Abstract
Background
The widespread use of indoor-based malaria vector control interventions has been shown to alter the behaviour of vectors in Africa. There is an increasing concern that such changes could sustain residual transmission. This study was conducted to assess vector species composition, feeding behaviour and their contribution to indoor and outdoor malaria transmission in western Kenya.
Methods
Anopheles mosquito collections were carried out from September 2015 to April 2016 in Ahero and Iguhu sites, western Kenya using CDC light traps (indoor and outdoor), pyrethrum spray catches (PSCs) (indoor) and pit shelters (outdoor). Species within Anopheles gambiae s.l. and Anopheles funestus s.l. were identified using polymerase chain reaction (PCR). Enzyme-linked immunosorbent assay (ELISA) was used to determine mosquito blood meal sources and sporozoite infections.
Results
A total of 10,864 female Anopheles mosquitoes comprising An. gambiae s.l. (71.4%), An. funestus s.l. (12.3%), Anopheles coustani (9.2%) and Anopheles pharoensis (7.1%) were collected. The majority (61.8%) of the anopheline mosquitoes were collected outdoors. PCR result (n = 581) revealed that 98.9% An. arabiensis and 1.1% An. gambiae s.s. constituted An. gambiae s.l. in Ahero while this was 87% An. gambiae s.s. and 13% An. arabiensis in Iguhu. Of the 108 An. funestus s.l. analysed by PCR, 98.1% belonged to An. funestus s.s. and 1.9% to Anopheles leesoni. The human blood index (HBI) and bovine blood index (BBI) of An. arabiensis was 2.5 and 73.1%, respectively. Anopheles gambiae s.s. had HBI and BBI of 50 and 28%, respectively. The HBI and BBI of An. funestus was 60 and 22.3%, respectively. Forage ratio estimate revealed that An. arabiensis preferred to feed on cattle, An. gambiae s.s. showed preference for both human and cattle, while An. funestus preferred human over other hosts. In Ahero, the sporozoite rates for An. arabiensis and An. funestus were 0.16 and 1.8%, respectively, whereas in Iguhu, the sporozoite rates for An. gambiae s.s. and An. funestus were 2.3 and 2.4%, respectively. In Ahero, the estimated indoor and outdoor entomological inoculation rate (EIR) was 108.6 infective bites/person/year (79.0 from An. funestus and 29.6 from An. arabiensis) and 43.5 infective bites/person/year (27.9 from An. arabiensis and 15.6 from An. funestus), respectively. In Iguhu, the estimated indoor and outdoor EIR was 24.5 infective bites/person/year (18.8 from An. gambiae s.s. and 5.7 from An. funestus) and 5.5 infective bites/person/year (all from An. gambiae s.s.), respectively.
Conclusion
Anopheles gambiae s.s. showed an increasing tendency to feed on cattle. Anopheles arabiensis was highly zoophagic, whereas An. funestus showed anthropophagic behaviour. While the majority of malaria transmission occurred indoor, the magnitude of outdoor transmission was considerably high. Additional control tools that complement the existing interventions are required to control residual transmission.
Keywords: Malaria vectors, Surveillance, Behavior, Residual transmission, Kenya
Background
Malaria is a serious vector-borne disease affecting hundreds of millions of people in Africa. In the past decade, a substantial reduction in malaria incidence has been observed in Africa, including Kenya, due to the scale-up of interventions. Vector control is one of the key elements in achieving the remarkable decline of malaria, with the scale-up of insecticide-treated nets (ITNs) and expansion of indoor residual spray (IRS) contributing significantly [1–4]. The proportion of households owning at least one ITN in sub-Saharan Africa is estimated to have risen from 3% in 2000 to 67% in 2015 [1]. In western Kenya, the ITN ownership rose from 12.8% in 2004 to over 80% in 2015 [5–7].
Despite the progress made in scaling-up of the interventions, malaria transmission continues to occur. Several factors are responsible for this transmission, including the spread of insecticide resistance [5, 8], shift in vector species composition [9–12] and increasing vector behavioural change towards more zoophagic, exophagic and/or exophilic tendencies following the widespread use of ITNs and IRS [13, 14].
Recent reports from East Africa showed strong evidence for shifts in Anopheles gambiae sensu lato (s.l.) sibling species composition from predominantly endophagic An. gambiae sensu stricto (s.s.) to predominantly exophagic Anopheles arabiensis following the scale-up of ITNs [9, 11–13, 15]. In the lowlands of western Kenya, the proportion of An. gambiae s.s. declined from about 85% in 1998 to 1% in 2009 following massive distribution of ITNs, whereas An. arabiensis population showed proportionate increment [9]. While malaria transmission by An. gambiae s.s. declined significantly, residual transmission continued to occur by An. arabiensis. Similarly, the proportion of An. arabiensis in the highlands of western Kenya has been increasing gradually [5].
Vector behavioural modifications including changes in host-preference, biting locations (indoor or outdoor) and resting behaviours have been reported following the long-term use of ITNs. For instance, ITN use was associated with shift in host preference of An. gambiae s.s. from human to cattle in Burkina Faso [16]. The long-term use of ITN increased the outdoor feeding proportion of An. gambiae s.s. in Bioko Island [17, 18] and Anopheles funestus in Tanzania [13]. However, these changes are not universal. A recent study in Asembo district of western Kenya showed that the majority of biting by An. arabiensis, An. gambiae s.s. and An. funestus occurred indoors despite high ITN coverage in the area [19].
Malaria is mesoendemic and holoendemic in the highland and lowland areas of western Kenya, respectively [20]. The transmission is maintained by An. gambiae s.s., An. funestus and An. arabiensis. Anopheles gambiae s.s and An. funestus are considered as highly endophagic and anthropophagic, while An. arabiensis is considered as zoophagic and endophilic. However, most of the studies on their feeding and resting behaviour were conducted before the scale-up of vector control interventions [21–23]. It is possible that the anthropophagic and endophilic individuals could shift to zoophagic and exophilic tendencies or be reduced to leave zoophagic and exophilic sibling species following the scale-up of ITNs as has been observed elsewhere.
In view of the increasing concern about residual malaria transmission in Africa, there is a pressing need to enhance our understanding about vector behaviours to evaluate the likely success of the current vector control tools. The main aim of this study was to assess vector species composition, feeding behaviour and their contribution to indoor and outdoor malaria transmission in western Kenya.
Methods
Study sites
The study was conducted in lowland and highland settings of western Kenya. Two sites were selected (Fig. 1): Ahero (0°.11′S, 34°.55′E, altitude 1162 m) in Kisumu county and Iguhu (0°.17′N; 34°.74′E, altitude 1430–1580 m a.s.l) in Kakamega county. Iguhu site is highland characterized by valleys and depressions surrounded by densely populated hills whereas Ahero is lowland plain area. The sites have bimodal pattern of rainfall, with long rainy season from April to June, which triggers peak malaria transmission period and short rainy season from October to November with minimal transmission [24]. The hot and dry season is from January to March and this marks the lowest transmission [5]. Plasmodium falciparum is the predominant malaria species in the area and is transmitted by An. gambiae s.s., An. arabiensis and An. funestus [5, 25].
Fig. 1.
Map of the study sites
Mosquito collections
Adult mosquito collections were carried out monthly during the short rainy season (September to November) in 2015 and dry season (February to April) in 2016. Indoor and outdoor host-seeking mosquitoes were collected using Centers for Disease Control and Prevention (CDC) light traps (John W. Hock Ltd, Gainesville, FL., USA). For indoor host-seeking mosquito collection, CDC light traps were set inside houses near the bed at a height of 1.5 m from 18:00 to 06:00 h in twenty randomly selected houses per month in each study site. For the outdoor host-seeking mosquito sampling, CDC light traps were set outdoor in the vicinity (within 2 m) of sentinel houses. The same houses were used for mosquito collections each month.
