SUMMARY
The human growth hormone (hGH) minigene is frequently used in the derivation of transgenic mouse lines to enhance transgene expression. Although this minigene is present in the transgenes as a second-cistron, and thus not thought to be expressed, we found that three commonly used lines, Pdx1-CreLate, RIP-Cre, and MIP-GFP, each expressed significant amounts of hGH in pancreatic islets. Locally secreted hGH binds to prolactin receptors on β cells, activates STAT5 signaling, and induces pregnancy-like changes in gene expression, thereby augmenting pancreatic β cell mass and insulin content. In addition, islets of Pdx1-CreLate mice have lower GLUT2 expression and reduced glucose-induced insulin release and are protected against the β cell toxin streptozotocin. These findings may be important when interpreting results obtained when these and other hGH minigene-containing transgenic mice are used.
INTRODUCTION
Conditional inactivation of genes in mice by specific DNA recombination in target tissues was developed in the early 90s (Orban et al., 1992). By then, it was already known that intronic sequences and a polyadenylation signal are essential to achieve efficient expression of the transgene (Brinster et al., 1988). The entire human growth hormone (hGH) coding region, including introns and polyadenylation signal (also called hGH minigene), was oftentimes inserted downstream of coding regions, such as that of Cre recombinase, to generate transgenic mouse models (Orban et al., 1992), some of which are in wide use today.
Prolactin (PRL), placental lactogen (PL), and GH are homologous proteins that display overlapping structure (Goffin et al., 1996) and biological activities (Soares, 2004). The lactogens PRL and PL have a profound effect on the pancreatic islet phenotype of pregnant females (Parsons et al., 1992; Sorenson et al., 1987). During pregnancy, insulin resistance is induced in the mother, which facilitates nutrient flow toward the fetus (Freemark, 2006). To compensate for this increased metabolic demand and to prevent hyperglycemia, maternal pancreatic β cells undergo several structural and functional changes. These changes have been under investigation for several decades and involve multiple β cell parameters, such as increased glucose-stimulated insulin secretion (GSIS) (Green and Taylor, 1972), enhanced β cell proliferation (Van Assche, 1974), accelerated proinsulin biosynthesis (Bone and Taylor, 1976), a higher rate of glucose oxidation and glucose utilization (Green et al., 1978; Weinhaus et al., 1996), and increased gap-junctional coupling of β cells (Sheridan et al., 1988). The importance of lactogens in this phenotypic switch has been demonstrated in mouse models in which β cell-specific overexpression of PL leads to enhanced insulin secretion and increased β cell mass (Fleenor et al., 2000; Vasavada et al., 2000). These signals are mediated by prolactin receptors (PRLR), as was demonstrated in Prlr+/− mice (Huang et al., 2009). Growth hormones of primates, but not of other vertebrates, are also able to activate PRLR, a property that is maintained in several heterologous systems (Goffin et al., 1996). Accordingly, it was shown that hGH can mimic the effects of lactogens on mouse and rat β cells (Parsons et al., 1995).
To define the molecular basis of lactogen signaling during pregnancy, we and others recently explored the changes that occur in the gene expression using genome-wide mRNA expression analysis (Kim et al., 2010; Rieck et al., 2009; Schraenen et al., 2010a, 2010b). The largest cluster of upregulated genes during pregnancy regulates β cell mass (Schraenen et al., 2010a). In addition, a strong induction of the serotonin biosynthetic pathway was found in a subset of β cells (Schraenen et al., 2010b). Genes encoding tryptophan hydroxylase-1 (TPH1) and tryptophan hydroxylase-2 (TPH2), catalyzing the rate-limiting step of serotonin biosynthesis, were found to be vastly upregulated (Kim et al., 2010; Schraenen et al., 2010b), resulting in an increase in islet serotonin content greater than 100-fold. The responsible mechanism involved PRLR and activation of its canonical Janus kinase 2 (JAK2)/signal transducer and activator of transcription 5 (STAT5) signaling pathway (Schraenen et al., 2010b). An autocrine/paracrine role for serotonin has been suggested in activating β cell proliferation via serotonin receptor 2B (Kim et al., 2010).
Here we describe unexpected functional consequences resulting from the placement of hGH minigene in the transgenic constructs. The principal mouse strain used for these studies was the Tg(Pdx1-cre)1Herr mouse strain, also known as the Pdx1-CreLate model (Herrera, 2000). This transgenic line contains a 4.5 kb fragment of the Pdx1 promoter inserted upstream of the Cre recombinase-coding region and an hGH minigene in order to achieve efficient transgene expression. We studied the expression of hGH at the mRNA and protein level and the consequences for islets from Pdx1-CreLate mice, such as changes in β cell mass and insulin secretion. In addition, we examined hGH expression and serotonin biosynthesis in two other lines that are frequently used for pancreatic 25Mgn/J line (Postic et al., 1999), which is frequently used to generate β cell conditional gene knockouts, and the B6.Cg-Tg(Ins1-EGFP)1Hara/J line, which is often used to visualize and/or purify pancreatic β cells (Hara et al., 2003), from now on referred to as RIP-Cre and MIP-GFP, respectively.
RESULTS
Pancreata from Pdx1-CreLate Mice Have Increased β Cell Mass and Insulin Content
While working with the Pdx1-CreLate driver line to generate a new β cell-specific knockout strain, we found that circulating blood glucose, in both the random-fed (Figure 1A) and fasted state (Figure 1B), was lower in the Pdx1-CreLate mice than in littermate controls. This difference could not be explained by enhanced insulin sensitivity of Pdx1-CreLate mice (Figure 1C). Instead, we observed an increase in pancreatic β cell mass (Figure 1D), which was accompanied by a nearly doubled pancreatic insulin content (Figure 1E, Pdx1-CreLate 390 ± 19 μg/g versus WT 204 ± 52 μg/g, n = 9–10/genotype, p = 0.006).
Figure 1. Increased β Cell Mass and Insulin Content in Nonpregnant Pdx1-CreLate Mice.
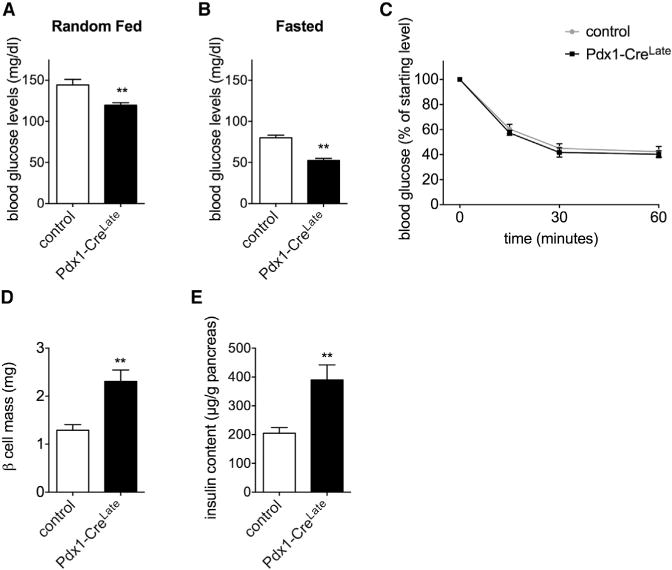
(A and B) Blood glucose levels in random-fed (A) and fasted (B) Pdx1-CreLate versus control mice. Data are represented as mean ± SEM, n = 7 mice per genotype, **p < 0.01.
(C) Insulin tolerance test (ITT) on 10-week-old Pdx1-CreLate versus control mice. Values are presented as a percentage compared to the starting glucose level. Data are represented as mean ± SEM, n = 7–8 mice per genotype.
(D) Quantification of β cell mass in 24-week-old Pdx1-CreLate versus control mice, performed as described in the Experimental Procedures. Data are represented as mean ± SEM, n = 4 mice per genotype, **p < 0.01.
(E) Pancreatic insulin content in 24-week-old Pdx1-CreLate versus control mice. Data are represented as mean ± SEM, n = 9–10 mice per genotype, **p < 0.01.
