Abstract
Stroke is considered to be an acute cerebrovascular disease, including ischemic stroke and hemorrhagic stroke. The high incidence and poor prognosis of stroke suggest that it is a highly disabling and highly lethal disease which can pose a serious threat to human health. Nitric oxide (NO), a common gas in nature, which is often thought as a toxic gas, because of its intimate relationship with the pathological processes of many diseases, especially in the regulation of blood flow and cell inflammation. However, recent years have witnessed an increased interest that NO plays a significant and positive role in stroke as an essential gas signal molecule. In view of the fact that the neuroprotective effect of NO is closely related to its concentration, cell type and time, only in the appropriate circumstances can NO play a protective effect. The purpose of this review is to summarize the roles of NO in ischemic stroke and hemorrhagic stroke.
Keywords: nitric oxide, neuronal nitric oxide synthase, inducible nitric oxide synthase, endothelial nitric oxide synthase, ischemia stroke, hemorrhagic stroke, neuroprotection, neurotoxicity
INTRODUCTION
Stroke has a high morbidity and mortality, which is defined as a kind of acute cerebrovascular disease.1,2 According to the existing data, ischemic stroke and hemorrhagic stroke have a record of more than one million new cases each year. These new cases bring a heavy burden on the family and society because of a substantial expenditure for complication treatment in healthcare systems.3 With the aging of population and the change of diet structure, the incidence of stroke increases year by year, which further aggravates the burden. A large number of animal experiments and clinical studies have shown primary brain injury and secondary brain injury caused by stroke are complex pathophysiological processes, involving inflammatory reaction, neuronal apotosis/death, ischemia-reperfusion injury, blood-brain barrier damage, neurotoxic substance release, the generation of free radical, oxidative stress and brain edema.4,5,6,7,8,9,10,11 At present, ischemic stroke therapies mainly concentrate on translator mechanical thrombectomy, stenting and angioplasty, surgical treatment (decompressive craniectomy and carotid endarterectomy), thrombolytic agents, neuroprotective drugs12 and rehabilitation training.13 The comprehensive therapy may also improve the prognosis and quality of life to a certain extent, and early thrombolysis or mechanical thrombectomy may also improve the prognosis of stroke. However, the therapeutic effect of stroke is still unsatisfactory. Therefore, some new treatment strategies and the pathogenesis of stroke need to be further studied.
Nitric oxide (NO) is commonly considered as a toxic gas, but it was found to transmit biological information as a signal molecule 40 years ago. At first, it was recognized that the endothelium released a factor which relaxed vascular smooth muscle cells and subsequently caused vasodilatation in the late 1970s.14,15 During that time, as the molecular structure of this factor was unknown, it was named endothelium-derived relaxing factor (EDRF). Furchgott and his colleagues16 conformed that EDFR was NO, a colorless, odorless gas until 10 years later. Since then, NO has been gradually recognized as a gas signaling molecule and its mechanisms of action in the laboratory animals and humans have been extensively researched. The main physiological functions of NO include the maintenance of vascular tone, the reduction of inflammation response, the balance of thrombotic-thrombolytic homeostasis and the regulation cell growth.
NO has a close relationship with stroke. There are three kinds of NO synthases (NOS) produced by NO during the stroke. Inducible NOS (iNOS)-derived NO and neuronal NOS (nNOS)-derived NO play neurotoxicity, but endothelial NOS (eNOS)-derived NO plays a neuroprotective role in acute ischemic stroke. The toxic effects of NO produced by iNOS and nNOS are mainly due to the production of nitrates and the release of free radicals, which directly damage mitochondrial enzymes and genetic materiasupls.17,18,19,20 On the contrary, neuroprotective effects of NO produced by eNOS are achieved primarily by regulating vascular bed and peripheral nerve tissue.21,22 In hemorrhagic stroke, NO is extensively studied in subarachnoid hemorrhage (SAH). The poor prognosis of SAH is due to cerebral vasospasm and delayed ischemic neurologic deficits (DIND).23,24,25 Cerebral vasospasm and DIND are related to complex pathophysiological processes. For exemple, DIND is involved in ruptured aneurysm, cerebral ischemia, blood-brain barrier dysfunction, increased intracranial pressure, and macro- and microcirculatory embolism and spasm.26,27,28,29 Some studies have suggested that NOS dysfunction in the vicinity of cerebral vascular beds leads to cerebral vasospasm, DIND and clearance of deoxyhemoglobin.30,31,32 Some studies have shown that the concentration of NO is associated with cerebral vasospasm.33,34 Current research shows that the activation of the NO may improve vascular diameter, but it remains unclear in regard to the survival of patients.
MECHANISMS OF NO IN STROKE
Mechanisms of NO in ischemic stroke
NO has a dual identity including neuroprotectiion and neurotoxicity during ischemia reperfusion. The distribution and concentration of NO in brain tissue was significantly changed after cerebral ischemia. NO is mainly synthesized by three subtypes of NOS in brain tissue: nNOS, eNOS and iNOS. Among them, nNOS and eNOS are calcium-dependent NOS, iNOS is a calcium-independent.35,36 In the acute phase of ischemic stroke, the increase of NO was mainly caused by nNOS, followed by eNOS, but the formation of NO mediated by iNOS did not increase significantly, especially in 30 minutes after ischemia stroke.37
In general, nNOS and iNOS play a neuronal injury role in the early and late stage of ischemic stroke, while the activation of eNOS mainly exerts neuroprotection effects. NO is produced in several different types of cells, such as endothelial cells, neurons, glial cells and neutrophils. It plays a dual role in different time and space in ischemic stroke.38 The beneficial or harmful role NO played in brain tissue of ischemic stroke depending on the cell type, the concentration of NO and microenvironment of ischemia.39,40,41
nNOS derived NO
In ischemic stroke, the concentration of NO decreases rapidly due to blocked blood flow.37 Once the blood flow is restored, the production of NO will increase, which is mainly mediated by nNOS. Scientists Used nNOS gene-deficient mice and nNOS-specific inhibitors to verify above view.39 The synthesis of NO through nNOS is mainly related to calcium overload induced by glutamate in ischemic neurons.42 Within one hour after reperfusion, the concentration of NO returned to physiological level. However, the defect of nNOS gene or the inhibition of nNOS can reduce the area of ischemic penumbra and the number of neuronal necrosis.39,43,44 Inhibition of nNOS is also able to produce oxygen free radicals45 and nitrosative stress,46 reduce excitotoxicity and down regulate the expression caspase-3 in ischemic stroke.44
iNOS derived NO
The activation of iNOS increases from 12 hours after the onset of ischemic stroke and lasts for 1 week.47 At this stage, iNOS is mainly produced by microglia, astrocytes, endothelial cells and infiltrating lymphocytes. The amount of NO released by iNOS is 1,000 times than that by nNOS.48 Additionally, the production of NO induced by iNOS leads to brain damage during ischemia reperfusion.49 The overexpression of iNOS can promote the secretion of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), and subsequently induce secondary inflammatory reaction and the generation of oxygen free radicals.50,51 After ischemic stroke, iNOS produces a large amount of NO, and NO elevates nitrous oxide levels and causes nitrosation damage within 12 hours to 8 days.52,53
eNOS derived NO
Unlike the other two subtypes of NOS, NO derived from eNOS often plays a neuroprotective role in ischemic stroke. In brain tissue, eNOS is mainly produced by the vascular endothelial cells and the choroid.54 Although eNOS generates a small amount of NO, it plays a critical role in the regulation of cerebral microvascular tone, the protection of the blood-brain barrier, the reduction of oxidative stress and the alleviation of procoagulant stimulation. It has been proved that NO released by eNOS can scavenge oxygen free radicals, inhibit the expression of adhesion molecules, and promote the aggregation of platelet and the adhesion of lymphocyte.55,56,57,58 the Inhibition of eNOS Activity Achieved by Employing Knockout Mice (eNOS–/–) and eNOS-specific inhibitors leads to hypertensive-prone organism, and more severe ischemia-reperfusion injury, significantly reduced cerebral blood flow, and thus subsequently result in greater infarct size.21,39,59 On the contrary, flavonoids induced overexpression of enos and therefore exerted neuroprotection effects.60
Non-Selective inhibition of NOS did not significantly alter the infarct volume in the permanent model, but the total infarct volume in the transient ischemic model was reduced. Although inhibition of NOS may have a negative effect on cerebral blood flow,61 Further investigations are required. Selective nNOS and iNOS inhibitors can be candidates for acute ischemic stroke treatment.61
No plays a dual role in ischemic stroke, and the production of NO in the early stage of transient cerebral ischemia has a positive effect on the neuroprotection of stroke, but nNOS and iNOS play a negative role in the later stage.62,63 eNOS plays a key role in the protection of neurovascular system. The production of NO derived from eNOS around the nerve vessels is capable to regulate the tension between the cerebral vessels and plays a positive role in improving the blood supply of the brain tissue (Figure 1).64
Figure 1.