Indoor resting mosquitoes were sampled using pyrethrum spray catches (PSCs) from another twenty randomly selected houses from 06:00 to 09:00 h following standard protocol [26]. Outdoor resting mosquitoes were collected monthly in the mornings (06:00 to 09:00 h.) from twenty artificial outdoor pit shelters constructed according to the method of Muirhead-Thomson [27], in the compound of 20 selected houses in each study site. The collections were repeated using the same pit shelters each month.
Along with mosquito collection, data on the numbers of potential hosts in the study area including human, bovine, goat, dog and chicken were collected using questionnaire surveys. All collected mosquitoes were identified morphologically to species using keys [28]. Female Anopheles mosquitoes were further classified as unfed, blood fed, half-gravid and gravid. Each mosquito was kept in a labelled 1.5 ml Eppendorf tube containing silica gel desiccant and cotton wool. Samples were stored at − 20 °C refrigerator at Climate and Human Health Research Laboratory of Kenya Medical Research Institute until used for further processing.
Identification of vector species complexes
Members of An. gambiae s.l. and An. funestus s.l. groups were identified to species by polymerase chain reaction (PCR), following the protocols developed by Scott et al. for An. gambiae s.l. [29] and Koekemoer et al. for An. funestus s.l. [30].
Detection of blood meal sources
The blood meal sources of freshly fed Anopheles mosquitoes were analyzed by a direct enzyme-linked immunosorbent assay (ELISA) [31] using human, bovine, goat, chicken and dog antibodies. Positive controls were included for each host during the assay. Laboratory reared unfed An. gambiae was used as negative control.
Sporozoite ELISA
Dried head and thorax of the preserved Anopheles mosquito specimens were carefully separated from the abdomen and tested for P. falciparum circumsporozoite proteins (CSPs) using sand-witch ELISA method [32, 33].
Data analysis
The density of adult anopheline mosquitoes was calculated as the number of female mosquitoes per trap/night for each collection method. Analysis of variance (ANOVA) was used to compare malaria vector density between indoor and outdoor locations. χ2-test was employed to test the difference in vector species composition between indoor and outdoor.
Human blood index (HBI) was calculated as the proportion of Anopheles mosquitoes that fed on human over the total Anopheles tested for blood meal origins [34]. Bovine, goat, dog and chicken blood indices were also calculated in similar way. Mixed blood meals were included in the calculation of blood meal indices [35]. The forage ratio (FR), a measure of host preference by mosquitoes, was determined as the percent of engorged Anopheles mosquitoes which have fed on a given host (human, bovine, goat, dog or chicken) divided by the percent which it comprises in the total population of hosts available in the study area [36]. The FR w i for species i was calculated as:
where w i is the FR for mosquito species i, o i is the proportion of host species i in the blood meals, and p i is the proportion of host species i available in the environment.
Statistical significance of the FR estimate for each host was based on overlap of the 95% confidence interval (CI) of the estimate with the value one [37]. A host was considered to have been preferred if the lower 95% confidence limit for the FR estimate was greater than one. A host was inferred to have been avoided if the upper 95% confidence limit for the FR estimate was less than one. A host for which the 95% CI for its FR included one was considered to have been feed on opportunistically [37].
The sporozoite rate was estimated as the proportion of mosquitoes positive for P. falciparum CSPs over the total number tested. Annual entomological inoculation rate (EIR) was calculated from mosquito collections by CDC light traps using the formula, 1.605 × (no. CSP-positive ELISA results from CDC light traps/no. mosquitoes tested) × (no. mosquitoes collected from CDC light traps/no. trap-nights) × 365 [38, 39]. The multiplication factor 1.605 is a conversion factor for CDC light trap catches vs. man biting catches [38]. The annual EIR of Anopheles mosquitoes collected by PSCs was determined as: (no. fed mosquitoes caught by PSC/no. human occupants who spent the night in the sprayed house) × (no. mosquitoes fed on human/no. mosquitoes tested for human blood meal) × (PSC based sporozoite rate) × 365 [40].
The annual EIR for Anopheles mosquitoes collected from pit shelters was also estimated as (no. fed mosquitoes caught in the pit shelters/no. human occupants who spent the night in a house nearest to the pit shelter) × (no. human fed mosquitoes/no. mosquitoes tested for human blood meal) × (sporozoite rate from pit shelters) × 365. This formula was employed based on the assumption that all Anopheles mosquitoes collected from pit shelters have got their human blood meals from occupants of the nearest house, either indoor or outdoor.
Data were analyzed using STATISTICA 8.0 (StatSoft, Tulsa, USA) and SPSS version 20.0 (SPSS, Chicago, IL, USA) software packages. p < 0.05 was considered statistically significant during the analysis.
Results
Anopheline mosquito species composition and abundance
A total of 10,864 female Anopheles mosquitoes belonging to four species were collected during the study period (Table 1). Anopheles gambiae s.l. was the predominant species accounting for 71.4% of the total captures, followed by An. funestus s.l. (12.3%), Anopheles coustani complex (9.2%) and Anopheles pharoensis (7.1%). In addition, 3263 male anopheline mosquitoes and 5206 Culex species (males and females together) were collected over the study period. There was a significant difference in anopheline mosquito species co-occurrence between the study sites (F 1, 952 = 423.02, p < 0.0001). There was also significant difference in anopheline mosquito species co-occurrence between indoor and outdoor locations (F 1, 956 = 29.44, p < 0.0001). The majority (61.8%) of the anopheline mosquitoes were collected outdoors.
Table 1.
Summary of female Anopheles mosquitoes collected from indoor and outdoor in lowland (Ahero) and highland (Iguhu) settings of western Kenya (n = 120 trap-nights for each trap)
| Study sites and Anopheles spp. | Indoor | Outdoor | Total | ||
|---|---|---|---|---|---|
| Light trap | PSC | Light trap | Pit shelter | ||
| Ahero | |||||
| An. gambiae s.l. | 1592 | 1009 | 1636 | 3262 | 7499 |
| An. funestus s.l. | 628 | 204 | 270 | 142 | 1244 |
| An. coustani | 321 | 2 | 652 | 15 | 990 |
| An. pharoensis | 78 | 0 | 688 | 0 | 766 |
| Iguhu | |||||
| An. gambiae s.l. | 108 | 51 | 56 | 41 | 256 |
| An. funestus s.l. | 49 | 30 | 13 | 4 | 96 |
| An. coustani | 3 | 0 | 10 | 0 | 13 |
| Total | 2779 | 1296 | 3325 | 3464 | 10,864 |
PSC, pyrethrum spray catch
Indoor and outdoor Anopheles mosquito density
Figure 2 shows the mean indoor and outdoor density of host-seeking and resting female Anopheles mosquitoes. In Ahero, the mean outdoor resting density of An. gambiae s.l. was significantly higher than indoor resting density (t 238 = 8.45, p < 0.0001), whereas the difference in mean indoor and outdoor resting density of An. funestus s.l. was not significant (p > 0.05). The mean outdoor host-seeking density of An. gambiae s.l. was also higher than indoor, although the difference was not statistically significant (t 238 = 0.14, p = 0.889). The mean indoor host-seeking density of An. funestus s.l. was significantly higher than outdoor (t 238 = 2.37, p = 0.019). Significantly higher outdoor host-seeking density than indoor was observed for An. coustani (t 238 = 2.589, p = 0.01) and An. pharoensis (t 238 = 4.923, p < 0.0001).
Fig. 2.
