Abstract
Background
Prophylaxis for high-risk populations, such as forest workers, could be one component for malaria elimination in the Greater Mekong Sub-region. A study was conducted to assess the malaria incidence in forest rangers and the feasibility of malaria prophylaxis for rangers sleeping in forest camps.
Methods
Forest rangers deployed in the Bu Gia Map National Park, Vietnam were invited to participate in the study. Plasmodium infections were cleared using presumptive treatment, irrespective of malaria status, with a 3-day course dihydroartemisinin/piperaquine (DP) and a 14-day course of primaquine. Before returning to the forest, study participants were randomly allocated to a 3-day course of DP or placebo. Fifteen days after returning from their forest deployment the participants were tested for Plasmodium infections using uPCR.
Results
Prior to treatment, 30 of 150 study participants (20%) were found to be infected with Plasmodium. Seventeen days (median) after enrolment the rangers were randomized to DP or placebo 2 days before returning to forest camps where they stayed between 2 and 20 days (median 9.5 days). One ranger in the DP-prophylaxis arm and one in the placebo arm were found to be infected with Plasmodium falciparum 15 days (median) after returning from the forest. The evaluable P. falciparum isolates had molecular markers indicating resistance to artemisinins (K13-C580Y) and piperaquine (plasmepsin), but none had multiple copies of pfmdr1 associated with mefloquine resistance.
Conclusion
Anti-malarial prophylaxis in forest rangers is feasible. The findings of the study highlight the threat of multidrug-resistant malaria.
Trial registration NCT02788864
Electronic supplementary material
The online version of this article (10.1186/s12936-017-2091-6) contains supplementary material, which is available to authorized users.
Keywords: Plasmodium falciparum, Plasmodium vivax, An. dirus, An. maculatus, An. barbirostris, Anti-malarial resistance, Forest, Prophylaxis, DHA, Piperaquine, Kelch K13-C580Y, Plasmepsin
Background
Falciparum and vivax malaria cases have dropped in Vietnam from over one million resulting in 4500 deaths in 1991 to 19,252 confirmed malaria cases with 25 recorded deaths in 2015 [1]. The goal of malaria elimination has been endorsed by the Vietnamese Government, but this goal is jeopardized by the emergence and spread of Plasmodium falciparum resistance to artemisinins and partner drugs [2–5]. Several new anti-malarial drugs are in development but the availability of a new first line drug is not expected within this decade [6]. Accelerated elimination before the current arsenal of artemisinin combination therapy loses efficacy is probably the only way to stop the spread of anti-malarial resistance.
A large proportion of malaria transmission in Vietnam occurs in forested areas, which serve as perpetual sources of transmission [7–11]. The population attributable fraction of malaria cases acquired by forest-related activities has been estimated as high as 53% [12]. Malaria epidemiology is compounded by poverty, a risk factor in Vietnam for malaria like many other malaria-endemic countries [10]. Vietnamese ethnic minorities carry a disproportionately large share of the malaria burden related to their remote settlements and the need to make a living through forest-related activities [10].
Rural villagers tend to refer to the land surrounding their village as forest. Forest-related activities can range from hunting in deep primary forest to work in plantations and rice paddies. Forest-related work tends to be seasonal, does not follow a regular schedule, and is difficult for outsiders to predict. Some villagers spend months on farms miles away from their villages while others tend to return at nightfall to their homes in their villages. Some villagers are accompanied by their families while other families remain in their villages and only men work in the forest for extended periods. One unifying feature of forest work is the basic character of overnight accommodation [13] and exposure to the Anopheles vectors (e.g. Anopheles dirus), which tend to bite outdoors and during the day. Insecticide-treated bed nets have a high protective efficacy against nocturnal, indoor malaria transmission [14] but are less protective against daytime, outdoor-biting vectors like An. dirus. The improvised housing of forest workers is frequently poorly suited to hanging bed nets [15].
In Binh Phuoc province, forest rangers are considered a high-risk population due to their work-related exposure. 150 forest rangers are permanently employed and twice that number are intermittently employed as part-time rangers by Bu Gia Map National Park. The self-reported malaria incidence in this population is very high. Up to 80% of forest rangers state they had malaria in the previous year but no accurate incidence estimates exist. Strategies are needed to protect these forest rangers who could be an ongoing source of malaria transmission. The purpose of the study was to: (1) measure the malaria incidence in forest rangers to determine the number of participants required in future studies to estimate the efficacy of anti-malarial prophylaxis in forest rangers; and, (2) assess the feasibility of malaria prophylaxis for rangers based in forest camps.
Methods
Study site
Bu Gia Map National Park is dedicated to the conservation of rare and precious species of Vietnamese fauna and flora [16]. The park encompasses 26,032 ha (260 km2) located in an area overlapping Binh Phuoc and Dak Nong Province (Fig. 1). The park is part of the South-Central Highlands; the highest elevation is 700 m above sea level and enjoys a year-round, hot-humid, tropical climate. Entry in the park requires a permit from the park service/Ministry of Forestry. The forest and its wildlife are protected by approximately 150 permanent and 300 short-time forest rangers. The permanent rangers stay in 12 stations dispersed throughout the park, each of which accommodates 3–6 staff members. The stations are wood constructions elevated from the ground with one or two floors. Permanent rangers stay for several consecutive months in the forest. They are supported by part-time rangers recruited from hamlets surrounding the park. The short-term rangers stay in 13 camp sites some of which adjacent to the permanent stations. The accommodation of the short-term rangers is more temporary, tents and basic wooden ground floor structures. The short-time rangers spend between 2 and 20 days in the forest before returning home. All rangers tend to sleep in hammocks with or without bed nets. Malaria transmission occurs throughout the year and is slightly increased from September to March.