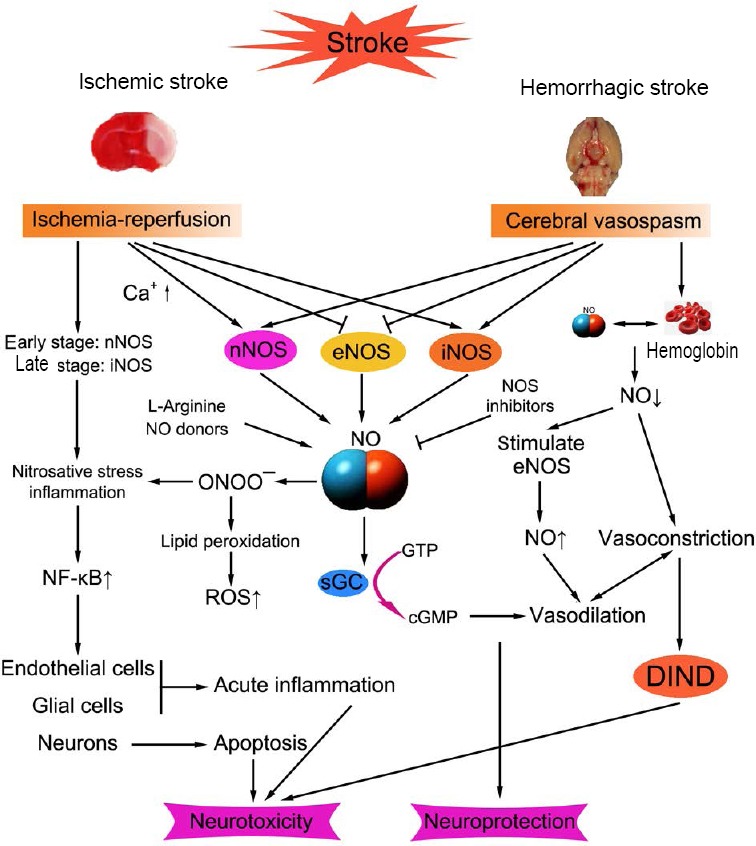
Schematic diagram of NO synthesis and action on ischemic stroke and hemorrhagic stroke.
Note: NO: Nitric oxide; NOS: nitric oxide synthase; nNOS: neuronal NOS; eNOS: endothelial NOS; iNOS: inducible NOS; sGC: soluble guanylate cyclase; ROS: reactive oxygen species; DIND: delayed ischemic neurological deficits; onoo-: peroxynitrite anion; NF-κB: nuclear factor-κB; GTP: guanosine triphosphate; cGMP: cyclic guanosine monophosphate.
Mechanisms of NO in hemorrhagic stroke
At Present, the study of NO in hemorrhagic stroke is mainly focused on SAH. We further discuss the case of SAH. About 1/4 of SAH patients in the first week will produce vascular spasm, which makes blood flow reduce to half of the normal blood flow.65 Although there are many studies on dind around the world last century, its pathological mechanism remains to be further explored.66 In any case, it is thought that hemoglobin may be the cause of cerebral vasospasm because the affinity of eNOS-derived no and hemoglobin is 1,000 times than that of oxygen and hemoglobin.14,67 The amount of NO produced by eNOS is decreased after SAH and thereby reduced the affinity between NO and hematoma in the cerebrospinal fluid, reduced the concentration of NO around the blood vessel, and increased cerebral vasospasm.66 Recent research has suggested that there is a close relationship between NO and cerebral vasospasm after SAH, and it is of great significance to study the basic role of NO after SAH.68
nNOS derived NO
As to whether SAH would change the expression of nNOS and therefore affect the production of NO, there were some related researches dedicated to explore this pathophysiological effect. The expression of nNOS was decreased from the onset of vasospasm, it resulted the decrease of the concentration of NO in the arterial adventitia and ultimately leaded to vasoconstriction. The latest data suggest that elevated intracranial pressure may cause transient cerebral ischemia after SAH, which may subsequently promoted the phosphorylation of Ser847 of nNOS via Ca2+/calmodulin-dependent protein kinase IIα (CamkIIα) pathway in the hippocampus. The phosphorylation of nNOS reduces ischemic injury and plays a neuroprotective effect in early brain injury.69
iNOS derived NO
In the early study of human SAH, it was found that the genenation of iNOS is a consequence of SAH and plays a major role in the pathogenesis of vasospasm.70 Hyperglycemia increases the chance of cerebral vasospasm after SAH, mainly through the NO pathway as a potential underlying mechanism via the dysregulation of eNOS and iNOS.71 A study found that aminoguanidine inhibits iNOS activity and reduces cerebral vasospasm after SAH in rabbits after abnormal endothelial cell repair.72
eNOS derived NO
Cerebral vasospasm is a common complication of SAH, and eNOS has the effect of regulating vascular tone. At present, the study of NOS after SAH is mainly focused on eNOS subtype. Early stenosis of the spastic artery was able to stimulate eNOS due to increased shear stress.73 Therefore, the production of NO in the early stage counteracts the decrease of no and leads to vasodilation. However, the persistence of delayed cerebral vasospasm in the arterial wall lowered the levels of cyclic guanosine monophosphate (GMP) and nitrites in the cerebrospinal fluid (CSF), which companied with the dysfunction of vascular endothelial cell and the reduction of eNOS and the decreased levels of NO around the arterial wall.66,74,75,76,77 The functional defect of eNOS may be due to the increased activation of phosphodiesterase and the quick elimination of 3′,5′-cGMP, which may activate endogenous inhibitors of eNOS through asymmetric dimethylarginine, an endogenous inhibitor of NOS that produced by the fault of the oxidative cleavage fragment of bilirubin in haemorrhagic cerebrospinal fluid.74,78,79 The interaction between asymmetric dimethyl-L-arginine (ADMA) and bilirubin-oxidation products (BOXes) in the CSF is related to the degree and time course of vasospasm in patients with SAH.78,80 The levels of ADMA in late CSF are reduced by the clearance of BOXes, and increased NO levels resulting from eNOS ultimately lead to the relaxation of vascular endothelial.66,74,81
The levels of NO are closely related to cerebral vasospasm after SAH, and more and more studies have assumed that low levels of NO could contribute to cerebral vasospasm.66,82 Increased NO levels and increased NO donors (NODs) can reverse cerebral vasospasm.83 In conclusion, the present studies suggest that increased concentration of NO after SAH is expected to improve the prognosis of patients with cerebral vasospasm after SAH (Figure 1).
