Abstract
Objective:
This study aims to describe the overall cumulative effect of sevoflurane on kidney function in healthy patients in terms of mean plasma creatinine, blood urea nitrogen (BUN), creatinine clearance, urinary protein, and glucose excretion at 24 and 72 hours post-anesthesia.
Data retrieval:
A systematic literature search using MEDLINE and EMBASE as primary search engines was conducted. Articles, relevant abstracts, and citations dated January 1, 1995 to June 30, 2016 were retrieved.
Data selection:
Search terms included the pharmacological generic name sevoflurane. Search was expanded using the terms “renal function” OR “kidney” function AND “creatinine” OR “blood urea nitrogen” OR “creatinine clearance” OR “proteinuria” OR “glucosuria” OR “nephrotoxicity.” Limitations included randomized controlled trial, humans, and ages 19 and above, to include English and non-English text formats. All bibliographic indices for the relevant journals identified were also searched and collated according to relevance.
Main outcome measures:
Changes in serum/plasma creatinine, BUN, urinary protein, and glucose excretion of sevoflurane at 24 and 72-hours were determined.
Results:
Six relevant studies were qualified by both the inclusion criteria and inclusion dates. This review consists of 873 patients, 65% are males and 35% are females, with mean age of 56 ± 3 years. Sevoflurane was compared to isoflurane with regard to its nephrotoxic potential. Analyses on the effects of sevoflurane were performed on serum/plasma creatinine, BUN, urinary protein, and glucose excretion at 24 and 72 hours which showed no statistical difference between sevoflurane and isoflurane.
Conclusion:
In an apparently healthy adult without coexisting renal disorder, sevoflurane does not produce elevations in creatinine and BUN above the established upper limit of the reference range.
Keywords: sevoflurane, isoflurane, nephrotoxicity, creatinine, blood urea nitrogen, meta-analysis, glucosuria, proteinuria, creatinine clearance, compound A
INTRODUCTION
Inhalational anesthesia has always been the most widespread technique in maintaining general anesthesia for nearly all types of surgeries. Sevoflurane and isoflurane are two of the most popular potent inhaled anesthetics used in adult surgical procedures. Isoflurane is a halogenated anesthetic that has low biotransformation and low toxicity. It is a very stable molecule that undergoes little metabolism; hence, it is believed to be less tissue-toxic.1 Sevoflurane as a volatile halogenated ether anesthetic agent is nonirritant hence inhalation is less likely to produce coughing and breath holding. Likewise, having low solubility allows it to achieve a rapid and effective brain concentration.1 Isoflurane is an ether derivative commonly used as an inhalational agent to provide general anesthesia. It undergoes minimal biotransformation thus is safe to use in patients with known hepatic or renal impairment. Propofol is an intravenous anesthetic used for maintenance of general anesthesia. It is highly protein-bound and metabolized by conjugation in the liver. It is rapidly distributed into the peripheral tissues, making its clinical effects shorter.1,2
Sevoflurane is degraded to Compound A (CpA) by carbon dioxide absorbents containing a strong base.2 It has been found that low flow fresh gas rates, increase in temperature, reduced water content of the absorber, the use of baralyme as an absorbent, and a high sevoflurane concentration predispose to CpA could cause dose-dependent renal injury and death in rats but not in humans.3
Several studies have shown no statistical difference in measured parameters of renal function in humans.2,4,5 These studies have been the subject of discourse since the parameters used (blood urea nitrogen (BUN), creatinine) were not sensitive indicators of renal impairment. In a rat study by Keller et al.,6 necrosis seen on histology was not predictive of the creatinine level. Even with normal serum creatinine levels, necrosis occurred.7 Another study utilizing albuminuria, glucosuria, and enzyme markers such as alpha-glutathione-S-transferase (GST) and pi-GST found in tubular injuries failed to predict the presence or absence of renal injury.8 Moreover, the validity of these markers and cost-effectiveness has been questioned by experts.
To date, the issue regarding the occurrence of sevoflurane-related renal injury has yet to be settled. There have been no long-term renal functional effects reported in literature regarding sevoflurane. The cumulative effect of sevoflurane on kidney function has been discussed in detail in several randomized and quasi-randomized trials. No pooled evidence exists regarding its safety in patients with normal or pre-existing stable renal disease.