Fig. 1.
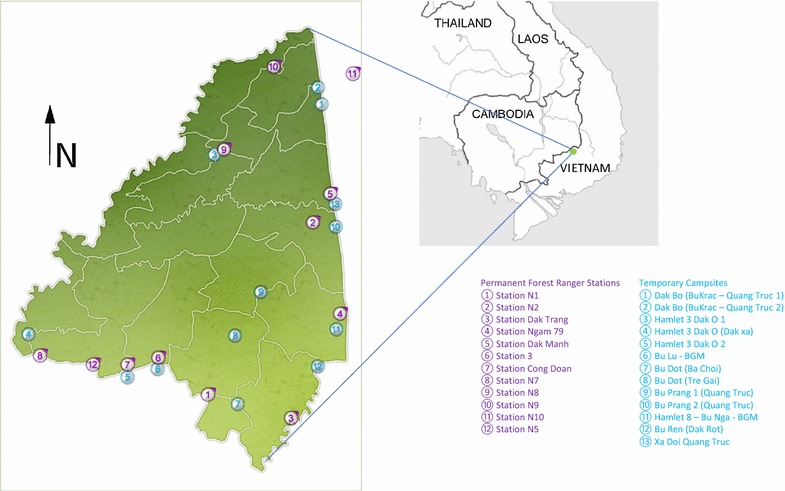
Map of National Park Bu Gia, Binh Phuoc Province, Vietnam, with forest ranger stations and campsites
Participants
Male forest rangers over 18 years of age were invited to participate in the study. Rangers who consented were enrolled at Bu Gia Map Health Station and the Office of the Forest Rangers. Candidates with anaemia (Hb < 9 mg/dl), G6PD deficiency diagnosed by fluorescent spot test or a previous history of adverse reactions to the study drugs were excluded.
Study design and procedures
Study participants were asked to provide a blood sample on enrolment to assess parasite infections using ultrasensitive (u)PCR, and to assess the presence of G6PD deficiency. All enrolled participants received a 3-day course of dihydroartemisinin (DHA)/piperaquine (7 mg/kg/day dihydroartemisinin; 55 mg/kg/day piperaquine phosphate (Arterakine©; Pharbaco Central Pharmaceutical JSC No. 1, Hanoi, Vietnam) and 14 days of 0.25 mg/kg/day primaquine (Danaphar Pharmaceutical JSC; Da Nang City, Vietnam). The DHA/piperaquine tables were split into half tablets as needed for precise dosing. The administration of study drugs was directly observed by study staff.
Two days prior to their assignment in the forest the study participants were randomized 1:1 (block size 4) using a computer-generated schedule to receive either a 3-day course of DHA/piperaquine (Arterakine©; 7 mg/kg/day dihydroartemisinin; 55 mg/kg/day piperaquine phosphate; Pharbaco Central Pharmaceutical JSC No. 1, Hanoi, Vietnam) or placebo with identical physical properties (also Pharbaco Central Pharmaceutical JSC No. 1). Study participants and staff were blinded to the identity of the study drugs.
A venous blood sample was collected from all participants on enrolment, prior to the trip to forest trip and 14 days after their return from the forest, to detect infections still incubating on return from the forest trip.
Detection and quantification of malaria parasitaemia
Standard microscopy was performed by microscopists who had at least 5 years’ experience and/or were confirmed to be Level 2 or higher with a WHO 55 slide set. Thick and thin blood films were examined at 3 visits during the study period (base line, pre-forest and post-forest). Specimens were labelled to maintain anonymity (study number, day of visit, and date). Thick and thin blood smears were examined were stained with 10% Giemsa in pH 7.2 buffer solution for 15 min. Parasitaemia was estimated by counting the number of asexual parasites per 500 leucocytes in the thick blood film or per 1000 red blood cells on a thin film then calculated followed WHO guideline. All slides were read by two qualified microscopists independently, and the average of the two counts was taken as the parasite density. Blood smears with discordant results (differences in assessments of species, parasite density > 50%, or the presence of parasites) were re-examined by a third independent microscopist, and parasite densities were calculated by averaging the two closest counts. For rapid diagnosis, SD Bioline Malaria Ag Pf/Pv RDTs were used (SD Standard Diagnostics, Gyeonggi-do, Republic of Korea).
Sub-microscopic parasitaemia was detected by high-volume ultrasensitive qPCR (uPCR) method reported recently [17]. In summary, the parasite DNA were extracted by MagNa Pure 96 Instrument automated extraction system using MagNa Pure 96 DNA and Viral NA Small Volume Kit (Roche, Switzerland) from the thawed packed red blood cells samples. Purified DNA was dehydrated and then suspended in a small volume of PCR grade water. Two µl of resuspended DNA were used as template in the qPCR reaction. The presence of malaria parasites and an estimate of the parasite numbers (genomes) in each sample were assessed by an absolute quantitative real-time PCR (qPCR) method (Roche, Switzerland). The 18S rRNA-targeting primers and hydrolysis probes used in the assay have been validated and are highly specific for Plasmodium species [18]. The lower limit of accurate quantitation of this method is 22 parasites/ml of whole blood [19]. For samples where the uPCR was positive an attempt was made to determine the Plasmodium species present using qPCR protocols specific to P. falciparum (18s rRNA) and Plasmodium vivax (18s rRNA) described previously [18, 20–22]. Samples for which there was insufficient DNA to do this, or where no amplification was obtained in this step were reported as being of indeterminate species (Plasmodium spp.). uPCR results became available after study completion and did not trigger the treatment of study participants.
