Abstract
Purpose
To analyze the renal function outcomes in patients undergoing radiation therapy for neuroblastoma.
Methods and Materials
The clinical metrics of renal function were analyzed in patients undergoing radiation therapy for high-risk neuroblastoma from 2000 to 2015. The blood urea nitrogen (BUN) and creatinine values before radiation therapy were compared with last available follow-up values and analyzed with the clinical circumstances, including follow-up length, age at primary irradiation, nephrectomy, and radiation technique. The creatinine clearance was estimated using the Shull method.
Results
With a median follow-up period of 3.5 years, none of the 266 patients studied developed a chronic renal insufficiency. For all patients, the creatinine level increased from 0.44 to 0.51 mg/dL and the BUN increased from 10.53 to 15.52 mg/dL. Three patients required antihypertensive medication. The patients who underwent intensity modulated radiation therapy did not experience increased creatinine levels during the follow-up period; however, they had a reduced median follow-up length compared with patients treated with anteroposterior/posteroanterior beams (4.7 vs 3.3 years). A longer follow-up length was associated with an increased creatinine level. The preradiation therapy creatinine level increased with patient age, similar to that of the last follow-up creatinine level, suggesting that the changes in creatinine could likely be explained by physiologic increases associated with aging rather than radiation-induced renal damage. The creatinine clearance did not decrease in any circumstance.
Conclusions
The present cohort had excellent renal outcomes after radiation therapy for neuroblastoma. No patient developed chronic renal insufficiency, and the small increases in BUN and creatinine we observed correlated, as expected, with increases in patient age. The results of the present study revealed a possible advantage for intensity modulated radiation therapy in preserving renal function; however, the follow-up length is a recognized confounding variable. The kidneys are vital structures to consider when planning radiation therapy for neuroblastoma patients, and we have found encouraging evidence that modern techniques to spare them in the setting of multiple treatment-related insults have been successful.
Introduction
Neuroblastoma is the third most common malignancy of childhood and the most common malignancy of infants, with the vast majority of cases developing before age 5 years (1). Treatment of high-risk disease is multidisciplinary, usually requiring surgery, aggressive chemotherapy, and radiation therapy (RT) to achieve the optimal response. However, the use of RT in young children, paired with the critical structures at risk in the abdomen, where most neuroblastomas arise, necessitates firm consideration of the potential adverse effects of therapy, particularly in the setting of additional nephrotoxic insults resulting from surgery, chemotherapy, and management of treatment-associated conditions such as infection.
The kidneys are the most important organs at risk when treating abdominal cancer with RT (2, 3). In infants and children, greater consideration is required to ensure the damage to the developing organs is minimal (4). Experiences in Wilms tumor have given some insight into renal damage from abdominal RT in the pediatric population. In a study of 108 patients undergoing whole kidney RT after contralateral nephrectomy in Wilms tumor in the 1960s, reduced creatinine clearance (CrCl) was observed with an increasing radiation dose, with 73% of patients receiving ≥23 Gy experiencing a CrCl of <54 mg/mL (5). Doses of ≤12 Gy were well tolerated, with 70% of patients studied retaining a normal CrCl. Six patients in the study developed overt renal failure, all of whom had received >23 Gy. The incidence of end-stage renal disease in patients treated for Wilms tumor has been remarkably low (6). Total body irradiation of children in preparation for stem cell transplantation could similarly show evidence of renal insufficiency owing to the significant doses to the whole kidneys bilaterally. In a study of 9 patients who had undergone 8 to 12 Gy total body irradiation, 4 developed a reduced estimated glomerular filtration rate (eGFR) at 5 years of follow-up (7). Although Wilms tumor and total body irradiation patients undergoing RT represent an opportunity for understanding the potential renal risks in neuroblastoma patients, differences in the RT technique, tumor locations, surgical procedures, and systemic therapy limit extrapolation of the findings.
Relatively few data are available on the renal outcomes in neuroblastoma patients. A small study of 3 patients aged <6 months who had received 12 to 14 Gy to the entire kidneys bilaterally showed reductions in the eGFR at 6 months and 2 years after treatment, and 1 patient had abnormal findings on a pyelogram thought to be consistent with radiation damage (8). Other studies of renal outcomes in pediatric malignancy did not consider neuroblastoma specifically, limiting disease-specific interpretations. The present study was conducted to elucidate the efficacy of modern RT for neuroblastoma in preserving renal function.
