Abstract
Increasing evidence suggests that abnormal brain accumulation of amyloid-β1–42 (Aβ1–42) oligomers plays a causal role in Alzheimer’s disease (AD), and in particular may cause the cognitive deficits that are the hallmark of AD. In vitro, Aβ1–42 oligomers impair insulin signaling and suppress neural functioning. We previously showed that endogenous insulin signaling is an obligatory component of normal hippocampal function, and that disrupting this signaling led to a rapid impairment of spatial working memory, while delivery of exogenous insulin to the hippocampus enhanced both memory and metabolism; diet-induced insulin resistance both impaired spatial memory and prevented insulin from increasing metabolism or cognitive function. Hence, we tested the hypothesis that Aβ1–42 oligomers could acutely impair hippocampal metabolic and cognitive processes in vivo in the rat. Our findings support this hypothesis: Aβ1–42 oligomers impaired spontaneous alternation behavior while preventing the task-associated dip in hippocampal ECF glucose observed in control animals. In addition, Aβ1–42 oligomers decreased plasma membrane translocation of the insulin-sensitive glucose transporter 4 (GluT4), and impaired insulin signaling as measured by phosphorylation of Akt. These data show in vivo that Aβ1–42 oligomers can rapidly impair hippocampal cognitive and metabolic processes, and provide support for the hypothesis that elevated Aβ1–42 leads to cognitive impairment via interference with hippocampal insulin signaling.
Keywords: Amyloid, glucose, glucose transporter type 4, hippocampal, insulin, memory
INTRODUCTION
Alzheimer’s disease (AD) is characterized by progressive impairment in cognitive function, decreased brain glucose metabolism, and both cortical and limbic amyloid deposition (in addition to other pathologies such as abnormal tau phosphorylation). Several recent imaging studies showed that brain glucose hypometabolism is correlated with total amyloid-β (Aβ) levels [1, 2], and the cognitive deficits caused by acute reductions in brain glucose metabolism are well known [3–13]. Abnormal accumulation of Aβ1–42 is considered a causative agent in the pathologies of AD [14]; recently, attention has focused specifically on oligomeric Aβ1–42, which has deleterious effects on neuronal function in vitro [15–18]. However, it is unknown whether oligomeric Aβ regulates brain glucose metabolism in vivo.
Chronic elevation of brain Aβ causes cognitive impairments and in vitro studies show extensive neurotoxicity of Aβ [19–24]. Neurotoxic Aβ1–42 oligomers appear to act, at least in part, via inhibition of insulin signaling, which promotes reduced synaptic function [17, 18].Aβ1–42 oligomers are known to bind and inactivate insulin receptors, which may not only impair synaptic function but potentially also impair brain glucose metabolism [25, 26]. Conversely, insulin can attenuate synaptic deficits caused by Aβ1–42 oligomers [17, 18]. Importantly, we recently showed that hippocampal insulin signaling is a critical component of both mnemonic processing and local metabolic regulation [27]. Hence, interference with insulin signaling may be the mechanism by which Aβ oligomers impair neural function. One component of this action may be impairment of translocation of the insulin-sensitive glucose transporter GluT4 to the plasma membrane of hippocampal neurons. GluT4 is heavily expressed in the hippocampus [28–30] and has been suggested as a mediator of on-demand glucose supply during memory processing [28, 31, 32]; provision of glucose to the hippocampus is well-established to regulate mnemonic performance [33], and hippocampal glucose transport deficits may lead to hippocampal hypometabolism during AD [34–36]. Because Aβ1–42 oligomers impair the phosphoinositide 3-kinase (PI3K) pathway [16, 17, 37, 38], which directly regulates GluT4 translocation in the CNS and periphery [30, 39, 40], we hypothesized that Aβ1–42 oligomers would impair translocation of GluT4.
A further link between insulin signaling and Aβ is suggested by the comorbidity seen between AD and type 2 diabetes mellitus, a disease characterized by impaired insulin sensitivity [13, 41–44]. Postmortem analysis of brain tissue from AD patients shows several features associated with insulin resistance, prompting speculation that AD might be a type of brain diabetes [45]. Rodents placed on diabetogenic diets increase brain Aβ accumulation [46, 47], and have impairments to both hippocampally-mediated memory and hippocampalmetabolism [27].Collectively, these findings suggest that cognitive impairment in AD may be mediated through effects of Aβ on brain insulin signaling.
In the current study, we sought to determine the effects of acute intrahippocampal Aβ1–42 oligomer administration on hippocampal metabolism, memory processes, and insulin signaling in vivo. Our primary hypothesis, that acute cognitive impairment would occur, was confirmed. Further, Aβ1–42 oligomer administration prevented the task-associated dip in hippocampal extracellular fluid (ECF) glucose seen in untreated animals, attenuated hippocampal insulin signaling, and lowered hippocampal GluT4 translocation. These data provide support for the hypothesis that Aβ1–42 oligomer accumulation leads to cognitive decline and hypometabolism because of interference with central insulin signaling, and suggests that impairment of GluT4 translocation may play a potentially critical role in the cognitive and metabolic deficits seen in AD patients.
METHODS
Subjects
Juvenile male Sprague-Dawley rats (Charles River, Wilmington, MA), approximately 300 g at time of arrival, were housed in pairs on a 12:12 h light:dark schedule with food and water available ad libitum. Following surgery, rats were housed singly. Rats were given at least one week to acclimate prior to any surgery or testing, during which time they were handled extensively. Rats were randomly assigned to one of three groups: vehicle control, scrambled Aβ42-1, or Aβ42-1 oligomers. Each rat was used only once. All procedures were approved by the University at Albany Institutional Animal Care and Use Committee.
Surgeries
Rats were anaesthetized with isoflurane and a single microinjection (Plastics One) or dual mD/microinjection cannula (BASi Cat. No. MD-2250) was stereotaxically implanted in the dorsal hippocampus using aseptic surgical technique. For microinjection cannula, the nose bar was set at 4.6 mm above the interaural line. Cannulae coordinates were 5.6 mm posterior to bregma, +4.6 lateral, and 3.3 ventral from dura. For experiments involving mD/microinjections, cannulae were implanted at 5.6 mm posterior to bregma, +5.0 lateral, and 3.0 ventral from dura. The difference in cannullae placement was to account for the 4 mm probe used in microdialysis experiments. Rats received acetaminophen in their drinking water following surgery and were allowed a two-week long recovery period prior to testing.