Therapeutic approaches of NO donors and inhibitors in stroke
NOD is a class of drugs which is generally characterized by the production of NO or NO-related substances independently in vivo or in vitro, such as nitro anions (NO–) or nitroonium ions (NO+).84 NODs are the most commonly used donors in basic and clinical studies: organic nitrate, S-nitrosothiols, sydnonimines, NONOates and sodium nitroprusside.85 NOD has many neurotoxic effects that are not associated with NO and the neurotoxicity of the media molecules carried by the NOD themselves.86 Therefore, it is important to avoid the adverse influence of NOD medium on the treatment of stroke.
At present, some common inhibitors include: NOS inhibitors (e.g., Nω-nitro-L-arginine methyl ester hydrochloride, pan-NOS inhibitors, 7-nitroindazole); statins (HMG-CoA-reductase inhibitors); Rho kinase (ROCK)-inhibitors; and phosphodiesterase inhibitors. However, most of the NOS inhibitors are nonselective and may cause toxic side effects to eNOS, so they have not been applied clinically. It is necessary to develop highly selective inhibitors of NOS that will be better applied clinically.87 Statins do improve cerebral perfusion during the acute phase of ischemia stroke, but it may increase the risk of infection.88 The limiting factor for ROCK inhibitors in stroke treatment is that it has the potential to cause hypotension. Thus, the development of ROCK inhibitors with selectively targeted cerebral blood flow may improve ROCK therapeutic value in stroke.89
No and neonatal hypoxia-ischemia (HI) brain injury
In neonatal HI brain injury, NO plays a different role in different studies. Some studies suggest that NO has neuroprotective effects in neonatal rat brain hypoxia. In 2012, Zhu et al.90 reported that inhalation of NO in neonatal mice with HI brain damage had protective effects on male mice, but had no protective effect on female mice. The neuroprotective effect of NO on neonatal HI rat model after helium pretreatment (He-PC) suggests that the treatment of He-PC may induce the production of NO and activation of nuclear factor erythroid 2-related factor 2 (Nrf2), which plays a neuroprotective role on neonatal HI.91 Study on the relationship between low dose lipopolysaccharide (LPS) pretreatment and neonatal HI showed that low-dose LPS-mediated neuronal activation and enhanced endothelial cell eNOS activity can improve hypoxia tolerance through AKT pathway and thus play a neuroprotective effect.92
In addition, some studies about the negative effects of NO on neonatal HI are reported. NO is responsible for the death of neuronal cells in neonatal HI brain injury by disrupting the homeostasis of iron metabolism and generating more free radicals.93 In a recent study, it has been shown that the activation of nNOS leads to microcirculation injury and the reduction of blood flow after recanalization and exacerbates brain damage.94 Neonatal HI brain damage leads to overexpression of iNOS and cause white matter damage. The expression of iNOS may be involved in the ischemic cellular events including apoptosis, and play a role in the pathophysiological process of white matter damage.95
NEUROPROTECTION AND NEUROTOXIC STUDIES
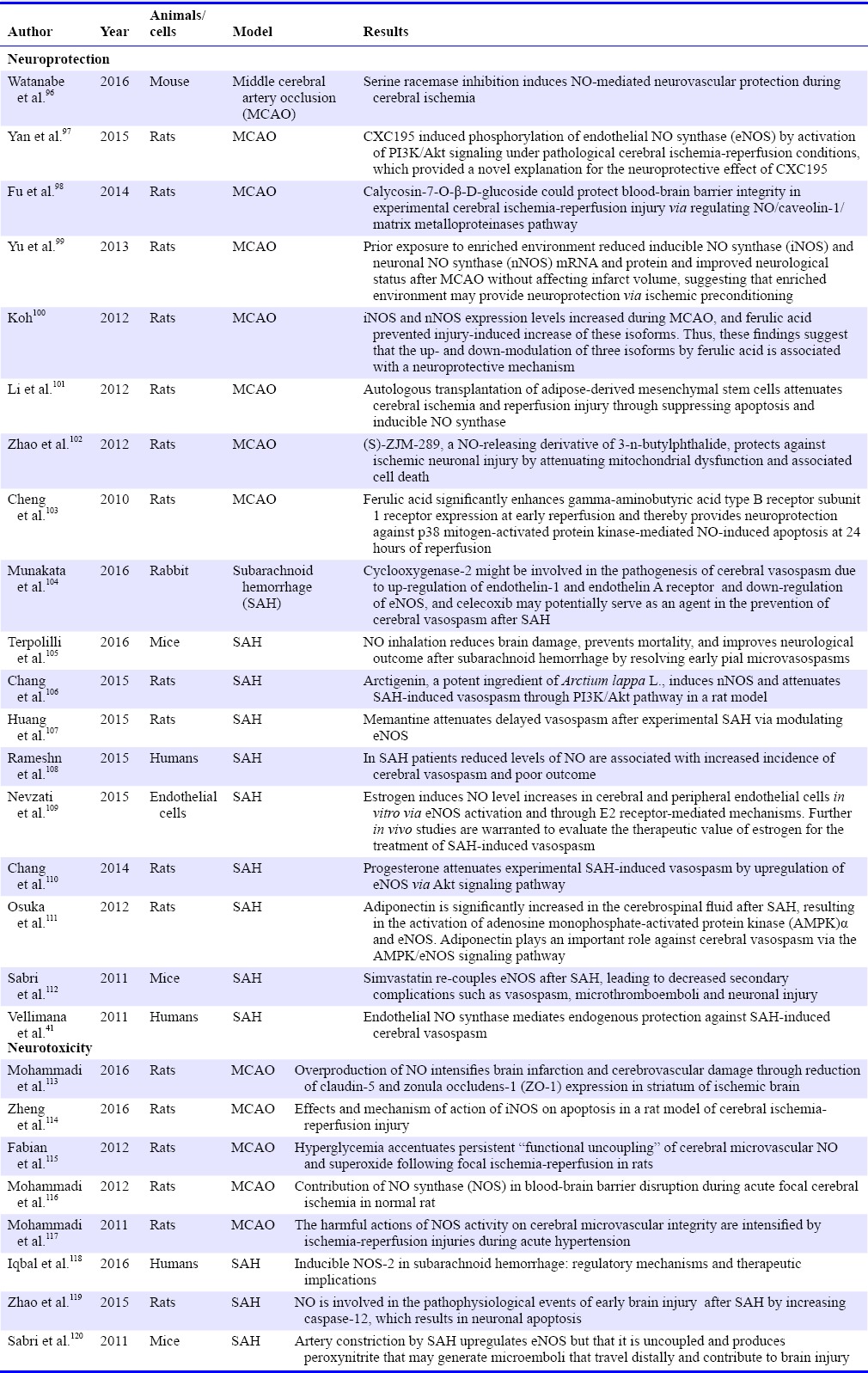
It is well known that a large number of animals and cell studies must be tested before NO enters the clinical application. These studies further elaborate on the molecular mechanisms of NO from several aspects such as neuroprotection, neurotoxicity and biological effects. The study summarizes that most of the neuroprotective effects of NO are associated with eNOS, and the neurotoxicity is primarily related to nNOS and iNOS. In our review we will systematically summarize the existing animal and human studies on the role of NO in stroke (Table 1).
Table 1.