Methods and Materials
Patient selection
A retrospective review with institutional review board approval was conducted to evaluate the renal function outcomes in International Neuroblastoma Staging System high-risk stage 3 and 4 neuroblastoma patients who received primary RT at Memorial Sloan Kettering Cancer Center from 2000 to 2015. No limitations were included for age or minimum post-treatment survival. A total of 267 patients were identified; 1 patient who did not have blood urea nitrogen (BUN) or creatinine data identifiable in the medical record before RT was excluded.
Biochemical endpoints
Renal function was assessed using available biochemical markers of renal function, including serum creatinine, BUN, and CrCl. The BUN and creatinine values closest to before the start of RT were used as the “pre-RT” values. The most recent BUN and creatinine values available in the patient medical record were used as the “last follow-up” values. Aberrant values during acute illness at the time of death that represented a clear departure from the relative baseline were not considered. In these cases, the values before the onset of the acute illness consistent with the patient’s recent baseline levels were used. CrCl was calculated using the Shull formula: (Creatinine excretion rate × 100)/(serum creatinine), where Creatinine excretion rate = (0.035 × age) + 0.236 (9). Other CrCl estimations in children were evaluated; however, these generally require precise weight or height measurements, which were not available for many patients (10).
Radiographic endpoints
Each patient’s last available cross-sectional imaging study addressing the kidneys was compared against the study most recently available before RT. This was a contrast-enhanced computed tomography scan for the vast majority of patients; however, a few had undergone magnetic resonance imaging. Comments pertaining to kidney abnormalities were recorded. When an anomaly was present, imaging studies using the same modality were evaluated before RT to determine whether the anomaly predated RT.
Statistical analysis
Differences in creatinine, BUN, and CrCl before RT and at the last follow-up examination were analyzed using a paired Student t test and considered significant at P≤.05.
Results
Patient and treatment characteristics
A total of 267 neuroblastoma patients were identified who had undergone primary RT at our institution from 2000 to 2015. One patient was excluded because no post-RT creatinine values were available in the medical record; thus, 266 patients were included in the present analysis. Of these 266 patients, 148 (55.6%) were male. The median follow-up time for all patients was 3.5 years (range 0.04–14.3), at which point, the median age was 8.0 years (range 1.2–22.6). The patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics (n=266)
| Characteristic | n (%) |
|---|---|
| Sex | |
| Male | 148 (55.6) |
| Female | 118 (44.4) |
| Age at primary RT (y) | |
| Median | 4.1 |
| Mean | 4.7 |
| Age at last follow-up visit (y) | |
| Median | 8.0 |
| Mean | 8.9 |
| Follow-up duration (y) | |
| Median | 3.5 |
| Mean | 4.2 |
Abbreviation: RT = radiation therapy.
All but 14 patients, 5% of this cohort, were treated with the anti-GD2 antibody 3F8 or iodine-131-3F8. All patients had undergone chemotherapy before RT, as previously described (11). The systemic therapy exposures are summarized in Table 2. Eighty patients (30.1%) had undergone autologous stem cell transplantation as a part of their therapy. All but 5 patients had undergone surgery before RT. Twenty nephrectomies were performed, including 2 partial nephrectomies. The RT was either intensity modulated RT (IMRT) (34.6%) or anteroposterior/posteroanterior (AP/PA) beams (65.4%), and 248 patients (93.2%) underwent primary abdominal RT. The radiation dose was 21 to 22 Gy for 97.1% of the patients; 2 patients received <21 Gy (11 and 13 Gy, respectively) and 6 received a boost to 30 to 36 Gy. All but 8 patients received hyperfractionated RT in 1.5-Gy doses twice daily. The goal for kidney constraints was to keep the dose to approximately two-thirds of each kidney to <18 Gy. For patients with a single kidney, ≥80% was kept to <18 Gy. We analyzed the mean dose and volume of kidney receiving 20% (V20), 15% (V15), 10% (V10), and 5% (V5) of the prescription dose in a subset of patients with 2 intact kidneys who had undergone IMRT (Table 3). The median age at primary RT was 4.1 years (range 1.1–17.9).
Table 2.
Systemic therapy exposure
| Therapy | Patients (n) |
|---|---|
| 3F8 | 231 |
| I-131-3F8 | 31 |
| Cyclophosphamide | 265 |
| Etoposide | 263 |
| Cisplatin | 208 |
| Isotretinoin | 162 |
| Carboplatin | 129 |
| Vincristine | 120 |
| Doxorubicin | 113 |
| Melphalan | 110 |
| Thiotepa | 57 |
| Ifosfamide | 10 |
| Busulfan | 2 |
| Incomplete records | 14 |
Table 3.