Anti-malarial drug resistance markers detection
K13 gene amplification and sequencing K13 encoding gene was amplified by nested PCR then genotyped by capillary sequencing as described previously [23]. The K13 sequences were assembled by ContigExpress and aligned with the K13 gene sequence of 3D7 clone (PF3D7_1343700) using MEGA 5.0 to identify any SNPs present. Piperaquine resistance marker testing DNA samples were also sequenced to detect mutation at position 415 in putative exonuclease gene (exoE415G) following a protocol described recently [24]. Copy number of plasmepsin 2 encoding gene was detected by real time qPCR following a procedure shared by the same group [24]. Multidrug resistance marker testing copy number of pfmdr1 gene were detected by real time qPCR following a procedure published previously [25].
Entomology
Entomological studies using CDC light traps and human landing catches were conducted for 3 nights in camps 2 and 79 and for 4 nights in camp 8. Light traps were set up following the instructions provided by the producer [26]. A single light-trap was suspended about 1.5 m above ground near a sleeping ranger. The light traps were used from sunset (18:00) to sunrise (06:00). The traps were collected in the morning by project staff. For human landing catches, approaching mosquitoes were detected by flashlight, aspirated and placed in screened, pint-sized containers. Mosquito collections were performed from 18.00 to 06:00. The light traps were used only indoors while human landing catches were conducted indoors and outdoors. All anopheline mosquitoes caught were sorted and morphologically identified using the taxonomic keys of Rattanarithikul and Panthusiri [27–33]. PCR was used to detect Plasmodium infections in mosquitoes [34]. Mosquitoes other than anophelines were discarded.
Data management and analysis
Data were recorded on case report forms and double-entered in a custom-designed Access database (Microsoft, Redmond, WA, USA). Line listings of the study participants were analysed in Excel (Microsoft, Redmond, WA, USA). Proportions were compared using Chi squared or Fisher’s exact test. Stata 14.2 was used for statistical testing (StataCorp, College Station, TX, USA).
This was the first study of malaria in the forest rangers working in Bu Gia Map National Park. Neither the duration of forest stays, nor an estimate of malaria incidence or the prevalence of subclinical Plasmodium infections in forest rangers has been reported prior to this study. In the absence of prior estimates of prevalence and incidence of Plasmodium infections in this population it was not possible to estimate the sample size required to demonstrate the protective efficacy of anti-malarials.
Results
The study was conducted between May and September 2016. 157 forest rangers were screened. Six rangers were excluded due to G6PD deficiency and one due to anaemia (Fig. 2); 150 rangers were enrolled, gave a venous blood sample and received 3 days’ DP combined with 14 days’ primaquine. The study drugs were well tolerated; none of the participants reported adverse reactions to the study drugs. After a median time span of 17 days (IQR 16–17; Table 1) the 150 enrolled rangers were randomized to receive either DP or placebo and departed for the forest after completing 3 days of directly observed drug administration. The median duration of forest stay was 9.5 days (IQR 5–12 days). The median duration of study participation was 47 days (IQR 42–54). No study participant was lost to follow-up.
Fig. 2.
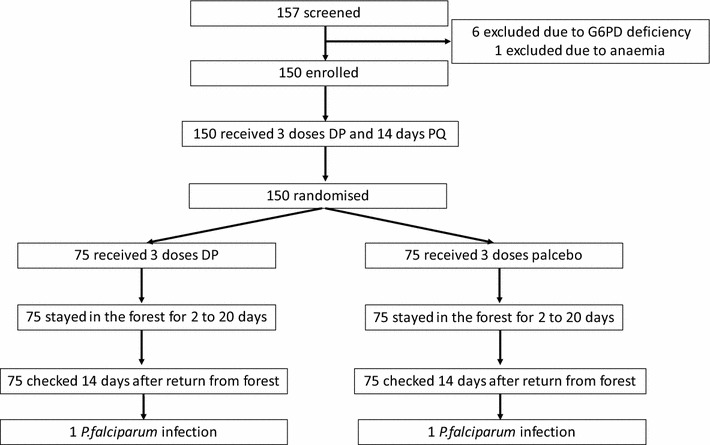
Assembly and flow of study participants
Table 1.