The objective of this study is to describe the overall cumulative effect of sevoflurane on kidney function in healthy patients in terms of mean plasma creatinine, BUN, creatinine clearance, urinary protein, and glucose excretion at 24 and 72 hours post-anesthesia.
DATA AND METHODS
Literature retrieval
A systematic literature search using MEDLINE and EMBASE as primary search engines was conducted. Articles, relevant abstracts and citations dated January 1, 1995 to June 30, 2016 were retrieved. On-Line journal websites such as Anesthesiology (http://anesthesiology.pubs.asahq.org/journal.aspx); Anesthesia & Analgesia (http://journals.lww.com/anesthesia-Analgesia/pages/default.aspx); Journal of Clinical Anesthesia (https://www.journals.elsevier.com/journal-of-clinical-anesthesia); Anaesthesia and Intensive Care (http://www.aaic.net.au); British Journal of Anaesthesia (https://academic.oup.com/bja); and Canadian Journal of Anesthesia (https://link.springer.com/journal/12630) were also searched.
Search terms utilized using the boolean approach included the pharmacological generic name sevoflurane or its market name depending on the country of origin. Search was expanded using the terms “renal function” or “kidney function” and “creatinine” or “blood urea nitrogen” or “creatinine clearance” or “proteinuria” or “glucosuria” or “nephrotoxicity.” limitations included randomized controlled trial, humans, and ages 19 and above, to include english and non-english text formats. All bibliographic indices for the relevant journals identified were also searched and collated according to relevance.
To provide a substantial update, a reference search on the studies published on all available meta-analysis concerning the pharmacologic profile of sevoflurane in humans was performed.
Eligibility criteria of studies
According to trial design and comparator arms: Studies involving the randomization of patients to either low-flow anesthesia or high-flow anesthesia depending on the criteria utilized per protocol were included. Comparator arms included all other types of inhalational anesthesia. Quasi-randomized studies and before-and-after trial designs were automatically excluded.
According to Nature of Participant: Studies which dealt with healthy adults aged 18 and above, without significant co-morbidities, were included. Studies dealing with patients with end-stage renal disease subjects as well as those with hepatic derangement were excluded.
According to Nature and Time of Surgery and Interventions Received: Standardized, step-by-step inhalational anesthetic procedures were described and compared. Low-flow anesthesia with sevoflurane was defined as that which received a mean flow of 1 L/min while high-flow would mean a flow of 6 L/min for body surface area. The contemplated total time for surgery (of at least 2 hours), minimum alveolar concentrations, and baseline biochemical markers were likewise accounted for.
According to Primary Outcomes: Primary endpoints were the 24- and 72-hour post-anesthesia values of creatinine (plasma or serum), BUN, creatinine clearance, and urine glucose and protein excretion. Studies which published the exact proportion of subjects achieving clinically and statistically significant increase or decrease in renal function values as established per protocol were included.
According to the Combinability of Statistical Data: Units of laboratory values were pegged at milligrams per deciliter for serum creatinine and BUN, while milliliters per minute for creatinine clearance was determined by either the Cockroft-Gault formulae or nuclear glomerular filtration rate (GFR) studies. Urine protein and glucose were expressed as milligrams collected per 24 hours. All values were subtracted and compared from the reported baseline results. The International System of Units and World Health Organization (WHO) standards for reference range of all laboratory results were utilized.
Validity and methodological assessment
Critical appraisal of all relevant literature was performed using the users' Guides to the medical literature: How to use an article about therapy or prevention,9 and users' Guides to the Medical Literature: How to Use an Article about Harm.10
A total of three raters (including the principal investigator) performed an independent and blinded review using a standardized checklist which examined the criteria of randomization, blinding, intention-to-treat analysis, sensitivity analysis, applicability of results, and relevance. For all the studies, subset representativeness, similarities of sample at the beginning of the observation, sufficiency and completeness of follow-up were rated. The raters were blinded as to the author, institution, and publication date. Another unblinded review followed to check whether the authors agreed with the given ratings.
Studies were categorized as “high,” “satisfactory,” and “fair” depending on the composite score of the assigned points on each of the criteria set by the authors. The quality scores ranged from 0 to 4 points per criteria. The summed scores were then compared across raters. The computed inter-rater variability was kappa-value = 0.79 (high reliability) while the computed intra-rater reliability was = 0.85 (high internal consistency.)