Treatment preparation
Soluble oligomers were made from synthetic human Aβ1–42 using a slight modification of the procedure of Lambert et al. [20, 48]. Aβ1–42 or scrambled Aβ1–42 was reconstituted with ice-cold hexafluoroisopropanol (HFIP) and allowed to evaporate overnight in nonsiliconized microcentrifuge tubes. The next day, tubes were dried by vacuum centrifugation to remove remaining HFIP, leaving monomeric Aβ. Tubes were then stored over dessicant at −80°C until oligomer preparation. On the day prior to testing, stock preps were reconstituted in anhydrous DMSO and brought to 100 µMin either artificial extracellular fluid (aECF; 153.5 mM Na, 4.3 mM K, 0.41 mM Mg, 0.71 mM Ca, 139.4 mMCl, buffered at pH 7.4) or F12 medium without phenol red (Sigma), incubated at 4°C for 24 h, centrifuged for 14,000 g at 4°C for 10 min, and the supernatant collected for use as oligomers for animal treatment. Preparation of the scrambled control peptide (synthetic Aβ42-1) and vehicle control followed the same protocol. All treatments were filtered with a 0.2 µm filter prior to microinjections to prevent clogging of the 40 µm pore of the dual mD/microinjection cannulae. Oligomerization was verified by silver staining using a commercially available kit (Pierce). Silver staining represents a quick and efficient way to folding states of isolated proteins following SDS-PAGE. It was previously found that Aβ1–42 oligomers are SDS stable and detectable by the silver staining procedure using a 4–20% SDS-PAGE gel under non-denaturing conditions [20, 48]. Preparations of Aβ1–42 oligomers in both aECF and F12 medium, but not scrambled Aβ42-1, contained prominent bands consistent with the known molecular weights of monomers, dimers, and trimers, respectively [20, 48].
Behavioral testing
To assess spatial working memory we used spontaneous alternation (SA) in a 4-arm plus-maze. Rats perform this task using spatial working memory to remember the least-recently visited arms. Animals are placed into the maze and allowed to explore freely for 20 min and will spontaneously tend to visit the least-recently visited arm, using spatial working memory to recall arm-visit history; alternation scores are calculated by dividing the percentage of alternations (defined as a visit to each of the 4 arms within each span of 5 consecutive entries), with chance performance being 44%. Others and we have used this task extensively over the past decade to examine brain metabolism, insulin signaling, and spatial working memory in rats [27, 49–52]. Data are the combination of two independent replications.
Microinjections and in vivo microdialysis
Microdialysis was performed as previously described [53], with samples collected before, during, and after microinjection and subsequent behavioral testing. On the day of testing, a fresh 4 mm probe (BASi Cat. No. MD-2264) was placed into the guide cannula and the animal was allowed to acclimate for at least 1 h while perfused with aECF including 1.25 mM glucose at a rate of 1.5 µl/min. Perfusate was collected in 20-min samples. Two baseline samples were collected prior to microinjection. Microinjections were administered at a rate of 0.125 µl/min over 4 min for a total volume of 0.5 µl, 10 min prior to behavioral testing. This volume corresponds to approximately 200 ng of either Aβ1–42 oligomers or scrambled Aβ42-1. Rats in the no maze condition were left in the control chamber during the ‘testing’ period. Glucose and lactate were measured in a CMA600 analyzer (Stockholm, Sweden). Sample measurements were corrected for in vivo probe recovery using a zero-net-flux plot for ECF glucose measurements under the same experimental conditions.
Histology
Immediately following experimentation, rats were sacrificed. Probe placement was verified using Nissl staining on coronal sections in half of the subjects to ensure consistency. In the other half of subjects, whole hippocampi were removed, frozen on dry ice, and stored at −80°C until plasma membrane extraction. In animals that underwent mD, all brains were checked by Nissl staining for probe placement. Data from animals that had misplaced cannulae or evidence of excessive inflammation were excluded (n = 4).
Cell fractionation
Plasma membrane (PM) fractions were collected using a commercially available kit (Biovision). Whole hippocampi were homogenized with a Polytron hand-held electric homogenizer in 3 volumes of (1 mg/3 µl) lysis buffer with lyophilized protease inhibitors (Bio-vision). Following the initial homogenization 30 µl of the total lysate was added to 200 µL ice-cold RIPA buffer (50 mM Tris, 150 mM NaCl, 0.1% SDS, 0.5% sodium deoxycholate,1%Triton) containing fresh protease and phosphatase inhibitors (Pierce) and stored at −80°C until protein quantification. For the remaining lysate, PM separation proceeded according to the kit’s instructions.
Immunoblotting
Protein concentration was determined using a commercially available kit for the bicinchoninic assay (Pierce). 10–20 µg of protein was placed in sample buffer in denaturing and reducing conditions. Samples tested for total protein content were heated at 95°C for 5 min prior to sample loading. Samples analyzed for plasma membrane associated proteins were not heated prior to gel loading. Samples were resolved on 8% or 12% SDS polyacrylamide minigels (Pierce or Bio-Rad) and transferred onto PVDF membranes (Bio-Rad) followed by detection with appropriate primary antibodies. Antibodies used were anti-rabbit directed against GluT4 (Millipore Cat. No. 07-1404), GluT1 (Abcam Cat. No. ab652.), GluT3 (Abcam Cat. No. ab41525), phosphorylated Akt at serine 473 (Cell Signaling Technology Cat. No 4060L), and total Akt (Cell Signaling Cat. No. 4691L). Monocolonal anti-mouse directed against Na+/K+ ATPase (Abcam Cat. No. ab7671) or β-actin (Sigma Cat. No. A2228) were used as loading controls for PM and total protein samples, respectively. Following wet-transfer at 350 mA in the cold specify for 60 min, PVDF membranes were blocked for 1 h with 5% milk or 5% BSA in tris-buffered saline containing 0.1% tween-20. Membranes were incubated with primary antibodies in blocking solution overnight at 4°C in conditions optimized for immunodection by each antibody. Secondary incubation in biotin-conjugated goat anti-mouse (Pierce) or goat anti-rabbit (Vector) IgG was followed by incubation in HRP strepdavidin (Pierce) for enhanced immunoreactivity, both for 1 h at room temperature. Immunoblots were developed with chemiluminscent detection using the SuperSignal West Pico Chemiluminescent Kit (Pierce). Images were taken in a Bio-rad ChemiDoc XRS Image Analyzer. Membranes were then stripped with Restore Plus Stripping Buffer (Pierce) and reprobed for loading controls or total protein content. Band intensity was measured by densitometry in ImageQuant software and normalized to loading controls or total protein content. To control for between gel variations in band intensity, vehicle control values were normalized to 100% and scrambled Aβ42-1 and Aβ1–42 samples were analyzed as percents of control values.
Statistical analyses
Statistics are reported as means ± standard error of the mean. For statistical comparisons of two groups, we used the student’s t test, and for comparisons of more than two groups we used one-way ANOVA followed by Tukey’s post hoc test. To test for the equality of group variances assumption the Levene test was used in all analyses. An α level of 0.05 was set for significance. All statistical analyses were made in SPSS version 17.