Actual renal dose exposure in a subset of patients who underwent IMRT (n=49)
| Kidney | ||
|---|---|---|
|
|
||
| Dose | 1 | 2 |
| Mean (Gy) | 14.8 ± 2.8 (14.2) | 10.7 ± 2.9 (10.4) |
| V20 | 21.7 ± 9.7 (22.3) | 7.0 ± 6.7 (8.4) |
| V15 | 47.5 ± 18.3 (49.5) | 21.7 ± 13.8 (22.7) |
| V10 | 78.3 ± 21.3 (74.5) | 46.1 ± 23.9 (47.2) |
| V5 | 99.2 ± 14.8 (92.1) | 88.7 ± 21.2 (83.3) |
Abbreviations: IMRT = intensity modulated radiation therapy; Vx % = volume of kidney receiving x% of the prescription dose.
Data presented as mean ± standard deviation (median).
Change in renal function after RT
Statistically significant increases in serum creatinine and BUN were observed in all patients and in most clinical circumstances considered comparing the pre-RT values to the last available follow-up values (Table 4). The absolute increase for all patients in serum creatinine and BUN was 0.07 mg/dL and 4.99 mg/dL, respectively. The calculated CrCl had increased significantly at the last follow-up examination in most clinical circumstances, including all patients with an absolute increase of 8.0 mL/min.
Table 4.
Renal outcomes with associated clinical scenarios
| Creatinine (mg/dL) | BUN (mg/dL) | CrCl (mg/mL) | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| Variable | Pre-RT | Last FU | Pre-RT | Last FU | Pre-RT | Last FU |
| All patients (n=266) | 0.44 | 0.51 (P=.0005) | 10.53 | 15.52 (P=.000) | 107.91 | 115.91 (P=.0122) |
| Nephrectomy | ||||||
| Yes (n=20) | 0.66 | 0.58 (P=.3343) | 10.6 | 14.65 (P=.0189) | 92.56 | 103.13 (P=.085) |
| No (n=246) | 0.43 | 0.5 (P=.0000) | 10.52 | 15.59 (P=.000) | 109.16 | 116.95 (P=.0201) |
| FU length (y) | ||||||
| 0–4.9 (n=164) | 0.43 | 0.47 (P=.0542) | 10.78 | 14.46 (P=.000) | 115.9 | 109.89 (P=.0928) |
| 5–9.9 (n=76) | 0.45 | 0.54 (P=.0027) | 9.01 | 17.47 (P=.000) | 100.23 | 125.02 (P=.0989) |
| 10–14.9 (n=26) | 0.52 | 0.62 (P=.0133) | 12.92 | 17.19 (P=.0016) | 76.4 | 130.75 (P=.000) |
| Age at RT (y) | ||||||
| <5 (n=178) | 0.41 | 0.48 (P=.0025) | 10.94 | 15.3 (P=.000) | 105.74 | 112.14 (P=.0687) |
| >5 (n=88) | 0.52 | 0.55 (P=.0230) | 9.69 | 15.97 (P=.000) | 112.31 | 123.54 (P=.0378) |
| RT technique | ||||||
| AP/PA (n=174) | 0.46 | 0.53 (P=.000) | 10.47 | 15.49 (P=.000) | 101.66 | 116.22 (P=.0007) |
| IMRT (n=92) | 0.41 | 0.46 (P=.1345) | 10.69 | 15.47 (P=.000) | 119.66 | 114.40 (P=.1670) |
| Abdominal RT | ||||||
| No (n=18) | 0.46 | 0.48 (P=.2760) | 13.33 | 14.28 (P=.2854) | 115.42 | 131.76 (P=.1433) |
| Yes (n=248) | 0.44 | 0.51 (P=.0006) | 10.32 | 15.61 (P=.000) | 107.37 | 114.76 (P=.0217) |
| Radiographic evidence of RT-induced renal damage | ||||||
| Yes (n=35) | 0.42 | 0.47 (P=.0172) | 8.86 | 15.89 (P=.000) | 112.1 | 123.93 (P=.1723) |
| No (n=231) | 0.45 | 0.51 (P=.0015) | 10.78 | 15.47 (P=.000) | 107.28 | 114.69 (P=.0187) |
Abbreviations: AP/PA = anteroposterior/posteroanterior; BUN = blood urea nitrogen; CrCl = creatinine clearance; FU = follow-up; IMRT = intensity modulated radiation therapy; Last FU = most recent values available in patients’ medical records; Pre-RT = values closest to before the start of RT; RT = radiation therapy.