Assessments of confounding variables were done for each study. Cox proportional hazards model as well as multivariate logistic regression was determined. Meta-regression for individual patient characteristics possibly influencing the primary endpoints was done.
Data abstraction using another standardized data collection form was performed. Pertinent data collected were author, date of publication, population size, population characteristics—age, sex, presence or absence of post-operative anesthesia complications, type of comparator anesthesia, its dose and minimum alveolar concentration, duration of administration, induction time, duration of surgery and recovery time. All biochemical markers were collated and summarized. Exact mean values were obtained by either writing the authors whenever feasible, otherwise, mathematical derivation and extrapolation from graphical data was performed.
Statistical analysis
Description of the population, interventions, primary endpoints, and critique are tabulated. Descriptive statistics were used to summarize the pooled participant population.
For all renal function tests, the standard mean difference was utilized to arrive at a summary estimate. The random effects model was utilized as the strategy to evaluate the effect size. Sensitivity analyses were carried out to compare the post 24 and 72 hours values while subgroup analysis was performed by temporarily deleting some studies and examining the effect of their absence in the overall estimate.
Testing for significant study heterogeneity was carried out using the cochran Q Test.
The pooled weighted mean difference was derived utilizing the licensed statistical software Review Manager (REVMAN 4.1, http://handbook.cochrane.org) with the effect size falling at 95% confidence interval (CI) pegged to 0.05 alpha level of significance.
RESULTS
Description of trials
A total of six relevant studies qualified by both the inclusion criteria and during the inclusion dates January 1, 1995 to June 30, 2016. The search process and yield of studies are summarized as Figure 1.
Figure 1.
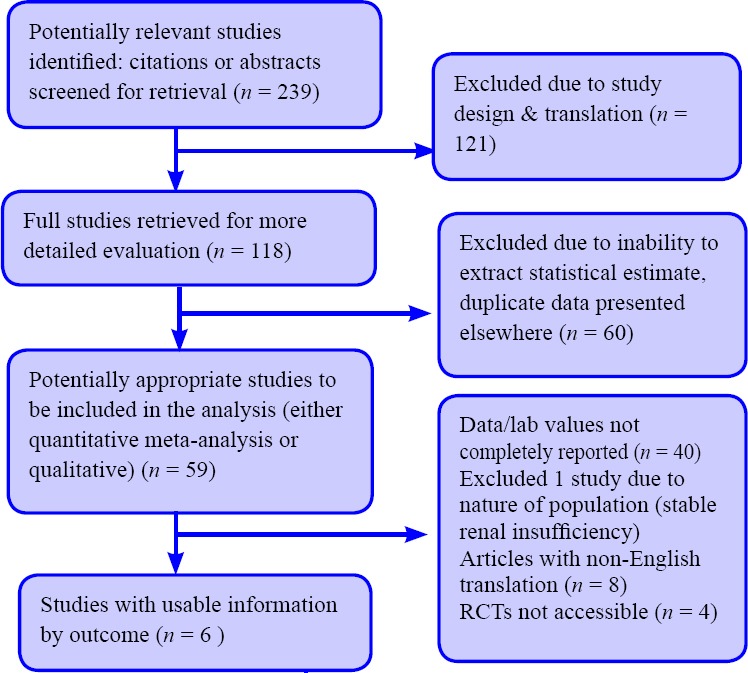
Flow diagram of study screening.
Table 1 summarizes the general description of the six studies included in this review.
Table 1.