RESULTS
Intrahippocampal administration of Aβ1–42 oligomers impaired spatial working memory
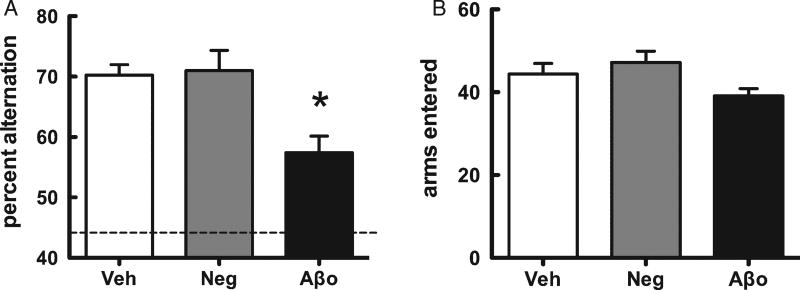
A single administration of Aβ1–42 oligomers to the left hippocampus significantly impaired SA behavior, tested 10 min later, when compared to vehicle or scrambled Aβ42-1 control groups (Fig. 1A). Scrambled Aβ42-1 did not affect SA behavior. There was no significant difference in the number of arm entries made between groups, a measure which controls for nonspecific effects of treatment on motor activity or motivation (Fig. 1B).
Fig. 1.
Spatial memory (spontaneous alternation performance, SA) following administration of Aβ1–42 oligomers to dorsal hippocampus 10 min prior to behavioral testing. A) SA performance scores (mean ± SEM). Dotted line represents chance performance (44%). B) The number of arms entered during the SA task was not significantly different across treatment groups (mean ± SEM). *p < 0.05.
Aβ1–42 oligomers prevented the task-associated dip in extracellular glucose during the working memory task
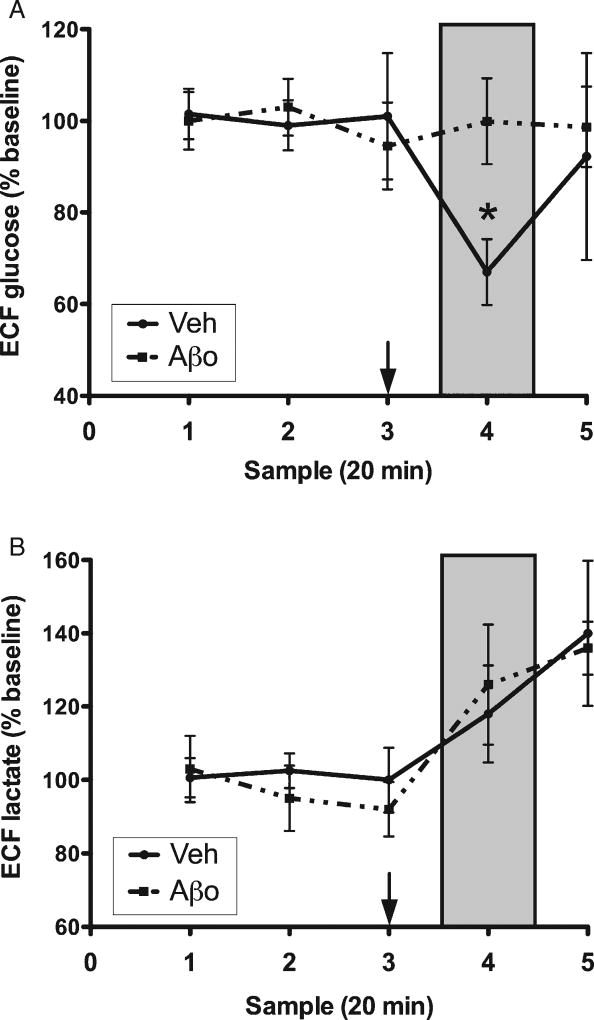
Rats in the scrambled Aβ and vehicle control groups showed an approximately 30% dip in hippocampal ECF glucose, highly consistent with our previous work [51, 53, 54], which has shown increased hippocampal metabolic demand during SA testing. No such dip was seen during maze testing in rats treated with Aβ1–42 oligomers (Fig. 2A). Aβ1–42 oligomer treatment did not affect the rise in lactate levels that occurred during SA testing (Fig. 2B).
Fig. 2.
Hippocampal extracellular fluid (ECF) glucose (A) and lactate (B). Samples 1–3 are baseline measurements, sample 4 (shaded box) was collected during spatial memory testing, and sample 5 is post-maze. Treatment was administered 10 min prior to maze testing (arrow). Each data point is mean ± SEM. *p < 0.05.
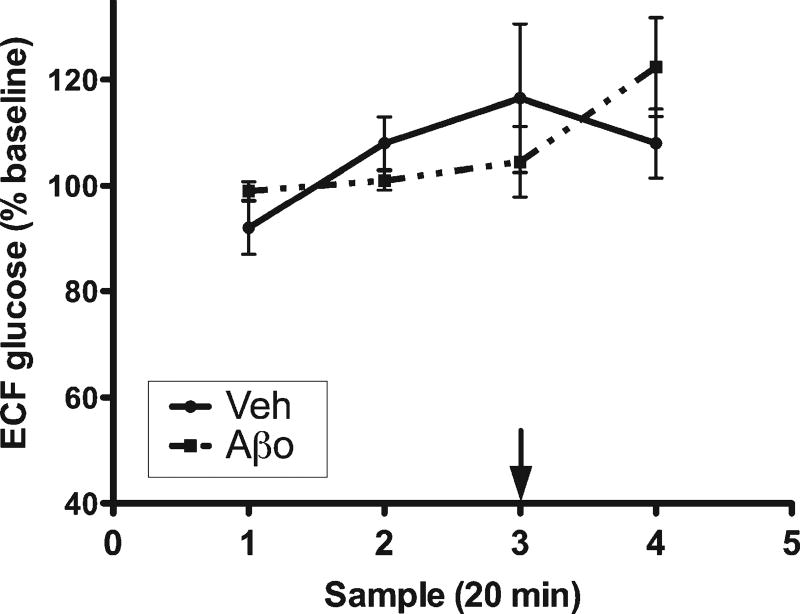
Aβ1–42 oligomers did not affect hippocampal ECF glucose in the absence of cognitive load
Animals were treated and handled identically to maze-tested animals, but were returned to their home chamber rather than being placed into the maze. Hippocampal ECF glucose was unchanged following administration of either vehicle control or Aβ1–42 oligomers (Fig. 3.
Fig. 3.