The neuroprotective and neurotoxic effects of hydrogen nitric oxide (NO) in stroke
No and neuroprotection
The neuroprotection of NO in the model of middle cerebral artery occlusion (MCAO) is exhibited as follows: (1) Watanabe and his colleagues96 reported that the inhibition of serine racemases induce NO-mediated neurovascular protection in cerebral ischemia. (2) Yan et al.97 reported that CXC195 induced phosphorylation of eNOS by the activation of PI3K/Akt signaling pathway under pathological cerebral ischemia-reperfusion conditions, which provided a novel explanation for the neuroprotective effect of CXC195. (3) Calycosin-7-O-β-D-glucoside could protect BBB integrity in experimental cerebral ischemia-reperfusion injury by regulating NO/caveolin-1/matrix metalloproteinases pathway.98 (4) Neuroprotection was achieved by reducing the mRNA and protein levels of iNOS and nNOS.99 (5) Ferulic acid can inhibit the expression of nNOS and iNOS, prevent the increase of isomer and therefore achieve neuroprotection.100 (6) Mutologous adipose derived from mesenchymal stem cells (MSCs) transplantation can inhibit brain injury by inhibiting apoptosis and iNOS in ischemia-reperfusion injury.101 (7) (S)-ZJM-289, a NOD, reduces neuronal mitochondrial dysfunction and reduces cell death in ischemic stroke.102 (8) Ferulic acid significantly enhances the expression of gamma-aminobutyric acid type B receptor subunit 1 receptor during early reperfusion and thereby provides neuroprotection against p38 mitogen-activated protein kinase-mediated and NO-induced apoptosis at 24 hours of reperfusion.103
The neuroprotection of NO in the SAH is exhibited as follows: (1) The pathophysiological mechanism of Cyclooxygenase-2 may be involved in cerebral vasospasm. The upregulation of endothelin-1, down regulation of eNOS and ETAR and the regulation of celecoxib might contribute to the prevention of cerebral vasospasm after SAH.104 (2) Inhaled NO can alleviate the early cerebral microvasospasms, reduce brain injury and improve the prognosis of neurological impairment after SAH.105 (3) Arctigenin induced eNOS and alleviated vasospasm after SAH through PI3K/Akt signaling pathway.106 (4) Memantine alleviates cerebral vasospasm by regulating the eNOS in experimental SAH.107 (5) Reduced levels of NO are associated with increased incidence of cerebral vasospasm and poor outcome in SAH patients.108 (6) Estrogen increases the level of NO in brain and peripheral vascular endothelial cells, and alleviates the vascular spasm after SAH.109 (7) Progesterone reduces cerebral vasospasm induced by SAH via upregulating eNOS through Akt signaling pathway.110 (8) Adiponectin was significantly increased in the cerebrospinal fluid after SAH. It results in the activation of AMPKα and eNOS, which played an important role in antagonizing cerebral vasospasm.111 (9) Simvastatin improves the expression of eNOS after SAH and leads to reduced complications such as cerebral vasospasm, thrombosis, and neuronal injury.112 (10) eNOS mediates endogenous protection against SAH-induced cerebral vasospasm.41
No and neurotoxicity
However, the role of NO in different concentrations, different environments is quite different, even in different time periods and different cells will get different or even the opposite consequences. The neurotoxicity of NO in the model of MCAO is exhibited as follows: (1) Excess NO increases infarct size and cerebral vascular injury.113 (2) The activation of iNOS induced cell apoptosis in a rat model of cerebral ischemia-reperfusion injury.114,121 (3) Persistent hyperglycemia after ischemic stroke is associated with an excess of NO and peroxides which leads to microvascular dysfunction and poor prognosis.115 (4) NOS leads to the damage of blood brain barrier in acute ischemic stroke.116 (5) The activation of NOS leads to the damage of blood-brain barrier and brain edema in acute ischemic stroke.117 The neurotoxicity of NO in the SAH is exhibited as follows: (1) Imbalance of NOS and its product NO leads to adverse factors in SAH.118 (2) NO increases the production of caspase-12 which induced neuronal apoptosis in early brain injury after SAH.119 (3) SAH leads to the upregulation of eNOS and results in the generation of microemboli. It reaches to the end of blood vessels and leads to brain injury.120
CONCLUSION
There is growing evidence that NO has an important role in neuroprotection in stroke, even if NO is usually considered as a toxic gas. Therefore, we need to dialectically treat NO, and further research including animal and clinical research can provide us with a new insight into the treatment of stroke and other central nervous system diseases such as multiple sclerosis, Parkinson's disease and traumatic brain injury.
Footnotes
Conflicts of interest
The authors declare that they have no competing interests.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open peer reviewer
Wen-wu Liu, Second Military Medical University, China.
Funding: This work was supported by Suzhou Key Medical Center (No. Szzx201501), grants from the National Natural Science Foundation of China (No. 81571115, 81422013, and 81471196), Scientific Department of Jiangsu Province (No. BL2014045), Suzhou Government (No. SZS201413, SYS201608, and LCZX201601), Jiangsu Province (No. 16KJB320008).
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rolfs A, Fazekas F, Grittner U, et al. Acute cerebrovascular disease in the young: the stroke in young Fabry patients study. Stroke. 2013;44:340–349. doi: 10.1161/STROKEAHA.112.663708. [DOI] [PubMed] [Google Scholar]
- 3.Pucciarelli G, Vellone E, Savini S, et al. Roles of changing physical function and caregiver burden on quality of life in stroke: a longitudinal dyadic analysis. Stroke. 2017;48:733–739. doi: 10.1161/STROKEAHA.116.014989. [DOI] [PubMed] [Google Scholar]
- 4.Li SH, Chen L, Pang XM, et al. Decreased miR-146a expression in acute ischemic stroke directly targets the Fbxl10 mRNA and is involved in modulating apoptosis. Neurochem Int. 2017;107:156–167. doi: 10.1016/j.neuint.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Zhao X, Sun G, Ting SM, et al. Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J Neurochem. 2015;133:144–152. doi: 10.1111/jnc.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zeynalov E, Jones SM, Elliott JP. Therapeutic time window for conivaptan treatment against stroke-evoked brain edema and blood-brain barrier disruption in mice. PLoS One. 2017;12:e0183985. doi: 10.1371/journal.pone.0183985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kikuchi K, Miura N, Kawahara KI, et al. Edaravone (Radicut), a free radical scavenger, is a potentially useful addition to thrombolytic therapy in patients with acute ischemic stroke. Biomed Rep. 2013;1(1):7–12. doi: 10.3892/br.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bustamante A, Simats A, Vilar-Bergua A, García-Berrocoso T, Montaner J. Blood/brain biomarkers of inflammation after stroke and their association with outcome: from C-reactive protein to damage-associated molecular patterns. Neurotherapeutics. 2016;13:671–684. doi: 10.1007/s13311-016-0470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidale S, Consoli A, Arnaboldi M, Consoli D. Postischemic inflammation in acute stroke. J Clin Neurol. 2017;13:1–9. doi: 10.3988/jcn.2017.13.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang MD, Wang Y, Xia YP, et al. High serum miR-130a levels are associated with severe perihematomal edema and predict adverse outcome in acute ICH. Mol Neurobiol. 2016;53:1310–1321. doi: 10.1007/s12035-015-9099-0. [DOI] [PubMed] [Google Scholar]