Paracrine/Autocrine PRLR Activation by hGH in Islets from Nonpregnant Pdx1-CreLate Mice
In the original description of the Pdx1-CreLate mouse model (Her-rera, 2000), the complete coding hGH gene sequence, including exons, introns, and its polyadenylation signal, is present downstream of the Pdx1 promoter and Cre recombinase (Figure 2A). Results from Figures 2B–2F demonstrate that the hGH minigene is specifically and highly expressed in mouse β cells of the Pdx1-CreLate strain. We quantified hGH expression at both the mRNA level (Figure 2B) and protein level (Figures 2C–2E). We found significant hGH expression only in pancreatic islets, but not in the other tissues (Figure 2B), including the exocrine part of the pancreas (Figure 2D). Western blots (Figure 2C) indicated that the mature hormone of the expected molecular weight (22 kDa) was produced in Pdx1-CreLate islets. Quantification of hGH in extracts from isolated islets and in conditioned medium (Figure 2F) shows that approximately 4% of cellular hGH content is released per hour from 20 mM glucose-stimulated Pdx1-CreLate islets. Figure 2G supports the idea that locally secreted hGH has functional effects on pancreatic islet in vivo. Indeed, a significant increase (3.7-fold, n = 3, p = 0.005) in STAT5 phosphorylation was detected in freshly isolated Pdx1-CreLate islets compared to control islets (Figure 2G). Because hGH can bind to GH receptors (GHR) and to PRLR (Goffin et al., 1996), and since both receptors are strongly expressed in rodent β cells (Brelje et al., 2002; Møldrup et al., 1993), we next investigated the mechanism by which hGH causes phenotypic changes in β cells. We first used MIN6 cells as a surrogate model for mouse β cells (Miyazaki et al., 1990) and quantified Tph1 as a marker for lactogen-mediated PRLR activation, taking ovine PL (oPL) as a positive control (Schraenen et al., 2010b). As is shown in Figure 2H, both hGH and oPL were very potent ligands in this assay, as a significant induction of Tph1 mRNA was already observed with 25 ng/ml (~1 nM). In comparison, the minimal effective concentration of mouse (m)GH, which only binds to GHR, was 500 ng/ml, indicating that synthetic hGH was at least 20-fold more potent. Similar results were obtained with primary islets: a significant induction of Tph1 mRNA was found in islet monolayers treated with 500 ng/ml oPL or hGH, but not after treatment with mGH (Figure 2I). To confirm that hGH stimulation of Tph1 expression is mediated by PRLR signaling, MIN6 cells stimulated or not with 25 ng/ml hGH were cotreated with increasing concentrations of the synthetic PRLR antagonist Δ1-9-G129R-hPRL (Bernichtein et al., 2003). A concentration-dependent antagonism was observed (Figure 2I). Together, our data indicate that lactogen-like increment of pancreatic β cell mass and insulin content in Pdx1-CreLate mice is initiated by local hGH release and mediated by activation of PRLR.
Figure 2. Pregnancy-Related Phenotypic Changes Are Caused by Local Production and Secretion of hGH in Islets from Nonpregnant Pdx1-CreLate Mice.
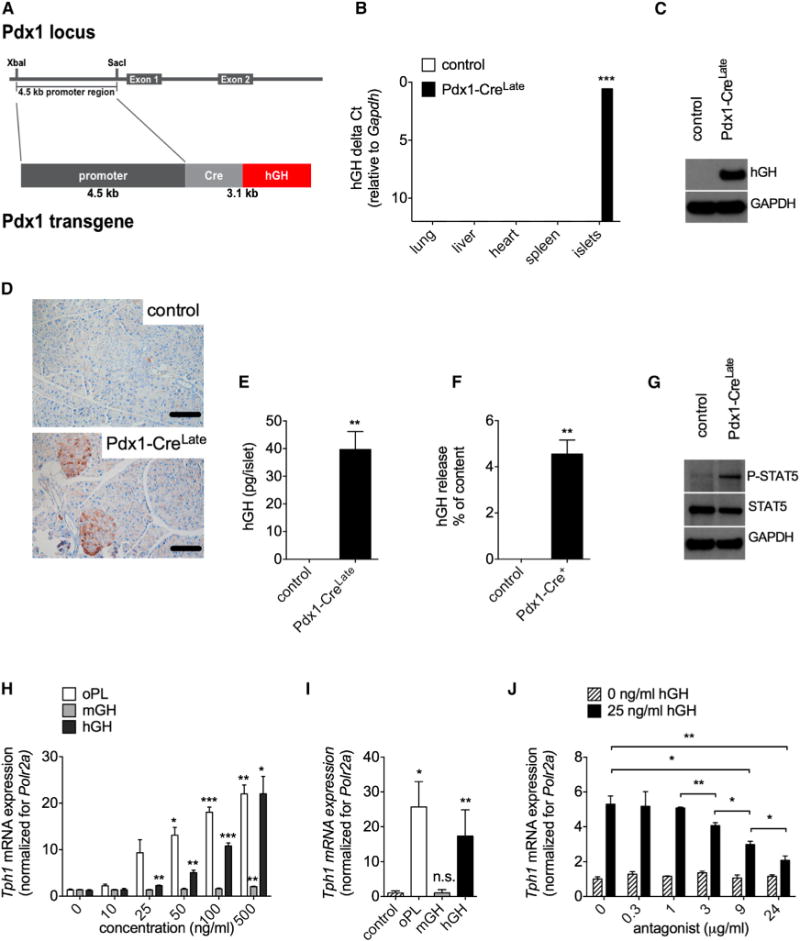
(A) Schematic representation of the Pdx1-Cre construct used to generate the Pdx1-CreLate mouse model. A 4.5 kb Pdx1 promoter fragment was inserted upstream of the Cre transgene. The hGH minigene, containing introns, exons, and polyadenylation signal, is located directly downstream of the Cre fragment and indicated in red. This figure was based on the materials and methods from Herrera (2000).
(B) qRT-PCR analysis of hGH mRNA in different tissues, including isolated islets from control and nonpregnant Pdx1-CreLate mice. Data were quantified as delta Ct values (relative to Gapdh). Data are represented as mean ± SEM, n = 3–6 per condition, ***p < 0.001.
(C) Western blot analysis of hGH expression in islets of control and nonpregnant Pdx1-CreLate mice. GAPDH was used as a loading control.
(D) Immunoreactive hGH in pancreatic sections from control and nonpregnant Pdx1-CreLate mice. The hGH signal is most intense in pancreatic islets and not uniformly distributed over their different islet cells. Scale bar, 100 μm.
(E and F) Quantification of hGH content (E) and release (F) (measured as the percentage of cellular content per hour) in islets isolated from Pdx1-CreLate mice. For the release experiments, isolated islets were incubated with 20 mM D-glucose for 1 hr. Data are represented as mean ± SEM, n = 3 per condition, **p < 0.01.
(G) Representative immunoblot of islet phospho-STAT5 (P-STAT5) and total STAT5 protein in islets from control and nonpregnant Pdx1-CreLate mice. GAPDH was used as a loading control. Mean density ratios of P-STAT5/total STAT5 were increased 3.72 ± 0.38-fold in Pdx1-CreLate islets compared to controls. Data are represented as mean ± SEM, n = 3 per condition, p = 0.005.
(H and I) Induction of Tph1 expression in MIN6 cells (H) and primary islet monolayers (I) by hGH and oPL. Cells were treated with the indicated concentrations of oPL, mGH, or hGH, and islet monolayers with vehicle (control) or 500 ng/ml oPL, mGH, or hGH. Expression of Tph1 was quantified by qRT-PCR. Polr2a was used as a reference gene. Data were calculated via the Pfaffl method, and the ratio of the control sample was normalized to 1. Data are represented as mean ± SEM, n = 3–5 independent measurements per condition; *p < 0.05, **p < 0.01, ***p < 0.001.
(J) Inhibition of hGH-induced Tph1 expression in MIN6 cells by a specific PRLR inhibitor. MIN6 cells preincubated (30 min) with different concentrations of PRLR-antagonist Δ1-9-G129R-hPRL were stimulated with 0 or 25 ng/ml hGH; Tph1 mRNA expression was used as readout for the pregnancy signature, and Polr2a was used as a reference gene. Data were calculated via the Pfaffl method, and the average ratio of 0 ng/ml hGH was set to 1. Data are represented as mean ± SEM, n = 3 per condition; *p < 0.05, **p < 0.01.
Activation of a Pregnancy-Related Phenotypic Switch in Islets from Pdx1-CreLate Mice
The autocrine/paracrine PRLR stimulation by local release of hGH in pancreatic islets not only enhanced the β cell mass but also upregulated more than 100 genes that are also upregulated in islets from C57BL/6J pregnant mice (Figure 3A). This was measured by comparison of the global islet gene expression profile in 12-week-old Pdx1-CreLate mice and control nonpregnant littermates as well as islet from C57BL/6J pregnant mice not carrying the Pdx1-CreLate insertion. More than 50% (121/218) of the upregulated genes in nonpregnant Pdx1-CreLate islets were also upregulated during pregnancy (Figure 3A). Overlap was complete for a pregnancy gene expression signature, consisting of 12 known genes described previously to be highly up-regulated during pregnancy (fold increase ≥ 3 and p < 0.001 at pregnancy day 12.5) (Figure S1, available online). For this strongly induced pregnancy gene expression signature, we confirmed by quantitative RT-PCR (qRT-PCR) analysis that the mRNA signal in Pdx1-CreLate islets was significantly higher than in control islets (Figure 3B). The two transcripts that encode the nonallelic paralogs of tryptophan hydroxylase, Tph1 and Tph2, were highly induced in Pdx1-CreLate islets: 346.3 ± 69.4-fold (p = 0.004) and 14.3 ± 3.2-fold (p = 0.011), respectively. To assess the importance of the PRLR in the pregnancy switch in vivo, expression of the 12 genes in the gene expression signature was quantified in islets from Pdx1-CreLate;PRLR−/− mice versus Pdx1-CreLate littermates by qRT-PCR. A significant reduction in gene expression was observed for most of the 12 genes in the Pdx1-CreLate;PRLR−/− mouse model as compared to Pdx1-CreLate controls (Figure 3C), again stressing the crucial role of the PRLR in the pregnancy switch.