Hippocampal ECF glucose under control (no-testing) conditions. Samples 1–2 are baseline measurements. Treatment was administered 50 min into collection (arrow).
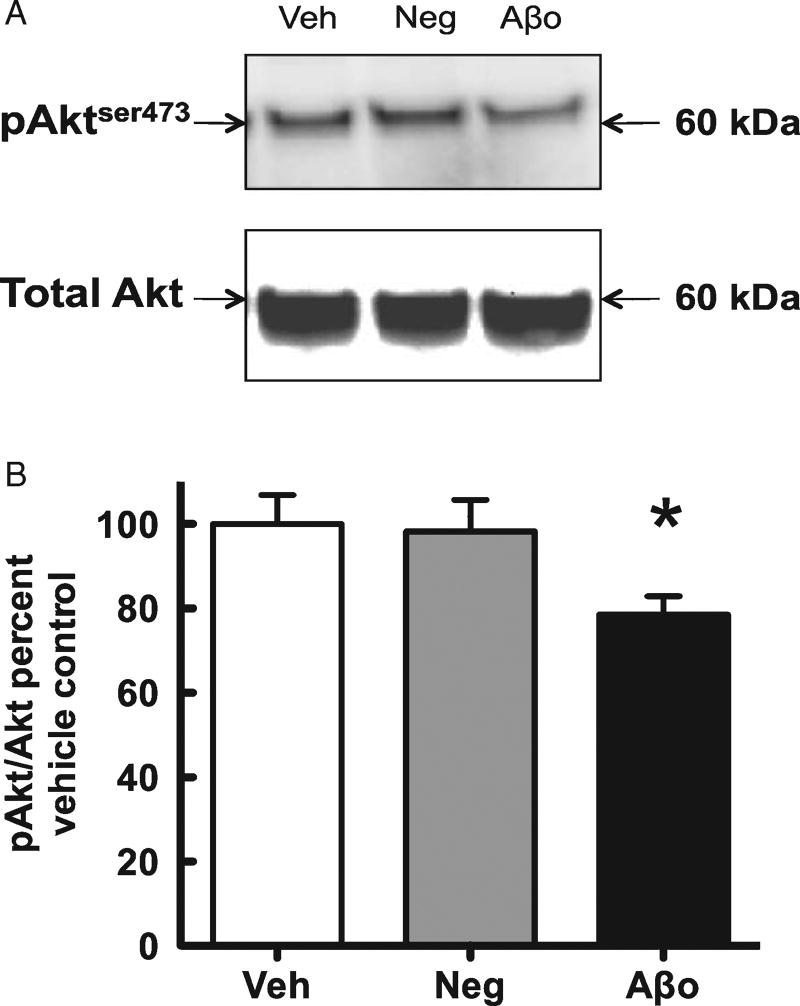
Aβ1–42 oligomers attenuated Akt activity and plasma membrane levels of the insulin-sensitive glucose transporter GluT4
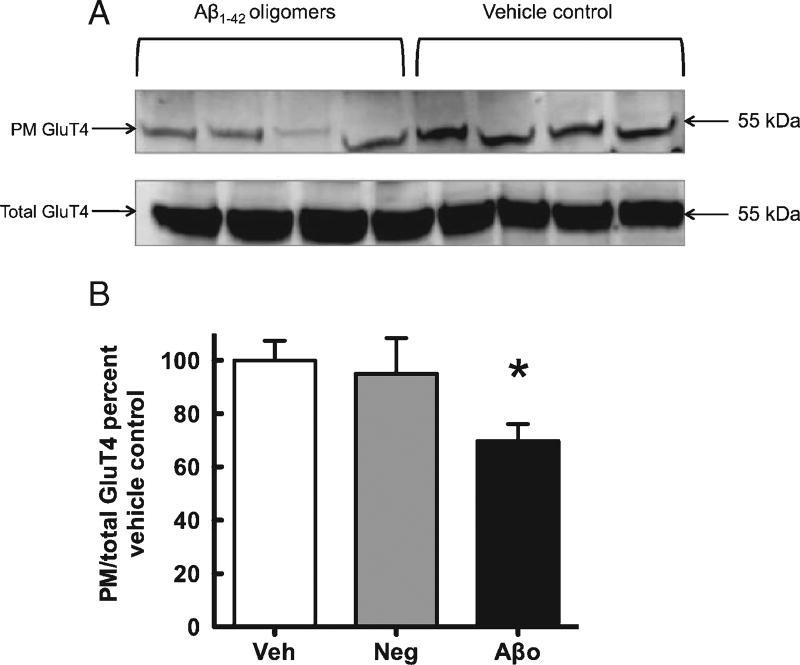
Treatment with Aβ1–42 oligomers caused a significant reduction in the ratio ofPMto total GluT4, but did not alter total GluT4 (Fig. 4, suggesting interference with translocation rather than synthesis or degradation, as would be expected from the acute treatment. Akt, a downstream intermediate in the phosphatidyl-inositol-3-kinase (PI3K) signaling cascade, can be phosphorylated at either serine 473 (pAktser473) or threonine 408. Phosphorylation at serine 473 is necessary for full activation of Akt and signaling to downstream protein activity, including GluT4 translocation to the plasma membrane (64), and we hence examined the effect of Aβ1–42 oligomer administration on pAktser473. Our western blot analyses revealed a significant reduction in pAktser473 (normalized to total Akt) following Aβ1–42 oligomer treatment, but no significant effect on total Akt protein (Fig. 5).
Fig. 4.
A) Representative western blot images for PM and total GluT4. B) Analysis of western blots comparing plasma membrane GluT4 to total GluT4. Vehicle control = Veh, Aβ42-1 = Neg, Aβ1–42 oligomers =Aβo. Veh normalized to 100% and compared to Neg and Aβo. Each data point is mean ± SEM. *p < 0.05.
Fig. 5.
A) Representative western blot images for phosphorylated Akt at serine 473 (pAkt) and Akt. B) Analysis of western blots comparing pAkt to Akt. Vehicle control = Veh, Aβ42-1 = Neg, Aβ1–42 oligomers =Aβo.Veh normalized to 100% and compared to Neg and Aβo. *p < 0.05.