General description of included studies, meta-analysis on the effects of sevoflurane (Sevo) on renal function
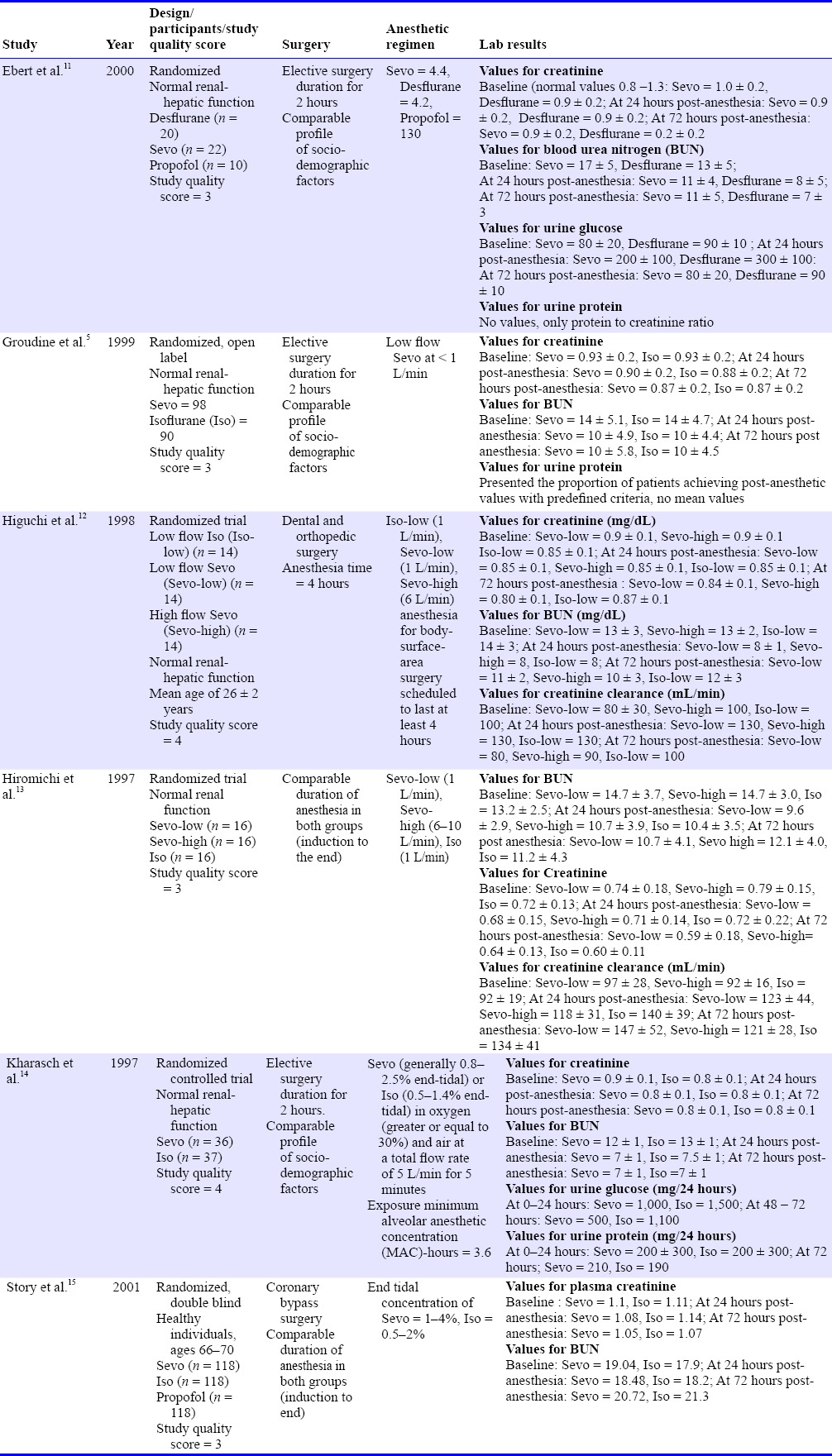
All six studies are randomized controlled trials, utilizing isoflurane as comparator arm studies while one study used desflurane,11 and two studies used propofol.14 All trials had intention-to-treat analysis mentioned.
This review consists of 873 patients, of whom 65% are males and 35% are females with age ranging from 19 to 67 (56 ± 3) years. All of the surgical procedures were done on an elective basis with a mean surgical time ranging from 2.5 to 5 hours. All six studies reported values for creatinine and BUN. Only one study reported 24-hour urine protein and urine glucose excretion.11 Therefore, definite conclusions using these parameters are confined to the studies which reported them. Only one study performed the renal function tests up to 5 days postoperatively.11
Analyses on the effects of sevoflurane were performed on renal function tests serum/plasma creatinine, BUN, urinary protein, and glucose excretion at 24 and 72 hours. Only one study presented creatinine clearance as a primary endpoint.11
Meta-analysis results
Serum creatinine
At 24 hours post-maintenance anesthesia, no statistical difference in the values existed between sevoflurane and isoflurane. The mean difference between the groups was –0.14, 95% CI –0.45, 0.17 (Figure 2). This obtained difference was below normal range (normal value: 0.8 to 2.0 mg/dL). There were no individual study values for creatinine that exceeded the upper limit of the normal range (2.0 mg/dL) (Figure 2).