Figure 3. Pregnancy-Related Phenotypic Switch in Islets Isolated from Nonpregnant Pdx1-CreLate Mice.
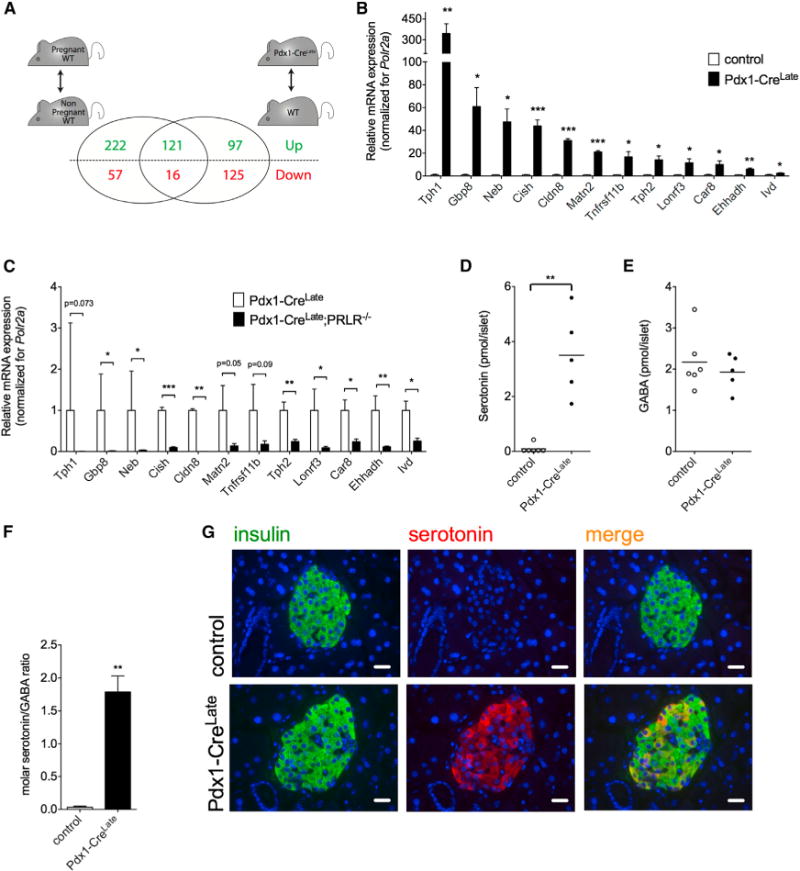
(A) Microarray analysis of islet mRNA expression. A substantial overlap of >100 differentially expressed genes (p < 0.001 and fold change of ≥1.5) was found when comparing pregnant versus nonpregnant mice on the one hand (left) and nonpregnant Pdx1-CreLate mice versus nonpregnant control mice on the other hand (right). This overlap contains almost 10-fold more upregulated than downregulated genes.
(B) qRT-PCR analysis of the pregnancy gene signature in nonpregnant Pdx1-CreLate versus control mice. For each of the top 12 upregulated genes during pregnancy at P12.5, we observed significant upregulation in islets from nonpregnant Pdx1-CreLate mice. Polr2a was used as a reference gene. Data were calculated via the Pfaffl method, and the average ratio of each gene was set to 1 for the control mice. Two strongly induced genes in islets from Pdx1-CreLate mice encode the two isoforms of tryptophan hydroxylase (TPH1 and TPH2). Data are represented as mean ± SEM, n = 4–6 per group; *p < 0.05, **p < 0.01, ***p < 0.001.
(C) qRT-PCR analysis on all 12 genes from the pregnancy gene expression signature, in islets from Pdx1-CreLate;PRLR−/−mice versus Pdx1-CreLate littermates, n = 3 mice per genotype. Data are represented as mean ± SEM, n = 3 per group, *p < 0.05, **p < 0.01, ***p < 0.001.
(D–F) Quantification of serotonin and GABA in islets isolated from nonpregnant control and nonpregnant Pdx1-CreLate mice. (D) Basal islet serotonin levels are near the detection limit of the assay in control mice and dramatically upregulated in nonpregnant Pdx1-CreLate mice. (E) In contrast, GABA is as abundant in islets of control as in islets of nonpregnant Pdx1-CreLate mice. (F) Consequently, the molar serotonin/GABA ratio is about 100-fold higher in islets from Pdx1-CreLate mice compared to control islets. Data are represented as mean ± SEM, n = 5–6 per group; *p < 0.05, **p < 0.01, ***p < 0.001.
(G) Heterogeneous serotonin immunoreactivity in islets from nonpregnant Pdx1-CreLate mice. No serotonin immunoreactivity is detected in control mice, while in nonpregnant Pdx1-CreLate mice serotonin is only present in islet β cells with marked differences between neighboring β cells. Data are representative sections from pancreata analyzed from five nonpregnant control mice and four nonpregnant Pdx1-CreLate mice (scale bar, 20 μm). See also Figure S1 and Table S1.
Because DOPA decarboxylase, the second enzyme needed for serotonin biosynthesis from tryptophan, is constitutively and highly expressed in mouse pancreatic islets, the strong up-regulation of the tryptophan hydroxylase step indicates that islets of Pdx1-CreLate mice are competent to synthesize serotonin under nonpregnant conditions. This is in contrast to control mice, which only produce serotonin during pregnancy. Therefore, we quantified islet serotonin content of control and Pdx1-CreLate mice under nonpregnant conditions and observed a striking difference (Figure 3D). This difference between mouse genotypes contrasted with islet gamma-amino butyric acid (GABA) (Figure 3E), a neurotransmitter that is constitutively produced by decarboxylation of glutamate in rodent and human β cells (Sorenson et al., 1991). Consequently, the islet serotonin/GABA molar ratio increased by at least one order of magnitude in islet extracts from the nonpregnant Pdx1-CreLate strain (Figure 3F). To analyze the serotonin production at the cellular level in nonpregnant Pdx1-CreLate mice, we performed immunostaining on pancreatic islet sections. A heterogeneous pattern of serotonin immunoreactivity was found in islet β cells of nonpregnant Pdx1-CreLate mice (Figure 3G), similar to the production observed in wild-type C57BL/6J mice during pregnancy (Schraenen et al., 2010b). No serotonin immunoreactivity was detected in nonpregnant control mice. Together, these data show that the normal serotonin biosynthetic pathway, observed in a subpopulation of β cells in the pancreas of pregnant mice, is induced independently of pregnancy in islets from Pdx1-CreLate mice.
Impaired Glucose Tolerance and Reduced Islet GLUT2 Expression in Pdx1-CreLate Mice
Overlap between genes that are downregulated in islets from pregnant wild-type C57BL/6J mice versus nonpregnant Pdx1-CreLate mice was much weaker (Figure 3A). One example was the mRNA encoding the glucose transporter GLUT2, which was repressed in Pdx1-CreLate islets (Figure 4A), but not in islets from pregnant mice. GLUT2 acts as a glucose sensor protein in rodent pancreatic β cells and is essential for normal glucose homeostasis in mice (Guillam et al., 1997; Thorens et al., 1988). This reduction in Glut2 mRNA expression correlated with a strong reduction in immunoreactive protein on islet β cell membranes (Figure 4B) but contrasted with that of other genes involved in GSIS, which remained at the control level in islets isolated from Pdx1-CreLate mice (Figure S2A). The reduction of GLUT2 expression also coincided with a partial loss of GSIS with either 20 mM glucose or 20 mM glucose plus the phosphodiesterase inhibitor 3-isobutyl-1-methylxanthine (IBMX) as well as higher basal release (Figure 4C). This abnormality was associated with a slightly decreased glucose tolerance (Figure 4D) and lower circulating insulin levels (Figure 4E) after 2.5 mg/g body weight (BW) intraperitoneal (i.p.) glucose injection. Glucose intolerance was even more pronounced in older Pdx1-CreLate mice (20 weeks old) (Figure S2B). However, glycemia turned back to starting levels at the same time point as that for control mice. Together, these data indicate that in addition to some pregnancy-related phenotypic changes, local release of hGH induces other changes in pancreatic islets, as exemplified by reduced GLUT2 and partial loss of GSIS in Pdx1-CreLate islets.
Figure 4. Reduced Islet GLUT2 Expression, Loss of GSIS, and Protection against STZ in Pdx1-CreLate Mice.