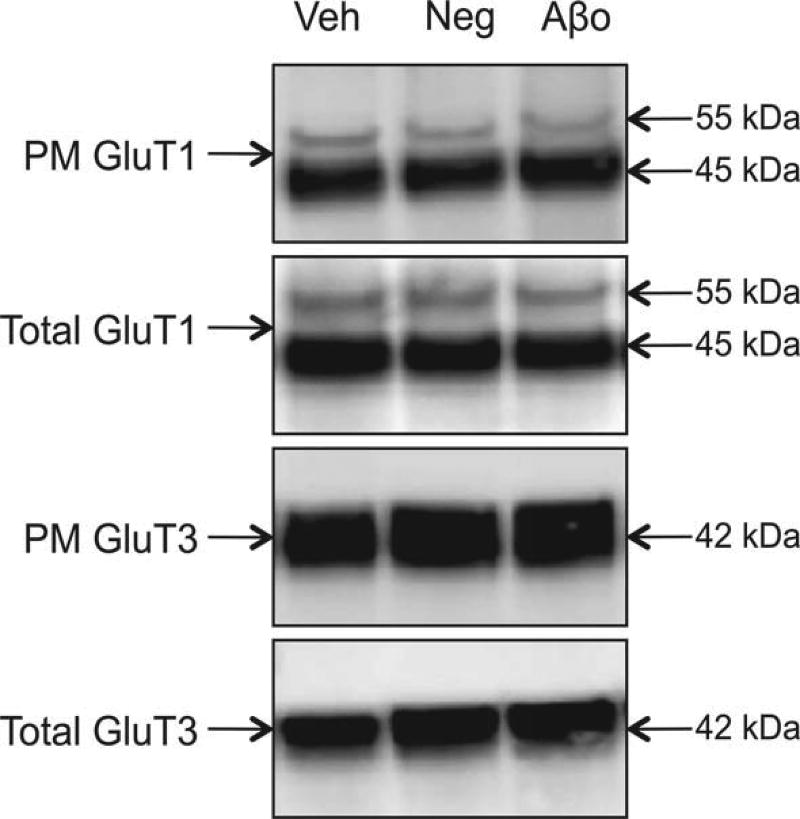
Aβ1–42 oligomers had no effect on GluT1 or GluT3
We examined both isoforms of GluT1 and found no change in the ratio of plasma membrane to total expression following treatment with Aβ1–42 oligomers, nor in plasma membrane or total expression of GluT1. Similarly, expression of neuronal GluT3 was not significantly affected by treatment with Aβ1–42 oligomers, There was no difference in total GluT1 or GluT3 following Aβ1–42 oligomer treatment (Fig. 6).
Fig. 6.
A) Representative western blot images for PM and total GluT1 and GluT3. There were no statistically significant differences between treatment groups. Vehicle control Veh, Aβ42-1 = Neg, Aβ1–42 oligomers = Aβo. Veh normalized to 100% and compared to Neg and Aβo. *p < 0.05.
DISCUSSION
The results of this study show that Aβ1–42 oligomers acutely impair both cognitive and metabolic processes within the hippocampus, with these effects likely being mediated (at least in part) through interference with insulin-regulated glucose transport and metabolism, a key modulator of hippocampal function. Attenuation of brain glucose metabolism is characteristic of AD; several studies have found decreased glucose transport and phosphorylation in the brains of AD patients [35, 36, 55]. In the periphery, insulin plays a key role in glucose uptake through regulation of GluT4 activity. Because insulin regulates glucose uptake in discrete brain areas including the hippocampus [27, 56], which expresses both insulin receptors and GluT4 at high levels [29–32], it is reasonable to suggest that insulin may support neuronal functioning by increasing local glucose metabolism. Indeed, we previously showed, using in vivo microdialysis, that intrahippocampal insulin delivery, at a dose that enhanced spatial working memory, caused a decrease in hippocampal extracellular glucose and a concomitant increase in lactate, consistent with increased local glycolysis [27, 57]. Aβ1–42 oligomers’ role as synaptic pathogens may stem, at least partially, from their detrimental effects on insulin signaling, which could lead to attenuated glucose metabolism and synaptic activity. In the present study, delivery of Aβ1–42 oligomers to the hippocampus had effects very similar to those produced by other memory-impairing agents such as morphine: worsened spatial memory accompanied by an absence of task-associated dip in ECF glucose [58]. Previous studies of Aβ on cognitive impact have largely focused on chronic effects following up to several weeks of treatment, generally given i.c.v. and hence affecting much of the brain [19–24]. The fact that Aβ1–42 oligomers impair cognitive and metabolic processes very rapidly after intrahippocampal administration is consistent with the immediate impact of Aβ1–42 oligomers on insulin-mediated PI3K activity previously shown [15–18], and is potentially necessary for the hypothesis that amyloid-induced cognitive impairments occur due to blockade of insulin signaling: we previously showed that cognitive deficits emerge within 10 min of hippocampal insulin signaling blockade [27].
We also show that administration of Aβ1–42 oligomers acutely impairs translocation of the insulin-sensitive glucose transporter GluT4, as well as impairing PI3K signaling; as far as we know, this is the first in vivo confirmation that acute elevation in Aβ1–42 oligomers regulates pAktser473. GluT4 expression is abundant throughout the hippocampus. Moreover, GluT4 immunoreactivity colocalizes with cholinergic markers [59]. These data are thus relevant for AD pathology, which shows extensive damage to synapses and cholinergic neurons [15, 60]. Although some studies have examined glucose transporters in postmortem analysis of the brains of human AD patients, relatively little is known about the physiological role of hippocampal GluT4 during AD. Measurements of total protein have suggested that there may be little or no change in the total amount of GluT4, although total GluT1 and GluT3 were decreased [61, 62]. The present data illustrate the need for in vivo measurement of both total and plasma-membrane GluT4: we also found no change in total GluT4 protein, following Aβ administration, but the impact of Aβ1–42 oligomers was clearly seen as a reduction in translocation to the plasma membrane (and hence a functional impairment). One might speculate that if anything, the brains of AD patients might show normal or slightly elevated total GluT4 protein, as possible compensation for impaired trafficking to the plasma membrane.
No effect on hippocampal metabolism was seen following administration of Aβ1–42 oligomers in the absence of cognitive testing, suggesting that the effects of Aβ may be seen only at times of elevated cognitive demand. This pattern is highly consistent with a large literature showing that alterations in glucose supply to the hippocampus also affect function only under conditions of high cognitive load [58, 63, 64]. The metabolic data presented here are also consistent with our protein-level measurements of the impact of Aβ administration on glucose transporters: no effect was seen on the constitutively membrane-located transporters GluT1 or GluT3, but Aβ oligomers markedly impaired translocation of the insulin-sensitive transporter GluT4, which could provide additional neuronal glucose transport capacity when hippocampal mnemonic processes, of which insulin signaling is a key component, are upregulated [27]. Additionally or alternatively, it is possible that Aβ1–42 oligomers may impair glucose supply within the hippocampus through vascular effects. The fact that ECF lactate levels were not altered by Aβ administration, despite decreased glucose usage, might suggest that the impact of Aβ administration is at least in part on oxidative rather than non-oxidative metabolism; in that case, reduction in oxygen supply (presumably via impaired neurovascular coupling) might also contribute to the cognitive impairments observed. We are currently investigating whether hippocampal blood flow is altered following Aβ administration. Our data are consistent with PET studies in humans, where measurements taken at baseline (low cognitive load) in AD patients show that Aβ levels can be elevated without observable decrease in brain glucose metabolism [65, 66].
