Abstract
Background
Prostate cancer (PCa) is the second most commonly diagnosed cancer in males worldwide. This study aimed to identify differentially expressed genes and to investigate the potential correlation between gene abnormalities and clinical features in PCa to evaluate disease progression and prognosis.
Material/Methods
A total of 4 independent microarrays of PCa patients from the Oncomine database were used to identify differences in expression of genes contributing to cancer progression. Quantitative real-time polymerase chain reaction (RT-qPCR) analysis was used to evaluate the mRNA expression of the target in human prostate cancer cells. To explore the relationship between the DNA copy number alteration and mRNA expression changes, dataset containing copy number alteration, DNA methylation, and gene expression in PCa were obtained from the cBioPortal online platform (n=273).
Results
We identified 40 genes that were significantly dysregulated in PCa from 4 independent microarrays. Among these, 3 genes showed a consistent change of over 2-fold in the 4 microarrays. The mRNA expression of C10orf116 showed consistent expression in prostate cancer cells compared with that in prostate gland cells as assessed by RT-qPCR. Moreover, C10orf116 loss was associated with poor distant relapse-free survival (DFS) by analyzing data of 273 PCa patients, but it was not identified as an independent prognostic risk factor for DFS. In addition, we found that C10orf116 loss was associated with higher pathological stage, higher clinical stage, and lymph node metastasis in PCa, and that C10orf116 copy number was highly correlated with PTEN copy number and mRNA expression.
Conclusions
As a predictive indicator, C10orf116 loss contributes to our understating of the biology of aggressive changes in PCa and also helps evaluate the prognosis of patients.
MeSH Keywords: Computational Biology, Disease Progression, Prognosis, Prostatic Neoplasms
Background
Prostate cancer (PCa) is the second most commonly diagnosed cancer in males worldwide [1]. At present, PCa treatments are primarily made on the basis of tumor stage, prostate-specific antigen (PSA) level, histological grading, Gleason score, and lymph node metastasis [2]. Due to the relatively small tumor size and complex structure that requires careful pathologist-guided dissection, novel biomarkers are needed to evaluate disease progression and prognosis to reduce the risk of overdiagnosis and overtreatment.
Significant efforts have been focused on genomics analysis, including copy number, methylation, and sequence classification. In recent years, there has been a rapid increase of information about prostate cancer genomics. Genomic instability, such as losses or gains of copy number, are a hallmark of PCa cells and are speculated to reflect the potential of malignancy of PCa and to influence patient outcome. Previous studies have shown the gain of MYC and the losses of PTEN and TP53 to be of prognostic value in prostate cancer [3–5]. Hieronymus [6] showed that DNA copy number alteration (CNA) burden in prostate cancer genomes, defined as the percentage of the tumor genome affected by CNA, was associated with recurrence and metastasis. There has been little progress in discovering the other prognostic genomic markers targeted by various common amplifications and deletions in recent years, in part due to limited prostate cancer cohorts with complete genomic and clinical outcome data.
C10orf116 is overexpressed in obese individuals and in human adipose tissues. There are few reports on the functional study of this gene in the literature. C10orf116 has been shown to differ in different pathological grades of ovarian carcinoma and nonmuscle-invasive bladder cancer [7,8]. Moreover, germline polymorphisms at rs4934282 (C10orf116) are associated with ovarian cancer survival [9]. In prostate cancer, only 1 study showed that C10orf116 is a potential prostate cancer biomarker in urinary exosomes [10]. Thus, it is necessary to explore the biological significance of C10orf116 (mRNA expression and CNA) in PCa.
In this study, we identified differently expressed genes and investigated the potential correlation between C10orf116 CNA and clinical features in PCa by using the public databases Oncomine, cBioPortal, and TCGA. We found that C10orf116 loss was associated with high pathological stage, high clinical stage, and lymph node metastasis in PCa, and that the C10orf116 copy number was highly correlated with PTEN copy number and mRNA expression. Our study shows that the copy number loss of C10orf116 is a promising biomarker for assessing prostate cancer progression and recurrence.
Material and Methods
Cell culture
The human prostate cancer cell lines PC-3, DU145, LNCaP, and C42B were purchased from the American Type Culture Collection (Manassas, VA) and maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA). RWPE-1, the normal prostate cell line, was purchased from ATCC and cultured in a complete medium (keratinocyte serum-free medium (K-SFM) with bovine pituitary extract and epidermal growth factor).