Figure 2.
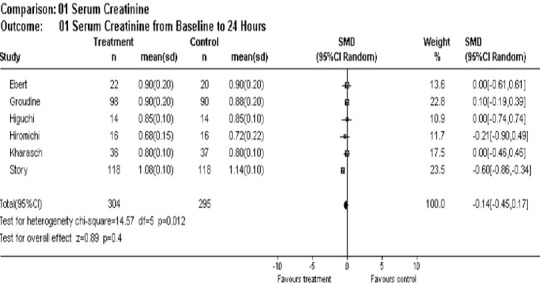
Overall effect of sevoflurane on serum creatinine post 24 hours of anesthesia.
At 72 hours post-maintenance anesthesia, no individual study values exceeded the established normal reference range and the pooled estimate difference was still within normal range (mean difference = 0.35, 95% CI –0.24, 0.94) favoring isoflurane slightly over sevoflurane (Figure 3).
Figure 3.
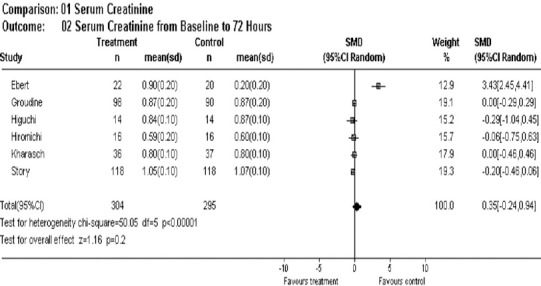
Overall effect of sevoflurane on serum creatinine post 72 hours of anesthesia.
BUN
There was no statistically significant difference in the BUN levels at 24 hours between control and sevoflurane (mean difference = 0.06, 95% CI –0.23, 0.34). This pooled estimate for difference was low when compared to established normal reference standards (8–20 mg/dL) (Figure 4).
Figure 4.
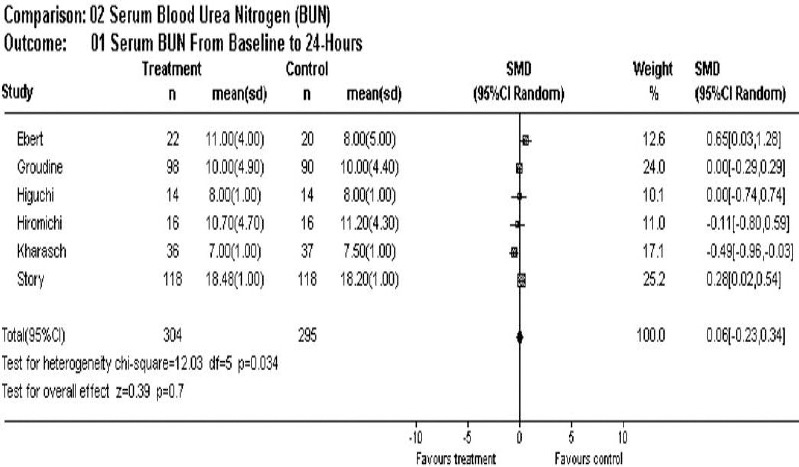
Overall effect of sevoflurane on blood urea nitrogen post 24 hours of anesthesia.
At 72 hours, BUN levels showed no statistical difference between the two groups (mean difference = –0.05, 95% CI –0.44, 0.34) (Figure 5).
Figure 5.
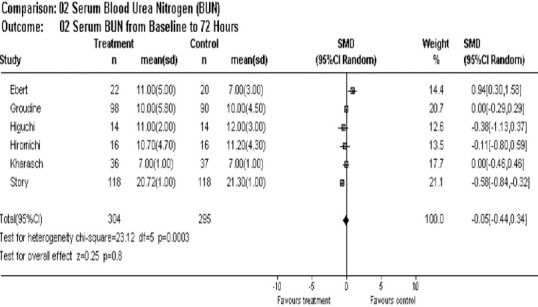
Overall effect of sevoflurane on blood urea nitrogen post 72 hours of anesthesia.