Previous studies found that impaired glucose metabolism and PI3K activity is associated with chronically increased Aβ levels [46, 67], but the current study demonstrates that even acute elevation in hippocampal Aβ1–42 oligomers is sufficient to attenuate Akt phosphorylation and GluT4 translocation. These data are consistent with prior reports that Aβ1–42 oligomers can directly attenuate the insulin-mediated PI3K pathway [37, 38]. Acute hyperinsulinemia, including intranasal administration of insulin, improves cognition in the elderly [68–70], possibly by increasing insulin-mediated glucose transport through GluT4 [71]. Activation of PI3K by insulin promotes α-secretase mediated cleavage of AβPP leading to the production of soluble AβPPα, which has neuroprotective properties [37]. Insulin is also a known regulator of Aβ degradation and clearance from the brain [72, 73]. Because Aβ1–42 monomers and insulin compete for breakdown by insulin-degrading enzyme, which shows a stronger affinity for insulin than Aβ1–42 monomers [72], chronic long-term hyperinsulinemia during insulin resistance may promote Aβ aggregation by preventing the breakdown of Aβ1–42 monomers. Taken together with the present data, these studies offer further support for the suggestion that insulin and oligomeric Aβ mutually oppose, with intact insulin signaling protecting against Aβ toxicity but elevated Aβ impairing insulin signaling, consistent with the clinical finding that insulin resistance is a risk factor for AD [13, 27, 41–44, 46, 47]. It is possible, and indeed likely, that the effects of chronic Aβ1–42 elevation on brain metabolism and cognitive function may vary across time; future studies aimed at clarifying the timeline along which Aβ1–42 oligomers impair hippocampal function by various potential mechanisms may build on the present work.
Acknowledgments
We thank Andrew C. Byrne, Michael Vidal, and Lauren Moria for assistance with performing these experiments. This work was funded by National Institutes of Health grant NIHR01DK077106, Alzheimer’s Association grant NIRG-10-176609, and internal funding through the University at Albany.
Footnotes
Authors’ disclosures available online (http://www.j-497alz.com/disclosures/view.php?id=1177).
References
- 1.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstroöm M, Savitcheva I, Huang GF, Estrada S, Ausén B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Långström B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 2.Shin J, Tsui W, Li Y, Lee S-Y, Kim SJ, Cho S-J, Kim Y-B, de Leon MJ. Resting-state glucose metabolism level is associated with the regional pattern of amyloid pathology in Alzheimer’s disease. Int J Alzheimers Dis. 2011;2011:759780. doi: 10.4061/2011/759780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalimo H, Olsson Y. Effects of severe hypoglycemia on the human brain. Neuropathological case reports. Acta Neurol Scand. 1980;62:345–356. doi: 10.1111/j.1600-0404.1980.tb03047.x. [DOI] [PubMed] [Google Scholar]
- 4.Pramming S, Thorsteinsson B, Theilgaard A, Pinner EM, Binder C. Cognitive function during hypoglycaemia in type I diabetesmellitus. BrMed J (Clin Res Ed) 1986;292:647–650. doi: 10.1136/bmj.292.6521.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon RP, Schmidley JW, Swan JH, Meldrum BS. Neuronal alterations in hippocampus following severe hypoglycaemia: A light microscopic and ultrastructural study in the rat. Neuropathol Appl Neurobiol. 1986;12:11–26. doi: 10.1111/j.1365-2990.1986.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 6.Jones TW, McCarthy G, Tamborlane WV, Caprio S, Roessler E, Kraemer D, Starick-Zych K, Allison T, Boulware SD, Sherwin RS. Mild hypoglycemia and impairment of brain stem and cortical evoked potentials in healthy subjects. Diabetes. 1990;39:1550–1555. doi: 10.2337/diab.39.12.1550. [DOI] [PubMed] [Google Scholar]
- 7.Draelos MT, Jacobson AM, Weinger K, Widom B, Ryan CM, Finkelstein DM, Simonson DC. Cognitive function in patients with insulin-dependent diabetes mellitus during hyperglycemia and hypoglycemia. Am J Med. 1995;98:135–144. doi: 10.1016/S0002-9343(99)80397-0. [DOI] [PubMed] [Google Scholar]
- 8.Weinger K, Jacobson AM, Draelos MT, Finkelstein DM, Simonson DC. Blood glucose estimation and symptoms during hyperglycemia and hypoglycemia in patients with insulin-dependent diabetes mellitus. Am J Med. 1995;98:22–31. doi: 10.1016/S0002-9343(99)80077-1. [DOI] [PubMed] [Google Scholar]
- 9.Auer RN, Anderson LG. Hypoglycaemic brain damage: Effect of a dihydropyridine calcium channel antagonist in rats. Diabetologia. 1996;39:129–134. doi: 10.1007/BF00403954. [DOI] [PubMed] [Google Scholar]
- 10.Kim WH, Lee JW, Suh YH, Hong SH, Choi JS, Lim JH, Song JH, Gao B, Jung MH. Exposure to chronic high glucose induces beta-cell apoptosis through decreased interaction of glucokinase with mitochondria: Downregulation of glucokinase in pancreatic beta-cells. Diabetes. 2005;54:2602–2611. doi: 10.2337/diabetes.54.9.2602. [DOI] [PubMed] [Google Scholar]
- 11.Suh SW, Aoyama K, Matsumori Y, Liu J, Swanson RA. Pyruvate administered after severe hypoglycemia reduces neuronal death and cognitive impairment. Diabetes. 2005;54:1452–1458. doi: 10.2337/diabetes.54.5.1452. [DOI] [PubMed] [Google Scholar]
- 12.Suh SW, Fan Y, Hong SM, Liu Z, Matsumori Y, Weinstein PR, Swanson RA, Liu J. Hypoglycemia induces transient neurogenesis and subsequent progenitor cell loss in the rat hippocampus. Diabetes. 2005;54:500–509. doi: 10.2337/diabetes.54.2.500. [DOI] [PubMed] [Google Scholar]
- 13.Irie F, Fitzpatrick AL, Lopez OL, Kuller LH, Peila R, Newman AB, Launer LJ. Enhanced risk for Alzheimer disease in persons with type 2 diabetes and APOE epsilon4: The Cardiovascular Health Study Cognition Study. Arch Neurol. 2008;65:89–93. doi: 10.1001/archneurol.2007.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardy J. Has the amyloid cascade hypothesis for Alzheimers disease been proved? Curr Alzheimer Res. 2006;3:71–73. doi: 10.2174/156720506775697098. [DOI] [PubMed] [Google Scholar]