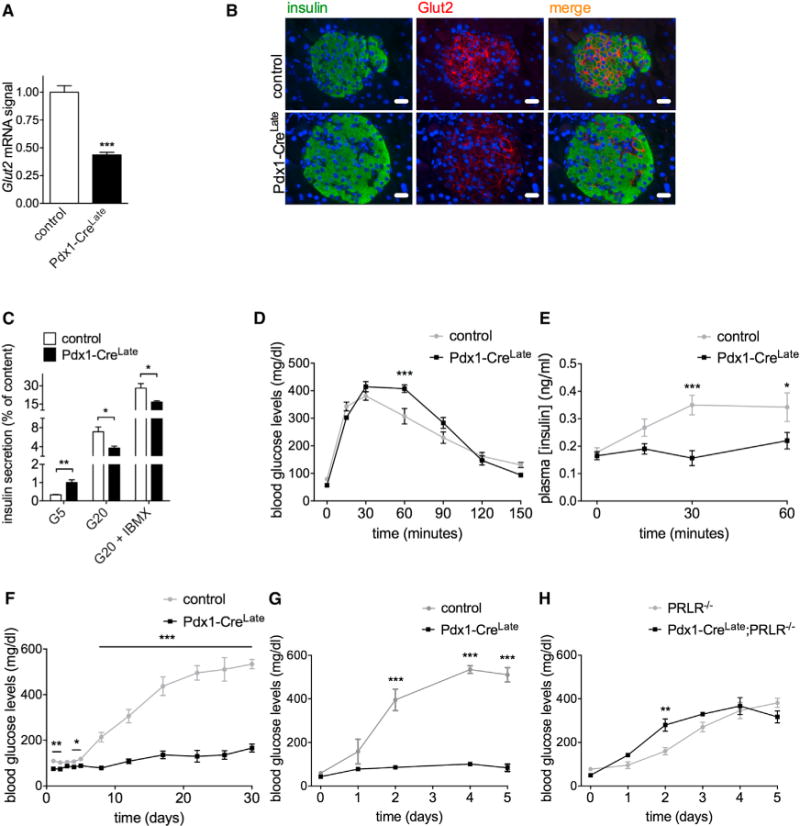
(A) Glut2 mRNA signal in control versus Pdx1-CreLate islets. Gapdh was used as a reference gene. Data are represented as mean ± SEM, n = 3–4 mice per genotype, ***p < 0.001.
(B) Representative immunofluorescence staining for insulin (green signal), GLUT2 (red signal), and merge on pancreatic sections from control versus Pdx1-CreLate mice. Overall, the GLUT2 signal is much weaker in Pdx1-CreLate islets compared to controls. Scale bar, 20 μm.
(C) Ex vivo insulin secretion assay. Islets from Pdx1-CreLate and control mice were isolated and incubated for 1 hr with 5 mM (G5), 20 mM (G20), or 20 mM D-glucose with the phosphodiesterase inhibitor IBMX (G20 + IBMX) Insulin release was quantified as the percentage of insulin secreted compared to islet insulin content. Data are represented as mean ± SEM, n = 6 mice per genotype; *p < 0.05, **p < 0.01.
(D) Intraperitoneal glucose tolerance test (IPGTT) on 10-week-old Pdx1-CreLate versus control mice, n = 5 per genotype. Overnight fasted mice were injected i.p. with 2.5 mg/g BW D-glucose and blood glucose levels were measured at the indicated time points. Data are represented as mean ± SEM, n = 5 mice per genotype; ***p < 0.001, repeated measures ANOVA.
(E) Circulating plasma insulin levels in blood samples obtained at the indicated time points from IPGTT. Data are represented as mean ± SEM, n = 5 mice per genotype; *p < 0.05, ***p < 0.001, repeated measures ANOVA.
(F) Multiple low-dose (MLD) treatment of the diabetogenic agent streptozotocin (STZ) in Pdx1-CreLate versus control mice. Mice aged 12 weeks were injected i.p. with 50 mg STZ/kg BW for 5 consecutive days, and random-fed blood glucose levels were measured at the indicated time points. Data are represented as mean ± SEM, n = 5 mice per genotype; *p < 0.05, **p < 0.01, ***p < 0.001, repeated measures ANOVA.
(G) Single high dose of STZ in Pdx1-CreLate versus control mice. Mice were injected i.p. with 150 mg STZ/kg BW, and fed blood glucose levels were measured. Data are represented as mean ± SEM, n = 5–6 mice per genotype; ***p < 0.001, repeated measures ANOVA.
(H) Single high dose (150 mg/kg BW) of STZ in Prlr−/− versus Pdx1-CreLate; Prlr−/− mice. Data are represented as mean ± SEM, n = 3–4 mice per genotype; **p < 0.01, repeated measures ANOVA. See also Figure S2.
Pdx1-CreLate Mice Are Protected against the Diabetogenic Toxin Streptozotocin
The GLUT2 transporter on the β cell surface is also responsible for the uptake of the β cell toxin streptozotocin (STZ) (Schnedl et al., 1994). Consequently, in control mice, which have very high levels of GLUT2 expression on β cells, STZ can induce an acute β cell necrosis after single injection of a high dose or chronically after multiple low-dose injections (Lenzen, 2008). As the Glut2 gene expression is reduced in Pdx1-CreLate islets, the effect of STZ might be diminished. Moreover, PRL-induced activation of the JAK2/STAT5 pathway was reported to protect mice against multiple low doses of STZ (Holstad and Sandler, 1999; Jackerott et al., 2006). The effect of these two protective changes in islets of Pdx1-CreLate mice on blood glucose levels was tested in the multiple low-dose STZ model (50 mg/kg BW for 5 consecutive days and 1 month follow up of blood glucose levels; Figure 4F) as well as in the single high-dose STZ model (150 mg/kg BW and 5 days of follow up; Figure 4G). Pdx1-CreLate mice remained normoglycemic during the whole follow-up period in both models, whereas all control animals developed diabetes. However, the protection of Pdx1-CreLate mice against a single high-dose STZ injection was completely lost when Pdx1-CreLate mice were crossed to Prlr−/−, further emphasizing the involvement of the PRLR in the hGH-induced phenotypic changes observed in Pdx1-CreLate mice (Figure 4H).
hGH and Serotonin Production Also Occurs in Islets from RIP-Cre and MIP-GFP Mice
Since many transgenic mouse models, in addition to the Pdx1-CreLate animals, incorporated a hGH minigene in their design (see Table 1), we also examined whether hGH protein is produced in MIP-GFP and RIP-Cre islets, two transgenic lines that are commonly used in studies of β cell biology and cellular mechanisms of diabetes. While expression of the hGH mRNA was expected given the placement of the gene sequences in the transgene construct (Figure 5A), hGH protein immunoreactivity was also observed (Figures 5B and S3). Furthermore, we found evidence for a pregnancy-related phenotypic switch as illustrated by a strong induction of Tph1 mRNA (Figure 5A) and immunoreactive serotonin in islets from MIP-GFP and RIP-Cre islets (Figure 5C and quantified in Figure S4). Therefore, the presence and functional activity of hGH protein is not limited to Pdx1-CreLate mice, but also occurs in these two other lines. Similar to what was observed in the Pdx1-CreLate mice, 9-week-old MIP-GFP mice had lower blood glucose levels after an overnight fast (Figure S5A) and lower plasma insulin levels 30 min after the start of the glucose tolerance test (Figures S5B and S5C), whereas insulin sensitivity was normal (Figure S5D).
Table 1.
β Cell-Specific Transgenic Mice Generated Based on a Similar Strategy, Namely Using the hGH Minigene as Transgene Enhancer
| Common Strain Name | Reference |
|---|---|
| Ins-rtTA/TetO-RXRβΔC2 | Miyazaki et al., 2010 |
| MIP-CFP | Hara et al., 2006 |
| MIP-Cy3.3er | Hara et al., 2004 |
| MIP-GFP | Hara et al., 2003 |
| MIP-HIMP1 | Zhang et al., 2012 |
| MIP-hProCpepGFP | Hodish et al., 2010 |
| MIP-Luc | Park et al., 2005 |
| MIP-Phogrin-pHluorin-mCherryhip-rxr | Lu et al., 2009 |
| MIP-RFP | Hara et al., 2006 |
| MIP-sr39tk | McGirr et al., 2011 |
| Pdx1-CreLate | Herrera, 2000 |
| Pdx1-eGFP | Sylvestersen et al., 2011 |
| RIP-Cx32 | Charollais et al., 2000 |
| RIP-Cx43 | Klee et al., 2011 |
| RIP-HGF | Garcia-Ocaña et al., 2000 |
| RIP-PL | Vasavada et al., 2000 |
| RIP-PTHrP | Vasavada et al., 1996 |
| RIP-TGFb1 | Sanvito et al., 1995 |
| RIP-VegfA165 | Gannon et al., 2002 |
| RIP1-Podo | Wicki et al., 2006 |
| RIP1-VEGFD | Kopfstein et al., 2007 |
| RIP2-Cre | Postic et al., 1999 |
This table includes the names of the strains that were identified and the references of the first report.
Figure 5. Growth Hormone and Tph1 Expression and Islet Serotonin Immunoreactivity in Pancreatic Islets from Two Other Commonly Used Mouse Driver Strains, MIP-GFP and RIP-Cre.
