Abstract
Peripheral neuropathy and nerve compression syndromes lead to substantial morbidity following burn injury. Patients present with pain, paresthesias, or weakness along a specific nerve distribution or experience generalized peripheral neuropathy. The symptoms may manifest at various times from within one week of hospitalization to many months after wound closure. While current treatments require surgical release of entrapped or compressed nerves, additional studies are necessary to develop therapies for peripheral neuropathy when no detectable signs of nerve compression are present. Studies have shown that peripheral neuropathy may also be due to vascular occlusion of vasa nervorum, inflammation, neurotoxin production leading to apoptosis, and direct destruction of nerves from the burn injury. A better understanding of the molecular and cellular mechanisms underlying the pathogenesis of neuropathic pain following burn injury is essential to the development of novel interventions. Early and effective treatment to minimize the long-term sequela of peripheral neuropathy and nerve compression syndromes will lead to improved outcomes. In this review, we discuss the natural history, diagnosis, current treatments, and future directions for potential interventions for peripheral neuropathy and nerve compression syndromes as they relate to burn injury.
Keywords: Nerve compression syndromes, Peripheral neuropathy
Introduction
Peripheral neuropathy and nerve compression syndromes lead to post-burn morbidity that can often be difficult to recognize and manage. Entrapment or compression of the peripheral nerves is associated with common symptoms of pain, weakness, and paresthesia, often requiring acute decompressive fasciotomies or escharotomies. Muscle wasting and weakness are late symptoms of nerve compression that yield distinct symptoms based on the nerve affected. Post-burn peripheral neuropathy and nerve compression syndromes can also be present in a delayed fashion by the formation of scar tissue or heterotopic bone.1 Heterotopic ossification (HO) is the formation of new bone in non-osseous tissue. While HO is a rare but well-known complication of burns, it results in decreased range of motion, painful and/or swollen joints, or nerve deficits. It most commonly involves the elbow joint, often leading to symptoms of ulnar nerve compression, regardless of the location of the burn.2 Irrespective of the cause, the development of peripheral neuropathy or nerve compression syndromes is often recognized late after the burn injury and results in substantial impairment on the activities of daily living in patients who may already be functionally limited by their burns. Thus, post-burn peripheral neuropathy and nerve compression syndromes remain an unmet challenge that requires adequate diagnosis and treatment in a timely fashion.
Epidemiology
The association between peripheral neuropathy and burns has been found to vary widely from 2% to 52% of patients depending on the study methodology.3–10 Studies surveying burn patients with electrodiagnostic evaluations and clinical assessments demonstrated higher rates of peripheral neuropathy in the range of 11 to 52% (Table 1).3–6 The incidence of neuropathy after burn injury was first well described by Henderson and colleagues in 1971.3 Of the 249 hospitalized burn patients who underwent electrodiagnostic testing, 44 patients demonstrated conduction slowing in two or more nerves.3 In 1977, a prospective study analyzing a cohort of burn patients, the authors found peripheral neuropathy in 24 of the 66 patients (36%) with clinical evidence of weakness.4 Helm and colleagues evaluated an additional 88 burn patients and confirmed peripheral neuropathy with electrodiagnostic testing in 46 patients (52%).5 More recently, Kowalske et al. found the incidence of mononeuropathy and/or generalized peripheral neuropathy totaled 11%.6 The large variability in the incidence in these prospective studies is likely attributed to the significant variability in total body surface area (TBSA) injured and depth of the burn, as more severe burns have been shown to correlate with a higher incidence of peripheral neuropathy.
Table 1.
Prevalence of peripheral neuropathy following burn injury.
| Study | Year | Study Type | Findings |
|---|---|---|---|
| Margherita et al. | 1995 | Prospective | At 6 weeks, peripheral neuropathy persistent in 27% of patients. |
|
| |||
| Hayes et al. | 2002 | Prospective | After 6 months, 78% of patients still had peripheral neuropathy. |
| After 12 months, 56% of patients still had peripheral neuropathy. | |||
|
| |||
| Schneider et al. | 2006 | Retrospective | Of the patients with peripheral neuropathy, improvement in symptoms was noted starting around 7 ± 0.8 months. |
|
| |||
| Wu et al. | 2013 | Retrospective | The majority of the patients reported subjective improvement of symptoms (82%), while 18% of patients showed no improvement by 4 months. |
In contrast, studies that rely on clinical diagnosis followed by electrodiagnostic testing, including nerve conduction studies, demonstrated much lower incidence, ranging from 2 to 7% (Table 1). In these studies, Dagum et al. found that 9 out of the 121 burn patients (7.4%) analyzed were found to have severe peripheral neuropathy.7 In 1993, Marquez et al. performed a retrospective study that demonstrated peripheral neuropathy in 19 out of 800 patients (2%).8 The patients studied in this retrospective study were referred to a tertiary center for electrodiagnostic testing following initial complaints of neuropathic pain. Additional retrospective studies performed by Lee et al. and Tamam et al. demonstrated an incidence of 4% and 7% respectively.9,10 The incidence in these retrospective studies may be lower as they rely on clinical diagnosis, which may not be as sensitive as electrodiagnostic studies.
The exact quantification of the true incidence of peripheral neuropathy remains a challenge as it requires diagnostic testing at the time of injury and at different intervals during healing, which is time consuming, expensive, and may be uncomfortable for the patient. Additional variables such as TBSA burned and depth of burn injury adds an additional layer of difficulty in assessing the true incidence. Furthermore, neuropathic pain may not be clinically evident due to the comprehensive pain control regimens administered to the burn patients.
Onset of disease
Neuropathic pain following burn injuries has been reported to develop as early as the first week of hospitalization.11–14 However, other reports have demonstrated nerve compression syndromes developing between 50 and 130 days, following the initial injury.15,16 Marquez and colleagues postulated that the delay in diagnosis may be related to several factors including the severity of their medical condition initially, the level of sensitivity to neuropathic pain as the patient recovers from the original burn injury, and the increasing pain experienced when the patient begins to return to their activities of daily living.8 The second argument is that the compression neuropathy is progressive.6,8 Together, identifying neuropathic pain is essential in the early stages in order to appropriately treat patients and minimize the sequela of long-term pain.