- 11.Chen Y, Tong H, Pan Z, et al. Xuebijing injection attenuates pulmonary injury by reducing oxidative stress and proinflammatory damage in rats with heat stroke. Exp Ther Med. 2017;13(6):3408–3416. doi: 10.3892/etm.2017.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venti M, Parnetti L, Silvestrelli G, Gallai V. Role of neuroprotective drugs in acute ischemic stroke. Cerebrovasc Dis. 2000;10(Suppl 4):24–26. doi: 10.1159/000047589. [DOI] [PubMed] [Google Scholar]
- 13.Jin XF, Wang S, Shen M, et al. Effects of rehabilitation training on apoptosis of nerve cells and the recovery of neural and motor functions in rats with ischemic stroke through the PI3K/Akt and Nrf2/ARE signaling pathways. Brain Res Bull. 2017;134:236–245. doi: 10.1016/j.brainresbull.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann J, Lerman A. The endothelium: dysfunction and beyond. J Nucl Cardiol. 2001;8:197–206. doi: 10.1067/mnc.2001.114148. [DOI] [PubMed] [Google Scholar]
- 16.Furchgott RF, Carvalho MH, Khan MT, Matsunaga K. Evidence for endothelium-dependent vasodilation of resistance vessels by acetylcholine. Blood Vessels. 1987;24:145–149. doi: 10.1159/000158689. [DOI] [PubMed] [Google Scholar]
- 17.Hirvonen MR, Brüne B, Lapetina EG. Heat shock proteins and macrophage resistance to the toxic effects of nitric oxide. Biochem J. 1996;315:845–849. doi: 10.1042/bj3150845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002;40:511–526. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 19.Hämäläinen M, Nieminen R, Vuorela P, Heinonen M, Moilanen E. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007;2007:45673. doi: 10.1155/2007/45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao X, Haensel C, Araki E, Ross ME, Iadecola C. Gene-dosing effect and persistence of reduction in ischemic brain injury in mice lacking inducible nitric oxide synthase. Brain Res. 2000;872:215–218. doi: 10.1016/s0006-8993(00)02459-8. [DOI] [PubMed] [Google Scholar]
- 21.Huang Z, Huang PL, Ma J, et al. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Zhang F, Iadecola C. Reduction of focal cerebral ischemic damage by delayed treatment with nitric oxide donors. J Cereb Blood Flow Metab. 1994;14:574–580. doi: 10.1038/jcbfm.1994.71. [DOI] [PubMed] [Google Scholar]
- 23.Pluta RM, Hansen-Schwartz J, Dreier J, et al. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res. 2009;31:151–158. doi: 10.1179/174313209X393564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin CO, Rymer MM. Hemorrhagic stroke: aneurysmal subarachnoid hemorrhage. Mo Med. 2011;108:124–127. [PMC free article] [PubMed] [Google Scholar]
- 25.Tseng MY, Czosnyka M, Richards H, Pickard JD, Kirkpatrick PJ. Effects of acute treatment with pravastatin on cerebral vasospasm, autoregulation, and delayed ischemic deficits after aneurysmal subarachnoid hemorrhage: a phase II randomized placebo-controlled trial. Stroke. 2005;36(8):1627–1632. doi: 10.1161/01.STR.0000176743.67564.5d. [DOI] [PubMed] [Google Scholar]
- 26.Kubo Y, Ogasawara K, Kakino S, et al. Serum inflammatory adhesion molecules and high-sensitivity C-reactive protein correlates with delayed ischemic neurologic deficits after subarachnoid hemorrhage. Surg Neurol. 2008;69:592–596. doi: 10.1016/j.surneu.2008.02.014. discussion 596. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256–263. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Martin RD, Zhang JH. Advances in experimental subarachnoid hemorrhage. Acta Neurochir Suppl. 2011;110:15–21. doi: 10.1007/978-3-7091-0353-1_3. [DOI] [PubMed] [Google Scholar]
- 29.Stienen MN, Smoll NR, Weisshaupt R, et al. Delayed cerebral ischemia predicts neurocognitive impairment following aneurysmal subarachnoid hemorrhage. World Neurosurg. 2014;82(5):e599–605. doi: 10.1016/j.wneu.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Pluta RM, Oldfield EH. Analysis of nitric oxide (NO) in cerebral vasospasm after aneursymal bleeding. Rev Recent Clin Trials. 2007;2(1):59–67. doi: 10.2174/157488707779318062. [DOI] [PubMed] [Google Scholar]
- 31.Pluta RM. Dysfunction of nitric oxide synthases as a cause and therapeutic target in delayed cerebral vasospasm after SAH. Acta Neurochir Suppl. 2008;104:139–147. doi: 10.1007/978-3-211-75718-5_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pluta RM. Dysfunction of nitric oxide synthases as a cause and therapeutic target in delayed cerebral vasospasm after SAH. Neurol Res. 2006;28:730–737. doi: 10.1179/016164106X152052. [DOI] [PubMed] [Google Scholar]
- 33.Durmaz R, Ozkara E, Kanbak G, et al. Nitric oxide level and adenosine deaminase activity in cerebrospinal fluid of patients with subarachnoid hemorrhage. Turk Neurosurg. 2008;18:157–164. [PubMed] [Google Scholar]
- 34.Pluta RM. Dysfunction of nitric oxide synthases as a cause and therapeutic target in delayed cerebral vasospasm after SAH. Acta Neurochir Suppl. 2008;104:139–147. doi: 10.1007/978-3-211-75718-5_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guix FX, Uribesalgo I, Coma M, Muñoz FJ. The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol. 2005;76:126–152. doi: 10.1016/j.pneurobio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Casado M, D-iaz-Guerra MJ, Rodrigo J, Fernández AP, Boscá L, Martín-Sanz P. Expression of the calcium-independent cytokine-inducible (iNOS) isoform of nitric oxide synthase in rat placenta. Biochem J. 1997;324:201–207. doi: 10.1042/bj3240201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adachi N, Lei B, Soutani M, Arai T. Different roles of neuronal and endothelial nitric oxide synthases on ischemic nitric oxide production in gerbil striatum. Neurosci Lett. 2000;288:151–154. doi: 10.1016/s0304-3940(00)01222-2. [DOI] [PubMed] [Google Scholar]
- 38.Iadecola C, Zhang F, Casey R, Nagayama M, Ross ME. Delayed reduction of ischemic brain injury and neurological deficits in mice lacking the inducible nitric oxide synthase gene. J Neurosci. 1997;17:9157–9164. doi: 10.1523/JNEUROSCI.17-23-09157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ito Y, Ohkubo T, Asano Y, et al. Nitric oxide production during cerebral ischemia and reperfusion in eNOS- and nNOS-knockout mice. Curr Neurovasc Res. 2010;7:23–31. doi: 10.2174/156720210790820190. [DOI] [PubMed] [Google Scholar]
- 40.Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28:1283–1288. doi: 10.1161/01.str.28.6.1283. [DOI] [PubMed] [Google Scholar]