Patients who had undergone nephrectomy had an increased pre-RT creatinine baseline value (0.66 mg/dL with nephrectomy vs 0.43 mg/dL without nephrectomy). However, they did not have a change in serum creatinine at the last follow-up examination (0.58 mg/dL with nephrectomy vs 0.5 mg/dL without nephrectomy).
No statistically significant increase in creatinine was seen in patients with <5 years of follow-up after RT. In contrast, creatinine increases were manifest in patients with >5 years of follow-up. Patients aged <5 years during primary RT had a larger magnitude increase in creatinine at the last follow-up examination compared with those aged >5 years (0.07 mg/dL vs 0.03 mg/dL).
Patients who underwent IMRT did not have a significant increase in serum creatinine; however, patients treated with AP/PA beams had a significant increase of 0.07 mg/dL. The median follow-up time for the patients who received IMRT was 3.25 years compared with 4.70 years for patients treated with AP/PA beams. The difference resulted more from the recent adoption of IMRT as the standard of care for neuroblastoma patients. No change was found in the creatinine level in the 6 patients who had received a boost to 30 to 36 Gy.
BUN increased in all clinical circumstances considered, with the exception of the group of 18 patients who did not receive abdominal RT. The BUN increases were largely uniform in all groups, with a range of 3.68 to 8.46 mg/dL (Table 4).
Chronic renal dysfunction after RT
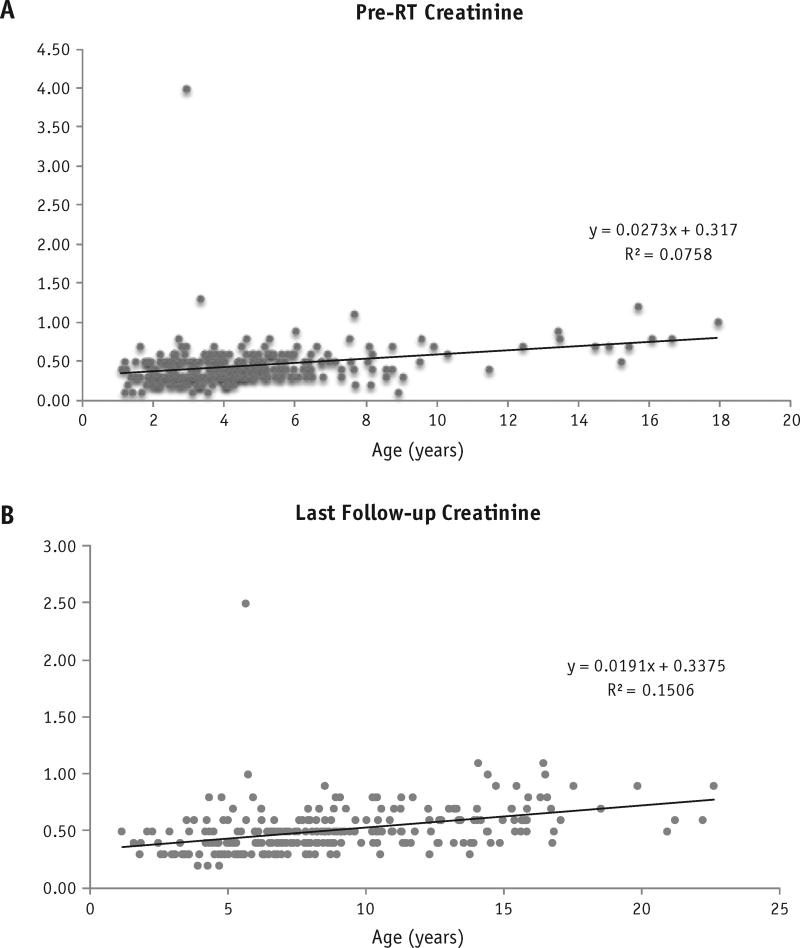
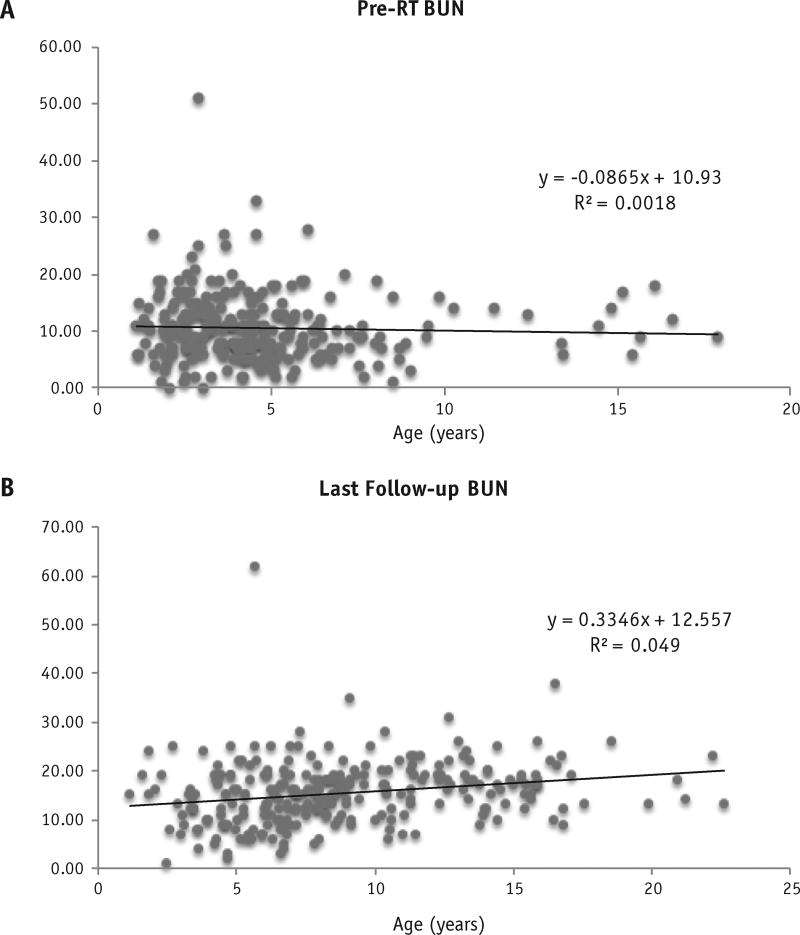
No patients experienced chronic renal dysfunction after RT. The maximum creatinine value observed at the last follow-up examination for any patient was 1.1 mg/dL, considered within the normal range for the patient’s age. The pre-RT creatinine values were plotted against patient age (Fig. 1A) to give an estimate of the expected increase in creatinine with normal physiologic aging in this patient population. The slope of the trend line was 0.0273, indicating