Time spans (median and interquartile range)
| ACT | IQR | Placebo | IQR | All | IQR | |
|---|---|---|---|---|---|---|
| Duration of last forest stay before enrolment (days) | 8 | 4–13 | 8 | 5–14 | 8 | 5–12 |
| Days since last forest stay | 7 | 7–28 | 7 | 7–28 | 7 | 7–14 |
| Days between start drug administration* and return to forest | 19 | 19–28 | 19 | 18–20 | 19 | 18–19 |
| Days in forest | 8 | 5–12 | 10 | 3–10 | 9.5 | 5–10 |
| Days between return to forest and last screening | 28 | 23–34 | 28 | 23–34 | 28 | 23–34 |
| Duration of study participation (days) | 47 | 42–54 | 47 | 42–54 | 47 | 42–54 |
* 3 days DHA/piperaquine and 14 days PQ
Baseline characteristics
Except for one permanent ranger, all participants were short-time forest rangers. Nearly all participants (148/150; 99%) worked as farmers when not employed as forest rangers (Table 2). There were no statistically significant differences between demographic, clinical and parasitological characteristics of participants in the artemisinin-based combination therapy (ACT) and placebo arm. The rangers had returned from the forest 14 days earlier (median; range 10–20 days) and the previous forest visit had lasted 8 days (median; IQR 5–10 days). 30/150 study participants (20%; 95% CI 13–27%) were found to be infected with Plasmodium at enrolment (P. falciparum 12/150 = 8% 95% CI 4–14%; P. vivax 9/150 = 6%; P. falciparum/P. vivax mix 2/150 = 1%; Plasmodium spp. 7/150 = 5%). Four of the infected participant had parasitaemia detectable by microscopy and 3 by rapid diagnostic tests (RDT). The positive results detected by RDT, microscopy and uPCR were concordant. The mean haemoglobin (Hb) of the participants was 14.7 g/dl. Eleven P. falciparum infections had K13 C580Y mutations in the K-13 propeller region associated with artemisinin resistance; the remaining infection could not be genotyped due to low DNA quantities. Two of the 12 P. falciparum infections had multiple copies of P14 associated with piperaquine resistance, 7 had single copies, and 4 samples had insufficient DNA material for testing. Eleven of the 12 P. falciparum infections had an exo E415G mutation associated with piperaquine resistance, one was a wild type; the other P. falciparum infection could not be tested due to low sample volume. None of the P. falciparum isolates had multiple copies of pfmdr1.
Table 2.
Demographic, clinical, and parasitological characteristics of 150 study participants
| ACT | %, 95% CI | Placebo | %, 95% CI | Total | P value | |
|---|---|---|---|---|---|---|
| n | 75 | 50% | 75 | 50% | 150 | |
| Baseline | ||||||
| Male | 75 | 100% | 75 | 100% | 150 | 1 |
| Age (years; mean; 95% CI) | 30.6 | 28.4–32.9 | 33.1 | 30.6–35.7 | 32.9 | 0.9 |
| Weight (kg, mean; 95% CI) | 58.3 | 56.5–60.2 | 58.4 | 56.5–60.4 | 58.4 | 0.9 |
| Temperature (celsius; mean; 95% CI) | 36.2 | 36.1–36.3 | 36.2 | 36.1–36.3 | 36.2 | 0.9 |
| Fever (temp > 37.4) | 0 | 0% | 1 | 1% | 1 | 0.3 |
| Employment: farmer | 73 | 97% | 75 | 100% | 148 | 0.4 |
| uPCR positive | 18 | 24% | 12 | 16% | 30 | 0.2 |
| Pf | 8 | 11% | 4 | 5% | 12 | 0.4 |
| Pv | 7 | 9% | 2 | 3% | 9 | 0.2 |
| Pf/Pv mix | 1 | 1% | 1 | 1% | 2 | 1 |
| P spp. | 2 | 3% | 5 | 7% | 7 | 0.4 |
| RDT positive | 2 | 3% | 1 | 1% | 3 | 0.6 |
| Microscopy positive | 3 | 4% | 1 | 1% | 4 | 0.3 |
| Hb (mg/dl; mean; 95% CI) | 14.6 | 14.2–14.9 | 14.7 | 14.3–15.0 | 14.6 | 0.7 |
| Pre-forest entry | ||||||
| Fever (temp > 37.4) | 0 | 0% | 0 | 0% | 0 | 1 |
| uPCR pos | 1 | 1% | 4 | 5% | 5 | 0.4 |
| Pf | 1 | 1% | 2 | 3% | 3 | 1 |
| Pv | 0 | 0% | 0 | 0% | 0 | na |
| Pspp | 0 | 0% | 2 | 3% | 2 | 0.5 |
| RDT positive | 0 | 0% | 0 | 0% | 0 | na |
| Microscopy positive | 0 | 0% | 0 | 0% | 0 | na |
Pre-forest entry
Two out of 150 participants (1%) were found to be infected with P. falciparum after treatment and before returning to the forest (Table 3). The parasite densities of both infections were low (663, 6412/ml). Two days before returning to the forest all 150 study participants received either a 3-day course DHA/piperaquine (DP) or placebo.
Table 3.
Key data of 3 study participants infected on enrolment and 2 participants who acquired Plasmodium infections during the study period
| Participant | #107 | #124 | #006 |
|---|---|---|---|
| Baseline | |||
| Date 1 | 21/7/16 | 24/8/16 | 20/5/16 |
| Species | Pf | Pv | Not infected |
| K13 | C580Y | Not tested | |
| ExoE415G | E415G | ||
| P14 CNV | Single copy | ||
| Pfmdr1 CNV | Single copy | ||
| Parasites/ml | 22,225 | 1505 | |
| Treatment 1 | ACT + 14 days primaquine (15 mg/day) | ||
| Pre-forest entry | |||
| Date 2 | 7/8/16 | 9/10/16 | 6/6/16 |
| Species | Pf | Not infected | Pf |
| K13 | C580Y | Wild type | |
| ExoE415G | E415G | Insufficient DNA sample for testing | |
| P14 CNV | Multicopies | ||
| Pfmdr1 CNV | Single copy | Single copy | |
| Parasites/ml | 6412 | 663 | |
| Treatment 2 | ACT | ACT | Placebo |
| Forest days | 10 | 5 | 20 |
| Camp | Not known | Campsitea | Camp 8 Tram 7 |
| 15 days after return from forest | |||
| Date 3 | 6/9/16 | 4/11/16 | 12/7/16 |
| Species | Not infected | Pf | Pf |
| K13 | C580Y | C580Y | |
| ExoE415G | E415G | E415G | |
| P14 CNV | Multicopies | Multicopies | |
| Pfmdr1 CNV | Single copy | Single copy | |
| Parasites/ml | 5142 | 111,500 | |
aCamp: “campsite” refers to an overnight campsite while the rangers are moving through the forest
Post-forest visit
The 150 forest rangers stayed a total of 1523 days or a median of 9.5 days (IQR 5–10) in the forest. Fifteen days after their return from the forest the 150 participants were evaluated (Table 4). One asymptomatic participant in the treatment arm and one in the control arm (each 1.3%; 95% CI 0.03–7%; P = 1) was parasitaemic after returning from the forest. The incidence of P. falciparum infections in this population was 479/1000/year (95% CI 12–2666). Two study participants became infected while staying in the forest (Table 3). A participant in the treatment arm (#124) was uninfected prior to forest entry and had a P. falciparum parasitaemia of 5142/ml after staying in the forest for 5, 26 days after DP administration. A participant in the placebo arm (#006) had a P. falciparum parasitaemia of 111,500/ml after spending 20 days in the forest, and 36 days after DP administration. The participant was infected with wild type isolate of P. falciparum (K13 wild type; Pfmdr1 CNV single copy) prior to forest entry. When returning from forest work the participant was infected with a mutant P. falciparum isolate (PfPailin; K13 C580Y; Exo E415G; P14 CNV multi-copy).