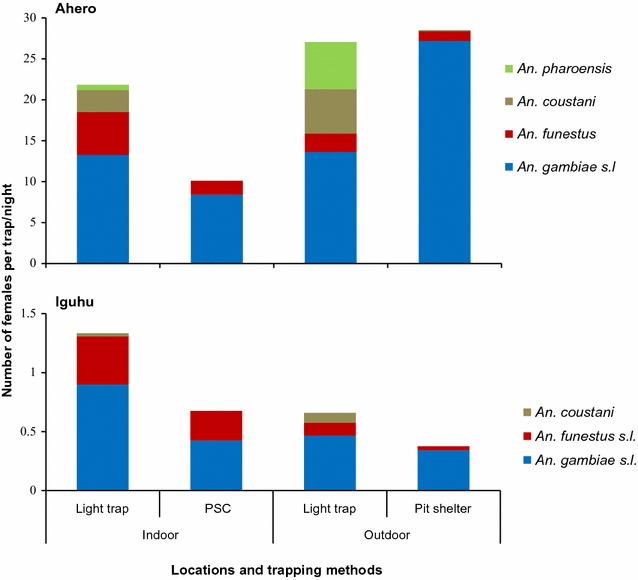
Indoor and outdoor host-seeking and resting density of female Anopheles mosquitoes collected from Ahero and Iguhu, western Kenya
In Iguhu, the indoor host-seeking densities of An. gambiae s.l. and An. funestus s.l. were significantly higher than outdoor (An. gambiae s.l., t 238 = 2.12, p = 0.034; An. funestus s.l., t 238 = 3.09, p = 0.002). The difference in mean indoor and outdoor resting density of An. gambiae s.l. was not significant (t 238 = 0.97, p = 0.335), while the mean indoor resting density of An. funestus s.l. was significantly higher (t 238 = 3.23, p = 0.001) than outdoor.
Composition of Anopheles gambiae and Anopheles funestus sibling species
A total of 750 specimens (628 An. gambiae s.l. and 122 An. funestus s.l.) were analysed for identification of their respective sibling species. Of theses, 581 An. gambiae s.l. and 108 An. funestus s.l. specimens were successfully amplified and identified to species by PCR. Figure 3 shows member species of An. gambiae s.l. In Ahero, of the An. gambiae s.l. assayed, An. arabiensis and An. gambiae s.s accounted for 98.9 and 1.1%, respectively. In contrast in Iguhu, An. gambiae s.s. and An. arabiensis constituted 87 and 13%, respectively of the assayed An. gambiae s.l. specimens. Overall, there was significant difference between indoor and outdoor locations in terms of An. gambiae s.l. species composition (χ2 = 26.443, df = 1, p < 0.0001). The proportion of An. arabiensis was higher outdoors than indoors. Of the 108 An. funestus s.l. confirmed by PCR, An. funestus s.s. (hereafter An. funestus) and Anopheles leesoni, accounted for 98.1 and 1.9%, respectively. All the An. leesoni were from outdoor CDC light traps. The member species of An. funestus s.l. did not vary between the study sites.
Fig. 3.
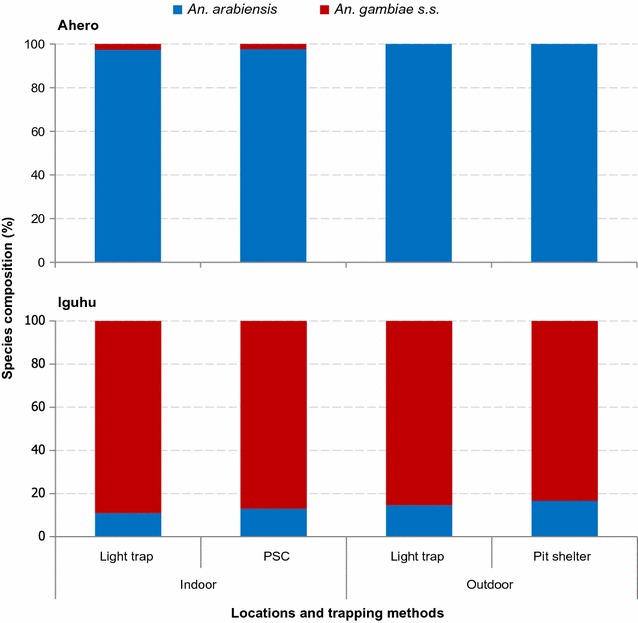
Composition of Anopheles gambiae sibling species in Ahero and Iguhu, western Kenya
Physiological status
In both indoor and outdoor collections, the majority (> 70%) of the host-seeking anophelines were unfed. About 55% of the indoor resting and 39% of the outdoor resting An. arabiensis were blood fed. One-third of the indoor resting and 31.7% of the outdoor resting An. gambiae s.s. were blood fed. About half of the indoor resting An. funestus were blood fed, while this was 11.6% for the outdoor resting An. funestus.
Blood meal indices
Table 2 shows the host blood indices of An. arabiensis and An. funestus in Ahero. The HBI of An. arabiensis from indoor CDC light traps and PSCs was 8.2 and 1.2%, respectively, whereas the HBI of An. arabiensis from outdoor CDC traps and pit shelters was 3.4 and 0.7%, respectively. The overall HBI of An. arabiensis was 2.5%. The HBI of An. funestus from indoor CDC light traps and PSCs was 72.7 and 63.6%, respectively, while the HBI of An. funestus from both outdoor CDC light traps and pit shelters was 50%. In Ahero, the overall HBI for An. funestus was 62%.
Table 2.
Blood meal origins of An. arabiensis and An. funestus from indoor and outdoor collections in Ahero, western Kenya
| Blood-meal origins | An. arabiensis | An. funestus | ||||||
|---|---|---|---|---|---|---|---|---|
| Indoor | Outdoor | Indoor | Outdoor | |||||
| Light trap | PSC | Light trap | Pit shelter | Light trap | PSC | Light trap | Pit shelter | |
| Number tested | 122 | 165 | 59 | 298 | 11 | 44 | 4 | 12 |
| Human | 7 (5.7) | 1 (0.6) | 2 (3.4) | 2 (0.7) | 8 (72.7) | 23 (52.3) | 2 (50.0) | 6 (50.0) |
| Bovine | 74 (60.7) | 108 (65.5) | 30 (50.8) | 251 (84.2) | 0 | 10 (22.7) | 1 (25.0) | 5 (41.7) |
| Goat | 5 (4.1) | 5 (3.0) | 1 (1.7) | 4 (1.3) | 0 | 0 | 0 | 0 |
| Dog | 1 (0.8) | 5 (3.0) | 1 (1.7) | 5 (1.7) | 0 | 0 | 0 | 0 |
| Chicken | 2 (1.6) | 0 | 0 | 1 (0.3) | 0 | 0 | 0 | 0 |
| Human + bovine | 1 (0.8) | 1 (0.6) | 0 | 0 | 0 | 2 (4.6) | 0 | 0 |
| Human + dog | 2 (1.6) | 0 | 0 | 0 | 0 | 3 (6.8) | 0 | 0 |
| Bovine + dog | 1 (0.8) | 1 (0.6) | 0 | 4 (1.3) | 0 | 0 | 0 | 0 |
| Goat + dog | 0 | 1 (0.6) | 0 | 0 | 0 | 0 | 0 | 0 |
| Dog + chicken | 0 | 0 | 0 | 1 (0.3) | 0 | 0 | 0 | 0 |
| Unknown | 29 (23.8) | 43 (26.1) | 25 (42.4) | 30 (10.1) | 3 (27.3) | 6 (13.6) | 1 (25.0) | 1 (8.3) |
| HBI | 8.2 | 1.2 | 3.4 | 0.7 | 72.7 | 63.6 | 50.0 | 50.0 |
HBI was calculated as the number of mosquito positive for human (including mixed blood meal) divided by the total number tested
HBI, human blood index; PSC, pyrethrum spray catches
In contrast, the bovine blood index (BBI) of An. arabiensis from indoor CDC light traps, PSCs, outdoor CDC light traps and pit shelters was 62.3, 66.7, 50.8, and 85.6%, respectively. Overall, the BBI of An. arabiensis was 73.1%. The BBI of An. funestus from PSCs, outdoor CDC light traps and pit shelters was 27.3, 22.7 and 41.7%, respectively. None of the An. funestus from indoor CDC light traps was positive for bovine blood meal. In Ahero, the overall BBI of An. funestus was 25.4%. Blood meal indices for other vertebrate hosts (goat, dog and chicken) were low (< 4%).