an expected increase in serum creatinine with age in our patient population before RT. Similar trends were observed for BUN (Fig. 2). Similarly, the last follow-up creatinine values against age at follow-up were plotted, giving an estimation of the rate of creatinine increase in our patients after RT (Fig. 1B). The slope of this line was 0.0191, indicating a positive association between age and serum creatinine but less steep than our pre-RT values.
Fig. 1.
Creatinine was plotted against age at measurement for (A) pre-RT patients and (B) last follow-up after RT patients. Linear regression analysis was used to estimate R2, and linear line of best fit with associated best-fit equation is presented.
Fig. 2.
Blood urea nitrogen (BUN) was plotted against age at measurement for (A) pre-RT patients and (B) last follow-up after RT patients. Linear regression analysis was used to estimate R2, and linear line of best fit with associated best-fit equation is presented.
Of the 266 patients, 3 required initiation or escalation of antihypertensive therapy. One patient underwent dialysis for acute renal failure in the setting of dehydration and shortly after her first cycle of Hu3F8. This patient recovered fully with respect to her renal function and did not have subsequent episodes of renal failure with Hu3F8 therapy.
Radiographic evidence of renal damage
Renal atrophy was the most common renal anomaly noted by the diagnostic radiologists and was reported 25 times before RT and another 16 times after RT (Table 5). Renal scarring, cortical thinning, and low attenuation were noted on both the pre-RT and the post-RT images; however, these abnormalities tended to present after RT. Nonspecific “postoperative changes” were noted in 9 patients. Patients with radiographic evidence of renal damage, whether before or after RT, did not have worse biochemical outcomes than patients without radiographic evidence, with absolute creatinine increases at the last follow-up visit of 0.05 and 0.06 mg/dL, respectively.
Table 5.
