FM, Zahra F, Esmaeel F, Hossein A. Preemptive analgesic effect of ketamine in patients undergoing elective cesarean section. Clin J Pain. 2010;26:223–6. doi: 10.1097/AJP.0b013e3181bff86d. [DOI] [PubMed] [Google Scholar]
- 29.Helmy N, Badawy AA, Hussein M, Reda H. Comparison of the preemptive analgesia of low dose ketamine versus magnesium sulfate on parturient undergoing cesarean section under general anesthesia. Egypt J Anaesth. 2015;31:53–8. [Google Scholar]