Many microbes undergo transformations from planktonic swimmer cells to sessile surface-attached cells. This is the first step in bacterial biofilm formation. Biofilm-associated microorganisms are responsible for ~80% of human bacterial infections according to the U.S. National Institutes of Heath and are associated with antibiotic resistance (1). Thus, methods to disrupt biofilms are actively pursued. Biofilm formation is initiated by the ability of bacteria to sense surfaces, resulting in metabolic responses that allow bacteria to adhere and colonize the surface. Yet, how bacteria sense surfaces at the onset of biofilm formation has remained elusive (2). Differentiation from swimmer to swarmer cells in Vibrio parahaemolyticus was shown to occur simply by increasing the viscosity of the liquid growth medium (3). This suggested that some kind of mechanosensing mechanism was involved in surface recognition, but how? On pages 535 and 531 of this issue, Ellison et al. (4) and Hug et al. (5), respectively, discover separate mechanisms that allow bacteria to recognize a surface by mechanosensation and initiate a cellular response that allows them to attach and multiply.
Two candidate organelles present in bacteria that could facilitate mechanosensation are the rotary flagellum and the retractable Type IV pilus (6–8). Bacteria swim by rotating propellar-like organelles, called flagella, which extend from the cell surface (9). Pioneering work on the bacterium V. parahaemolyticus revealed that surface-sensing results in the differentiation from free-swimming bacteria that use the rotation of single polar flagellum to elongated bacteria that produce dozens of lateral flagella that facilitate surface colonization and spreading (10). Pili, also called fimbriae, are hair-like structures that extend from the cell surface and are usually associated with surface attachment. However, Type IV pili are retractable and are used to crawl across surfaces by a process called twitching motility (11). Inhibition of flagellar rotation or pilus retraction upon surface contact could contribute to the mechanosensing of surfaces.
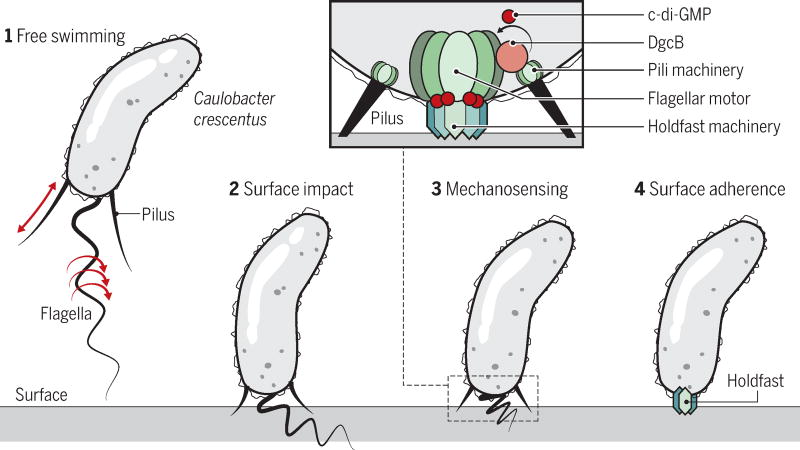
Ellison et al. and Hug et al. report separate mechanosensing mechanisms associated with surface recognition and induction of an adherence organelle in Caulobacter crescentus. This bacterium has a long history as a model system to study fundamental mechanisms of developmental biology (12). This is because this organism undergoes a programmed cell cycle in which a motile, flagellated swimmer cell differentiates into a sessile stalked cell, which is glued to surfaces via an exopolysaccharide adhesin, the holdfast (see the figure). Although holdfast production is part of a slower developmental program, its production is triggered rapidly when motile cells experience surface contact. Newborn motile cells are equipped with a single flagellum and adjacent pili at one pole of the cell. Although the Caulobacter pili were known to facilitate surface attachment, whether or not they were retractable and therefore candidates for mechanosensing of surfaces, was unclear. By fluorescently labeling the extracellular pili, Ellison et al. visualized pilus dynamics of extension and retraction, which is inhibited on surface contact. The authors demonstrate that this retraction generates a measurable force. In the absence of surface contact, blocking pilus retraction is enough to initiate holdfast synthesis. These authors conclude that it is the blocking of pilus retraction that facilitates surface sensing.
Figure 1.
Motile bacteria can become sessile cells that adhere to and colonize a surface.
Hug et al. provide evidence that inhibition of rotation of the Caulobacter flagellum can also work as a surface sensor. These authors developed a microfluidic assay that exposes newly divided flagellated daughter cells to a surface as a consequence of flow of the culture medium so that holdfast production and surface attachment occur before separation of daughter cell from mother cell. The ability to attach was enhanced in strains lacking outer parts of the flagellum, suggesting that the flagellum is not essential as a surface tether. However, mutants that were defective in rotor and stator components (which are required for flagellar rotation) were defective in attachment. The presence of the polar pili was also required for rapid attachment in these assays. The authors then specifically addressed the role of pili in surface sensing by designing thin, diffusion-controlled microchambers with a height of a single bacterium. Under this growth condition, cells were in constant surface exposure without the flow of medium, and pili were no longer required for holdfast synthesis, whereas flagellar motor rotation remained important. The authors conclude that the role of the pilus is to retract upon surface contact and bring the cell pole close to the surface to facilitate effective sensing by the flagellar motor.