Mononeuropathy, mononeuropathy multiplex, and polyneuropathy
Several factors have been associated with a significantly higher incidence of peripheral neuropathy after burn injury (Table 2). These factors include age above 20 years, injury resulting in full thickness burn wounds, and burns involving a surface area of more than 20%.6,17 The type of peripheral neuropathy experienced by burn patients have been further divided into mononeuropathy, mononeuropathy multiplex, and polyneuropathy. The development of the type of neuropathy has been shown to be related to the mechanism of injury, percent of TBSA injured, and percent of full thickness burn.
Table 2.
Risk factors for development of peripheral neuropathy.
| Study | Year | Study Type | No. with peripheral neuropathy (%) |
Diagnostic criteria | Findings |
|---|---|---|---|---|---|
| Henderson et al. | 1971 | Prospective | 36 of 249 (15%) | EMG/NCS | Polyneuropathy was more common in burns over 20% TBSA. |
|
| |||||
| Marquez et al. | 1993 | Retrospective | 19 of 800 (2%) | Clinical assessment followed by EMG/NCS | Most patients with peripheral neuropathy had multiple nerves affected (three or more). |
| Of the patients that presented with peripheral neuropathy, 69% were severely burned with TBSA greater than 20%. The number of nerves affected correlated with the full thickness burn area. | |||||
|
| |||||
| Khedr et al. | 1997 | Prospective | 16 of 55 (29%) | EMG/NCS followed by clinical assessment | Mononeuropathy multiplex was diagnosed in 56% of patients. Generalized peripheral neuropathy was noted in 31% of patients. |
| Higher prevalence of neuropathy associated with age > 20, burns involving full thickness wounds, and TBSA > 20%. | |||||
|
| |||||
| Kowlske et al. | 2001 | Retrospective | 64 of 572 (11%) | Clinical assessment | Of the patients that presented with peripheral neuropathy, 56 patients (88%) had mononeuropathy, while 18 patients (28%) had polyneuropathy. Of these patients, 10 (16%) had both mononeuropathy and polyneuropathy. |
| Higher prevalence of neuropathy associated with severe burn injury in patients who were older (>40 years), critically ill, had an electrical injury, or a history of alcohol abuse. | |||||
|
| |||||
| Lee et al. | 2009 | Retrospective | 35 of 868 (4%) | Clinical assessment followed by EMG/NCS | Flame injuries and full thickness burn injuries were most common in patients with peripheral neuropathy. |
|
| |||||
| Tamam et al. | 2013 | Retrospective | 47 of 648 (7%) | Clinical assessment followed by EMG/NCS | Of the patients with peripheral neuropathy, 68% of patients had mononeuropathy while 42% had polyneuropathy. |
| Most frequent etiology of mononeuropathy was low-voltage electrical injury (<1000V) (50%). | |||||
EMG, electromyography; NCS, nerve conduction study.
Mononeuropathy is characterized as weakness and sensory loss that fits a pattern of a specific peripheral nerve distribution. Marquez and colleagues demonstrated that the incidence of isolated mononeuropathy was 19%.8 Isolated mononeuropathy is caused by local factors injuring the nerve. Damage to individual nerves or nerve fascicles has been shown to occur iatrogenically through escharotomy, fasciotomy, or multiple intramuscular injections.18 Injury can also occur from compression by circumferential burns, bulky tight dressings, or HO.19 Faulty positioning during splinting, forceful exercise, or skeletal suspension have also been shown to cause mononeuropathy.19 Therefore, it is essential to be meticulous when performing these procedures to minimize the iatrogenic causes intra-operatively and post-operatively.
Electrical burns have also been associated with the development of mononeuropathy. Electrical burns cause the highest incidence of nerve injury, as electricity follows the path of least resistance with nerves having the least resistance followed by blood vessels, muscle, skin, tendon, fat, and bone (in order of increasing resistance).20 The heat generated by electrical current can cause direct nerve injury, scar tissue formation around nerves, and neuropathy from post-injury tissue edema.6,21 Tamam et al. found that 90% of patients with electrical injury presented with mononeuropathy.10 In a 17-year review of burn unit admissions, permanent nerve injuries were found in 22% of electrocuted patients. The upper limb was most commonly involved, with the median and ulnar nerves most commonly injured.22
Mononeuropathy multiplex is the simultaneous malfunction of two or more peripheral nerves in separate areas of the body. Mononeuropathy multiplex has been reported as the most common type of peripheral neuropathy, occurring in 56% to 69% of burn patients.8,17 Mononeuropathy multiplex has been shown to be more common in patients who sustained burns involving more than 20% of the TBSA and having greater than 15% full thickness burns.8,17 Patients with more extensive burns were more likely to develop mononeuropathy multiplex.