Urine glucose and protein excretion
Only two studies reported values for urine glucose and protein excretion.11,15 The latter study showed both at undetectable values during the 24 and 48 hours post-anesthesia for urine protein.15 At 72 hours, urine protein was noted to be higher with sevoflurane than isoflurane according to the Kharasch study,14 but this was not statistically significant.
Urine glucose excretion was not statistically different between the two groups.
Subgroup analysis
A subgroup analysis was performed by eliminating two studies in the main analysis frame. These two studies had utilized a high flow arm in comparison to the low flow arm plus low flow isoflurane. The resultant standard mean difference in creatinine values for 24 hours post anesthesia were (–0.15, 95% CI –0.55, 0.26) in favor for sevoflurane while 0.62 (95% CI –0.17, 1.41) in favor for isoflurane at 72 hours post anesthesia.
In terms of BUN, the pooled standard mean difference was also not significant between the two groups (0.09, 95% CI –0.28, 0.46) at 24 hours and at 72 hours (0.03, 95% CI –0.48, 0.54)
Analysis of publication bias
The funnel plots as shown in Figure 6 indicate the asymmetry of the individual studies around the pooled standard mean difference and away from the 95% CI limits of the difference. Sources of this bias may include failure to include two studies due to the language of publication and true heterogeneity of the individual studies included.
Figure 6.
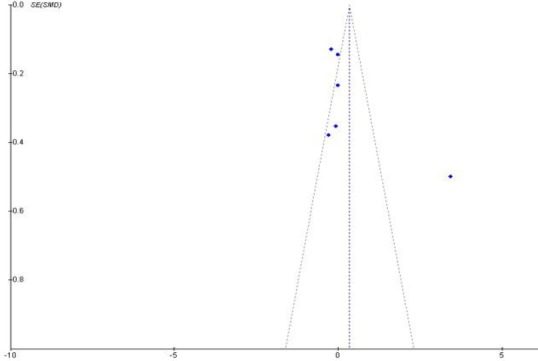
Funnel plots of the six included studies.
DISCUSSION
This meta-analysis revealed that sevoflurane is an anesthetic gas with minimal acute nephrotoxic potential. Although the estimates were focused on creatinine and BUN post 24 and 72 hours, an attempt to search for studies which utilized creatinine clearance and urinary protein and glucose excretion as indicators of kidney function was made. The conclusions in this meta-analysis are largely based on the parameters mentioned above and hence, evidence concerning the renal safety of sevoflurane as gauged by measurement of urinary glucose, protein excretion and creatinine clearance should be sought since the identified studies lacked these comparisons.
The results of this study have shown that creatinine change from baseline among patients receiving either low or high-flow sevoflurane did not statistically differ from those with isoflurane.
Although the standard mean difference in creatinine at 24 hours post anesthesia showed a negative value (in favor of treatment), the values were statistically imprecise probably owing to the inherently small sample size of the individual studies which are weighted. Alternately, positive values in the mean difference indicating that creatinine and BUN values were higher in the treatment arm versus control showed statistically insignificant results.
The individual studies given the largest weight in the statistical analysis5,15 had the highest number of subjects, 188 and 236 respectively, which revealed a zero or negative difference in terms of the two parameters mentioned. The overall effect size was dramatically influenced by the skewed data.