- 15.Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 16.Townsend M, Mehta T, Selkoe DJ. Soluble Aβ inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- 17.Zhao W-Q, De Felice FG, Fernandez S, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22:246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- 18.De Felice FG, Vieira MNN, Bomfim TR, Decker H, Velasco PT, Lambert MP, Viola KL, Zhao W-Q, Ferreira ST, Klein WL. Protection of synapses against Alzheimer’s-linked toxins: Insulin signaling prevents the pathogenic binding of Aβ oligomers. Proc Natl Acad Sci U S A. 2009;106:1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurice T, Lockhart BP, Privat A. Amnesia induced in mice by centrally administered [beta]-amyloid peptides involves cholinergic dysfunction. Brain Res. 1996;706:181–193. doi: 10.1016/0006-8993(95)01032-7. [DOI] [PubMed] [Google Scholar]
- 20.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Aβ-142 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephan A, Laroche S, Davis S. Generation of aggregated {beta}-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J Neurosci. 2001;21:5703–5714. doi: 10.1523/JNEUROSCI.21-15-05703.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-[beta] protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 23.Klyubin I, Walsh DM, Lemere CA, Cullen WK, Shankar GM, Betts V, Spooner ET, Jiang L, Anwyl R, Selkoe DJ, Rowan MJ. Amyloid [beta] protein immunotherapy neutralizes A[beta] oligomers that disrupt synaptic plasticity in vivo. Nat Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- 24.Yamada K, Takayanagi M, Kamei H, Nagai T, Dohniwa M, Kobayashi K, Yoshida S, Ohhara T, Takauma K, Nabeshima T. Effects of memantine and donepezil on amyloid [beta]-induced memory impairment in a delayed-matching to position task in rats. Behav Brain Res. 2005;162:191–199. doi: 10.1016/j.bbr.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 25.Zhao W-Q, Lacor PN, Chen H, Lambert MP, Quon MJ, Krafft GA, Klein WL. Insulin receptor dysfunction impairs cellular clearance of neurotoxic oligomeric Aβ. J Biol Chem. 2009;284:18742–18753. doi: 10.1074/jbc.M109.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao F-F, Xu H. Insulin signaling in sporadic Alzheimer’s disease. Sci Signal. 2009;2:pe36. doi: 10.1126/scisignal.274pe36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Memory. 2010;93:546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Messari S, Aït-Ikhlef A, Ambroise D-H, Penicaud L, Arluison M. Expression of insulin-responsive glucose transporter GLUT4 mRNA in the rat brain and spinal cord: An in situ hybridization study. J Chem Neuroanat. 2002;24:225–242. doi: 10.1016/s0891-0618(02)00058-3. [DOI] [PubMed] [Google Scholar]
- 29.Piroli GG, Grillo CA, Reznikov LR, Adams S, McEwen BS, Charron MJ, Reagan LP. Corticosterone impairs insulin-stimulated translocation of GLUT4 in the rat hippocampus. Neuroendocrinology. 2007;85:71–80. doi: 10.1159/000101694. [DOI] [PubMed] [Google Scholar]
- 30.Grillo CA, Piroli GG, Hendry RM, Reagan LP. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Res. 2009;1296:35–45. doi: 10.1016/j.brainres.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leloup C, Arluison M, Kassis N, Lepetit N, Cartier N, Ferré P, Pénicaud L. Discrete brain areas express the insulin-responsive glucose transporter GLUT4. Mol Brain Res. 1996;38:45–53. doi: 10.1016/0169-328x(95)00306-d. [DOI] [PubMed] [Google Scholar]
- 32.Alquier T, Leloup C, Lorsignol A, Pénicaud L. Translocable glucose transporters in the brain. Diabetes. 2006;55:S131–S138. [Google Scholar]
- 33.McNay EC, Gold PE. Age-related differences in hippocampal extracellular fluid glucose concentration during behavioral testing and following systemic glucose administration. J Gerontol A Biol Sci Med Sci. 2001;56:B66–B71. doi: 10.1093/gerona/56.2.b66. [DOI] [PubMed] [Google Scholar]
- 34.Duara R, Grady C, Haxby J, Sundaram M, Cutler NR, Heston L, Moore A, Schlageter N, Larson S, Rapoport SI. Positron emission tomography in Alzheimer’s disease. Neurology. 1986;36:879–887. doi: 10.1212/wnl.36.7.879. [DOI] [PubMed] [Google Scholar]
- 35.Piert M, Koeppe RA, Giordani B, Berent S, Kuhl DE. Diminished glucose transport and phosphorylation in Alzheimer’s disease determined by dynamic FDG-PET. J Nucl Med. 1996;37:201–208. [PubMed] [Google Scholar]
- 36.De Santi S, de Leon MJ, Rusinek H, Convit A, Tarshish CY, Roche A, Tsui WH, Kandil E, Boppana M, Daisley K, Wang GJ, Schlyer D, Fowler J. Hippocampal formation glucose metabolism and volume losses in MCI and AD. Neurobiol Aging. 2001;22:529–539. doi: 10.1016/s0197-4580(01)00230-5. [DOI] [PubMed] [Google Scholar]
- 37.Solano DC, Sironi M, Bonfini C, Solerte SB, Govoni S, Rachhi M. Insulin regulates soluble amyloid precursor protein release via phosphatidyl inositol 3 kinase-dependent pathway. FASEB J. 2000;14:1015–1022. doi: 10.1096/fasebj.14.7.1015. [DOI] [PubMed] [Google Scholar]
- 38.Chen T-J, Wang D-C, Chen S-S. Amyloid-β interrupts the PI3K-Akt-mTOR signaling pathway that could be involved in brain-derived neurotrophic factor-induced Arc expression in rat cortical neurons. J Neurosci Res. 2009;87:2297–2307. doi: 10.1002/jnr.22057. [DOI] [PubMed] [Google Scholar]
- 39.Thong FSL, Dugani CB, Klip A. Turning signals on and off: GLUT4 traffic in the insulin-signaling highway. Physiology. 2005;20:271–284. doi: 10.1152/physiol.00017.2005. [DOI] [PubMed] [Google Scholar]
- 40.Benomar Y, Naour N, Aubourg A, Bailleux V, Gertler A, Djiane J, Guerre-Millo M, Taouis M. Insulin and leptin induce Glut4 plasma membrane translocation and glucose uptake in a human neuronal cell line by a phosphatidylinositol 3-kinase-dependent mechanism. Endocrinology. 2006;147:2550–2556. doi: 10.1210/en.2005-1464. [DOI] [PubMed] [Google Scholar]
- 41.Luchsinger JA, Tang M-X, Stern Y, Shea S, Mayeux R. Diabetes mellitus and risk of Alzheimer’s disease and dementia with wtroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–641. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 42.Peila R, Rodriguez BL, Launer LJ. Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies. Diabetes. 2002;51:1256–1262. doi: 10.2337/diabetes.51.4.1256. [DOI] [PubMed] [Google Scholar]