In contrast, polyneuropathy or generalized peripheral neuropathy is characterized by distal sensory loss and weakness in a symmetrical pattern and is least common among burn patients. In the case of severe burns, the prevalence of peripheral neuropathy has not been shown to exceed 20%. Risk factors for the development of polyneuropathy following burn injury include older age (>40 years) and length of stay in the ICU for an extended period of time (>20 days)6. Interestingly, studies have found that symptoms of peripheral neuropathy often gradually decreased within a few months after the injury.5
Mechanism
Pain is processed in a neuronal network, and the interaction between neurons, microglia, and astrocytes is critical for the initiation and maintenance of chronic pain. Activation of glia cells, such as astrocytes and microglia, contributes to that pathogenesis of chronic pain through the interactions between glial cells and neurons.23–26 The activation of astrocytes results in the upregulation of nuclear factor-kappa B, extracellular regulated kinase and Jun N-terminal kinase signaling pathways, while the activation of spinal microglial results in the activation of p38 mitogen-activated protein kinase.27–33 The activation of these key signaling factors in astrocytes and microglial cells produce pro-inflammatory cytokines including TNF-α, interleukin-1 beta (IL-1β), nitric oxide, prostaglandin, and neurotropins following burn injury.25,26 These inflammatory mediators in turn increase cyclooxygenase-2 (COX-2) activity, causing sensitization of peripheral nociceptors and generation of chronic pain.34,35
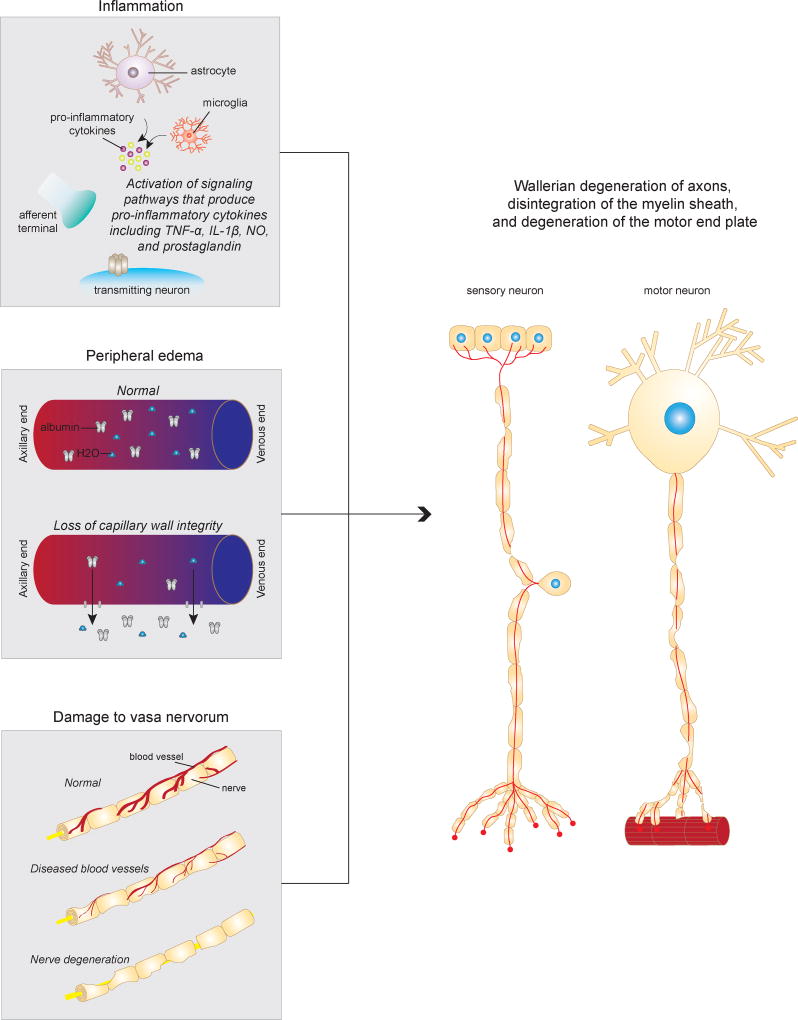
With respect to the reduction in motor and sensory conduction velocity following burn injury, several animal models have been instrumental in our understanding of the pathophysiology (Figure 1). In rats, histological evaluation of nerve fibers following burn injury have shown Wallerian degeneration of axons, disintegration of the myelin sheath, and degeneration of the motor end plate.36,37 A decrease in the caliber of large axons have been demonstrated with histological studies.36,37 Furthermore, increased platelet aggregation, accelerated fibrin deposition, and clot formation have been shown to occur following burn injury, leading to vascular occlusion of the vasa nervorum.38,39 Additional studies have demonstrated that cutaneous burns induce the release of large molecules from damaged epidermal and dermal cells, which increases interstitial oncotic pressure and stimulates fluid loss leading to edema formation.40,41 Furthermore, damaged cells secrete inflammatory cytokines that have been shown to activate inflammatory response pathways. Together, these local and systemic reactions have been postulated to affect changes in the conduction velocity of peripheral nerves leading to neuropathy in the short term and long term.38–41
Figure 1.
Potential mechanism of peripheral neuropathy following burn injury. Damaged cells release inflammatory cytokines that likely active the inflammatory response pathways. Large molecules from damaged cells increases interstitial oncotic pressure and stimulates fluid loss, leading to edema. The release of cytokines and chemokines due to burn injury also results in platelet aggregation, accelerated fibrin deposition, and clot formation follow burn injury, which can lead to vascular occlusion of the vasa nervorum. The cumulative effect is Wallerian degeneration of axons, disintegration of the myelin sheath, and degeneration of the motor end plate.
In addition to the local and systemic reactions that patients may experience from the burn injury, other patients may simply experience local symptoms of neuropathy in regions that are completely distinct and remotely separated from the site of the burn. In 1979, Sepulchre et al. postulated that circulating burn neurotoxin can lead to neuropathy at sites remote from the original burned area.42 Monafo and Eliasson further proved these findings in a thermal injury rat model. Burn injury led to the accumulation of neurotoxic factors that lead to progressive conduction block and ultimately led to peripheral neuropathy at sites distant to the burn.43 Additional animal models have shown that burns as small as 4% can affect nerves distant to the burn. Following a burn injury, inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) are released and have been shown to mediate systemic effects on nociception by altering the structural integrity of nociceptive fibers.44 Thus, the systemic effects have been postulated to result in neuropathy at distant sites.
Current Treatments and Future Directions
Peripheral Neuropathy
Currently, the gold standard for pain treatment is opioids for neuropathic pain. Morphine has been used since the 19th century and is today one of the most widely used treatments for burn pain, neuropathic pain, and inflammatory pain in both animal models and in humans45–47,48,49. A variety of other opioid-based options exist for the treatment of burn pain with varying degrees of potency and side effects. Fentanyl has been shown to effectively attenuate neuropathic pain in humans.50 Non-opioid based medications have also been used, including gabapentin, to reduce pain and minimize anxiety related to the burn injury. Combination therapy with morphine and gabapentin confers increased analgesia at lower dosages than using either drug alone.51 In addition, this combination also results in overall decreased opioid utilization and sensitization in burn patients. In a majority of cases, resolution of pain is observed 13 months post-burn. However, 40% of burn patients continue to have neuropathic pain many months following the original injury, indicating a need for further investigation to identify alternative treatment modalities other than the currently used medications. Current methods to manage peripheral neuropathy pain are designed to control symptoms, but these methods are unable to eliminate neuropathic pain in these patients.