- 41.Vellimana AK, Milner E, Azad TD, et al. Endothelial nitric oxide synthase mediates endogenous protection against subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 2011;42:776–782. doi: 10.1161/STROKEAHA.110.607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou L, Zhu DY. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Willing AE, Pennypacker KR. Alternate approach to understanding the molecular mechanisms of stroke-induced injury. Histol Histopathol. 2007;22:697–701. doi: 10.14670/HH-22.697. [DOI] [PubMed] [Google Scholar]
- 44.Sun M, Zhao Y, Gu Y, Xu C. Inhibition of nNOS reduces ischemic cell death through down-regulating calpain and caspase-3 after experimental stroke. Neurochem Int. 2009;54:339–346. doi: 10.1016/j.neuint.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 45.Gürsoy-Ozdemir Y, Can A, Dalkara T. Reperfusion-induced oxidative/nitrative injury to neurovascular unit after focal cerebral ischemia. Stroke. 2004;35:1449–1453. doi: 10.1161/01.STR.0000126044.83777.f4. [DOI] [PubMed] [Google Scholar]
- 46.Gürsoy-Ozdemir Y, Bolay H, Saribas O, Dalkara T. Role of endothelial nitric oxide generation and peroxynitrite formation in reperfusion injury after focal cerebral ischemia. Stroke. 2000;31:1974–1980. doi: 10.1161/01.str.31.8.1974. discussion 1981. [DOI] [PubMed] [Google Scholar]
- 47.Niwa M, Inao S, Takayasu M, et al. Time course of expression of three nitric oxide synthase isoforms after transient middle cerebral artery occlusion in rats. Neurol Med Chir (Tokyo) 2001;41:63. doi: 10.2176/nmc.41.63. [DOI] [PubMed] [Google Scholar]
- 48.Pannu R, Singh I. Pharmacological strategies for the regulation of inducible nitric oxide synthase: neurodegenerative versus neuroprotective mechanisms. Neurochem Int. 2006;49:170–182. doi: 10.1016/j.neuint.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 49.Danielisova V, Burda J, Nemethova M, Gottlieb M. Aminoguanidine administration ameliorates hippocampal damage after middle cerebral artery occlusion in rat. Neurochem Res. 2011;36:476–486. doi: 10.1007/s11064-010-0366-1. [DOI] [PubMed] [Google Scholar]
- 50.Foncea R, Carvajal C, Almarza C, Leighton F. Endothelial cell oxidative stress and signal transduction. Biol Res. 2000;33:89–96. doi: 10.4067/s0716-97602000000200008. [DOI] [PubMed] [Google Scholar]
- 51.Trickler WJ, Mayhan WG, Miller DW. Brain microvessel endothelial cell responses to tumor necrosis factor-alpha involve a nuclear factor kappa B (NF-kappaB) signal transduction pathway. Brain Res. 2005;1048:24–31. doi: 10.1016/j.brainres.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 52.ArunaDevi R, Ramteke VD, Kumar S, et al. Neuroprotective effect of s-methylisothiourea in transient focal cerebral ischemia in rat. Nitric Oxide. 2010;22:1–10. doi: 10.1016/j.niox.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Khan M, Sekhon B, Giri S, et al. S-Nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke. J Cereb Blood Flow Metab. 2005;25:177–192. doi: 10.1038/sj.jcbfm.9600012. [DOI] [PubMed] [Google Scholar]
- 54.Stanarius A, Topel I, Schulz S, Noack H, Wolf G. Immunocytochemistry of endothelial nitric oxide synthase in the rat brain: a light and electron microscopical study using the tyramide signal amplification technique. Acta Histochem. 1997;99:411–429. doi: 10.1016/S0065-1281(97)80034-7. [DOI] [PubMed] [Google Scholar]
- 55.Hossain M, Qadri SM, Liu L. Inhibition of nitric oxide synthesis enhances leukocyte rolling and adhesion in human microvasculature. J Inflamm (Lond) 2012;9:28. doi: 10.1186/1476-9255-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuhlencordt PJ, Rosel E, Gerszten RE, et al. Role of endothelial nitric oxide synthase in endothelial activation: insights from eNOS knockout endothelial cells. Am J Physiol Cell Physiol. 2004;286:C1195–1202. doi: 10.1152/ajpcell.00546.2002. [DOI] [PubMed] [Google Scholar]
- 57.Moore C, Sanz-Rosa D, Emerson M. Distinct role and location of the endothelial isoform of nitric oxide synthase in regulating platelet aggregation in males and females in vivo. Eur J Pharmacol. 2011;651:152–158. doi: 10.1016/j.ejphar.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Nabah YN, Mateo T, Cerda-Nicolas M, et al. L-NAME induces direct arteriolar leukocyte adhesion, which is mainly mediated by angiotensin-II. Microcirculation. 2005;12:443–453. doi: 10.1080/10739680590960962. [DOI] [PubMed] [Google Scholar]
- 59.Wei G, Dawson VL, Zweier JL. Role of neuronal and endothelial nitric oxide synthase in nitric oxide generation in the brain following cerebral ischemia. Biochim Biophys Acta. 1999;1455:23–34. doi: 10.1016/s0925-4439(99)00051-4. [DOI] [PubMed] [Google Scholar]
- 60.Li R, Guo M, Zhang G, Xu X, Li Q. Nicotiflorin reduces cerebral ischemic damage and upregulates endothelial nitric oxide synthase in primarily cultured rat cerebral blood vessel endothelial cells. J Ethnopharmacol. 2006;107:143–150. doi: 10.1016/j.jep.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 61.Willmot M, Gibson C, Gray L, Murphy S, Bath P. Nitric oxide synthase inhibitors in experimental ischemic stroke and their effects on infarct size and cerebral blood flow: a systematic review. Free Radic Biol Med. 2005;39:412–425. doi: 10.1016/j.freeradbiomed.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 62.Zhang F, White JG, Iadecola C. Nitric oxide donors increase blood flow and reduce brain damage in focal ischemia: evidence that nitric oxide is beneficial in the early stages of cerebral ischemia. J Cereb Blood Flow Metab. 1994;14:217–226. doi: 10.1038/jcbfm.1994.28. [DOI] [PubMed] [Google Scholar]
- 63.Zhang F, Xu S, Iadecola C. Time dependence of effect of nitric oxide synthase inhibition on cerebral ischemic damage. J Cereb Blood Flow Metab. 1995;15:595–601. doi: 10.1038/jcbfm.1995.73. [DOI] [PubMed] [Google Scholar]
- 64.Willmot MR, Bath PM. The potential of nitric oxide therapeutics in stroke. Expert Opin Investig Drugs. 2003;12:455–470. doi: 10.1517/13543784.12.3.455. [DOI] [PubMed] [Google Scholar]
- 65.Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;336:28–40. doi: 10.1056/NEJM199701023360106. [DOI] [PubMed] [Google Scholar]
- 66.Pluta RM. Delayed cerebral vasospasm and nitric oxide: review, new hypothesis, and proposed treatment. Pharmacol Ther. 2005;105:23–56. doi: 10.1016/j.pharmthera.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 67.Macdonald RL, Weir BK. A review of hemoglobin and the pathogenesis of cerebral vasospasm. Stroke. 1991;22:971–982. doi: 10.1161/01.str.22.8.971. [DOI] [PubMed] [Google Scholar]
- 68.Gabikian P, Clatterbuck RE, Eberhart CG, Tyler BM, Tierney TS, Tamargo RJ. Prevention of experimental cerebral vasospasm by intracranial delivery of a nitric oxide donor from a controlled-release polymer: toxicity and efficacy studies in rabbits and rats. Stroke. 2002;33:2681–2686. doi: 10.1161/01.str.0000033931.62992.b1. [DOI] [PubMed] [Google Scholar]