Table 4.
Exposure and parasitaemia detected after returning from forest at the end of the study
| ACT | %, 95% CI (n = 75) | Placebo | %, 95% CI (n = 75) | Total | P value | |
|---|---|---|---|---|---|---|
| Did you use a bed net? | ||||||
| Every night | 49 | 65% | 50 | 67% | 99 | 1.0 |
| Some nights | 16 | 21% | 11 | 15% | 27 | 0.4 |
| No bed net use | 10 | 13% | 14 | 19% | 24 | 0.5 |
| Did you use insect-repellent coil? | 18 | 24% | 8 | 11% | 26 | 0.05 |
| How many people did you meet while in forest (mean; 95% CI) | 5.0 | 4.5–5.5 | 4.8 | 4.6–5.1 | 4.9 | 0.7 |
| uPCR pos—P. falciparum | 1 | 1% | 1 | 1% | 2 | 1 |
| RDT positive | 0 | 0% | 0 | 0% | 0 | na |
| Microscopy positive | 0 | 0% | 1 | 1% | 1 | 1 |
Entomology
Entomological studies were conducted between 18:00 and 06:00 in 3 camps for 3 nights in camps 2 and 79 and for 4 nights in camp 8. Indoor light traps caught 99 Anopheles mosquitoes while outdoor and indoor human landing catches caught 6 and 4 Anopheles mosquitoes, respectively (Additional file 1: Table S1). Ninety-two of the 109 mosquitoes were Anopheles dirus (84%), 15 Anopheles maculatus (14%), and 2 Anopheles barbirostris (2%). PCR found Plasmodium-related infections in 6 of the 92 An. dirus (7%) and in one of the 2 An. barbirostris (50%). Of the two participants found infected after returning from the forest only one stayed in a camp where mosquitoes had been collected (camp 8).
Discussion
This is the first study investigating malaria in forest rangers and approaches to prevent Plasmodium infections during their stay in the forest. After clearing all Plasmodium infections with a schizonticidal and hypnocytocidal drug combination (DP and 14 days PQ) all but two participants had cleared Plasmodium infections. Nearly 3 weeks later, just before returning for their next forest rotation the participating rangers received either another course of 3 doses of DP, which can provide anti-malarial prophylaxis for up to 4 weeks, or placebo. The forest rangers stayed only for a median of 9.5 days in the forest and were screened a third time 28 days after the second anti-malarial prophylaxis had started. One participant in the placebo arm was infected and one participant who had received anti-malarial prophylaxis.
Prior to the study there were no data on the prevalence and incidence of Plasmodium infections in forest rangers in Bu Gia Map National Park. Using uPCR, which is more sensitive than microscopy, RDTs or qPCR on dried blood spots, the study found P. falciparum prevalence in forest rangers was 8% and incidence in this population was 479/1000/year (95% CI 12–2666). This compares to an incidence of 332/1000/year (95% CI 253–420) among farmers in Dak O commune, also in Binh Phuoc Province, estimated in a longitudinal study also using active surveillance with uPCR in 2014 [Nguyen et al., pers. comm.]. The study did not detect any impact of the prophylaxis; one infection was detected in both the treatment and in the placebo arm. This finding could be related to the limited sample size and number of infections. Based on the malaria incidence estimate from this study, 892 participants would have to spend 14 nights each in the forest to have sufficient power (90%) for demonstrating significant protection is afforded by DP prophylaxis (Box 1).
The entomological component of the study detected three Anopheles species. The large majority were An. dirus (84%), followed by An. maculatus (14%), and An. barbirostris (2%). These findings are consistent with earlier entomological studies in Binh Phuoc province. Ngo and coworkers found 17 Anopheles species in the province amongst which An. dirus was most prevalent [35]. Similarly Trung and coworkers found in 2005 that An. dirus was most prevalent in southern Vietnam [36]. They also found that An. dirus was preferentially anthropophilic, biting outdoors before 22:00. The biting rhythm and resting behaviour of An. dirus reduces the impact of the two most commonly employed control measures, insecticide-treated bed nets and indoor residual spraying. Imaginative interventions such as supplying forest workers with insecticide treated hammocks does not address the biting rhythm and resting behaviour and had a disappointing uptake in field studies [15, 37]. In the absence of simple, effective, and affordable vector control interventions providing forest rangers with effective anti-malarial prophylaxis seems a promising alternative approach to protect forest rangers against malaria.