Table 3 shows the host blood indices of An. gambiae s.s. and An. funestus in Iguhu. The HBI of An. gambiae s.s. from indoor CDC light traps and PSCs was 70.0 and 76.5%, respectively, whereas the HBI of An. gambiae s.s. from outdoor CDC light traps and pit shelters was 20.0 and 23.1% respectively. The overall HBI of An. gambiae s.s. was 50.0%. The HBI of An. funestus from indoor CDC light traps and PSCs was 53.8 and 61.1%, respectively. In outdoor CDC light traps, very small number of fed An. funestus was caught, which yielded a HBI of 50%. Hence, in Iguhu, the overall HBI of An. funestus was 55.9%.
Table 3.
Blood meal origins of An. gambiae s.s. and An. funestus from indoor and outdoor collections in Iguhu, western Kenya
| Blood-meal origins | An. gambiae s.s. | An. funestus | ||||||
|---|---|---|---|---|---|---|---|---|
| Indoor | Outdoor | Indoor | Outdoor | |||||
| Light trap | PSC | Light trap | Pit shelter | Light trap | PSC | Light trap | Pit shelter | |
| Number tested | 10 | 17 | 10 | 13 | 13 | 18 | 2 | 1 |
| Human | 7 (70) | 11 (64.7) | 2 (20) | 3 (23.1) | 7 (53.8) | 11 (61.1) | 1 (50.0) | 0 |
| Bovine | 0 | 3 (17.6) | 4 (40) | 6 (46.1) | 2 (15.4) | 3 (16.7) | 1 (50.0) | 0 |
| Goat | 0 | 0 | 0 | 0 | 1 (7.7) | 0 | 0 | 0 |
| Dog | 0 | 0 | 0 | 1 (7.7) | 1 (7.7) | 0 | 0 | 1 (100) |
| Human + bovine | 0 | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 |
| Human + dog | 0 | 1 (5.9) | 0 | 0 | 0 | 0 | 0 | 0 |
| Unknown | 3 (30) | 1 (5.9) | 4 (40) | 3 (23.1) | 2 (15.4) | 4 (22.2) | 0 | 0 |
| HBI | 70.0 | 76.5 | 20 | 23.1 | 53.8 | 61.1 | 50.0 | 0 |
HBI was calculated as the number of mosquito positive for human (including mixed blood meal) divided by the total number tested
HBI, human blood index; PSC, pyrethrum spray catches
The BBI of An. gambiae s.s. from PSCs, outdoor CDC light traps and pit shelters was 23.5, 40.0, and 46.1%, respectively. None of the tested An. gambiae s.s. from indoor CDC light traps was positive for bovine blood meal. The overall BBI of An. gambiae s.s. was 28%. The BBI of An. funestus from indoor CDC light traps, PSCs and outdoor CDC light traps was 15.4, 16.7, and 50%, respectively. In Iguhu, the overall BBI of An. funestus was 17.6%.
Feeding preference of malaria vectors
The overall blood meal indices and host preferences of Anopheles mosquitoes are shown in Table 4. Regardless of higher proportion of humans compared to domestic animals in Ahero, An. arabiensis showed a strong preference to feed on bovine (Forage ratio, FR = 3.9, 95% CI 3.7–4.9). Anopheles gambiae s.s. showed preference to both human (FR = 1.8, 95% CI 1.3–2.3) and bovine (FR = 2.3, 95% CI 1.3–3.3). Anopheles funestus showed a preference to human in both Ahero (FR = 2.2, 95% CI 1.8–2.6) and Iguhu (FR = 2.0, 95% CI 1.6–2.4).
Table 4.
Overall blood meal indices and host-preferences of malaria vectors from indoor and outdoor collections in Ahero and Iguhu, western Kenya
| Site and species | Parameters | Human | Bovine | Goat | Dog | Chicken |
|---|---|---|---|---|---|---|
| Ahero | ||||||
| Host abundance in the area (%) | 27.8 | 18.8 | 4.0 | 6.0 | 43.4 | |
| An. arabensis | Blood index | 2.5 | 73.1 | 2.5 | 3.4 | 0.6 |
| FR (95% CI) | 0.09 (0.05 to 0.13) | 3.9 (3.7 to 4.1)* | 0.6 (0.3 to 0.9) | 0.5 (0.3 to 0.7) | 0.01 (− 0.03 to 0.05) | |
| An. funestus | Blood index | 62.0 | 25.4 | 0 | 4.2 | 0 |
| FR (95% CI) | 2.2 (1.8 to 2.6)* | 1.4 (0.9 to 1.9) | 0 | 0.7 (− 0.1 to 1.5) | 0 | |
| Iguhu | ||||||
| Host abundance in the area (%) | 27.5 | 12.4 | 2.4 | 2.5 | 55.2 | |
| An. gambiae s.s. | Blood index | 50 | 28 | 0 | 2.0 | 0 |
| FR (95% CI) | 1.8 (1.3 to 2.3)* | 2.3 (1.3 to 2.3)* | 0 | 0.8 (− 0.7 to 2.3) | 0 | |
| An. funestus | Blood index | 55.9 | 17.6 | 2.9 | 2.9 | 0 |
| FR (95% CI) | 2.0 (1.6 to 2.4)* | 1.4 (0.4 to 2.4) | 1.2 (− 1.2 to 3.6) | 1.2 (− 1.1 to 3.5) | 0 | |
FR, forage ratio
* Indicates the preferred host
Sporozoite rates
Overall, 2608 Anopheles mosquitoes comprising An. arabiensis (n = 1280), An. gambiae s.s. (n = 214), An. funestus (n = 629), An. coustani (n = 255) and An. pharoensis (n = 230) were tested for P. falciparum CSPs. Of these, 20 specimens (2 An. arabiensis, 5 An. gambiae s.s., 12 An. funestus and 1 An. coustani) were positive for CSPs.
Table 5 shows the sporozoite rates of Anopheles mosquitoes collected from indoors and outdoors. In Ahero, the sporozoite rate of An. arabiensis from indoor and outdoor CDC light traps was 0.38 and 0.35%, respectively. However, none of the An. arabiensis tested from PSCs and pit shelters were positive. The overall sporozoite rate of An. arabiensis was 0.16%. The sporozoite rate of An. funestus from indoor CDC light traps and PSCs was 2.6 and 2.0%, respectively, while this was 1.2% from both outdoor CDC light traps and pit shelters. Hence, in Ahero, the overall spozoite rate of An. funestus was 1.8%. Moreover, one An. coustani specimen from outdoor CDC light trap was positive for CSP.
Table 5.