- 69.Makino K, Osuka K, Watanabe Y, et al. Increased ICP promotes CaMKII-mediated phosphorylation of neuronal NOS at Ser(8)(4)(7) in the hippocampus immediately after subarachnoid hemorrhage. Brain Res. 2015;1616:19–25. doi: 10.1016/j.brainres.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 70.Berra LV, Carcereri De Prati A, Suzuki H, Pasqualin A. The role of constitutive and inducible nitric oxide synthase in the human brain after subarachnoid hemorrhage. J Neurosurg Sci. 2007;51:1–9. [PubMed] [Google Scholar]
- 71.Huang YH, Chung CL, Tsai HP, et al. Hyperglycemia aggravates cerebral vasospasm after subarachnoid hemorrhage in a rat model. Neurosurgery. 2017;doi:10. doi: 10.1093/neuros/nyx016. 1093/neuros/nyx016. [DOI] [PubMed] [Google Scholar]
- 72.Zheng B, Zheng T, Wang L, Chen X, Shi C, Zhao S. Aminoguanidine inhibition of iNOS activity ameliorates cerebral vasospasm after subarachnoid hemorrhage in rabbits via restoration of dysfunctional endothelial cells. J Neurol Sci. 2010;295:97–103. doi: 10.1016/j.jns.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 73.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- 74.Jung CS, Iuliano BA, Harvey-White J, Espey MG, Oldfield EH, Pluta RM. Association between cerebrospinal fluid levels of asymmetric dimethyl-L-arginine, an endogenous inhibitor of endothelial nitric oxide synthase, and cerebral vasospasm in a primate model of subarachnoid hemorrhage. J Neurosurg. 2004;101:836–842. doi: 10.3171/jns.2004.101.5.0836. [DOI] [PubMed] [Google Scholar]
- 75.Kasuya H, Weir BK, Nakane M, et al. Nitric oxide synthase and guanylate cyclase levels in canine basilar artery after subarachnoid hemorrhage. J Neurosurg. 1995;82:250–255. doi: 10.3171/jns.1995.82.2.0250. [DOI] [PubMed] [Google Scholar]
- 76.Iuliano BA, Pluta RM, Jung C, Oldfield EH. Endothelial dysfunction in a primate model of cerebral vasospasm. J Neurosurg. 2004;100:287–294. doi: 10.3171/jns.2004.100.2.0287. [DOI] [PubMed] [Google Scholar]
- 77.Pluta RM, Thompson BG, Dawson TM, Snyder SH, Boock RJ, Oldfield EH. Loss of nitric oxide synthase immunoreactivity in cerebral vasospasm. J Neurosurg. 1996;84:648–654. doi: 10.3171/jns.1996.84.4.0648. [DOI] [PubMed] [Google Scholar]
- 78.Clark JF, Reilly M, Sharp FR. Oxidation of bilirubin produces compounds that cause prolonged vasospasm of rat cerebral vessels: a contributor to subarachnoid hemorrhage-induced vasospasm. J Cereb Blood Flow Metab. 2002;22:472–478. doi: 10.1097/00004647-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 79.Sobey CG. Cerebrovascular dysfunction after subarachnoid haemorrhage: novel mechanisms and directions for therapy. Clin Exp Pharmacol Physiol. 2001;28:926–929. doi: 10.1046/j.1440-1681.2001.03550.x. [DOI] [PubMed] [Google Scholar]
- 80.Pyne-Geithman GJ, Morgan CJ, Wagner K, et al. Bilirubin production and oxidation in CSF of patients with cerebral vasospasm after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:1070–1077. doi: 10.1038/sj.jcbfm.9600101. [DOI] [PubMed] [Google Scholar]
- 81.Rämet ME, Rämet M, Lu Q, et al. High-density lipoprotein increases the abundance of eNOS protein in human vascular endothelial cells by increasing its half-life. J Am Coll Cardiol. 2003;41(12):2288–2297. doi: 10.1016/s0735-1097(03)00481-9. [DOI] [PubMed] [Google Scholar]
- 82.Vijay A, Santhanam R, Katusic ZS. Genetic modification of cerebral arterial wall: implications for prevention and treatment of cerebral vasospasm. Neurol Res. 2006;28:759–768. doi: 10.1179/016164106X152034. [DOI] [PubMed] [Google Scholar]
- 83.Afshar JK, Pluta RM, Boock RJ, Thompson BG, Oldfield EH. Effect of intracarotid nitric oxide on primate cerebral vasospasm after subarachnoid hemorrhage. J Neurosurg. 1995;83:118–122. doi: 10.3171/jns.1995.83.1.0118. [DOI] [PubMed] [Google Scholar]
- 84.Miller MR, Megson IL. Recent developments in nitric oxide donor drugs. Br J Pharmacol. 2007;151:305–321. doi: 10.1038/sj.bjp.0707224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Scatena R, Bottoni P, Pontoglio A, Giardina B. Pharmacological modulation of nitric oxide release: new pharmacological perspectives, potential benefits and risks. Curr Med Chem. 2010;17:61–73. doi: 10.2174/092986710789957841. [DOI] [PubMed] [Google Scholar]
- 86.Mohanakumar KP, Hanbauer I, Chiueh CC. Neuroprotection by nitric oxide against hydroxyl radical-induced nigral neurotoxicity. J Chem Neuroanat. 1998;14:195–205. doi: 10.1016/s0891-0618(98)00032-5. [DOI] [PubMed] [Google Scholar]
- 87.Salerno L, Sorrenti V, Di Giacomo C, Romeo G, Siracusa MA. Progress in the development of selective nitric oxide synthase (NOS) inhibitors. Curr Pharm Des. 2002;8:177–200. doi: 10.2174/1381612023396375. [DOI] [PubMed] [Google Scholar]
- 88.Becker K, Tanzi P, Kalil A, Shibata D, Cain K. Early statin use is associated with increased risk of infection after stroke. J Stroke Cerebrovasc Dis. 2013;22:66–71. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei L, Roberts W, Wang L, et al. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development. 2001;128:2953–2962. doi: 10.1242/dev.128.15.2953. [DOI] [PubMed] [Google Scholar]
- 90.Zhu C, Sun Y, Gao J, Wang X, Plesnila N, Blomgren K. Inhaled nitric oxide protects males but not females from neonatal mouse hypoxia-ischemia brain injury. Transl Stroke Res. 2013;4:201–207. doi: 10.1007/s12975-012-0217-2. [DOI] [PubMed] [Google Scholar]
- 91.Li Y, Liu K, Kang ZM, Sun XJ, Liu WW, Mao YF. Helium preconditioning protects against neonatal hypoxia-ischemia via nitric oxide mediated up-regulation of antioxidases in a rat model. Behav Brain Res. 2016;300:31–37. doi: 10.1016/j.bbr.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 92.Lin HY, Wu CL, Huang CC. The Akt-endothelial nitric oxide synthase pathway in lipopolysaccharide preconditioning-induced hypoxic-ischemic tolerance in the neonatal rat brain. Stroke. 2010;41:1543–1551. doi: 10.1161/STROKEAHA.109.574004. [DOI] [PubMed] [Google Scholar]
- 93.Lu Q, Harris VA, Rafikov R, Sun X, Kumar S, Black SM. Nitric oxide induces hypoxia ischemic injury in the neonatal brain via the disruption of neuronal iron metabolism. Redox biology. 2015;6:112–121. doi: 10.1016/j.redox.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hsu YC, Chang YC, Lin YC, Sze CI, Huang CC, Ho CJ. Cerebral microvascular damage occurs early after hypoxia-ischemia via nNOS activation in the neonatal brain. J Cereb Blood Flow Metab. 2014;34:668–676. doi: 10.1038/jcbfm.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang HQ, Xiong Y, Guo WJ. Expression of iNOS protein and gliacyte apoptosis in neonatal rats with white matter damage. Zhongguo Dang Dai Er Ke Za Zhi. 2011;13:309–312. [PubMed] [Google Scholar]
- 96.Watanabe A, Sasaki T, Yukami T, et al. Serine racemase inhibition induces nitric oxide-mediated neurovascular protection during cerebral ischemia. Neuroscience. 2016;339:139–149. doi: 10.1016/j.neuroscience.2016.09.036. [DOI] [PubMed] [Google Scholar]
- 97.Yan S, Chen L, Wei X, et al. Tetramethylpyrazine analogue CXC195 ameliorates cerebral ischemia-reperfusion injury by regulating endothelial nitric oxide synthase phosphorylation via PI3K/Akt signaling. Neurochem Res. 2015;40:446–454. doi: 10.1007/s11064-014-1485-x. [DOI] [PubMed] [Google Scholar]