The study highlights the challenges of multidrug-resistant malaria to treatment and prophylaxis in the region. Treatment failures with ACT are an increasing challenge for healthcare providers treating falciparum malaria in Vietnam [3, 5]. All P. falciparum isolates evaluated at baseline carried genetic markers associated with artemisinin and resistance to the partner drug piperaquine. The long terminal half-life of piperaquine affords protection against infection with susceptible P. falciparum isolates for up to 4 weeks [38]. The presence of two P. falciparum infections after the directly observed administration of a course of DP in the first and second round is cause for concern. Three of the P. falciparum isolates carried markers for artemisinin and piperaquine resistance. The remaining 10 P. falciparum infections detected at baseline with K-13 and plasmepsin markers associated with artemisinin and piperaquine resistance had cleared after a course of DP due to the remaining therapeutic efficacy of DP or immunity. None of the P. falciparum isolates had multiple copy numbers of pfmdr1 associated with mefloquine resistance. This finding supports the notion of an inverse susceptibility between piperaquine and mefloquine and could suggest that the therapeutic efficacy of DP can be boosted by a triple therapy adding mefloquine to DP. Such a triple regimen is currently in clinical trials in the region [39]. Adapting triple therapy for prophylaxis may require co-formulation to achieve adequate adherence.
Box 1: Sample size required to estimate the protection afforded by antimalarial prophylaxis taken prior to says in the forest
2 infections acquired during 1000 nights spent in the forest.
25 infections would be needed to have sufficient power to show a 75% protection against infection during a forest visit. (20 infections in controls, 5 infections in participants receiving effective antimalarial prophylaxis).
12,500 forest nights should suffice to detect 25 infections acquired during stay in the forest.
892 participants would have to spent 14 nights each in the forest to demonstrate significant protection is afforded DP prophylaxis.
Conclusions
The findings of this study illustrate the challenges in preventing malaria in forest rangers in Bu Gia Map National Park. The impact of vector control approaches is limited by the biting behaviour of the dominant vector An. dirus and the impact of anti-malarial prophylaxis with DP is increasingly limited by the spread of anti-malarial resistance. Two populations of forest rangers, permanent and short time overlap temporarily in the park and sustain the transmission of Plasmodium infections. Breaking this cycle will require the targeting of all rangers in the forest at the same time. A mass anti-malarial administration campaign targeting all rangers in the National Park could provide a permanent solution. DP is probably no longer appropriate for such a campaign; new drug combinations will be needed. After the interruption of malaria transmission in the National Park preventing reintroduction of Plasmodium infections will remain a constant challenge until malaria is eliminated from Vietnam.
Authors’ contributions
DHS implemented the study, supervised the data collection and analysed the data. NT-N carried out data collection, laboratory work and contributed to the writing of the paper. LvS contributed to the design of the study, statistical analysis and the writing of the paper. TLPN performed the laboratory work. NTP, NHT, NVD, and BVQ contributed to the data collection. NTKT performed the laboratory work. NPJD, AMD and NJW contributed to the design of the study and the writing of the paper. GET contributed to the design of the study, revised and edited the paper. TTH contributed to the design of the study, supervised the implementation, obtained funding, contributed to the writing and revisions of the paper. All authors read and approved the final manuscript.
Acknowledgements
We thank the forest rangers for their participation and support of the study. We thank our colleagues at OUCRU and MORU for their suggestions and encouragement.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All the findings of this study are reported in the manuscript. The patient information file is available in institutional database at Oxford University Clinical Research Unit Vietnam (OUCRU). This contains a unique identification code and patient personal details. The datasets generated and analysed are not publicly available as the OUCRU policy does not permit this but the dataset would be available on reasonable request.
Consent for publication
All authors are reviewed the final version of the paper and have consented to the publication.
Ethics approval and consent to participate
Individual written consent was obtained from all trial participants. The protocol, informed consent documents, relevant supporting information and all patient recruitment information were approved by the Ethics Committee of Binh Phuoc Provincial Department of Health and the Oxford University Tropical Research Ethics Committee. The trial was registered NCT02788864.
Funding
The study was funded by the Wellcome Trust (B9R00770 to Tran Tinh Hien).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Table S1. Entomological data.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12936-017-2091-6) contains supplementary material, which is available to authorized users.
Contributor Information
Do Hung Son, Email: sondh@oucru.org.
Nguyen Thuy-Nhien, Email: nhienntt@oucru.org.
Lorenz von Seidlein, Email: Lorenz@tropmedres.ac.
Truong Le Phuc-Nhi, Email: nhitlp@oucru.org.
Ngo Thi Phu, Email: ngothiphu@yahoo.com.
Nguyen Thi Kim Tuyen, Email: tuyenntk@oucru.org.
Nguyen Huyen Tran, Email: trannh@oucru.org.
Nguyen Van Dung, Email: nguyendungsrbp@gmail.com.
Bui Van Quan, Email: bsquanbp@gmail.com.
Nicholas P. J. Day, Email: Nickd@tropmedres.ac
Arjen M. Dondorp, Email: arjen@tropmedres.ac
Nicholas J. White, Email: nickwdt@tropmedres.ac
Guy E. Thwaites, Email: gthwaites@oucru.org
Tran Tinh Hien, Email: hientt@oucru.org.