Radiographic evidence of kidney damage
| Kidney damage | Changes predating RT (n=39) |
Changes potentially attributable to RT (n=35) |
|---|---|---|
| Renal atrophy | 25 | 16 |
| Renal scarring | 1 | 6 |
| Cortical thinning | 2 | 8 |
| Low attenuation | 1 | 3 |
| Postoperative changes | 9 | NA |
| Other | 3 | 2 |
Abbreviations: NA = not applicable; RT = radiation therapy.
Discussion
Neuroblastoma patients endure numerous nephrotoxic insults in the course of curative therapy, including surgery, aggressive chemotherapy, and RT. The kidneys are among the most radiosensitive of organs, with a TD 5/5 (normal tissue complication probability of 5% at 5 years) of 2300 cGy and TD 50/5 of 2800 cGy (3); thus, RT strategies have continued to focus on minimizing the dose to the kidneys while maximizing the chances for local control (12). In the present study, we evaluated patients treated for neuroblastoma during the past 15 years at Memorial Sloan Kettering Cancer Center to determine whether current RT techniques, in the setting of aggressive multidisciplinary therapy, produce acceptable renal outcomes. We are encouraged to report evidence of excellent renal outcomes in high-risk neuroblastoma patients who underwent modern multidisciplinary treatment.
An estimate of the potential for renal dysfunction from RT for pediatric malignancy can be informed by studies evaluating whole abdominal RT. In 60 patients with a median follow-up period of 9 years, no clinically significant decline in renal function could be attributed to RT, with the only significant variable affecting a decline in CrCl being age, a position we support with our findings (13). In an analysis of the late effects in 42 patients treated for Wilms tumor with 10 to 40 Gy whole abdomen or hemi-abdomen RT, only 1 patient developed renal insufficiency after nephrectomy in the contralateral nonirradiated kidney and treatment with nephrotoxic ifosfamide (14). The median follow-up period was 15 years.
Renal dysfunction after RT is a late effect with an uncertain period for development, particularly in children (4, 7, 15). Although our study had a strong representation of patients with >10 years of follow-up and without renal damage, continued monitoring of these patients is warranted to determine whether late renal damage eventually manifests. Stage 3 and 4 neuroblastoma patients seen at our institution’s long-term follow-up clinic from 1991 to 2003 were previously studied for evidence of renal dysfunction. With a median follow-up of 7.6 years after diagnosis, 6 had renal dysfunction, as defined by the Common Terminology Criteria for Adverse Events, version 3. Of these 6 patients, 5 had received abdominal RT (16). The renal dysfunction was grade 3 or 4 in 2, including 1 patient with hypertension and 1 with tubular dysfunction. One patient had grade 1 or 2 chronic renal insufficiency. The focus of that study was not on the effect of RT specifically and had a strong possibility for selection bias, because only the patients who attended the long-term follow-up clinic were included. However, the findings from that study do suggest that longer follow-up of our cohort might reveal more clinically significant renal insufficiency.
Dekkers et al (17) conducted an important study evaluating renal injury in long-term survivors of childhood cancer, in which they estimated the GFR, measured tubular function and albuminuria, and tracked the development of hypertension in patients with a median follow-up duration of 18.3 years. In their evaluation of patients treated with abdominal, total body, or spinal irradiation, they found no decrease in eGFR, no increase in albuminuria, and no changes in tubular function. Although the population studied was not limited to neuroblastoma survivors, this cohort had also received modern multidisciplinary therapy. Renal atrophy after abdominal RT is a known complication of renal irradiation (18). In a study of adult patients after abdominal chemo-RT, V10, V15, and V20 were all predictive of renal atrophy 1 year after RT (19). In our patients, renal anomalies were reported in 39 kidneys before RT; the most common was renal atrophy, which was observed in 25 kidneys. The number of atrophic kidneys in our patients continued to increase after RT, just as did observations of scarring, cortical thinning, and low attenuation of contrast dye. Encouragingly, no renal dysfunction was observable in patients with radiographic findings of renal damage.
The GFR has been proposed as the standard by which radiation-induced renal damage should be evaluated (4). However, the measured GFR was not a feasible marker in the present study, because it requires measurement of an ideal filtration marker such as inulin and is rarely performed in real practice. Our chosen method of CrCl estimation was the Shull method, which relies only on patient age and incorporates assumptions about the ideal body surface area into its formulation (9). In a comparison of various methods of estimating CrCl, the Shull method had the highest correlation coefficient with measured CrCl (10). CrCl in our study was surprisingly found to be increased at the last follow-up visit in most clinical circumstances. However, the Shull method produced improbable results at the extremes of our study, reporting impossibly high CrCl values, as well as CrCl values consistent with end-stage renal disease in healthy patients with no clinical renal disease. We, therefore, included it as an estimate, with many limitations, that showed no signal for treatment-related renal insufficiency.