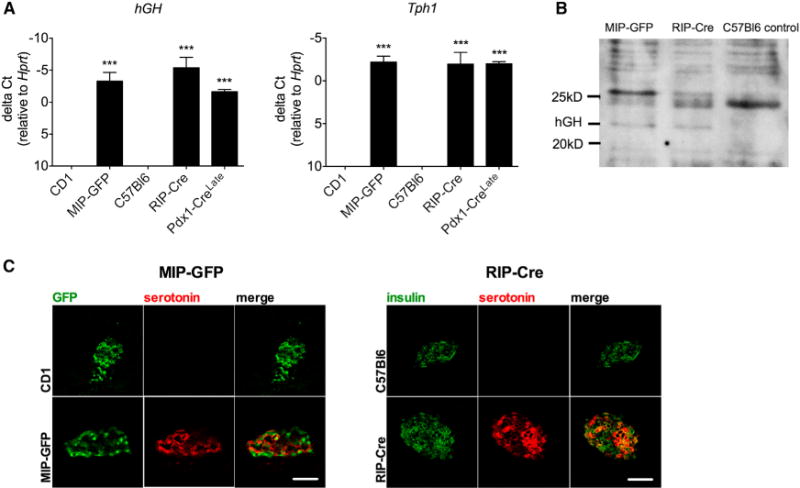
(A) hGH and Tph1 mRNA signal in isolated islets from MIP-GFP and RIP-Cre mice, quantified as delta Ct values relative to a housekeeping mRNA signal (Hprt). Data are represented as mean ± SEM, n = 3–6 mice per genotype, ***p < 0.001.
(B) Western blot analysis of hGH expression in islets from MIP-GFP, RIP-Cre, and C57Bl6 control mice. Predicted hGH weight = 22 kDa. GAPDH was used as a loading control. See also Figure S3.
(C) Representative immunofluorescence micrographs show serotonin immunoreactivity in islets from MIP-GFP and RIP-Cre mice, whereas no signal was observed in respective littermate controls. Scale bar, 50 μm. See also Figures S4 and S5.
DISCUSSION
The Cre/LoxP system is a powerful tool to conditionally inactivate or overexpress genes in transgenic animals with a number of specific advantages over whole-body knockouts. However, there have been many reports describing specific limitations of some Cre driver strains. These include variegated Cre expression in target organs (Gannon et al., 2000; Ryding et al., 2001), ectopic expression in undesired tissues (Delacour et al., 2004; Song et al., 2010; Wicksteed et al., 2010), and unwanted effects related to the integration site (Cartwright and Wang, 2009). For central nervous system and pancreas-specific Cre driver lines, these limitations and their likely causes have been reviewed recently (Harno et al., 2013; Magnuson and Osipovich, 2013). In particular for the RIP-Cre mouse model (Postic et al., 1999), a multicenter study showed glucose intolerance in these mice in the absence of genes targeted by loxP sites (Lee et al., 2006). Moreover, younger RIP-Cre mice exhibited β cell hypoplasia, whereas older mice showed β cell hyperplasia (Pomplun et al., 2007).
In the present study, we describe a mechanism whereby inclusion of an hGH minigene as a component of the transgene construct can impair β cell function. Since the hGH minigene is the second cistron in the transgene-encoded mRNA, it was believed for a long time that it was not expressed. Our studies provide compelling data otherwise. Expression of hGH may have a profound influence on the interpretation of certain types of experiments, especially those pertaining to the control of pancreatic β cell mass and the regulation of insulin secretion, both of which are very active fields of diabetes research.
In islets from Pdx1-CreLate, the MIP-GFP, and the RIP-Cre mouse models, we observed expression of hGH from the hGH minigene sequences placed downstream of the Cre or GFP coding regions (Brinster et al., 1988). As a result, hGH of the expected molecular weight (22 kDa) is synthesized and secreted from islets of these mice, causing Cre- or GFP-independent effects by autocrine/paracrine stimulation of β cells. An insertional effect has been postulated before (Lee et al., 2006; Pomplun et al., 2007) but is unlikely since we observe Tph1 expression and serotonin immunoreactivity in two other frequently used mouse lines (RIP-Cre and MIP-GFP). Figure 6 proposes a model in which locally secreted hGH acts as a lactogen by activating abundantly expressed PRLR on mouse β cells and initiating the JAK2/STAT5 signaling pathway. A first group of effects is therefore pregnancy like and includes induction of serotonin biosynthesis and a doubling of β cell mass and pancreatic insulin content. The most important of pregnancy-unrelated effects is a partial loss of GSIS, decreasing glucose tolerance. This loss of function could be partially caused by downregulation of GLUT2, a transporter responsible for the rapid equilibration between extra- and intracellular glucose concentration and thus the first step of glucose sensing in rodent islets (Guillam et al., 1997). This is in contrast with the pregnancy state, as β cell Glut2 levels have been reported to increase as part of the adaptive maternal response (Weinhaus et al., 1996). GLUT2 is also needed for rapid uptake of the β cell toxins STZ (Schnedl et al., 1994) and alloxan (De Vos et al., 1995), so it seems reasonable to attribute the protection of Pdx1-CreLate islets against STZ-induced diabetes to reduced toxin uptake rates caused by fewer GLUT2 channels (Figure 5). In addition, enhanced β cell mass and greater potential to regenerate new β cells may also be involved.
Figure 6. Model of hGH-Induced Phenotypic Changes in Pdx1-CreLate.
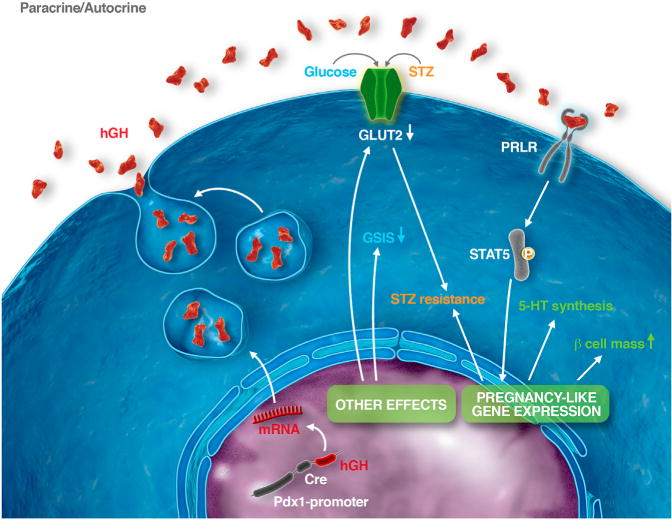
β Cells
Pdx1-promoter-driven expression of the hGH minigene causes biosynthesis and secretion of hGH, which exerts autocrine or paracrine effects after binding to PRLR on β cells. This causes STAT5 phosphorylation and pregnancy-like phenotypic changes, such as enhanced β cell mass and serotonin (5-HT) production. In addition, expression of the hGH minigene causes pregnancy-unrelated changes, such as reduction of GLUT2 expression and partial loss of glucose-induced insulin release. Lower GLUT2-mediated uptake and higher β cell mass protect against the diabetogenic effect of the β cell toxin STZ.
As the hGH minigene is frequently used to enhance expression of transgenes, we did a literature search for mouse lines generated using the same genetic strategy (Table 1). At the time of preparation of this manuscript, we listed a total of 22 mouse models. Due to the lack of information available for several other β cell-specific mouse strains, our table may be an underestima tion of the total amount of models involved. In some of the models listed in Table 1, expression of the hGH minigene was mentioned, at the level of either mRNA (Miyazaki et al., 2010; Postic et al., 1999; Sanvito et al., 1995) or immunoreactive protein (Klee et al., 2011). In none of them, however, was the potential influence on pancreatic islet morphology, β cell function, or glucose homeostasis considered.
The investigation of cellular and physiological mechanisms that regulate β cell mass and β cell function is currently a very active area of diabetes research that makes extensive use of genetically altered mice. Moreover, there is already a large body of experimental work that has made use of mice containing hGH minigene. Our current observations therefore seem to have potential implications on the interpretation of a large body of published data, in particular with respect to changes in functional β cell mass, insulin secretion, and protection against the diabetogenic effect of STZ.
EXPERIMENTAL PROCEDURES
Mice
Pdx1-CreLate transgenic mice (Herrera, 2000) (Dr. Herrera, University of Geneva, Switzerland) were crossed with C57BL/6J (Janvier) for at least eight generations. PRLR knockout mice (designated Prlr−/−) on a 129Sv background were described previously (Freemark et al., 2002). Pdx1-Crelate females were bred with Prlr+/−males to generate Pdx1-CreLate; Prlr+/−mice, which were subsequently crossed with Prlr+/− to obtain Pdx1-CreLate; Prlr−/− mice. Institutional guidelines for animal welfare and experimental conduct were followed. All experiments with laboratory animals were approved by the committee for animal welfare at the KU Leuven. RIP-Cre mice (Postic et al., 1999) and MIP-GFP mice (Hara et al., 2003) were maintained on C57Bl and CD1 backgrounds, respectively. All animal procedures and husbandry were approved by the Vanderbilt University Institutional Animal Care and Use Committee.