References
- 1.National Institute of Malariology, Parasitology and Entomology. Malaria Report Vietnam 2015. Hanoi; 2016.
- 2.Imwong M, Suwannasin K, Kunasol C, Sutawong K, Mayxay M, Rekol H, et al. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong Subregion: a molecular epidemiology observational study. Lancet Infect Dis. 2017;17:491–497. doi: 10.1016/S1473-3099(17)30048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thanh NV, Thuy-Nhien N, Tuyen NT, Tong NT, Nha-Ca NT, Dong LT, et al. Rapid decline in the susceptibility of Plasmodium falciparum to dihydroartemisinin-piperaquine in the south of Vietnam. Malar J. 2017;16:27. doi: 10.1186/s12936-017-1680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MalariaGEN Plasmodium falciparum Community Project Genomic epidemiology of artemisinin resistant malaria. Elife. 2016;5:e08714. doi: 10.7554/eLife.08714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Imwong M, Hien TT, Thuy-Nhien NT, Dondorp AM, White N. Spread of a single multidrug resistant malaria parasite lineage (PfPailin) to Vietnam. Lancet Inf Dis. 2017;17:1022–1023. doi: 10.1016/S1473-3099(17)30524-8. [DOI] [PubMed] [Google Scholar]
- 6.Phyo AP, von Seidlein L. Challenges to replace ACT as first-line drug. Malar J. 2017;16:296. doi: 10.1186/s12936-017-1942-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guerra CA, Snow RW, Hay SI. A global assessment of closed forests, deforestation and malaria risk. Ann Trop Med Parasitol. 2006;100:189–204. doi: 10.1179/136485906X91512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thanh PV, Hong NV, Van Van N, Van Malderen C, Obsomer V, Rosanas-Urgell A, et al. Epidemiology of forest malaria in Central Vietnam: the hidden parasite reservoir. Malar J. 2015;14:86. doi: 10.1186/s12936-015-0601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanh NH, Van Dung N, Thanh NX, Trung TN, Van Co T, Cooper RD. Forest malaria in central Vietnam. Am J Trop Med Hyg. 2008;79:652–654. [PubMed] [Google Scholar]
- 10.Erhart A, Ngo DT, Phan VK, Ta TT, Van Overmeir C, Speybroeck N, et al. Epidemiology of forest malaria in central Vietnam: a large scale cross-sectional survey. Malar J. 2005;4:58. doi: 10.1186/1475-2875-4-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thang ND, Erhart A, Speybroeck N, le Hung X, le Thuan K, Hung CT, et al. Malaria in central Vietnam: analysis of risk factors by multivariate analysis and classification tree models. Malar J. 2008;7:28. doi: 10.1186/1475-2875-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erhart A, Thang ND, Hung NQ, le Toi V, le Hung X, Tuy TQ, et al. Forest malaria in Vietnam: a challenge for control. Am J Trop Med Hyg. 2004;70:110–118. [PubMed] [Google Scholar]
- 13.Gryseels C, Peeters Grietens K, Dierickx S, Xuan XN, Uk S, Bannister-Tyrrell M, et al. High mobility and low use of malaria preventive measures among the Jarai male youth along the Cambodia–Vietnam border. Am J Trop Med Hyg. 2015;93:810–818. doi: 10.4269/ajtmh.15-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lengeler C. Insecticide-treated bednets and curtains for preventing malaria. Cochrane Database Syst Rev. 2000;2:CD000363. doi: 10.1002/14651858.CD000363. [DOI] [PubMed] [Google Scholar]
- 15.Thang ND, Erhart A, Speybroeck N, Xa NX, Thanh NN, Ky PV, et al. Long-lasting insecticidal hammocks for controlling forest malaria: a community-based trial in a rural area of central Vietnam. PLoS ONE. 2009;4:e7369. doi: 10.1371/journal.pone.0007369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.http://vietnamnationalpark.org/2819/bu-gia-map-national-park/.