Sporozoite rates of Anopheles mosquitoes from indoor and outdoor collections in Ahero and Iguhu, western Kenya
| Study site and Anopheles sp. | Parameters | Indoor | Outdoor | Total | ||
|---|---|---|---|---|---|---|
| Light trap | PSC | Light trap | Pit shelter | |||
| Ahero | ||||||
| An. arabiensis | No tested | 263 | 264 | 286 | 447 | 1260 |
| Pf +ve (%) | 1 (0.38) | 0 | 1 (0.35) | 0 | 2 (0.16) | |
| An. funestus | No tested | 194 | 100 | 169 | 84 | 547 |
| Pf +ve (%) | 5 (2.6) | 2 (2.0) | 2 (1.2) | 1 (1.2) | 10 (1.8) | |
| An. coustani | No tested | 50 | 0 | 200 | 0 | 250 |
| Pf +ve (%) | 0 | 0 | 1 (0.5) | 0 | 1 (0.4) | |
| An. pharoensis | No tested | 25 | 0 | 205 | 0 | 230 |
| Pf +ve (%) | 0 | 0 | 0 | 0 | 0 | |
| Iguhu | ||||||
| An. gambiae s.s. | No tested | 84 | 46 | 50 | 34 | 214 |
| Pf +ve (%) | 3 (3.6) | 0 | 1 (2.0) | 1 (2.9) | 5 (2.3) | |
| An. funestus | No tested | 42 | 25 | 13 | 2 | 82 |
| Pf +ve (%) | 1 (2.4) | 1 (4.0) | 0 | 0 | 2 (2.4) | |
| An. arabiensis | No tested | 8 | 5 | 2 | 5 | 20 |
| Pf +ve (%) | 0 | 0 | 0 | 0 | 0 | |
| An. coustani | No tested | 1 | 0 | 4 | 0 | 5 |
| Pf +ve (%) | 0 | 0 | 0 | 0 | 0 | |
Pf, Plasmodium falciparum; Pf +ve, number P. falciparum CSP positive (rate in percent)
In Iguhu, the sporozoite rate of An. gambiae s.s. from indoor CDC light traps was 3.6%, but none of the An. gambiae s.s. tested from PSCs was positive. In contrast, the sporozoite rate of An. gambiae s.s. from outdoor CDC light traps and pit shelters was 2.0 and 2.9%, respectively. Overall, the sporozoite rate of An. gambiae s.s. was 2.3%. The sporozoite rate of An. funestus from indoor CDC light traps and PSCs was 2.4 and 4%, respectively. No CSP was detected in An. funestus collected from outdoor CDC light traps and pit shelters. Thus, in Iguhu, the overall sporozoite rate of An. funestus was 2.4%.
Entomological inoculation rates (EIRs)
The EIRs of Anopheles mosquitoes are shown in Table 6. In Ahero, the estimated P. falciparum EIR of An. arabiensis from indoor and outdoor CDC light traps was 29.6 and 27.9 infective bites/person/year (ib/p/year), respectively, whereas the EIR of An. funestus from indoor and outdoor CDC light traps was 79.0 and 15.6 ib/p/year, respectively. The overall indoor and outdoor EIR was 108.6 and 43.5 ib/p/year, respectively. About 48% of the total infective bites by An. arabiensis and 16.5% by An. funestus occurred outdoor. The EIR of An. arabiensis and An. funestus from PSCs was 0 and 0.92 ib/p/year, respectively.
Table 6.
Entomological inoculation rates (EIR) of malaria vectors from indoor and outdoor collections in Ahero and Iguhu, western Kenya
| Site and species | Parameters | Indoor | Outdoor | ||
|---|---|---|---|---|---|
| Light trap | PSC | Light trap | Pit shelter | ||
| Ahero | |||||
| An. arabiensis | SR | 0.38 | 0 | 0.35 | 0 |
| EIR | 29.6 | 0 | 27.9 | 0 | |
| An. funestus | SR | 2.6 | 2.0 | 1.2 | 1.2 |
| EIR | 79.0 | 0.92 | 15.6 | 0.05 | |
| An. coustani | SR | 0 | 0 | 0.5 | 0 |
| EIR | 0 | 0 | 16.8 | 0 | |
| Iguhu | |||||
| An. gambiae s.s. | SR | 3.6 | 0 | 2.0 | 2.9 |
| EIR | 18.8 | 0 | 5.5 | 0.17 | |
| An. funestus | SR | 2.4 | 4.0 | 0 | 0 |
| EIR | 5.7 | 0.82 | 0 | 0 | |
| An. arabiensis | SR | 0 | 0 | 0 | 0 |
| EIR | 0 | 0 | 0 | 0 | |
SR, sporozoite rate in percent; EIR, annual entomological inoculation rate measured as the number of infective bites/person/year; PSC, pyrethrum spray catch
In Iguhu, the estimated P. falciparum EIR of An. gambiae s.s. from indoor and outdoor CDC light traps was 18.8 and 5.5 ib/p/year, respectively, whereas the EIR of An. funestus from indoor and outdoor CDC light traps was 5.7 and 0 ib/p/year, respectively. The overall indoor and outdoor EIR was 24.5 and 5.5 ib/p/year, respectively. About 22.6% of the total infective bites by An. gambiae s.s. occurred outdoor. The EIR of An. gambiae s.s. and An. funestus from PSCs was 0 and 0.82 ib/p/year, respectively.
Discussion
This study showed that An. arabiensis was the most abundant anopheline species in Ahero (lowland), whereas An. gambiae s.s. was the most abundant species in Iguhu (highland) sites of western Kenya. An. funestus was the second most abundant species in both sites, which is consistent with previous studies [5, 6].
Anopheles arabiensis showed increased exophagic tendency in the study area when compared with the findings of studies conducted before the scale up of vector control interventions [22, 41]. For instance, studies by Githeko et al. in 1990s, when ITN coverage was negligeable, showed that An. arabiensis was two times more likely to bite indoors than outdoors [22]. In the present study, the outdoor biting density of An. arabiensis was higher than indoor. The increased outdoor host-seeking tendency of An. arabiensis in this study compared to the previous reports might be due to the scale-up of ITNs. Bayoh et al. also noted that An. arabiensis was more likely to bite outdoors in western Kenya when compared with data collected before the scale-up of ITNs [19]. Moreover, An. arabiensis showed highly exophilic behaviour in this study, with significantly higher outdoor resting density than indoor resting density.
The proportion of An. arabiensis has been increasing in western Kenya highlands. Until 2002, An. gambiae s.s. was the only member of An. gambiae s.l. complex reported in western Kenya highlands > 1400 m a.s.l. [25, 42]. The proportion of An. arabiensis was reported to be 0.8% in 2003 [43] and reached 9.2% in 2010 [5]. In this study, the proportion of adult An. arabiensis increased to 13%. A recent study reported a higher proportion of An. arabiensis (38.2%) in larval population [44]. The continued proportional increase in An. arabiensis population might be due to the increased ITN coverage [9, 10] and/or the zoophilic and exophagic/exophilic behaviour of this species or due to species shift. Other factors such as climatic and environmental change, which resulted in increased temperature or availability of more habitats in the area, might have also contributed as this was found to favor An. arabiensis [45]. Such shift in vector species composition could undermine the efficacy of ITNs as the interventions do not target zoophilic and exophilic vector species which avoids the lethal effect of ITNs and sustain residual malaria transmission [46].
Anopheles gambiae s.s. showed endophagic behaviour, with higher indoor host-seeking density than outdoor. This is in agreement with the earlier reports by Githeko et al. [22]. Recent studies in western Kenya have also showed that An. gambiae s.s. was more likely to seek hosts indoor than outdoor [19, 47]. In contrast, studies in Bioko Island, Equatorial Guinea showed that An. gambiae s.s. seek hosts outdoor than indoor [48]. This difference might be due to the variation in molecular forms of An. gambiae s.s. (S and M/Anopheles coluzzii) from Kenya and Equatorial Guinea [49] although the variability in host-seeking behaviour between the two molecular forms is not yet explicitly described.