- 98.Fu S, Gu Y, Jiang JQ, et al. Calycosin-7-O-beta-D-glucoside regulates nitric oxide/caveolin-1/matrix metalloproteinases pathway and protects blood-brain barrier integrity in experimental cerebral ischemia-reperfusion injury. J Ethnopharmacol. 2014;155:692–701. doi: 10.1016/j.jep.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 99.Yu K, Wu Y, Hu Y, et al. Prior exposure to enriched environment reduces nitric oxide synthase after transient MCAO in rats. Neurotoxicology. 2013;39:146–152. doi: 10.1016/j.neuro.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 100.Koh PO. Ferulic acid modulates nitric oxide synthase expression in focal cerebral ischemia. Lab Anim Res. 2012;28:273–278. doi: 10.5625/lar.2012.28.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li D, Fang Y, Wang P, Shan W, Zuo Z, Xie L. Autologous transplantation of adipose-derived mesenchymal stem cells attenuates cerebral ischemia and reperfusion injury through suppressing apoptosis and inducible nitric oxide synthase. Int J Mol Med. 2012;29:848–854. doi: 10.3892/ijmm.2012.909. [DOI] [PubMed] [Google Scholar]
- 102.Zhao Q, Zhang C, Wang X, Chen L, Ji H, Zhang Y. (S)-ZJM-289, a nitric oxide-releasing derivative of 3-n-butylphthalide, protects against ischemic neuronal injury by attenuating mitochondrial dysfunction and associated cell death. Neurochem Int. 2012;60:134–144. doi: 10.1016/j.neuint.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 103.Cheng CY, Su SY, Tang NY, Ho TY, Lo WY, Hsieh CL. Ferulic acid inhibits nitric oxide-induced apoptosis by enhancing GABA(B1) receptor expression in transient focal cerebral ischemia in rats. Acta Pharmacol Sin. 2010;31:889–899. doi: 10.1038/aps.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Munakata A, Naraoka M, Katagai T, Shimamura N, Ohkuma H. Role of cyclooxygenase-2 in relation to nitric oxide and endothelin-1 on pathogenesis of cerebral vasospasm after subarachnoid hemorrhage in rabbit. Transl Stroke Res. 2016;7:220–227. doi: 10.1007/s12975-016-0466-6. [DOI] [PubMed] [Google Scholar]
- 105.Terpolilli NA, Feiler S, Dienel A, et al. Nitric oxide inhalation reduces brain damage, prevents mortality, and improves neurological outcome after subarachnoid hemorrhage by resolving early pial microvasospasms. J Cereb Blood Flow Metab. 2016;36:2096–2107. doi: 10.1177/0271678X15605848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang CZ, Wu SC, Chang CM, Lin CL, Kwan AL. Arctigenin, a potent ingredient of Arctium lappa L., induces endothelial nitric oxide synthase and attenuates subarachnoid hemorrhage-induced vasospasm through PI3K/Akt pathway in a rat model. Biomed Res Int 2015. 2015:490209. doi: 10.1155/2015/490209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang CY, Wang LC, Shan YS, Pan CH, Tsai KJ. Memantine attenuates delayed vasospasm after experimental subarachnoid hemorrhage via modulating endothelial nitric oxide synthase. Int J Mol Sci. 2015;16:14171–14180. doi: 10.3390/ijms160614171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ramesh SS, Prasanthi A, Bhat DI, Devi BI, Cristopher R, Philip M. Correlation between plasma total nitric oxide levels and cerebral vasospasm and clinical outcome in patients with aneurysmal subarachnoid hemorrhage in Indian population. J Neurosci Rural Pract. 2014;5:S22–27. doi: 10.4103/0976-3147.145196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nevzati E, Shafighi M, Bakhtian KD, Treiber H, Fandino J, Fathi AR. Estrogen induces nitric oxide production via nitric oxide synthase activation in endothelial cells. Acta Neurochir Suppl. 2015;120:141–145. doi: 10.1007/978-3-319-04981-6_24. [DOI] [PubMed] [Google Scholar]
- 110.Chang CM, Su YF, Chang CZ, Chung CL. Progesterone attenuates experimental subarachnoid hemorrhage-induced vasospasm by upregulation of endothelial nitric oxide synthase via Akt signaling pathway. Biomed Res Int. 2014;2014:207616. doi: 10.1155/2014/207616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Osuka K, Watanabe Y, Yasuda M, Takayasu M. Adiponectin activates endothelial nitric oxide synthase through AMPK signaling after subarachnoid hemorrhage. Neurosci Lett. 2012;514:2–5. doi: 10.1016/j.neulet.2011.12.041. [DOI] [PubMed] [Google Scholar]
- 112.Sabri M, Ai J, Marsden PA, Macdonald RL. Simvastatin re-couples dysfunctional endothelial nitric oxide synthase in experimental subarachnoid hemorrhage. PLoS One. 2011;6:e17062. doi: 10.1371/journal.pone.0017062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mohammadi MT. Overproduction of nitric oxide intensifies brain infarction and cerebrovascular damage through reduction of claudin-5 and ZO-1 expression in striatum of ischemic brain. Pathol Res Pract. 2016;212:959–964. doi: 10.1016/j.prp.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 114.Zheng L, Ding J, Wang J, Zhou C, Zhang W. Effects and mechanism of action of inducible nitric oxide synthase on apoptosis in a rat model of cerebral ischemia-reperfusion injury. Anat Rec (Hoboken) 2016;299:246–255. doi: 10.1002/ar.23295. [DOI] [PubMed] [Google Scholar]
- 115.Fabian RH, Kent TA. Hyperglycemia accentuates persistent “functional uncoupling” of cerebral microvascular nitric oxide and superoxide following focal ischemia/reperfusion in rats. Transl Stroke Res. 2012;3:482–490. doi: 10.1007/s12975-012-0210-9. [DOI] [PubMed] [Google Scholar]
- 116.Mohammadi MT, Shid-Moosavi SM, Dehghani GA. Contribution of nitric oxide synthase (NOS) in blood-brain barrier disruption during acute focal cerebral ischemia in normal rat. Pathophysiology. 2012;19:13–20. doi: 10.1016/j.pathophys.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 117.Mohammadi MT, Shid Moosavi SM, Dehghani GA. Contribution of nitric oxide synthase (NOS) activity in blood-brain barrier disruption and edema after acute ischemia/reperfusion in aortic coarctation-induced hypertensive rats. Iran Biomed J. 2011;15:22–30. [PMC free article] [PubMed] [Google Scholar]
- 118.Iqbal S, Hayman EG, Hong C, et al. Inducible nitric oxide synthase (NOS-2) in subarachnoid hemorrhage: Regulatory mechanisms and therapeutic implications. Brain Circ. 2016;2:8–19. doi: 10.4103/2394-8108.178541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao D, Liu Q, Ji Y, et al. Correlation between nitric oxide and early brain injury after subarachnoid hemorrhage. Int J Neurosci. 2015;125:531–539. doi: 10.3109/00207454.2014.951442. [DOI] [PubMed] [Google Scholar]
- 120.Sabri M, Ai J, Knight B, et al. Uncoupling of endothelial nitric oxide synthase after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2011;31:190–199. doi: 10.1038/jcbfm.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu L, Li Y, Fu Q, Ma S. Perillaldehyde attenuates cerebral ischemia-reperfusion injury-triggered overexpression of inflammatory cytokines via modulating Akt/JNK pathway in the rat brain cortex. Biochem Biophys Res Commun. 2014;454:65–70. doi: 10.1016/j.bbrc.2014.10.025. [DOI] [PubMed] [Google Scholar]