Cell Cultures
MIN6 cells were incubated in Dulbecco’s modified Eagle’s medium (DMEM; 25 mmol/l glucose, 2% fetal calf serum [FCS], 4 mmol/l glutamax) (Invitrogen, Gibco) with hGH (Calbiochem), mGH, or oPL (Prospec). For the experiments with the PRLR antagonist Δ1-9-G129R-hPRL MIN6 cells were preincubated for 30 min with the antagonist. Thereafter, recombinant hGH (from Vincent Goffin) was added to the medium. RNA was extracted 24 hr later.
Islet Isolation
Pancreatic islets were isolated after infusion and digestion of the pancreata by collagenase P (Roche) as described previously (Lemaire et al., 2009).
Islet Monolayers
Islet monolayers were performed as described previously (Schraenen et al., 2010b). Isolated islets were cultured for 7 days in RPMI medium (10% [v/v] de-complemented FCS, 100 U/ml penicillin, 100 μg/ml streptomycin, 4 mmol/l glutamax, 10 mM HEPES [pH 7.4]) to form monolayers. On day 7, they were stimulated with 0 or 500 ng/ml oPL, mGH, or hGH.
Microarray Expression Analysis
Microarray analysis was performed on RNA of isolated islets using MoGene_1.0_ST arrays (Affymetrix). For RNA extraction, see Supplemental Experimental Procedures. Total islet RNA (100 ng) was used to hybridize the arrays according to manufacturer’s manual 701880Rev4 as described previously (Lemaire et al., 2009). Samples were analyzed pairwise, using p < 0.001 and fold change ≥ 1.5 as selection criteria. A list of up- and downregulated genes in islets isolated from Pdx1-CreLate transgenic mice versus control mice is provided as an excel table (see Table S1), and genes related to pregnancy are marked.
Quantitative RT-PCR
Following cDNA synthesis using a reverse transcriptase kit (RevertAid H Minus; Fermentas), qRT-PCR (Absolute QPCR mix; Abgene, Thermo Fisher Scientific) was performed on a Rotorgene (Corbett Research). For primers and probes, see Supplemental Experimental Procedures. For Tph2, a Taqman gene expression assay (Mm00557717_m1; Applied Biosystems) was used. When a probe was used, data were analyzed according to the Pfaffl method; without a probe, delta Ct was used.
Histology
Pancreata were fixed overnight in 4% formaldehyde and embedded in paraffin. Sections were rehydrated and heated for 20 min in Target Retrieval Solution (pH 6.1, Dako). After blocking with 20% normal goat serum (Dako) in PBS, slides were incubated with 1/1,000 anti-hGH monoclonal antibody (ab15317, Abcam) in Antibody Diluent (Dako). For double immunofluorescent labeling, 1/50,000 rabbit anti-serotonin (Immunostar, #20080) or 1/2,000 polyclonal anti-GLUT2 (07-1402, Millipore) was combined with 1/10,000 diluted guinea pig anti-insulin antibody (a gift of Dr. Van Schravendijk, VUB, Brussels) and detected with anti-rabbit Cy3 and anti-guinea pig FITC, respectively (both from Jackson ImmunoResearch Laboratories). For MIP-GFP and RIP-Cre mice, frozen sections were stained with 1/10,000 rabbit anti-serotonin or 1/200 mouse anti-hGH (blocking with Mouse on Mouse [M.O.M.] Basic Kit from Vector Laboratories) and costained with 1/2,000 guinea pig anti-insulin in sections from RIP-Cre mice. For MIP-GFP, direct fluorescence for GFP was used to detect β cells.
Glucose and Insulin Tolerance Tests
Overnight (glucose tolerance test [GTT]) or 6 hr fasted (insulin tolerance test [ITT]) mice were injected i.p. with 2.5 mg/g BW D-glucose or 0.75 mU/g BW human insulin, respectively, and glycemia was measured by tail-blood analysis using a Contour glucose meter (Bayer). For GTT, tail blood was collected at the indicated time points, and plasma was analyzed.
Islet Serotonin and GABA Content
A total of 80 islets were homogenized by 3 min sonication in a buffer containing 0.01 mol/l HCl, 1 mmol/l EDTA, and 4 mmol/l sodium metabisulfite. After centrifugation (20,000 × g) for 15 min at 4°C and addition of 0.1% (w/v) ascorbic acid (Acros), lysates were stored at −80°C. Serotonin and GABA concentrations were determined via high-performance liquid chromatography (HPLC) (see Supplemental Experimental Procedures).
Islet hGH Content and Release
Freshly isolated islets were incubated in batches of 100 at 37°C. Incubation was in HEPES Krebs buffer (20 mmol/l HEPES [pH 7.4], 119 mmol/l NaCl, 4.75 mmol/l KCl, 2.5 mmol/l CaCl2, 1.2 mmol/l MgSO4, 1.2 mmol/l KH2PO4, 5 mmol/l NaHCO3, 0.5% [w/v] BSA) containing 20 mmol/l glucose. After 1 hr, half of the medium was removed for measurement of hGH release. For the content, Triton X-100 (final concentration: 0.5%) was added to the other half of the medium with the islets. Islets were sonicated for 3 min, and lysates were stored at −20°C. To measure concentrations, an hGH ELISA was used (Invitrogen).
Islet Insulin Content and Release
For insulin secretion measurements, size-matched islets (n = 5 per tube) were placed in glass tubes containing HEPES Krebs solution containing 0.5% BSA supplemented with glucose 5 mM (G5), 20 mM (G20), or G20 with 250 μM IBMX. Supernatant was collected after 1 hr incubation at 37°C. The islets were sonicated for 3 min after adding acid ethanol (final concentration: 75% EtOH, 0.1 N HCl, 1% Triton). Samples were stored at −20°C, and the ELISA kit used for insulin determination was from Crystal Chem.
Total Pancreas Insulin Content
Pancreata were dissected, and acid-ethanol extracts were diluted 1/1,000 in PBS and analyzed for insulin using an insulin high-range ELISA (Mercodia). Absolute insulin content per pancreas as well as relative content (corrected for pancreas weight) were quantified.
β Cell Mass Quantification
Total pancreas from 24-week-old Pdx1-Crelate and littermate control mice (four males per group) was processed, and six sections separated by 200 μm were stained for insulin as described above. The total surface area of insulin-positive cells (in pixels) was quantified with Zeiss Axiovision software (Micro Imaging). The relative insulin surface area per section (total insulin area [pixels]/total pancreas area [pixels]) was multiplied by the pancreas weight (mg) to obtain the β cell mass (mg).
Western Blot
Islets were isolated as described above and homogenized in lysis buffer (Cell Signaling Technology) by sonication. Protein extracts were separated by SDS-PAGE (10% [v/v] Bis/Tris gel; Life Technologies), blotted on a nitrocellulose membrane, blocked in 4% (w/v) milk, and incubated with primary antibody (anti-hGH, ab15317, 1/1,000; anti-GAPDH, 1/15,000; clone 6C5, both from Abcam). The blot was subsequently incubated with peroxidase-conjugated secondary antibody (Dako), and proteins were detected using the Western Lightning ECL System (PerkinElmer). For hGH staining on islets from MIP-GFP and RIP-Cre mice, a similar protocol was used with adjustments: 10 μg of islet protein sample in 1× Laemmli sample buffer was resolved on 15% SDS-PAGE and transferred to polyvinylidene difluoride membranes (EMD Millipore).
Statistical Methods
When not differently stated in the legend or text, data are presented as mean ± SEM, and significance is shown on graphs as *p < 0.05, **p < 0.01, or p < 0.001.
Supplementary Material
Acknowledgments
This work was financially supported by the Juvenile Diabetes Research Foundation (JDRF grant 1_2010_393), the Flemish Fund for Scientific Research (FWO grants G.0672.12 and G.A103.11 and fellowship to A.S.), and the Katholieke Universiteit Leuven (GOA/14/010 and GOA/12/016). B.B., V.P.E.G.P., and J.V.S. are fellows of “IWT-Vlaanderen.” The authors thank Ria Berckmans, Gino De Smet, Florence Boutillon, and Nicole Buelens for technical support, Pedro Herrera (University of Geneva) for supplying the Pdx1-CreLate strain, Jegan Iyyathurai for isolating bovine corneal endothelial cells, and Idoya Lahortiga and Luk Cox from somersault18:24 for Figure 6.
Footnotes
ACCESSION NUMBERS
Microarray data were deposited in the Gene Expression Omnibus Database of the National Centre for Biotechnology Information under the accession number GSE50851.
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, five figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.cmet.2014.11.004.