The nephrotoxic potential of sevoflurane is attributed to the so called degradation product of sevoflurane known as CpA. Carbon dioxide absorbents degrade sevoflurane, particularly at low-gas flow rates, to fluoromethyl-2,2-difluoro-1-(trifluoromethyl) vinyl ether (CpA). CpA causes renal proximal tubular injury in rats but has had no effect on BUN or creatinine concentrations in patients.16
In a study involving healthy volunteers, sevoflurane was associated with transient injury to: 1) the glomerulus, as revealed by post-anesthetic albuminuria; 2) the proximal tubule, as revealed by post-anesthetic glucosuria and increased urinary alpha-GST; and 3) the distal tubule, as revealed by post-anesthetic increased urinary pi-GST.8 This study by Eger et al.16 involving healthy volunteers exposed to 8 hours of sevoflurane theorized that a relatively low mean arterial blood pressure was not the cause of transient renal tubular injury (mean arterial blood pressure (MABP) = 56 mmHg) and attributed it solely to sevoflurane. However, given the recent clinical reports in which higher levels of CpA were inspired by patients (who were not hypotensive) without evidence of renal injury and given the higher blood pressures in the present study, persistent low blood pressure should not be ruled out as a contributor or cofactor in the renal dysfunction reported by Eger et al.16 The tubular dysfunction produced was notably reversible even if total preoperative anesthesia time was extended to 3 more hours.17
Contrary to the Eger study, the nephrotoxic potential of sevoflurane was disproved by Ebert et al.11 The latter study concluded that prolonged (8 hours), high concentration (3%) sevoflurane anesthesia administered to volunteers in a fresh gas flow of 2 L/min with a resultant minimum alveolar anesthetic concentration of 1.25 does not result in clinically significant changes in biochemical markers of renal or hepatic dysfunction.18
The BUN and creatinine levels revealed by the individual studies were consistently seen across each study. The observed mean difference in the values were obtained with all sources of potential bias excluded, e.g., co-intervention with other drugs and the effects produced by contrasting the doses of sevoflurane and isoflurane in another arm of at least two studies.
This comparable renal safety of both sevoflurane and isoflurane should be taken in the light of the degree of statistical heterogeneity seen in this review. Foremost, although the patients enrolled were homogenously distributed in terms of gender, age, baseline renal function test results, and anesthesia exposure, the individual studies were small scale and majority did not reach minimum sample size requirements to prove definite study power. Secondly, although most studies did account for differences in anesthesia administration techniques, inherently, a varied data regarding minimum alveolar concentration for sevoflurane was seen. We do not underestimate the effect of this factor in the individual study outcomes and hence we propose that more studies be identified and analyzed that control for this factor.
Limitations
The result of this meta-analysis extends to healthy subjects without apparent renal disease. However, even in patients with renal insufficiency (creatinine ≥ 1.5 mg/dL), low flow sevoflurane is shown to be safe.2,4,19 Low-flow sevoflurane is as safe as low-flow isoflurane and does not alter renal function in patients with preexisting renal disease. These results amplify previous studies in patients with renal insufficiency conducted at higher flow rates which showed no significant differences in the renal effects of sevoflurane and other volatile anesthetics. In lieu of the traditional markers for renal function assessment, studies utilizing the other sensitive markers can be employed in a separate analysis.
The comparisons dealt with low-flow sevoflurane only. Although two of the included studies had included a “high-flow arm,” these did not permit statistical combination and thus, one can only rely on the results of the individual trials.
CONCLUSION
In an apparently healthy adult void of coexisting renal disorders who is contemplating elective surgery, sevoflurane does not produce elevations in creatinine and BUN above the established upper limit of the reference range. The safety of sevoflurane on the kidneys as assessed by urinary protein and glucose excretion as well as creatinine clearance can only be deduced from two previous studies existed.
Acknowledgments
The authors would like to acknowledge the support of Dr. Ozan Akca (Department of Anesthesiology and Perioperative Medicine, University of Louisville, Louisville, KY, USA) in reviewing this paper and encouraging to submit to Medical Gas Research.