It is unusual that An. gambiae s.s. showed similar feeding preference to human and bovine. Two decades ago, the HBI of indoor resting An. gambiae s.s. in western Kenya and other parts of the country was 96–97%, an indication that they had fed exclusively on humans [21, 23, 50]. In this study, the overall HBI of An. gambiae s.s. was only 50.0% although predominantly from indoor collection. Compared to the earlier studies conducted in western Kenya before ITNs were used in large scale [21, 23], the HBI of indoor resting An. gambiae s.s. has significantly dropped by 20% and the drop was entirely replaced by BBI. For outdoor resting An. gambiae s.s., the BBI reached up to 46%. Similar reduction in HBI and increment in BBI has also been reported recently [7, 15]. This suggests an increasing tendency of An. gambiae s.s. to feed on bovine following the increased ITN coverage in the western Kenya highlands.
Anopheles funestus s.s. was the predominant species among Anopheles funestus group in the study area. Similar findings were reported in Tanzania [51]. Kweka et al. [52] also found that An. funestus s.s. was the predominant sibling species in larvae population in western Kenya. However, there was significant difference in terms of the relative proportion of An. funestus s.s. between adult and larvae population. In this study, An. funestus s.s. accounted for 98.1% of the adult An. funestus s.l. population. In contrast, Kweka et al. found only 32.9% An. funestus s.s. in larvae population. This difference could be due to the presence of other zoophilic and exophilic sibling species of An. funestus s.l. in the larvae that do not bite or rest indoor or around human dwellings.
Anopheles funestus showed anthropophagic behaviour in both study sites, feeding predominantly on human. The anthrophagic behaviour of An. funestus was frequently observed in Kenya [21, 50] and elsewhere in Africa [53–56]. Nevertheless, they also fed on bovine, with higher BBI than the previous reports [21, 50, 53–56].
The secondary vectors, An. pharoensis and An. coustani showed exophagic behaviour, with significantly higher outdoor host-seeking density than indoor. Other studies in Kenya [41, 57] and elsewhere in Africa [58–60] reported similar phenomenon for these species. It is worth mentioning that both An. pharoensis and An. coustani were very rare in both indoor resting collections and pit shelters despite their preponderance in CDC light traps. Hence, further studies are required to find out the potential resting places of An. pharoensis and An. coustani.
The EIR data showed that the majority of malaria transmission by An. gambiae s.s. and An. funestus occurred indoors, while An. arabiensis contributed almost equally to both outdoor and indoor transmission. The higher indoor EIRs despite high ITN coverage could be attributed to inconsistent ITN use [61], increasing insecticide resistance among vectors [5, 8], and shifts in malaria vector biting times from mid-night to early evening and morning when people are still indoor but unprotected by ITNs [47, 62]. However, the magnitude of the outdoor EIRs was also considerably high compared to previous reports [19]. The ongoing shifts in vector species composition and changes in vector behaviours might have contributed to the high outdoor EIRs.
In addition to the primary vectors, a single specimen of An. coustani from outdoor CDC light trap was found to be positive for P. falciparum CSP based on ELISA, although not yet confirmed by PCR. Studies are increasingly reporting the importance of the secondary vectors in residual malaria transmission [57, 60, 63–65]. Several studies have demonstrated the susceptibility of An. coustani to P. falciparum infection [57, 59, 60, 66]. Although ELISA technique is not specific enough to incriminate zoophagic mosquitoes as a vector [33], a recent study in Madagascar confirmed the presence of Plasmodium CSP in An. coustani by both ELISA and PCR [60], suggesting that this species could play a role in outdoor malaria transmission.
Conclusion
Anopheles arabiensis was highly exophilic and zoophagic. Anopheles gambiae s.s. showed an increasing tendency to feed on bovine while An. funestus showed anthropophagic behaviour. While most of malaria transmission occurred indoor, the magnitude of outdoor transmission was considerably high. Additional control tools that complement the existing interventions are required to control residual transmission. Further studies are required to comprehend the role of secondary vectors in malaria transmission.
Authors’ contributions
TD, DY, AKG and GY designed the study protocol. TD involved in data collection, laboratory work and data analysis. GZ and ML participated in data analysis. HA supervised data collection and laboratory work. TD drafted the manuscript. DY, AKG, GY critically reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors would like to acknowledge all entomology technicians of Climate and Human Health Research Unit, Kenya Medical Research Institute for their technical support in the field and laboratory. We thank the communities of Ahero and Iguhu for their willingness to participate in the study. This study is published with the permission of the Director, Kenya Medical Research Institute.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used for the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Ethical approval for the study was obtained from Ethical Review Board of Kenya Medical Research Institute and University of California, Irvine. Permission was sought from chief of each study site. Informed consent was obtained from heads of the households.
Funding
This work was supported by Grants from the National Institutes of Health (R01 AI050243, U19 AI129326 and D43 TW001505).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- BBI
bovine blood index
- CDC
Centers for Disease Control and Prevention
- CSP
circumsporozoite protein
- EIR
entomological inoculation rate
- ELISA
enzyme-linked immunosorbent assay
- FR
forage ratio
- HBI
human blood index
- IRS
indoor residual spray
- ITN
insecticide-treated net
- PCR
polymerase chain reaction
- PSC
pyrethrum spray catch
Contributor Information
Teshome Degefa, Email: teshedege@gmail.com.
Delenasaw Yewhalaw, Email: delenasawye@yahoo.com.
Guofa Zhou, Email: zhoug@uci.edu.
Ming-chieh Lee, Email: mingchil@uci.edu.
Harrysone Atieli, Email: etemesi2012@yahoo.com.
Andrew K. Githeko, Email: githeko@yahoo.com
Guiyun Yan, Email: guiyuny@uci.edu.
References
- 1.WHO . World Malaria Report 2015. Geneva: World Health Organization; 2015. [Google Scholar]
- 2.Shargie EB, Ngondi J, Graves PM, Getachew A, Hwang J, Gebre T, et al. Rapid increase in ownership and use of long-lasting insecticidal nets and decrease in prevalence of malaria in three regional States of Ethiopia (2006–2007) J Trop Med. 2010;2010:e750978. doi: 10.1155/2010/750978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattarai A, Ali AS, Kachur SP, Mårtensson A, Abbas AK, Khatib R, et al. Impact of artemisinin-based combination therapy and insecticide-treated nets on malaria burden in Zanzibar. PLoS Med. 2007;4:e309. doi: 10.1371/journal.pmed.0040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otten M, Aregawi M, Were W, Karema C, Medin A, Bekele W, et al. Initial evidence of reduction of malaria cases and deaths in Rwanda and Ethiopia due to rapid scale-up of malaria prevention and treatment. Malar J. 2009;8:14. doi: 10.1186/1475-2875-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou G, Afrane YA, Vardo-Zalik AM, Atieli H, Zhong D, Wamae P. Changing patterns of malaria epidemiology between 2002 and 2010 in Western Kenya: the fall and rise of malaria. PLoS ONE. 2011;6:e20318. doi: 10.1371/journal.pone.0020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ototo EN, Mbugi JP, Wanjala CL, Zhou G, Githeko AK, Yan G. Surveillance of malaria vector population density and biting behaviour in western Kenya. Malar J. 2015;14:244. doi: 10.1186/s12936-015-0763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ndenga BA, Mulaya NL, Musaki SK, Shiroko JN, Dongus S, Fillinger U. Malaria vectors and their blood-meal sources in an area of high bed net ownership in the western Kenya highlands. Malar J. 2016;15:76. doi: 10.1186/s12936-016-1115-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochomo EO, Bayoh NM, Walker ED, Abongo BO, Ombok MO, Ouma C, et al. The efficacy of long-lasting nets with declining physical integrity may be compromised in areas with high levels of pyrethroid resistance. Malar J. 2013;12:368. doi: 10.1186/1475-2875-12-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayoh MN, Mathias DK, Odiere MR, Mutuku FM, Kamau L, Gimnig JE, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province, Kenya. Malar J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mwangangi JM, Mbogo CM, Orindi BO, Muturi EJ, Midega JT, Nzovu J, et al. Shifts in malaria vector species composition and transmission dynamics along the Kenyan coast over the past 20 years. Malar J. 2013;12:13. doi: 10.1186/1475-2875-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell TL, Lwetoijera DW, Maliti D, Chipwaza B, Kihonda J, Charlwood JD, et al. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar J. 2010;9:187. doi: 10.1186/1475-2875-9-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Derua YA, Alifrangis M, Hosea KM, Meyrowitsch DW, Magesa SM, Pedersen EM, et al. Change in composition of the Anopheles gambiae complex and its possible implications for the transmission of malaria and lymphatic filariasis in north-eastern Tanzania. Malar J. 2012;11:188. doi: 10.1186/1475-2875-11-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80. doi: 10.1186/1475-2875-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durnez L, Coosemans M. Residual transmission of malaria: an old issue for new approaches. In: Manguin S, editor. Anopheles mosquitoes—new insights into malaria vectors; 2013. p. 671–704.