References
- Bernichtein S, Kayser C, Dillner K, Moulin S, Kopchick JJ, Martial JA, Norstedt G, Isaksson O, Kelly PA, Goffin V. Development of pure prolactin receptor antagonists. J Biol Chem. 2003;278:35988–35999. doi: 10.1074/jbc.M305687200. [DOI] [PubMed] [Google Scholar]
- Bone AJ, Taylor KW. Mitabolic adaptation to pregnancy shown by increased biosynthesis of insulin in islets of Langerhans isolated from pregnant rat. Nature. 1976;262:501–502. doi: 10.1038/262501a0. [DOI] [PubMed] [Google Scholar]
- Brelje TC, Svensson AM, Stout LE, Bhagroo NV, Sorenson RL. An immunohistochemical approach to monitor the prolactin-induced activation of the JAK2/STAT5 pathway in pancreatic islets of Langerhans. J Histochem Cytochem. 2002;50:365–383. doi: 10.1177/002215540205000308. [DOI] [PubMed] [Google Scholar]
- Brinster RL, Allen JM, Behringer RR, Gelinas RE, Palmiter RD. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci USA. 1988;85:836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright EJ, Wang X. In: Transgenesis. Walker JM, Rapley R, editors. Cambridge: Royal Society of Chemistry; 2009. pp. 390–491. [Google Scholar]
- Charollais A, Gjinovci A, Huarte J, Bauquis J, Nadal A, Martín F, Andreu E, Sánchez-Andrés JV, Calabrese A, Bosco D, et al. Junctional communication of pancreatic beta cells contributes to the control of insulin secretion and glucose tolerance. J Clin Invest. 2000;106:235–243. doi: 10.1172/JCI9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos A, Heimberg H, Quartier E, Huypens P, Bouwens L, Pipeleers D, Schuit F. Human and rat beta cells differ in glucose transporter but not in glucokinase gene expression. J Clin Invest. 1995;96:2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacour A, Népote V, Trumpp A, Herrera PL. Nestin expression in pancreatic exocrine cell lineages. Mech Dev. 2004;121:3–14. doi: 10.1016/j.mod.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Fleenor D, Petryk A, Driscoll P, Freemark M. Constitutive expression of placental lactogen in pancreatic beta cells: effects on cell morphology, growth, and gene expression. Pediatr Res. 2000;47:136–142. doi: 10.1203/00006450-200001000-00023. [DOI] [PubMed] [Google Scholar]
- Freemark M. Regulation of maternal metabolism by pituitary and placental hormones: roles in fetal development and metabolic programming. Horm Res. 2006;65(3):41–49. doi: 10.1159/000091505. [DOI] [PubMed] [Google Scholar]
- Freemark M, Avril I, Fleenor D, Driscoll P, Petro A, Opara E, Kendall W, Oden J, Bridges S, Binart N, et al. Targeted deletion of the PRL receptor: effects on islet development, insulin production, and glucose tolerance. Endocrinology. 2002;143:1378–1385. doi: 10.1210/endo.143.4.8722. [DOI] [PubMed] [Google Scholar]
- Gannon M, Herrera PL, Wright CV. Mosaic Cre-mediated recombination in pancreas using the pdx-1 enhancer/promoter. Genesis. 2000;26:143–144. doi: 10.1002/(sici)1526-968x(200002)26:2<143::aid-gene13>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Gannon G, Mandriota SJ, Cui L, Baetens D, Pepper MS, Christofori G. Overexpression of vascular endothelial growth factor-A165 enhances tumor angiogenesis but not metastasis during beta-cell carcinogenesis. Cancer Res. 2002;62:603–608. [PubMed] [Google Scholar]
- Garcia-Ocaña A, Takane KK, Syed MA, Philbrick WM, Vasavada RC, Stewart AF. Hepatocyte growth factor overexpression in the islet of transgenic mice increases beta cell proliferation, enhances islet mass, and induces mild hypoglycemia. J Biol Chem. 2000;275:1226–1232. doi: 10.1074/jbc.275.2.1226. [DOI] [PubMed] [Google Scholar]
- Goffin V, Shiverick KT, Kelly PA, Martial JA. Sequence-function relationships within the expanding family of prolactin, growth hormone, placental lactogen, and related proteins in mammals. Endocr Rev. 1996;17:385–410. doi: 10.1210/edrv-17-4-385. [DOI] [PubMed] [Google Scholar]
- Green IC, Taylor KW. Effects of pregnancy in the rat on the size and insulin secretory response of the islets of Langerhans. J Endocrinol. 1972;54:317–325. doi: 10.1677/joe.0.0540317. [DOI] [PubMed] [Google Scholar]
- Green IC, Perrin D, Howell SL. Insulin release in isolated islets of Langerhans of pregnant rats. Relationship between glucose metabolism and cyclic AMP. Horm Metab Res. 1978;10:32–35. doi: 10.1055/s-0028-1093476. [DOI] [PubMed] [Google Scholar]
- Guillam MT, Hümmler E, Schaerer E, Yeh JI, Birnbaum MJ, Beermann F, Schmidt A, Dériaz N, Thorens B. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat Genet. 1997;17:327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- Hara M, Wang X, Kawamura T, Bindokas VP, Dizon RF, Alcoser SY, Magnuson MA, Bell GI. Transgenic mice with green fluorescent protein-labeled pancreatic beta -cells. Am J Physiol Endocrinol Metab. 2003;284:E177–E183. doi: 10.1152/ajpendo.00321.2002. [DOI] [PubMed] [Google Scholar]
- Hara M, Bindokas V, Lopez JP, Kaihara K, Landa LR, Jr, Harbeck M, Roe MW. Imaging endoplasmic reticulum calcium with a fluorescent biosensor in transgenic mice. Am J Physiol Cell Physiol. 2004;287:C932–C938. doi: 10.1152/ajpcell.00151.2004. [DOI] [PubMed] [Google Scholar]
- Hara M, Dizon RF, Glick BS, Lee CS, Kaestner KH, Piston DW, Bindokas VP. Imaging pancreatic beta-cells in the intact pancreas. Am J Physiol Endocrinol Metab. 2006;290:E1041–E1047. doi: 10.1152/ajpendo.00365.2005. [DOI] [PubMed] [Google Scholar]
- Harno E, Cottrell EC, White A. Metabolic pitfalls of CNS Cre-based technology. Cell Metab. 2013;18:21–28. doi: 10.1016/j.cmet.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Hodish I, Liu M, Rajpal G, Larkin D, Holz RW, Adams A, Liu L, Arvan P. Misfolded proinsulin affects bystander proinsulin in neonatal diabetes. J Biol Chem. 2010;285:685–694. doi: 10.1074/jbc.M109.038042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstad M, Sandler S. Prolactin protects against diabetes induced by multiple low doses of streptozotocin in mice. J Endocrinol. 1999;163:229–234. doi: 10.1677/joe.0.1630229. [DOI] [PubMed] [Google Scholar]
- Huang C, Snider F, Cross JC. Prolactin receptor is required for normal glucose homeostasis and modulation of beta-cell mass during pregnancy. Endocrinology. 2009;150:1618–1626. doi: 10.1210/en.2008-1003. [DOI] [PubMed] [Google Scholar]
- Jackerott M, Møldrup A, Thams P, Galsgaard ED, Knudsen J, Lee YC, Nielsen JH. STAT5 activity in pancreatic beta-cells influences the severity of diabetes in animal models of type 1 and 2 diabetes. Diabetes. 2006;55:2705–2712. doi: 10.2337/db06-0244. [DOI] [PubMed] [Google Scholar]
- Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, Fujitani Y, Kawamori R, Miyatsuka T, Kosaka Y, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee P, Lamprianou S, Charollais A, Caille D, Sarro R, Cederroth M, Haefliger JA, Meda P. Connexin implication in the control of the murine beta-cell mass. Pediatr Res. 2011;70:142–147. doi: 10.1203/PDR.0b013e318220f106. [DOI] [PubMed] [Google Scholar]
- Kopfstein L, Veikkola T, Djonov VG, Baeriswyl V, Schomber T, Strittmatter K, Stacker SA, Achen MG, Alitalo K, Christofori G. Distinct roles of vascular endothelial growth factor-D in lymphangiogenesis and metastasis. Am J Pathol. 2007;170:1348–1361. doi: 10.2353/ajpath.2007.060835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Ristow M, Lin X, White MF, Magnuson MA, Hennighausen L. RIP-Cre revisited, evidence for impairments of pancreatic beta-cell function. J Biol Chem. 2006;281:2649–2653. doi: 10.1074/jbc.M512373200. [DOI] [PubMed] [Google Scholar]
- Lemaire K, Ravier MA, Schraenen A, Creemers JWM, Van de Plas R, Granvik M, Van Lommel L, Waelkens E, Chimienti F, Rutter GA, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA. 2009;106:14872–14877. doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51:216–226. doi: 10.1007/s00125-007-0886-7. [DOI] [PubMed] [Google Scholar]
- Lu J, Gustavsson N, Li Q, Radda GK, Südhof TC, Han W. Generation of transgenic mice for in vivo detection of insulin-containing granule exocytosis and quantification of insulin secretion. J Innov Opt Health Sci. 2009;2:397–405. [Google Scholar]