- 17.Imwong M, Hanchana S, Malleret B, Renia L, Day NP, Dondorp A, et al. High throughput ultra-sensitive molecular techniques to quantify low density malaria parasitaemias. J Clin Microbiol. 2014;9:3003–3009. doi: 10.1128/JCM.01057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamau E, Tolbert LS, Kortepeter L, Pratt M, Nyakoe N, Muringo L, et al. Development of a highly sensitive genus-specific quantitative reverse transcriptase real-time PCR assay for detection and quantitation of Plasmodium by amplifying RNA and DNA of the 18S rRNA genes. J Clin Microbiol. 2011;49:2946–2953. doi: 10.1128/JCM.00276-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imwong M, Hanchana S, Malleret B, Rénia L, Day NP, Dondorp A, et al. High throughput ultra-sensitive molecular techniques to quantity low density malaria parasitaemias. J Clin Microbiol. 2014;52:3303–3309. doi: 10.1128/JCM.01057-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imwong M, Snounou G, Pukrittayakamee S, Tanomsing N, Kim JR, Nandy A, et al. Relapses of Plasmodium vivax infection usually result from activation of heterologous hypnozoites. J Infect Dis. 2007;95:927–933. doi: 10.1086/512241. [DOI] [PubMed] [Google Scholar]
- 21.Snounou G. Detection and identification of the four malaria parasite species infecting humans by PCR amplification. Methods Mol Biol. 1996;50:263–291. doi: 10.1385/0-89603-323-6:263. [DOI] [PubMed] [Google Scholar]
- 22.WHO . Methods and techniques for clinical trial on antimalarial drug efficacy: genotyping to identify parasite populations. Geneva: World Health Organization; 2007. [Google Scholar]
- 23.Thuy-Nhien N, Tuyen NK, Tong NT, Vy NT, Thanh NV, Van HT, et al. K13 propeller mutations in Plasmodium falciparum populations in regions of malaria endemicity in Vietnam from 2009 to 2016. Antimicrob Agents Chemother. 2017;61:e01578–e015616. doi: 10.1128/AAC.01578-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amato R, Lim P, Miotto O, Amaratunga C, Dek D, Pearson RD, et al. Genetic markers associated with dihydroartemisinin–piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype-phenotype association study. Lancet Infect Dis. 2017;17:164–173. doi: 10.1016/S1473-3099(16)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, Phaipun L, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odetoyinbo JA. Preliminary investigation on the use of a light-trap for sampling malaria vectors in the Gambia. Bull World Health Organ. 1969;40:547–560. [PMC free article] [PubMed] [Google Scholar]
- 27.Rattanarithikul R, Panthusiri P. Illustrated keys to the medically important mosquitos of Thailand. Southeast Asian J Trop Med Public Health. 1994;25(Suppl 1):1–66. [PubMed] [Google Scholar]
- 28.Rattanarithikul R, Harrison BA, Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand I. Background; geographic distribution; lists of genera, subgenera, and species; and a key to the genera. Southeast Asian J Trop Med Public Health. 2005;36(Suppl 1):1–80. [PubMed] [Google Scholar]
- 29.Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Jones JW, Coleman RE. Illustrated keys to the mosquitoes of Thailand. II. Genera Culex and Lutzia. Southeast Asian J Trop Med Public Health. 2005;36(Suppl 2):1–97. [PubMed] [Google Scholar]
- 30.Rattanarithikul R, Harrison BA, Panthusiri P, Peyton EL, Coleman RE. Illustrated keys to the mosquitoes of Thailand III. Genera Aedeomyia, Ficalbia, Mimomyia, Hodgesia, Coquillettidia, Mansonia, and Uranotaenia. Southeast Asian J Trop Med Public Health. 2006;37(Suppl 1):1–85. [PubMed] [Google Scholar]
- 31.Rattanarithikul R, Harrison BA, Harbach RE, Panthusiri P, Coleman RE, Panthusiri P. Illustrated keys to the mosquitoes of Thailand. IV. Anopheles. Southeast Asian J Trop Med Public Health. 2006;37(Suppl 2):1–128. [PubMed] [Google Scholar]
- 32.Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Coleman RE, Richardson JH. Illustrated keys to the mosquitoes of Thailand. VI. Tribe Aedini. Southeast Asian J Trop Med Public Health. 2010;41(Suppl 1):1–225. [PubMed] [Google Scholar]
- 33.Rattanarithikul R, Harbach RE, Harrison BA, Panthusiri P, Coleman RE. Illustrated keys to the mosquitoes of Thailand V. Genera Orthopodomyia, Kimia, Malaya, Topomyia, Tripteroides, and Toxorhynchites. Southeast Asian J Trop Med Public Health. 2007;38(Suppl 2):1–65. [PubMed] [Google Scholar]
- 34.Cassiano GC, Storti-Melo LM, Povoa MM, Galardo AK, Rossit AR, Machado RL. Development of PCR-RFLP assay for the discrimination of Plasmodium species and variants of P. vivax (VK210, VK247 and P. vivax-like) in Anopheles mosquitoes. Acta Trop. 2011;118:118–122. doi: 10.1016/j.actatropica.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Ngo CT, Dubois G, Sinou V, Parzy D, Le HQ, Harbach RE, et al. Diversity of Anopheles mosquitoes in Binh Phuoc and Dak Nong Provinces of Vietnam and their relation to disease. Parasit Vectors. 2014;7:316. doi: 10.1186/1756-3305-7-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trung HD, Bortel WV, Sochantha T, Keokenchanh K, Briet OJ, Coosemans M. Behavioural heterogeneity of Anopheles species in ecologically different localities in Southeast Asia: a challenge for vector control. Trop Med Int Health. 2005;10:251–262. doi: 10.1111/j.1365-3156.2004.01378.x. [DOI] [PubMed] [Google Scholar]
- 37.Grietens KP, Xuan XN, Ribera J, Duc TN, Bortel W, Ba NT, Van KP, et al. Social determinants of long lasting insecticidal hammock use among the Ra-glai ethnic minority in Vietnam: implications for forest malaria control. PLoS ONE. 2012;7:e29991. doi: 10.1371/journal.pone.0029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanachayangkul P, Lon C, Spring M, Sok S, Ta-Aksorn W, Kodchakorn C, et al. Piperaquine population pharmacokinetics and cardiac safety in Cambodia. Antimicrob Agents Chemother. 2017;61:e02000–e02016. doi: 10.1128/AAC.02000-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.clinicaltrials.gov. A Study by the Tracking Resistance to Artemisinin Collaboration (TRAC) (TRACII). 2017. https://www.clinicaltrialsgov/ct2/show/NCT02453308?term=Tracking+Resistance+to+Artemisinin+%28TRAC%29&rank=2.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the findings of this study are reported in the manuscript. The patient information file is available in institutional database at Oxford University Clinical Research Unit Vietnam (OUCRU). This contains a unique identification code and patient personal details. The datasets generated and analysed are not publicly available as the OUCRU policy does not permit this but the dataset would be available on reasonable request.