Recently, autologous fat grafting has gained significant attention due its regenerative properties to promote wound healing and its capacity to alleviate burn-induced neuropathic pain.52,53 The mechanism of fat grafting involves a combination of mechanical and endocrine effects. The mechanical effects release fibrotic tissue and cushion sensory nerves in and near the zone of injury, while the endocrine effects release growth factors and cytokines from stem cells and mature adipocytes to promote regeneration.54 The ultimate result is architectural remodeling of the scar bed, with regeneration of an improved interface between normal and abnormal tissue. In a rat burn model, autologous fat grafting significantly alleviated the burn-induced scarring lessening the mechanical allodynia.55 Furthermore, fat grafting was found to improve neuropathic pain through the secretion of anti-inflammatory factors, reducing COX-2, inducible nitric oxide synthase, IL-1β, and TNF-α in the spinal cord dorsal horns. This reduction in pro-inflammatory cytokines further reduced apoptosis within the spinal cord dorsal horn following delivery of fat grafts.56 However, additional large scale double-blinded clinical trials are necessary to demonstrate the efficacy of autologous fat grafting compared to the current standard of care.57
Other less invasive interventions including transcranial direct current stimulation (tDCS) and somatosensory rehabilitation have been proposed, but these interventions also require additional larger scale studies before they can be translated into clinical practice. Chronic neuropathic pain has been associated with defective inhibition of the motor cortex, as indexed by decreased intracortical inhibition.58,59 In a case series, three patients with chronic neuropathic pain following burn injury were randomly assigned to undergo a single session of transcranial direct current stimulation (tDCS).60 The principles of tDCS consist of applying a weak, constant, and direct current over the scalp to neuromodulate the motor cortex. While the single session of tDCS did not change clinical outcomes, an overall increase in intracortical inhibition was observed.60 In contrast, somatosensory rehabilitation has been proposed to recondition the patient to recognize normal sensation.61 Neuropathic pain has been described as a complication or misperception of sensory experiences perceived by individuals who have sustained damage to their nervous system, and in the case of burn patients, primarily their peripheral nervous system. In a case series, the majority of patients (76%) showed substantial improvement after somatosensory rehabilitation.61 These studies highlight not only the potential efficacy of less invasive interventions but also highlight the potential for combination therapy to treat neuropathy after burn injury.
Ulnar Nerve, Radial Nerve, and Median Nerve Decompression
For nerve compression or entrapment, early wound excision has been shown to prevent severe nerve injury, and proper supportive care in acute settings has significantly reduced the possibility of long term neurologic complication.37 Sheridan et al. reviewed 659 patients and found that of the patients with upper extremity nerve compressions or entrapment who required surgery, 81% ultimately regained normal function of the hand when they were treated aggressively and timely.62 Unfortunately, it can often be extremely difficult to make the diagnosis of compressive neuropathy or determine the cause of neuropathy in critically ill patients.
The nerve most commonly compressed following burn injury is the ulnar nerve at the level of the elbow. Paresthesia and hypesthesias can be noted in the distribution of the ulnar nerve and in severe cases, it can result in sensory loss in the small finger and the ulnar half of the ring finger. The ulnar nerve originates from the medial cord of the brachial plexus and travels distally and posteriorly, accompanied by the superior ulnar collateral artery, and passes through the medial intermuscular septum. Eight centimeters proximal to the medial epicondyle, the ulnar nerve crosses the arcade of Struthers, a fascial layer that extends from the medial head of the triceps to the medial intermuscular septum. As the ulnar nerve enters the cubital tunnel, the ulnar nerve is bordered by the medial epicondyle anteriorly, the ulnohumeral ligament laterally, and Osborne’s fascia posteriorly.
With ulnar nerve compression, a submuscular transposition with Z-lengthening of the flexor pronator muscle at its origin provides a spacious new tunnel for the ulnar nerve. With the patient positioned supine, the shoulder abducted and externally rotated, and the elbow extended, an 8-cm C-shaped incision should be made on the anteromedial aspect of the elbow anterior to the medial epicondyle. The incision should extend through the skin and subcutaneous tissue. Reflecting the skin flaps, the medial antebrachial cutaneous nerve can be identified and protected. At this point, the ulnar nerve should be located in the forearm posterior to the medial intermuscular septum proximally and followed behind the medial epicondyle distally. For nerve decompression and transposition, the arcade of Struthers, the medial intermuscular septum, the Osborne’s fascia, and the aponeurosis between the two heads of the flexor carpi ulnaris (FCU) should be divided. Nerve mobilization should be carried out at least 8 cm proximal and 5 cm distal to the medial epicondyle. A large hemostat can be passed beneath the flexor pronator origin, allowing the common flexor pronator aponeurosis to become more apparent. The common flexor pronator aponeurosis should be divided, allowing the nerve to be transposed anteriorly. The tendon should be repaired with non-absorbable sutures, forming of a new spacious tunnel without any compression. The subcutaneous tissue should be closed and the skin edges should be reapproximated. In burn patients with ulnar nerve compression at the level of the elbow, early recognition and surgical treatment is critical to address the acute symptoms and to prevent the long term sequelae.
The radial nerve has also been shown to be compressed in select cases. Patients generally present with deep pain over the radial tunnel, typically 5 cm distal to the lateral epicondyle, posterior to the brachioradialis (BR). Local anesthetic can aid in the diagnosis of radial nerve compression if there is uncertainty of which nerve is involved, as paralysis of the radially innervated muscles and relief of pain should follow after successful radial nerve block. The radial nerve arises from the posterior cord of the brachial plexus. It enters the forearm anterior to the lateral epicondyle. The radial nerve splits into a superficial, sensory branch and a deep, motor branch. The superficial branch lies under the BR and emerges between the BR and extensor carpi radialis longus (ECRL) tendons in the subcutaneous plane 7 cm proximal to the wrist. The deep motor nerve comes off of the main trunk prior to its division. The most common site of compression is at the proximal edge of the superficial portion of the supinator muscle where the supinator muscle forms a fibrous arch known as the arcade of Frohse. There are also crossing vessels proximal to the arcade of Frohse called the leash of Henry that may compress the radial nerve. The radial tunnel is 5 cm in length and is bordered by the biceps tendon medially and the ECRL, extensor carpi radialis brevis (ECRB), and BR located laterally. The floor of the tunnel is composed of the radial-capitellar joint capsule, and the BR forms part of the roof that crosses laterally to anteriorly. The radiohumeral joint makes the most proximal portion of the radial tunnel and ends just distal to the arcade of the Froshe. Within the radial tunnel, there are four potential sites for compression: fibrous bands lying anterior to the radial head, a fan shaped leash of vessels, the tendinous margin of the ECRB, and the arcade of Frohse.