- Magnuson MA, Osipovich AB. Pancreas-specific Cre driver lines and considerations for their prudent use. Cell Metab. 2013;18:9–20. doi: 10.1016/j.cmet.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr R, Hu S, Yee SP, Kovacs MS, Lee TY, Dhanvantari S. Towards PET imaging of intact pancreatic beta cell mass: a transgenic strategy. Mol Imaging Biol. 2011;13:962–972. doi: 10.1007/s11307-010-0435-5. [DOI] [PubMed] [Google Scholar]
- Miyazaki J, Araki K, Yamato E, Ikegami H, Asano T, Shibasaki Y, Oka Y, Yamamura K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: special reference to expression of glucose transporter isoforms. Endocrinology. 1990;127:126–132. doi: 10.1210/endo-127-1-126. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Taniguchi H, Moritoh Y, Tashiro F, Yamamoto T, Yamato E, Ikegami H, Ozato K, Miyazaki J. Nuclear hormone retinoid X receptor (RXR) negatively regulates the glucose-stimulated insulin secretion of pancreatic β-cells. Diabetes. 2010;59:2854–2861. doi: 10.2337/db09-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møldrup A, Petersen ED, Nielsen JH. Effects of sex and pregnancy hormones on growth hormone and prolactin receptor gene expression in insulin-producing cells. Endocrinology. 1993;133:1165–1172. doi: 10.1210/endo.133.3.8365359. [DOI] [PubMed] [Google Scholar]
- Orban PC, Chui D, Marth JD. Tissue- and site-specific DNA recombination in transgenic mice. Proc Natl Acad Sci USA. 1992;89:6861–6865. doi: 10.1073/pnas.89.15.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Wang X, Chen Z, Powers AC, Magnuson MA, Head WS, Piston DW, Bell GI. Optical imaging of pancreatic beta cells in living mice expressing a mouse insulin I promoter-firefly luciferase transgene. 2005:80–86. doi: 10.1002/gene.20157. [DOI] [PubMed] [Google Scholar]
- Parsons JA, Brelje TC, Sorenson RL. Adaptation of islets of Langerhans to pregnancy: increased islet cell proliferation and insulin secretion correlates with the onset of placental lactogen secretion. Endocrinology. 1992;130:1459–1466. doi: 10.1210/endo.130.3.1537300. [DOI] [PubMed] [Google Scholar]
- Parsons JA, Bartke A, Sorenson RL. Number and size of islets of Langerhans in pregnant, human growth hormone-expressing transgenic, and pituitary dwarf mice: effect of lactogenic hormones. Endocrinology. 1995;136:2013–2021. doi: 10.1210/endo.136.5.7720649. [DOI] [PubMed] [Google Scholar]
- Pomplun D, Florian S, Schulz T, Pfeiffer AFH, Ristow M. Alterations of pancreatic beta-cell mass and islet number due to Ins2-controlled expression of Cre recombinase: RIP-Cre revisited; part 2. Horm Metab Res. 2007;39:336–340. doi: 10.1055/s-2007-976538. [DOI] [PubMed] [Google Scholar]
- Postic C, Shiota M, Niswender KD, Jetton TL, Chen Y, Moates JM, Shelton KD, Lindner J, Cherrington AD, Magnuson MA. Dual roles for glucokinase in glucose homeostasis as determined by liver and pancreatic beta cell-specific gene knock-outs using Cre recombinase. J Biol Chem. 1999;274:305–315. doi: 10.1074/jbc.274.1.305. [DOI] [PubMed] [Google Scholar]
- Rieck S, White P, Schug J, Fox AJ, Smirnova O, Gao N, Gupta RK, Wang ZV, Scherer PE, Keller MP, et al. The transcriptional response of the islet to pregnancy in mice. Mol Endocrinol. 2009;23:1702–1712. doi: 10.1210/me.2009-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryding AD, Sharp MG, Mullins JJ. Conditional transgenic technologies. J Endocrinol. 2001;171:1–14. doi: 10.1677/joe.0.1710001. [DOI] [PubMed] [Google Scholar]
- Sanvito F, Nichols A, Herrera PL, Huarte J, Wohlwend A, Vassalli JD, Orci L. TGF-beta 1 overexpression in murine pancreas induces chronic pancreatitis and, together with TNF-alpha, triggers insulin-dependent diabetes. Biochem Biophys Res Commun. 1995;217:1279–1286. doi: 10.1006/bbrc.1995.2906. [DOI] [PubMed] [Google Scholar]
- Schnedl WJ, Ferber S, Johnson JH, Newgard CB. STZ transport and cytotoxicity. Specific enhancement in GLUT2-expressing cells. Diabetes. 1994;43:1326–1333. doi: 10.2337/diab.43.11.1326. [DOI] [PubMed] [Google Scholar]
- Schraenen A, de Faudeur G, Thorrez L, Lemaire K, Van Wichelen G, Granvik M, Van Lommel L, in’t Veld P, Schuit F. mRNA expression analysis of cell cycle genes in islets of pregnant mice. Diabetologia. 2010a;53:2579–2588. doi: 10.1007/s00125-010-1912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraenen A, Lemaire K, de Faudeur G, Hendrickx N, Granvik M, Van Lommel L, Mallet J, Vodjdani G, Gilon P, Binart N, et al. Placental lactogens induce serotonin biosynthesis in a subset of mouse beta cells during pregnancy. Diabetologia. 2010b;53:2589–2599. doi: 10.1007/s00125-010-1913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan JD, Anaya PA, Parsons JA, Sorenson RL. Increased dye coupling in pancreatic islets from rats in late-term pregnancy. Diabetes. 1988;37:908–911. doi: 10.2337/diab.37.7.908. [DOI] [PubMed] [Google Scholar]
- Soares MJ. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol. 2004;2:51. doi: 10.1186/1477-7827-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Xu Y, Hu X, Choi B, Tong Q. Brain expression of Cre recombinase driven by pancreas-specific promoters. Genesis. 2010;48:628–634. doi: 10.1002/dvg.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson RL, Brelje TC, Hegre OD, Marshall S, Anaya P, Sheridan JD. Prolactin (in vitro) decreases the glucose stimulation threshold, enhances insulin secretion, and increases dye coupling among islet B cells. Endocrinology. 1987;121:1447–1453. doi: 10.1210/endo-121-4-1447. [DOI] [PubMed] [Google Scholar]
- Sorenson RL, Garry DG, Brelje TC. Structural and functional considerations of GABA in islets of Langerhans. Beta-cells and nerves. Diabetes. 1991;40:1365–1374. doi: 10.2337/diab.40.11.1365. [DOI] [PubMed] [Google Scholar]
- Sylvestersen KB, Herrera PL, Serup P, Rescan C. Fgf9 signalling stimulates Spred and Sprouty expression in embryonic mouse pancreas mesenchyme. Gene Expr Patterns. 2011;11:105–111. doi: 10.1016/j.gep.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Thorens B, Sarkar HK, Kaback HR, Lodish HF. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988;55:281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Van Assche FA. Quantitative morphologic and histoenzymatic study of the endocrine pancreas in nonpregnant and pregnant rats. Am J Obstet Gynecol. 1974;118:39–41. doi: 10.1016/s0002-9378(16)33642-0. [DOI] [PubMed] [Google Scholar]
- Vasavada RC, Cavaliere C, D’Ercole AJ, Dann P, Burtis WJ, Madlener AL, Zawalich K, Zawalich W, Philbrick W, Stewart AF. Overexpression of parathyroid hormone-related protein in the pancreatic islets of transgenic mice causes islet hyperplasia, hyperinsulinemia, and hypoglycemia. J Biol Chem. 1996;271:1200–1208. doi: 10.1074/jbc.271.2.1200. [DOI] [PubMed] [Google Scholar]
- Vasavada RC, Garcia-Ocaña A, Zawalich WS, Sorenson RL, Dann P, Syed M, Ogren L, Talamantes F, Stewart AF. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation, and hypoglycemia. J Biol Chem. 2000;275:15399–15406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- Weinhaus AJ, Stout LE, Sorenson RL. Glucokinase, hexokinase, glucose transporter 2, and glucose metabolism in islets during pregnancy and prolactin-treated islets in vitro: mechanisms for long term up-regulation of islets. Endocrinology. 1996;137:1640–1649. doi: 10.1210/endo.137.5.8612496. [DOI] [PubMed] [Google Scholar]
- Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Wicksteed B, Brissova M, Yan W, Opland DM, Plank JL, Reinert RB, Dickson LM, Tamarina NA, Philipson LH, Shostak A, et al. Conditional gene targeting in mouse pancreatic β-Cells: analysis of ectopic Cre transgene expression in the brain. Diabetes. 2010;59:3090–3098. doi: 10.2337/db10-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Degenstein L, Cao Y, Stein J, Osei K, Wang J. β-Cells with relative low HIMP1 overexpression levels in a transgenic mouse line enhance basal insulin production and hypoxia/hypoglycemia tolerance. PLoS ONE. 2012;7:e34126. doi: 10.1371/journal.pone.0034126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