- 15.Mutuku FM, King CH, Mungai P, Mbogo C, Mwangangi J, Muchiri EM, et al. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malar J. 2011;10:356. doi: 10.1186/1475-2875-10-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefèvre T, Gouagna L-C, Dabiré KR, Elguero E, Fontenille D, Renaud F, et al. Beyond nature and nurture: phenotypic plasticity in blood-feeding behavior of Anopheles gambiae s.s. when humans are not readily accessible. Am J Trop Med Hyg. 2009;81:1023–1029. doi: 10.4269/ajtmh.2009.09-0124. [DOI] [PubMed] [Google Scholar]
- 17.Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10:184. doi: 10.1186/1475-2875-10-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyers JI, Pathikonda S, Popkin-Hall ZR, Medeiros MC, Fuseini G, Matias A, et al. Increasing outdoor host-seeking in Anopheles gambiae over 6 years of vector control on Bioko Island. Malar J. 2016;15:239. doi: 10.1186/s12936-016-1286-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayoh MN, Walker ED, Kosgei J, Ombok M, Olang GB, Githeko AK, et al. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasit Vectors. 2014;7:380. doi: 10.1186/1756-3305-7-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Githeko A, Ototo E, Guiyun Y. Progress towards understanding the ecology and epidemiology of malaria in the western Kenya highlands: opportunities and challenges for control under climate change risk. Acta Trop. 2012;121:19–25. doi: 10.1016/j.actatropica.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Githeko A, Service M, Mbogo C, Atieli F, Juma F. Origin of blood meals in indoor and outdoor resting malaria vectors in western Kenya. Acta Trop. 1994;58:307–316. doi: 10.1016/0001-706X(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 22.Githeko AK, Adungo NI, Karanja DM, Hawley WA, Vulule JM, Seroney IK, et al. Some observations on the biting behavior of Anopheles gambiae s.s, Anopheles arabiensis, and Anopheles funestus and their implications for malaria control. Exp Parasitol. 1996;82:306–315. doi: 10.1006/expr.1996.0038. [DOI] [PubMed] [Google Scholar]
- 23.Shililu J, Maier W, Seitz H, Orago A. Seasonal density, sporozoite rates and entomological inoculation rates of Anopheles gambiae and Anopheles funestus in a high altitude sugarcane growing zone in western Kenya. Trop Med Int Health. 1998;3:706–710. doi: 10.1046/j.1365-3156.1998.00282.x. [DOI] [PubMed] [Google Scholar]
- 24.Munyekenye OG, Githeko AK, Zhou G, Mushinzimana E, Minakawa N, Yan G. Plasmodium falciparum spatial analysis, western Kenya highlands. Emerg Infect Dis. 2005;11:1571–1577. doi: 10.3201/eid1110.050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Githeko AK, Ayisi JM, Odada PK, Atieli FK, Ndenga BA, Githure JI, et al. Topography and malaria transmission heterogeneity in western Kenya highlands: prospects for focal vector control. Malar J. 2006;5:107. doi: 10.1186/1475-2875-5-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO . Manual on practical entomology in malaria. Geneva: World Health Organization; 1995. [Google Scholar]
- 27.Muirhead-Thomson R. A pit shelter for sampling outdoor mosquito populations. Bull World Health Organ. 1958;19:1116–1118. [PMC free article] [PubMed] [Google Scholar]
- 28.Gillies M, Coetzee M. A supplement to the Anophelinae of Africa South of the Sahara. Public South Afr Instit Med Res. 1987;55:1–143. [Google Scholar]
- 29.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am J Trop Med Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 30.Koekemoer L, Kamau L, Hunt R, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66:804–811. doi: 10.4269/ajtmh.2002.66.804. [DOI] [PubMed] [Google Scholar]
- 31.Beier JC, Perkins PV, Wirtz RA, Koros J, Diggs D. Bloodmeal identification by direct enzyme-linked immunosorbent assay (ELISA), tested on Anopheles (Diptera: Culicidae) in Kenya. DTIC Doc. 1988;25:9–16. doi: 10.1093/jmedent/25.1.9. [DOI] [PubMed] [Google Scholar]
- 32.Beier JC, Perkins PV, Wirtz RA, Whitmire RE, Mugambi M. Field evaluation of an enzymelinked immunosorbent assay (ELISA) for Plasmodium falciparum sporozoite detection in anopheline mosquitoes from Kenya. DTIC Doc. 1987;36:459–468. doi: 10.4269/ajtmh.1987.36.459. [DOI] [PubMed] [Google Scholar]
- 33.Wirtz R, Burkot T, Graves P, Andre R. Field evaluation of enzyme-linked immunosorbent assays for Plasmodium falciparum and Plasmodium vivax sporozoites in mosquitoes (Diptera: Culicidae) from Papua New Guinea. J Med Entomol. 1987;24:433–437. doi: 10.1093/jmedent/24.4.433. [DOI] [PubMed] [Google Scholar]
- 34.Garrett-Jones C. The human blood index of malaria vectors in relation to epidemiological assessment. Bull World Health Organ. 1964;30:241–261. [PMC free article] [PubMed] [Google Scholar]
- 35.Pappa V, Reddy M, Overgaard HJ, Abaga S, Caccone A. Estimation of the human blood index in malaria mosquito vectors in Equatorial Guinea after indoor antivector interventions. Am J Trop Med Hyg. 2011;84:298–301. doi: 10.4269/ajtmh.2011.10-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hess A, Hayes RO, Tempelis C. The use of the forage ratio technique in mosquito host preference studies. Mosq News. 1968;28:386–389. [Google Scholar]
- 37.Manly B, McDonald L, Thomas D, McDonald TL, Erickson WP. Resource selection by animals: statistical design and analysis for field studies. New York: Springer Science & Business Media; 2007. [Google Scholar]
- 38.Lines J, Curtis C, Wilkes T, Njunwa K. Monitoring human-biting mosquitoes (Diptera: Culicidae) in Tanzania with light-traps hung beside mosquito nets. Bull Entomol Res. 1991;81:77–84. doi: 10.1017/S0007485300053268. [DOI] [Google Scholar]
- 39.Drakeley C, Schellenberg D, Kihonda J, Sousa C, Arez A, Lopes D, et al. An estimation of the entomological inoculation rate for Ifakara: a semi-urban area in a region of intense malaria transmission in Tanzania. Trop Med Int Health. 2003;8:767–774. doi: 10.1046/j.1365-3156.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 40.WHO. Malaria entomology and vector control: learner’s guide. Trial Edition HIV/AIDS, tuberculosis and malaria, roll back malaria. Geneva: World Health Organization; 2003.