The excision of the potential compression sites in the radial tunnel is essential to release the radial nerve. Begin by identifying the BR and ECRL preoperatively by having the patient flex at the elbow with the forearm in a neutral position. This maneuver will accentuate the BR muscle. The groove between the BR and ECRL is marked and should be identified through an 8 cm incision. The a vascular plane between the BR and ECRL should be used to expose the radial tunnel and lateral epicondyle. After splitting the interval between the BR and ECRL, place retractors to expose the underlying supinator muscle. The edge of the arcade of Frohse is identified and resected. Identify the crossing vessels (leash of Henry) and ligate these vessels to remove a potential source of compression on the radial nerve. The interval between the BR and ECRL is closed with absorbable sutures. No drains are necessary post-operatively, but the patient should be placed in a soft dressing.
In severe traumas, carpal tunnel releases may be necessary to release the median nerve from entrapment. Open carpal tunnel release remains the standard treatment, as it allows for direct visualization of anatomical structures and adequate decompression of the median nerve. The palmar cutaneous branch of the median nerve is located 2 mm radial to the thenar crease or 5 mm radial to the interthecal depression. This nerve originates 8 cm proximal to the wrist crease, courses immediately ulnar to the flexor carpi radialis (FCR) tendon, and terminates 4.5 cm distal to the wrist crease. The recurrent motor branch of the median nerve is also frequently located radially. Care should be taken to avoid injury to these two branches of the median nerve as sensory and motor deficits will be noted. The carpal canal is a rigid, confined, unyielding fibro-osseous space that contains nine flexor tendons and the median nerve. The canal has a floor that is made up of a concave arch of carpal bones and carpometacarpal joints with their overlying ligament. The roof of the canal is the transverse carpal ligament (TCL).
To release the median nerve within the carpal tunnel, a longitudinal, slightly curved incision parallel to the thenar crease can be used. With the patient in a supine position, a tourniquet can be applied to the proximal arm. Local anesthetic consisting of 1% lidocaine combined with intravenous sedation should be injected for anesthesia. General anesthetic is rarely needed but can be used upon the patient’s request. The incision should be placed in a safe zone, which is typically 5 mm ulnar to the interthecal depression, in line with the third web space. This incision reduces the incidence of injury to the palmar cutaneous branch of the median nerve. The incision should be extended further distally and proximal to the wrist crease into the distal forearm and through the skin and subcutaneous tissue, revealing the longitudinal fibers of the palmar fascia. The palmar fascia should be identified and divided longitudinally in line with the ulnar border of the palmaris longus, exposing the deeper TCL. The proximal portion of the flexor retinaculum should now be exposed up to 2 cm proximal to the wrist crease in the forearm and the TCL should be exposed. A longitudinal incision should be made in the proximal flexor retinaculum, using caution to protect the median nerve immediately deep to the retinaculum. Using iris scissors and with the median nerve under direct visualization and protected, the flexor retinaculum should be released as far proximally as possible. The release should then be completed distally to the level of the wrist crease. Next, the TCL should be divided sharply longitudinally from proximal to distal along the ulnar boarder of the median nerve. The incisions should be carried out distally until the superficial palmar arterial arch is encountered. Following the release of the median nerve, the tourniquet should be released and adequate homeostasis should be achieved. The palmar fascia should be repaired to create a barrier between the nerve and the skin. The skin should be reapproximated and closed with horizontal or vertical mattress sutures to evert the skin edges. Xeroform gauze and sterile dresses can be applied to cover the surgical wound before applying a split.
Heterotopic ossification
With respect to HO and its impact on neuropathy in the burn patient, early studies discouraged early operative intervention due to the high incidence of recurrence. These studies also recommended delay of surgical intervention to allow the bone mass to reach radiological maturation and biological silence prior to performing surgery.63,64 However, more recent studies have demonstrated faster recovery time and return of nerve function following early excision of the HO.65–67 The recurrence rate following early excision of HO was 4 out of 35 patients (11.4%), with only one patient (2.8%) experiencing true recurrence with complete block of flexion and extension of the elbow, whereas the other three patients (8.6%) experienced only partial loss of function from HO recurrence.65 The three patients who experienced partial loss still obtained better movement following recurrence than prior to their operation, representing a considerable functional improvement. Thus, recent studies encourage early intervention in order to minimize long-term dysfunction of the compressed nerve.
Conclusion
Peripheral neuropathy and nerve compression syndromes are well described complications of severe burn injury, especially in those who are critically ill or who have electrical injuries. Diligent neurological examinations in these patients, combined with early intervention, can minimize long-term sequelae of nerve injuries. Furthermore, meticulous care during escharotomies and fasciotomies are key to limiting iatrogenic nerve injury around the burn site. It is also important to position patients appropriately to avoid nerve compression, including techniques to minimize prolonged elbow flexion, positioning the lower extremities in neutral rotation and knee extension, and avoiding prone positions with arm overhead. Prolonged tourniquet times and tight dressings can also contribute to neuropathy and should likewise be avoided.
Future directions focused on early diagnosis and treatment will minimize the morbidity associated with post-burn neuropathic pain. Both clinical and electrophysiological examinations have aided in early diagnosis. These can be carried out at the time of admission, at regular intervals during the hospitalization, and at subsequent follow-up to diagnose peripheral neuropathy at the onset of disease. Additional studies to investigate the molecular and cellular alterations associated with neuropathy following burn injury will assist in the development of novel therapies. Larger scale clinical trials to determine the efficacy of novel therapeutic interventions will also be necessary prior to implementation of new therapies. Together, these studies will aid in minimizing the morbidity associated with burn-related peripheral neuropathy and nerve compression syndromes.