- 43.Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 44.Launer LJ. Diabetes: Vascular or neurodegenerative: An epidemiologic perspective. Stroke. 2009;40:S53–S55. doi: 10.1161/STROKEAHA.108.533075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease - is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 46.Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- 47.Julien C, Tremblay C, Phivilay A, Berthiaume L, Émond V, Julien P, Calon F. High-fat diet aggravates amyloid-beta and tau pathologies in the 3×Tg-AD mouse model. Neurobiol Aging. 2010;31:1516–1531. doi: 10.1016/j.neurobiolaging.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 48.Klein WL. A[beta] toxicity in Alzheimer’s disease: Globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 49.Richman C, Dember W, Kim P. Spontaneous alternation behavior in animals: A review. Curr Psychol. 1986;5:358–391. [Google Scholar]
- 50.McNay EC, Gold PE. Memory modulation across neural systems: Intra-amygdala glucose reverses deficits caused by intraseptal morphine on a spatial task but not on an aversive task. J Neurosci. 1998;18:3853–3858. doi: 10.1523/JNEUROSCI.18-10-03853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNay EC, Sherwin RS. Effect of recurrent hypoglycemia on spatial cognition and cognitive metabolism in normal and diabetic rats. Diabetes. 2004;53:418–425. doi: 10.2337/diabetes.53.2.418. [DOI] [PubMed] [Google Scholar]
- 52.Lalonde R. The neurobiological basis of spontaneous alternation. Neurosci Biobehav Rev. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 53.McNay EC, Fries TM, Gold PE. Decreases in rat extracellular hippocampal glucose concentration associated with cognitive demand during a spatial task. Proc Natl Acad Sci U S A. 2000;97:2881–2885. doi: 10.1073/pnas.050583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNay EC, Williamson A, McCrimmon RJ, Sherwin RS. Cognitive and neural hippocampal effects of long-term moderate recurrent hypoglycemia. Diabetes. 2006;55:1088–1095. doi: 10.2337/diabetes.55.04.06.db05-1314. [DOI] [PubMed] [Google Scholar]
- 55.Swerdlow R. Brain glucose metabolism in Alzheimer’s disease. Am J Med Sci. 1994;308:141–144. doi: 10.1097/00000441-199409000-00003. [DOI] [PubMed] [Google Scholar]
- 56.Bingham EM, Hopkins D, Smith D, Pernet A, Hallett W, Reed L, Marsden PK, Amiel SA. The role of insulin in human brain glucose metabolism. Diabetes. 2002;51:3384–3390. doi: 10.2337/diabetes.51.12.3384. [DOI] [PubMed] [Google Scholar]
- 57.McNay EC, McCarty RC, Gold PE. Fluctuations in brain glucose concentration during behavioral testing: Dissociations between brain areas and between brain and blood. Neurobiol Learn Mem. 2001;75:325–337. doi: 10.1006/nlme.2000.3976. [DOI] [PubMed] [Google Scholar]
- 58.McNay EC, Canal CE, Sherwin RS, Gold PE. Modulation of memory with septal injections of morphine and glucose: Effects on extracellular glucose levels in the hippocampus. Physiol Behav. 2006;87:298–303. doi: 10.1016/j.physbeh.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Choeiri C, Staines W, Messier C. Immunohistochemical localization and quantification of glucose transporters in the mouse brain. Neuroscience. 2002;111:19–34. doi: 10.1016/s0306-4522(01)00619-4. [DOI] [PubMed] [Google Scholar]
- 60.Davies P, Maloney AJF. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976;308:1403–1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 61.Harr SD, Simonian NA, Hyman BT. Functional alterations in Alzheimer’s disease: Decreased glucose transporter 3 immunoreactivity in the perforant pathway terminal zone. J Neuropathol Exp Neurol. 1995;54:38–41. [PubMed] [Google Scholar]
- 62.Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong C-X. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett. 2008;582:359–364. doi: 10.1016/j.febslet.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gold PE. Role of glucose in regulating the brain and cognition. Am J Clin Nutr. 1995;61:987S–995S. doi: 10.1093/ajcn/61.4.987S. [DOI] [PubMed] [Google Scholar]
- 64.Gold PE. Glucose and age-related changes in memory. Neurobiol Aging. 2005;26(Suppl 1):60–64. doi: 10.1016/j.neurobiolaging.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer’s disease. Eur J Nucl Med Mol Imag. 2005;32:486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 66.Mosconi L, Berti V, Glodzik L, Pupi A, De Santi S, de Leon MJ. Pre-clinical detection of Alzheimer’s disease using FDG-PET, with or without amyloid imaging. J Alzheimers Dis. 2010;20:843–854. doi: 10.3233/JAD-2010-091504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei Qiao Q, Marshal FF. Insulin, insulin-degrading enzyme and amyloid-β peptide in Alzheimer’s disease: Review and hypothesis. Neurobiol Aging. 2006;27:190–198. doi: 10.1016/j.neurobiolaging.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 68.Kern W, Peters A, Fruehwald-Schultes B, Deininger E, Born J, Fehm HL. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology. 2001;74:270–280. doi: 10.1159/000054694. [DOI] [PubMed] [Google Scholar]
- 69.Reger MA, Watson GS, Frey Ii WH, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S. Effects of intranasal insulin on cognition in memory-impaired older adults: Modulation by APOE genotype. Neurobiol Aging. 2006;27:451–458. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 70.Reger MA, Watson GS, Green PS, Wilkinson CW, Baker LD, Cholerton B, Fishel MA, Plymate SR, Breitner JCS, DeGroodt W, Mehta P, Craft S. Intranasal insulin improves cognition and modulates {beta}-amyloid in early AD. Neurology. 2008;70:440–448. doi: 10.1212/01.WNL.0000265401.62434.36. [DOI] [PubMed] [Google Scholar]
- 71.Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging. 1996;17:123–130. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 72.Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guénette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bates KA, Verdile G, Li QX, Ames D, Hudson P, Masters CL, Martins RN. Clearance mechanisms of Alzheimer’s amyloid-[beta] peptide: Implications for therapeutic design and diagnostic tests. Mol Psychiatry. 2008;14:469–486. doi: 10.1038/mp.2008.96. [DOI] [PubMed] [Google Scholar]