- 41.Githeko A, Mbogo C, Atieli F, Juma F. Sampling Anopheles arabiensis, A. gambiae sensu lato and A. funestus (Diptera: Culicidae) with CDC light-traps near a rice irrigation area and a sugarcane belt in western Kenya. Bull Entomol Res. 1994;84:319–324. doi: 10.1017/S0007485300032430. [DOI] [Google Scholar]
- 42.Minakawa N, Sonye G, Mogi M, Githeko A, Yan G. The effects of climatic factors on the distribution and abundance of malaria vectors in Kenya. J Med Entomol. 2002;39:833–841. doi: 10.1603/0022-2585-39.6.833. [DOI] [PubMed] [Google Scholar]
- 43.Ndenga B, Githeko A, Omukunda E, Munyekenye G, Atieli H, Wamai P, et al. Population dynamics of malaria vectors in western Kenya highlands. J Med Entomol. 2006;43:200–206. doi: 10.1093/jmedent/43.2.200. [DOI] [PubMed] [Google Scholar]
- 44.Kweka EJ, Munga S, Himeidan Y, Githeko AK, Yan G. Assessment of mosquito larval productivity among different land use types for targeted malaria vector control in the western Kenya highlands. Parasit Vectors. 2015;8:356. doi: 10.1186/s13071-015-0968-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Life-table analysis of Anopheles arabiensis in western Kenya highlands: effects of land covers on larval and adult survivorship. Am J Trop Med Hyg. 2007;77:660–666. [PubMed] [Google Scholar]
- 46.Okumu FO, Kiware SS, Moore SJ, Killeen GF. Mathematical evaluation of community level impact of combining bed nets and indoor residual spraying upon malaria transmission in areas where the main vectors are Anopheles arabiensis mosquitoes. Parasit Vectors. 2013;6:17. doi: 10.1186/1756-3305-6-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooke MK, Kahindi SC, Oriango RM, Owaga C, Ayoma E, Mabuka D, et al. ‘A bite before bed’: exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar J. 2015;14:259. doi: 10.1186/s12936-015-0766-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Overgaard HJ, Reddy VP, Abaga S, Matias A, Reddy MR, Kulkarni V, et al. Malaria transmission after five years of vector control on Bioko Island, Equatorial Guinea. Parasit Vectors. 2012;5:253. doi: 10.1186/1756-3305-5-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehmann T, Licht M, Elissa N, Maega B, Chimumbwa J, Watsenga F, et al. Population structure of Anopheles gambiae in Africa. J Hered. 2003;94:133–147. doi: 10.1093/jhered/esg024. [DOI] [PubMed] [Google Scholar]
- 50.Mwangangi JM, Mbogo CM, Nzovu JG, Githure JI, Yan G, Beier JC. Blood-meal analysis for anopheline mosquitoes sampled along the Kenyan coast. J Am Mosq Control Assoc. 2003;19:371–375. [PubMed] [Google Scholar]
- 51.Derua YA, Alifrangis M, Magesa SM, Kisinza WN, Simonsen PE. Sibling species of the Anopheles funestus group, and their infection with malaria and lymphatic filarial parasites, in archived and newly collected specimens from northeastern Tanzania. Malar J. 2015;14:104. doi: 10.1186/s12936-015-0616-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kweka EJ, Kamau L, Munga S, Lee M-C, Githeko AK, Yan G. A first report of Anopheles funestus sibling species in western Kenya highlands. Acta Trop. 2013;128:158–161. doi: 10.1016/j.actatropica.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tanga M, Ngundu W, Tchouassi P. Daily survival and human blood index of major malaria vectors associated with oil palm cultivation in Cameroon and their role in malaria transmission. Trop Med Int Health. 2011;16:447–457. doi: 10.1111/j.1365-3156.2011.02726.x. [DOI] [PubMed] [Google Scholar]
- 54.Das S, Henning TC, Simubali L, Hamapumbu H, Nzira L, Mamini E, et al. Underestimation of foraging behaviour by standard field methods in malaria vector mosquitoes in southern Africa. Malar J. 2015;14:12. doi: 10.1186/s12936-014-0527-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mzilahowa T, Hastings IM, Molyneux ME, McCall PJ. Entomological indices of malaria transmission in Chikhwawa district, Southern Malawi. Malar J. 2012;11:380. doi: 10.1186/1475-2875-11-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dadzie SK, Brenyah R, Appawu MA. Role of species composition in malaria transmission by the Anopheles funestus group (Diptera: Culicidae) in Ghana. J Vector Ecol. 2013;38:105–110. doi: 10.1111/j.1948-7134.2013.12015.x. [DOI] [PubMed] [Google Scholar]
- 57.Mwangangi JM, Muturi EJ, Muriu SM, Nzovu J, Midega JT, Mbogo C. The role of Anopheles arabiensis and Anopheles coustani in indoor and outdoor malaria transmission in Taveta District, Kenya. Parasit Vectors. 2013;6:114. doi: 10.1186/1756-3305-6-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taye B, Lelisa K, Emana D, Asale A, Yewhalaw D. Seasonal dynamics, longevity, and biting activity of anopheline mosquitoes in southwestern Ethiopia. J Insect Sci. 2016;16:6. doi: 10.1093/jisesa/iev150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Antonio-Nkondjio C, Kerah CH, Simard F, Awono-Ambene P, Chouaibou M, Tchuinkam T, et al. Complexity of the malaria vectorial system in Cameroon: contribution of secondary vectors to malaria transmission. J Med Entomol. 2006;43:1215–1221. doi: 10.1093/jmedent/43.6.1215. [DOI] [PubMed] [Google Scholar]
- 60.Nepomichene TN, Tata E, Boyer S. Malaria case in Madagascar, probable implication of a new vector, Anopheles coustani. Malar J. 2015;14:475. doi: 10.1186/s12936-015-1004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Atieli HE, Zhou G, Afrane Y, Lee M-C, Mwanzo I, Githeko AK, et al. Insecticide-treated net (ITN) ownership, usage, and malaria transmission in the highlands of western Kenya. Parasit Vectors. 2011;4:113. doi: 10.1186/1756-3305-4-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wamae P, Githeko A, Otieno G, Kabiru E, Duombia S. Early biting of the Anopheles gambiae s.s and its challenges to vector control using insecticide treated nets in western Kenya highlands. Acta Trop. 2015;150:136–142. doi: 10.1016/j.actatropica.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 63.Stevenson J, Laurent BS, Lobo NF, Cooke MK, Kahindi SC, Oriango RM, et al. Novel vectors of malaria parasites in the western highlands of Kenya. Emerg Infect Dis. 2012;18:1547–1550. doi: 10.3201/eid1809.120283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laurent BS, Cooke M, Krishnankutty SM, Asih P, Mueller JD, Kahindi S, et al. Molecular characterization reveals diverse and unknown malaria vectors in the western Kenyan highlands. Am J Trop Med Hyg. 2016;94:327–335. doi: 10.4269/ajtmh.15-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stevenson JC, Simubali L, Mbambara S, Musonda M, Mweetwa S, Mudenda T, et al. Detection of Plasmodium falciparum infection in Anopheles squamosus (Diptera: Culicidae) in an area targeted for malaria elimination, southern Zambia. J Med Entomol. 2016;53:1482–1487. doi: 10.1093/jme/tjw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Degefa T, Zeynudin A, Godesso A, Michael YH, Eba K, Zemene E, et al. Malaria incidence and assessment of entomological indices among resettled communities in Ethiopia: a longitudinal study. Malar J. 2015;14:24. doi: 10.1186/s12936-014-0532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used for the current study are available from the corresponding author on reasonable request.