Footnotes
Conflicts of interest
None declared.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
REFERENCES
- 1.Eger EI, Eisenkraft JB, Weiskopf RB. The Pharmacology of Inhaled Anesthetics. USA: Springer-Verlag; 2003. [Google Scholar]
- 2.Conzen PF, Kharasch ED, Czerner SF, et al. Low-flow sevoflurane compared with low-flow isoflurane anesthesia in patients with stable renal insufficiency. Anesthesiology. 2002;97:578–584. doi: 10.1097/00000542-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Morio M, Fujii K, Satoh N, et al. Reaction of sevoflurane and its degradation products with soda lime. Toxicity of the byproducts. Anesthesiology. 1992;77:1155–1164. doi: 10.1097/00000542-199212000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Higuchi H, Adachi Y, Wada H, Kanno M, Satoh T. The effects of low-flow sevoflurane and isoflurane anesthesia on renal function in patients with stable moderate renal insufficiency. Anesth Analg. 2001;92:650–655. doi: 10.1097/00000539-200103000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Groudine SB, Fragen RJ, Kharasch ED, Eisenman TS, Frink EJ, McConnell S. Comparison of renal function following anesthesia with low-flow sevoflurane and isoflurane. J Clin Anesth. 1999;11:201–207. doi: 10.1016/s0952-8180(99)00027-6. [DOI] [PubMed] [Google Scholar]
- 6.Keller KA, Callan C, Prokocimer P, et al. Inhalation toxicity study of a haloalkene degradant of sevoflurane, Compound A (PIFE), in Sprague-Dawley rats. Anesthesiology. 1995;83:1220–1232. doi: 10.1097/00000542-199512000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Bito H, Ikeda K. Long-duration, low-flow sevoflurane anesthesia using two carbon dioxide absorbents. Quantification of degradation products in the circuit. Anesthesiology. 1994;81:340–345. doi: 10.1097/00000542-199408000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Kharasch ED, Thorning D, Garton K, Hankins DC, Kilty CG. Role of renal cysteine conjugate beta-lyase in the mechanism of compound A nephrotoxicity in rats. Anesthesiology. 1997;86:160–171. doi: 10.1097/00000542-199701000-00020. [DOI] [PubMed] [Google Scholar]
- 9.Guyatt GH, Sackett DL, Cook DJ. Users’ guides to the medical literature. II. How to use an article about therapy or prevention. A. Are the results of the study valid? Evidence-Based Medicine Working Group. JAMA. 1993;270:2598–2601. doi: 10.1001/jama.270.21.2598. [DOI] [PubMed] [Google Scholar]
- 10.Levine M, Walter S, Lee H, Haines T, Holbrook A, Moyer V. Users’ guides to the medical literature. IV. How to use an article about harm. Evidence-Based Medicine Working Group. JAMA. 1994;271:1615–1619. doi: 10.1001/jama.271.20.1615. [DOI] [PubMed] [Google Scholar]
- 11.Ebert TJ, Arain SR. Renal responses to low-flow desflurane, sevoflurane, and propofol in patients. Anesthesiology. 2000;93:1401–1406. doi: 10.1097/00000542-200012000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi H, Sumita S, Wada H, et al. Effects of sevoflurane and isoflurane on renal function and on possible markers of nephrotoxicity. Anesthesiology. 1998;89:307–322. doi: 10.1097/00000542-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bito H, Ikeuchi Y, Ikeda K. Effects of low-flow sevoflurane anesthesia on renal function: comparison with high-flow sevoflurane anesthesia and low-flow isoflurane anesthesia. Anesthesiology. 1997;86:1231–1237. doi: 10.1097/00000542-199706000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kharasch ED, Frink EJ, Jr, Zager R, Bowdle TA, Artru A, Nogami WM. Assessment of low-flow sevoflurane and isoflurane effects on renal function using sensitive markers of tubular toxicity. Anesthesiology. 1997;86:1238–1253. doi: 10.1097/00000542-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Story DA, Poustie S, Liu G, McNicol PL. Changes in plasma creatinine concentration after cardiac anesthesia with isoflurane, propofol, or sevoflurane: a randomized clinical trial. Anesthesiology. 2001;95:842–848. doi: 10.1097/00000542-200110000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Eger EI, 2nd, Koblin DD, Bowland T, et al. Nephrotoxicity of sevoflurane versus desflurane anesthesia in volunteers. Anesth Analg. 1997;84:160–168. doi: 10.1097/00000539-199701000-00029. [DOI] [PubMed] [Google Scholar]
- 17.Hara T, Fukusaki M, Nakamura T, Sumikawa K. Renal function in patients during and after hypotensive anesthesia with sevoflurane. J Clin Anesth. 1998;10:539–545. doi: 10.1016/s0952-8180(98)00078-6. [DOI] [PubMed] [Google Scholar]
- 18.Ebert TJ, Frink EJ, Jr, Kharasch ED. Absence of biochemical evidence for renal and hepatic dysfunction after 8 hours of 1.25 minimum alveolar concentration sevoflurane anesthesia in volunteers. Anesthesiology. 1998;88:601–610. doi: 10.1097/00000542-199803000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Tsukamoto N, Hirabayashi Y, Shimizu R, Mitsuhata H. The effects of sevoflurane and isoflurane anesthesia on renal tubular function in patients with moderately impaired renal function. Anesth Analg. 1996;82:909–913. doi: 10.1097/00000539-199605000-00003. [DOI] [PubMed] [Google Scholar]