Key Points.
Peripheral neuropathy and nerve compression syndromes lead to substantial morbidity following burn injury.
Early and effective treatment to minimize the long-term sequela of peripheral neuropathy and nerve compression syndromes will lead to improved outcomes.
Future directions focused on early diagnosis and treatment will minimize the morbidity associated with post-burn neuropathic pain.
Acknowledgments
Dr B. Levi was funded by 1K08GM109105-01, Plastic Surgery Foundation, American Association of Plastic Surgery Award, American Association for the Surgery of Trauma, American College of Surgeons Clowes Award and the Association of Academic Surgery. Dr B. Levi collaborates on a project unrelated to this review with Boehringer Ingelheim and has a Patent application on Rapamycin for use in heterotopic ossification which has not been licensed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The other authors have nothing to disclose.
References
- 1.Vanden Bossche L, Vanderstraeten G. Heterotopic ossification: a review. Journal of rehabilitation medicine. 2005 May;37(3):129–136. doi: 10.1080/16501970510027628. [DOI] [PubMed] [Google Scholar]
- 2.Hunt JL, Arnoldo BD, Kowalske K, Helm P, Purdue GF. Heterotopic ossification revisited: a 21-year surgical experience. Journal of burn care & research : official publication of the American Burn Association. 2006 Jul-Aug;27(4):535–540. doi: 10.1097/01.BCR.0000226023.58438.14. [DOI] [PubMed] [Google Scholar]
- 3.Henderson B, Koepke GH, Feller I. Peripheral polyneuropathy among patients with burns. Archives of physical medicine and rehabilitation. 1971 Apr;52(4):149–151. [PubMed] [Google Scholar]
- 4.Helm PA, Johnson ER, Carlton AM. Peripheral neurological problems in the acute burn patient. Burns : journal of the International Society for Burn Injuries. 1977;3:123–125. [Google Scholar]
- 5.Helm PA, Pandian G, Heck E. Neuromuscular problems in the burn patient: cause and prevention. Archives of physical medicine and rehabilitation. 1985 Jul;66(7):451–453. [PubMed] [Google Scholar]
- 6.Kowalske K, Holavanahalli R, Helm P. Neuropathy after burn injury. The Journal of burn care & rehabilitation. 2001 Sep-Oct;22(5):353–357. doi: 10.1097/00004630-200109000-00013. discussion 352. [DOI] [PubMed] [Google Scholar]
- 7.Dagum AB, Peters WJ, Neligan PC, Douglas LG. Severe multiple mononeuropathy in patients with major thermal burns. The Journal of burn care & rehabilitation. 1993 Jul-Aug;14(4):440–445. doi: 10.1097/00004630-199307000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Marquez S, Turley JJ, Peters WJ. Neuropathy in burn patients. Brain : a journal of neurology. 1993 Apr;116(Pt 2):471–483. doi: 10.1093/brain/116.2.471. [DOI] [PubMed] [Google Scholar]
- 9.Lee MY, Liu G, Kowlowitz V, et al. Causative factors affecting peripheral neuropathy in burn patients. Burns : journal of the International Society for Burn Injuries. 2009 May;35(3):412–416. doi: 10.1016/j.burns.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Tamam Y, Tamam C, Tamam B, Ustundag M, Orak M, Tasdemir N. Peripheral neuropathy after burn injury. European review for medical and pharmacological sciences. 2013 Feb;17(Suppl 1):107–111. [PubMed] [Google Scholar]
- 11.Gray P, Kirby J, Smith MT, et al. Pregabalin in severe burn injury pain: a double-blind, randomised placebo-controlled trial. Pain. 2011 Jun;152(6):1279–1288. doi: 10.1016/j.pain.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 12.Searle RD, Howell SJ, Bennett MI. Diagnosing postoperative neuropathic pain: a Delphi survey. British journal of anaesthesia. 2012 Aug;109(2):240–244. doi: 10.1093/bja/aes147. [DOI] [PubMed] [Google Scholar]
- 13.Taverner T, Prince J. Nurse screening for neuropathic pain in postoperative patients. British journal of nursing (Mark Allen Publishing) 2014;23(2):76–80. doi: 10.12968/bjon.2014.23.2.76. Jan 23–Feb 12. [DOI] [PubMed] [Google Scholar]
- 14.Hayes C, Browne S, Lantry G, Burstal R. Neuropathic pain in the acute pain service: a prospective survey. Acute Pain. 2002;4(2):45–48. 11// [Google Scholar]
- 15.Ferguson JS, Franco J, Pollack J, Rumbolo P, Smock M. Compression neuropathy: a late finding in the postburn population: a four-year institutional review. Journal of burn care & research : official publication of the American Burn Association. 2010 May-Jun;31(3):458–461. doi: 10.1097/BCR.0b013e3181db5183. [DOI] [PubMed] [Google Scholar]
- 16.Schneider JC, Harris NL, El Shami A, et al. A descriptive review of neuropathic-like pain after burn injury. Journal of burn care & research : official publication of the American Burn Association. 2006 Jul-Aug;27(4):524–528. doi: 10.1097/01.BCR.0000226019.76946.5D. [DOI] [PubMed] [Google Scholar]
- 17.Khedr EM, Khedr T, el-Oteify MA, Hassan HA. Peripheral neuropathy in burn patients. Burns : journal of the International Society for Burn Injuries. 1997 Nov-Dec;23(7–8):579–583. doi: 10.1016/s0305-4179(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg DB, Nelson M. Rehabilitation concerns in electrical burn patients: a review of the literature. The Journal of trauma. 1988 Jun;28(6):808–812. doi: 10.1097/00005373-198806000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Salisbury RE, Dingeldein GP. Peripheral nerve complications following burn injury. Clinical orthopaedics and related research. 1982 Mar;(163):92–97. [PubMed] [Google Scholar]
- 20.Salisbury RE, Bevin AG, Steinkraus GE, Enterline DS. Burn wound sepsis: effect of delayed treatment with topical chemotherapy on survival. The Journal of trauma. 1980 Feb;20(2):120–122. [PubMed] [Google Scholar]