Our findings showed a possible benefit for IMRT compared with AP/PA beams in preserving renal function in neuroblastoma patients. This finding might be in agreement with the findings from Paulino et al (20), who showed that IMRT plans did deliver lower doses to the kidney in patients with midline tumors. The effect of this finding must be tempered, however, by the lower median follow-up duration for the IMRT patients, because we found that the increases in creatinine we observed were related to follow-up length and patient age. Moreover, because we found no evidence of renal dysfunction in any patient, we could not state that patients treated with AP/PA beams experience renal damage and that those undergoing IMRT do not. We analyzed the actual kidney dose exposure in a subset of patients undergoing IMRT. The lack of renal dysfunction in our patients suggests that, at least during the follow-up period in the present study, mean kidney doses of approximately 15 Gy to the more exposed kidney (kidney 1) and 11 Gy to the less exposed kidney (kidney 2) are safe. Large V5 (>90% of both kidneys) and V10 (>50% of both kidneys) also appear to be safe.
Although we did not consider patients who had received proton therapy in the present study, its use for pediatric malignancies is increasing rapidly. This raises concern for nephrotoxicity in patients with neuroblastoma. For many patients with typical retroperitoneal tumors, sparing of the anterior abdominal organs with proton therapy comes at the expense of higher doses to the kidneys. Long-term follow-up of patients will be required to determine how the choice of radiation technology affects the nephrotoxicity in children with neuroblastoma.
Patients with high-risk neuroblastoma undergo many treatments and endure numerous nephrotoxic insults. All patients in our cohort had received multiple chemotherapy agents, including significant exposure to known nephrotoxic chemotherapy agents such as cisplatin, carboplatin, and ifosfamide. Our study results provide encouraging evidence that these patients are able to sustain healthy renal function despite a high burden of nephrotoxic chemotherapy and abdominal RT. Most of the patients in the present study received murine 3F8, an anti-GD2 antibody immunotherapy that has been a part of the standard of care for high-risk neuroblastoma at our institution since 2000 (21). Hypertension of unknown etiology has been observed in patients receiving this therapy, with grade 3 hypertension occurring in 5.5% of patients (22). The risk of long-term renal dysfunction with anti-GD2 immunotherapy has been previously unknown, and the findings from the present report provide reassuring evidence that the transient hypertension observed in some patients does not lead to chronic renal disease.
Conclusions
Patients receiving multidisciplinary therapy for high-risk neuroblastoma at our institution had excellent renal outcomes. The measures of renal function increased after RT; however, the rate was consistent with normal physiologic creatinine and BUN elevations, rather than being attributable to chronic renal damage from RT. Patients who underwent IMRT did not have a significant increase in creatinine after RT; however, the shorter follow-up duration is a recognized confounding variable. No patients developed clinically significant renal dysfunction.
Summary.
High-risk neuroblastoma patients require nephrotoxic chemotherapy and undergo often complicated abdominal and retroperitoneal surgical procedures and radiation therapy to maximize their chances for durable control and cure. We analyzed the data from 276 high-risk neuroblastoma patients and found no evidence of chronic renal damage resulting from their aggressive therapies. The present report provides evidence that conventional chemotherapy, novel immunotherapies, and modern radiation therapy do not cause long-term renal damage in neuroblastoma patients. We also reported the dose exposure for a subset of patients who underwent IMRT to guide practitioners in the dose constraints for modern RT for neuroblastoma patients.
Acknowledgments
Supported by NIH/NCI core grant P30 CA008748.
Footnotes
Conflict of interest: none.