Hug et al. also characterized the regulatory cascade initiated by surface contact that leads to holdfast synthesis. In many bacteria, surface contact and biofilm production coincides with the production of the second messenger cyclic diguanylate (c-di-GMP). A Caulobacter strain unable to synthesize c-di-GMP did not assemble holdfast, whereas constitutive synthesis of c-di-GMP bypassed the requirement of flagellar rotation for attachment but not the requirement for pili. This suggests c-di-GMP functions downstream of motor rotation to effect holdfast synthesis. The authors identified a diguanylate cyclase gene, dgcB, which resulted in defective rapid attachment when deleted, providing a candidate regulatory element that responds to surface contact and initiates holdfast synthesis. The DgcB protein was shown to localize to the flagellated pole and to interact with the MotA component of the flagellar stator. The authors further identified a potential redox or energy-sensing domain in the N terminus of DgcB. Because flagellum rotation drains the local proton motive force (PMF) at the cell pole, inhibition of flagellar rotation would effectively increase the local PMF, which could be the mechanism DgcB uses to sense inhibition of flagellar rotation upon surface contact. Finally, the authors identified HfsJ, a glycosyltransferase that is essential for holdfast biogenesis and functions as a c-di-GMP binding protein, exposing a missing link between the motor-induced burst of the second messenger and holdfast synthesis.
How does one resolve the apparent contradictions between the roles of the flagellum and pili? Ellison et al. claim that pili are the mechanosensors, with loss of flagellar rotation having no effect. By contrast, Hug et al. assert that the flagellar motor is essential for surface sensing and that in their assays, pili play an important but indirect role. The difference is in the timescales and conditions in their respective measurements. Ellison et al. conduct their assays in the absence of fluid flow, whereas Hug et al. record holdfast synthesis under flow and in quasi–two-dimensional chambers, assays that allow recording holdfast production in real time. By contrast, Ellison et al. use assays that include labeling, washing and concentrating cells over timescales of minutes. Therefore, one can conclude that under static conditions, pilus retraction is the surface sensor, whereas under conditions of flow, inhibition of flagellum rotation is sensing surface contact. In native environments, both activities are likely coordinated.
There are aspects of the flagellar rotation story that are baffling. Mutants lacking outer components of the motor (the rod, hook and filament) remained fully responsive to a surface, whereas mutants that failed to assemble elements of the rotor or stator of the motor were defective. A motor lacking external components, were it to rotate at all, would be expected to do so at top speed near zero load, without means for increasing that load and slowing down. The inner parts of the flagellar motor span the inner cell membrane with the stator elements mounted on the inner surface of the peptidoglycan layer. The inner membrane is held against the peptidoglycan layer by a large turgor pressure. How can displacement of the outer membrane have any effect? Is there something peculiar about the cell pole? Further work is needed to investigate these questions.
Figure 2. Surface sensing and attachment by Caulobacter crescentus.
When a free-swimming Caulobacter bacterium encounters a surface, either the intact flagellar motor, acting as a mechanosensitive channel, or a pilus, inhibited from retraction upon surface contact, stimulates localized c-di-GMP production that results in holdfast production and surface adherence. For the flagellum, the DgcB protein is responsible for c-di-GMP production at the cell pole, which binds to the HfsJ component of the holdfast synthesis machinery.
References
- 1.Butler MT, Wang Q, Harshey RM. Proc. Natl. Acad. Sci. U.S.A. 2010;107:3776. doi: 10.1073/pnas.0910934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Toole GA, Wong GC. Curr. Opin. Microbiol. 2016;30:139. doi: 10.1016/j.mib.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belas R, et al. J. Bacteriol. 1986;167:210. doi: 10.1128/jb.167.1.210-218.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellison CK, et al. Science. 2017;358:535. doi: 10.1126/science.aan5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hug I, Deshpande S, Sprecher KS, Pfohl T, Jenal U. Science. 2017;358:531. doi: 10.1126/science.aan5353. [DOI] [PubMed] [Google Scholar]
- 6.Rossez Y, Wolfson EB, Holmes A, Gally DL, Holden NJ. PLOS Pathog. 2015;11:e1004483. doi: 10.1371/journal.ppat.1004483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo Y, et al. mBio. 2015;6:e02456–14. [Google Scholar]
- 8.Persat A, Inclan YF, Engel JN, Stone HA, Gitai Z. Proc. Natl. Acad. Sci. U.S.A. 2015;112:7563. doi: 10.1073/pnas.1502025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg HC, Anderson RA. Nature. 1973;245:380. doi: 10.1038/245380a0. [DOI] [PubMed] [Google Scholar]
- 10.McCarter L, Silverman M. Molec. Microbiol. 1990;4:1057. doi: 10.1111/j.1365-2958.1990.tb00678.x. [DOI] [PubMed] [Google Scholar]
- 11.Burrows LL. Annu. Rev. Microbiol. 2012;66:493. doi: 10.1146/annurev-micro-092611-150055. [DOI] [PubMed] [Google Scholar]
- 12.Ausmees N, Jacobs-Wagner C. Annu. Rev. Microbiol. 2003;57:225. doi: 10.1146/annurev.micro.57.030502.091006. [DOI] [PubMed] [Google Scholar]