- 21.Smith MA, Muehlberger T, Dellon AL. Peripheral nerve compression associated with low-voltage electrical injury without associated significant cutaneous burn. Plastic and reconstructive surgery. 2002 Jan;109(1):137–144. doi: 10.1097/00006534-200201000-00023. [DOI] [PubMed] [Google Scholar]
- 22.Skoog T. Electrical injuries. The Journal of trauma. 1970 Oct;10(10):816–830. doi: 10.1097/00005373-197010000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Tan AM, Samad OA, Liu S, Bandaru S, Zhao P, Waxman SG. Burn injury-induced mechanical allodynia is maintained by Rac1-regulated dendritic spine dysgenesis. Experimental neurology. 2013 Oct;248:509–519. doi: 10.1016/j.expneurol.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Liu T, Berta T, Xu ZZ, et al. TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. The Journal of clinical investigation. 2012 Jun;122(6):2195–2207. doi: 10.1172/JCI45414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacology & therapeutics. 2010 Apr;126(1):56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svensson CI, Brodin E. Spinal astrocytes in pain processing: non-neuronal cells as therapeutic targets. Molecular interventions. 2010 Feb;10(1):25–38. doi: 10.1124/mi.10.1.6. [DOI] [PubMed] [Google Scholar]
- 27.Gao YJ, Zhang L, Samad OA, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009 Apr 1;29(13):4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang J, Zhu C, Li Z-h, et al. Inhibition of the spinal astrocytic JNK/MCP-1 pathway activation correlates with the analgesic effects of tanshinone IIA sulfonate in neuropathic pain. Journal of Neuroinflammation. 2015;12(1):57. doi: 10.1186/s12974-015-0279-7. 2015// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma W, Quirion R. Partial sciatic nerve ligation induces increase in the phosphorylation of extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) in astrocytes in the lumbar spinal dorsal horn and the gracile nucleus. Pain. 2002;99 doi: 10.1016/s0304-3959(02)00097-0. 2002// [DOI] [PubMed] [Google Scholar]
- 30.Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109 doi: 10.1016/j.pharmthera.2005.07.001. 2006// [DOI] [PubMed] [Google Scholar]
- 31.Jha MK, Jeon S, Suk K. Glia as a link between neuroinflammation and neuropathic pain. Immune Netw. 2012;12 doi: 10.4110/in.2012.12.2.41. 2012// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji RR, Suter MR. p38 MAPK, microglial signaling, and neuropathic pain. Mol Pain. 2007;3 doi: 10.1186/1744-8069-3-33. 2007// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji RR, Gereau RW, Malcangio M, Strichartz GR. MAP kinase and pain. Brain Res Rev. 2009;60 doi: 10.1016/j.brainresrev.2008.12.011. 2009// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao YJ, Ji RR. Targeting astrocyte signaling for chronic pain. Neurotherapeutics. 2010;7 doi: 10.1016/j.nurt.2010.05.016. 2010// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29 doi: 10.1523/JNEUROSCI.3623-08.2009. 2009// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fan KW, Zhu ZX, Den ZY. An experimental model of an electrical injury to the peripheral nerve. Burns : journal of the International Society for Burn Injuries. 2005 Sep;31(6):731–736. doi: 10.1016/j.burns.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Higashimori H, Whetzel TP, Mahmood T, Carlsen RC. Peripheral axon caliber and conduction velocity are decreased after burn injury in mice. Muscle & nerve. 2005 May;31(5):610–620. doi: 10.1002/mus.20306. [DOI] [PubMed] [Google Scholar]
- 38.Drost AC, Burleson DG, Cioffi WG, Jr, Jordan BS, Mason AD, Jr, Pruitt BA., Jr Plasma cytokines following thermal injury and their relationship with patient mortality, burn size, and time postburn. The Journal of trauma. 1993 Sep;35(3):335–339. doi: 10.1097/00005373-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Drost AC, Larsen B, Aulick LH. The effects of thermal injury on serum interleukin 1 activity in rats. Lymphokine and cytokine research. 1993 Jun;12(3):181–185. [PubMed] [Google Scholar]
- 40.Ferguson JL, Hikawyj-Yevich I, Miller HI. Body fluid compartment changes during burn shock in the guinea pig. Circulatory shock. 1980;7(4):457–466. [PubMed] [Google Scholar]
- 41.Kiernan MC, Guglielmi JM, Kaji R, Murray NM, Bostock H. Evidence for axonal membrane hyperpolarization in multifocal motor neuropathy with conduction block. Brain : a journal of neurology. 2002 Mar;125(Pt 3):664–675. doi: 10.1093/brain/awf041. [DOI] [PubMed] [Google Scholar]
- 42.Sepulchre C, Moati F, Miskulin M, et al. Biochemical and pharmacological properties of a neurotoxic protein isolated from the blood serum of heavily burned patients. The Journal of pathology. 1979 Mar;127(3):137–145. doi: 10.1002/path.1711270306. [DOI] [PubMed] [Google Scholar]
- 43.Monafo WW, Eliasson SG. Sciatic nerve function following hindlimb thermal injury. The Journal of surgical research. 1987 Oct;43(4):344–350. doi: 10.1016/0022-4804(87)90091-6. [DOI] [PubMed] [Google Scholar]
- 44.Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. The Journal of clinical endocrinology and metabolism. 2009 Jun;94(6):2157–2163. doi: 10.1210/jc.2008-2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan Y, Johanek LM, Hartke TV, Shim B, Tao YX, Ringkamp M. Peripherally acting mu-opioid receptor agonist attenuates neuropathic pain in rats after L5 spinal nerve injury. Pain. 2008;138 doi: 10.1016/j.pain.2008.01.004. 2008// [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki R, Chapman V, Dickenson AH. The effectiveness of spinal and systemic morphine on rat dorsal horn neuronal responses in the spinal nerve ligation model of neuropathic pain. Pain. 1999 Mar;80(1–2):215–228. doi: 10.1016/s0304-3959(98)00208-5. [DOI] [PubMed] [Google Scholar]