References
- 1.Maris JM, Hogarty MD, Bagatell R, et al. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 2.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10–S19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 4.Dawson LA, Kavanagh BD, Paulino AC, et al. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S108–S115. doi: 10.1016/j.ijrobp.2009.02.089. [DOI] [PubMed] [Google Scholar]
- 5.Mitus A, Tefft M, Fellers FX. Long-term follow-up of renal functions of 108 children who underwent nephrectomy for malignant disease. Pediatrics. 1969;44:912–921. [PubMed] [Google Scholar]
- 6.Breslow NE, Collins AJ, Ritchey ML, et al. End stage renal disease in patients with Wilms tumor: Results from the National Wilms Tumor Study Group and the United States Renal Data System. J Urol. 2005;174:1972–1975. doi: 10.1097/01.ju.0000176800.00994.3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe Nemoto M, Isobe K, Togasaki G, et al. Delayed renal dysfunction after total body irradiation in pediatric malignancies. J Radiat Res. 2014;55:996–1001. doi: 10.1093/jrr/rru041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peschel RE, Chen M, Seashore J. The treatment of massive hepatomegaly in stage IV-S neuroblastoma. Int J Radiat Oncol Biol Phys. 1981;7:549–553. doi: 10.1016/0360-3016(81)90142-5. [DOI] [PubMed] [Google Scholar]
- 9.Shull BC, Haughey D, Koup JR, et al. A useful method for predicting creatinine clearance in children. Clin Chem. 1978;24:1167–1169. [PubMed] [Google Scholar]
- 10.Jacobson P, West N, Hutchinson RJ. Predictive ability of creatinine clearance estimate models in pediatric bone marrow transplant patients. Bone Marrow Transplant. 1997;19:481–485. doi: 10.1038/sj.bmt.1700688. [DOI] [PubMed] [Google Scholar]
- 11.Casey DL, Kushner BH, Cheung NK, et al. Local control with 21-Gy radiation therapy for high-risk neuroblastoma. Int J Radiat Oncol Biol Phys. 2016;96:393–400. doi: 10.1016/j.ijrobp.2016.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kushner BH, Wolden S, LaQuaglia MP, et al. Hyperfractionated low-dose radiotherapy for high-risk neuroblastoma after intensive chemotherapy and surgery. J Clin Oncol. 2001;19:2821–2828. doi: 10.1200/JCO.2001.19.11.2821. [DOI] [PubMed] [Google Scholar]
- 13.Irwin C, Fyles A, Wong CS, et al. Late renal function following whole abdominal irradiation. Radiother Oncol. 1996;38:257–261. doi: 10.1016/0167-8140(95)01702-x. [DOI] [PubMed] [Google Scholar]
- 14.Paulino AC, Wen BC, Brown CK, et al. Late effects in children treated with radiation therapy for Wilms’ tumor. Int J Radiat Oncol Biol Phys. 2000;46:1239–1246. doi: 10.1016/s0360-3016(99)00534-9. [DOI] [PubMed] [Google Scholar]
- 15.Knijnenburg SL, Mulder RL, Schouten-Van Meeteren AY, et al. Early and late renal adverse effects after potentially nephrotoxic treatment for childhood cancer. Cochrane Database Syst Rev. 2013;10:CD008944. doi: 10.1002/14651858.CD008944.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Laverdiere C, Cheung NK, Kushner BH, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer. 2005;45:324–332. doi: 10.1002/pbc.20331. [DOI] [PubMed] [Google Scholar]
- 17.Dekkers IA, Blijdorp K, Cransberg K, et al. Long-term nephrotoxicity in adult survivors of childhood cancer. Clin J Am Soc Nephrol. 2013;8:922–999. doi: 10.2215/CJN.09980912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran LK, Maturen KE, Feng MU, et al. Renal remodeling after abdominal radiation therapy: Parenchymal and functional changes. AJR Am J Roentgenol. 2014;203:W192–W198. doi: 10.2214/AJR.13.12149. [DOI] [PubMed] [Google Scholar]
- 19.Yang GY, May KS, Iyer RV, et al. Renal atrophy secondary to chemoradiotherapy of abdominal malignancies. Int J Radiat Oncol Biol Phys. 2010;78:539–546. doi: 10.1016/j.ijrobp.2009.07.1744. [DOI] [PubMed] [Google Scholar]
- 20.Paulino AC, Ferenci MS, Chiang KY, et al. Comparison of conventional to intensity modulated radiation therapy for abdominal neuroblastoma. Pediatr Blood Cancer. 2006;46:739–744. doi: 10.1002/pbc.20456. [DOI] [PubMed] [Google Scholar]
- 21.Cheung NK, Cheung IY, Kushner BH, et al. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol. 2012;30:3264–3270. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kushner BH, Modak S, Basu EM, et al. Posterior reversible encephalopathy syndrome in neuroblastoma patients receiving anti-GD2 3F8 monoclonal antibody. Cancer. 2013;119:2789–2795. doi: 10.1002/cncr.28137. [DOI] [PMC free article] [PubMed] [Google Scholar]