- 47.Fowler M, Clifford JL, Garza TH, et al. A rat model of full thickness thermal injury characterized by thermal hyperalgesia, mechanical allodynia, pronociceptive peptide release and tramadol analgesia. Burns : journal of the International Society for Burn Injuries. 2014 Jun;40(4):759–771. doi: 10.1016/j.burns.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 48.Patterson DR, Hofland HW, Espey K, Sharar S. Pain management. Burns : journal of the International Society for Burn Injuries. 2004 Dec;30(8):A10–15. doi: 10.1016/j.burns.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 49.Lilleso J, Hammer NA, Pedersen JL, Kehlet H. Effect of peripheral morphine in a human model of acute inflammatory pain. British journal of anaesthesia. 2000 Aug;85(2):228–232. doi: 10.1093/bja/85.2.228. [DOI] [PubMed] [Google Scholar]
- 50.Dellemijn PL, Vanneste JA. Randomised double-blind active-placebo-controlled crossover trial of intravenous fentanyl in neuropathic pain. Lancet (London, England) 1997 Mar 15;349(9054):753–758. doi: 10.1016/S0140-6736(96)09024-1. [DOI] [PubMed] [Google Scholar]
- 51.Gilron I, Bailey JM, Tu D, Holden RR, Weaver DF, Houlden RL. Morphine, gabapentin, or their combination for neuropathic pain. The New England journal of medicine. 2005 Mar 31;352(13):1324–1334. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- 52.Caviggioli F, Maione L, Klinger F, Lisa A, Klinger M. Autologous Fat Grafting Reduces Pain in Irradiated Breast: A Review of Our Experience. Stem cells international. 2016;2016:2527349. doi: 10.1155/2016/2527349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fredman R, Edkins RE, Hultman CS. Fat Grafting for Neuropathic Pain After Severe Burns. Annals of plastic surgery. 2016 Jun;76(Suppl 4):S298–303. doi: 10.1097/SAP.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 54.Vaienti L, Merle M, Battiston B, Villani F, Gazzola R. Perineural fat grafting in the treatment of painful end-neuromas of the upper limb: a pilot study. The Journal of hand surgery, European volume. 2013 Jan;38(1):36–42. doi: 10.1177/1753193412441122. [DOI] [PubMed] [Google Scholar]
- 55.Huang SH, Wu SH, Chang KP, et al. Autologous fat grafting alleviates burn-induced neuropathic pain in rats. Plastic and reconstructive surgery. 2014 Jun;133(6):1396–1405. doi: 10.1097/PRS.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 56.Huang SH, Wu SH, Lee SS, et al. Fat Grafting in Burn Scar Alleviates Neuropathic Pain via Anti-Inflammation Effect in Scar and Spinal Cord. PloS one. 2015;10(9):e0137563. doi: 10.1371/journal.pone.0137563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ranganathan K, Wong VW, Krebsbach PH, Wang SC, Cederna PS, Levi B. Fat grafting for thermal injury: current state and future directions. Journal of burn care & research : official publication of the American Burn Association. 2013 Mar-Apr;34(2):219–226. doi: 10.1097/BCR.0b013e318280e2dd. [DOI] [PubMed] [Google Scholar]
- 58.Schwenkreis P, Scherens A, Ronnau AK, Hoffken O, Tegenthoff M, Maier C. Cortical disinhibition occurs in chronic neuropathic, but not in chronic nociceptive pain. BMC neuroscience. 2010 Jun 11;11:73. doi: 10.1186/1471-2202-11-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology. 2006 Nov 14;67(9):1568–1574. doi: 10.1212/01.wnl.0000242731.10074.3c. [DOI] [PubMed] [Google Scholar]
- 60.Portilla AS, Bravo GL, Miraval FK, et al. A feasibility study assessing cortical plasticity in chronic neuropathic pain following burn injury. Journal of burn care & research : official publication of the American Burn Association. 2013 Jan-Feb;34(1):e48–52. doi: 10.1097/BCR.0b013e3182700675. [DOI] [PubMed] [Google Scholar]
- 61.Nedelec B, Calva V, Chouinard A, et al. Somatosensory Rehabilitation for Neuropathic Pain in Burn Survivors: A Case Series. Journal of burn care & research : official publication of the American Burn Association. 2016 Jan-Feb;37(1):e37–46. doi: 10.1097/BCR.0000000000000321. [DOI] [PubMed] [Google Scholar]
- 62.Sheridan RL, Hurley J, Smith MA, et al. The acutely burned hand: management and outcome based on a ten-year experience with 1047 acute hand burns. The Journal of trauma. 1995 Mar;38(3):406–411. doi: 10.1097/00005373-199503000-00022. [DOI] [PubMed] [Google Scholar]
- 63.Roberts JB, Pankratz DG. The surgical treatment of heterotopic ossification at the elbow following long-term coma. The Journal of bone and joint surgery. American volume. 1979 Jul;61(5):760–763. [PubMed] [Google Scholar]
- 64.Garland DE. Surgical approaches for resection of heterotopic ossification in traumatic brain-injured adults. Clinical orthopaedics and related research. Feb;1991(263):59–70. [PubMed] [Google Scholar]
- 65.Tsionos I, Leclercq C, Rochet JM. Heterotopic ossification of the elbow in patients with burns. Results after early excision. The Journal of bone and joint surgery. British volume. 2004 Apr;86(3):396–403. doi: 10.1302/0301-620x.86b3.14480. [DOI] [PubMed] [Google Scholar]
- 66.Vorenkamp SE, Nelson TL. Ulnar nerve entrapment due to heterotopic bone formation after a severe burn. The Journal of hand surgery. 1987 May;12(3):378–380. doi: 10.1016/s0363-5023(87)80009-6. [DOI] [PubMed] [Google Scholar]
- 67.Wu C, Calvert CT, Cairns BA, Hultman CS. Lower extremity nerve decompression in burn patients. Annals of plastic surgery. 2013 May;70(5):563–567. doi: 10.1097/SAP.0b013e31827aef9c. [DOI] [PubMed] [Google Scholar]