Quantitative real-time polymerase chain reaction (RT-qPCR)
Total RNAs were extracted from cells using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. A total of 2 μg RNA were subjected to cDNA synthesis using the SuperScript III reverse transcriptase kit (Invitrogen). RT-qPCR was performed using the Power SYBR Green PCR MasterMix (Applied Biosystems, Foster City, CA, USA) on an ABI 7300 thermocycler. The sequences of the primers were as follows: C10orf116(sense: 5′-CACAGATACCCCGAAGCCAT-3′, antisense: 5′-CCTGGTCCACCACT TGCTGA-3′). GAPDH (sense: 5′-CCCACTCCTCCACCTTTGAC-3′, antisense: 5′-TCCTCTTGTGCTCTTGCTGG-3′). Each sample was analyzed in triplicate and the relative expression of the target gene to GAPDH was calculated using the ΔΔCT method.
Data mining
The significantly deregulated genes were identified in PCa by 4 independent microarrays from the Oncomine database (https://www.oncomine.org/resource/main.html): Liu Protate microarray (44 PCa vs. 13 prostate gland tissues), Vanaja Prostate microarray (27 PCa vs. 40 prostate gland tissues), Varambally Prostate microarray (7 PCa vs. 6 prostate gland tissues), and Wallace Prostate microarray (69 PCa vs. 20 prostate gland tissues). Gene copy number, mRNA, DNA methylation, and clinical data of 499 PCa patients in the TCGA prostate cancer (Provisional) dataset were retrieved from the cBioPortal online platform [11,12], but only 273 samples with full prognosis data were used in further analyses.
Statistical analysis
The mRNA expression of a gene is presented as the mean ±SD. The difference between the 2 groups was analyzed using the t test. One-way analysis of variance was performed to detect differences among 3 or more groups. DNA methylation values were divided into high- and low-methylation groups using the median as a cutoff. Recurrence-free survival rates were calculated using the Kaplan-Meier method and log-rank test. Risk factors for disease recurrence were assessed using the Cox hazard ratio model. The correlation between CNA and clinicopathologic characteristics was evaluated by Pearson’s χ2 test (two-sided). Pearson correlation coefficient was calculated to evaluate the relationship between the DNA copy number or DNA methylation and gene expression. P<0.05 was considered statistically significant. Statistical analysis was conducted using SPSS 13.0 software.
Results
Identification of genes expressing abnormalities in PCa by bioinformatics
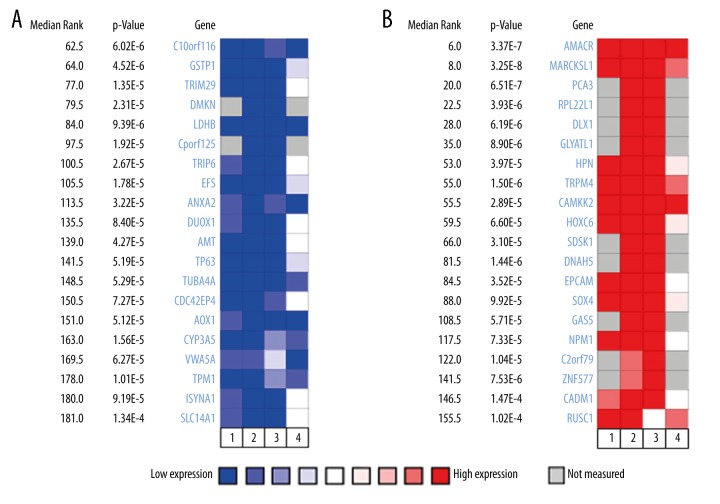
To identify dysregulated genes in PCa, we performed an analysis using 4 independent microarrays from the Oncomine database. The 4 microarrays were: Liu Prostate Statistics covering 44 cases of prostate carcinoma and 13 cases of prostate gland tissues, Vanaja Prostate Statistics covering 27 cases of prostate adenocarcinoma and 8 prostate gland tissues, Varambally Prostate Statistics covering 7 cases of prostate carcinoma and 6 prostate gland tissues, and Wallace Prostate Statistics covering 69 cases of prostate adenocarcinoma and 20 prostate gland tissues. According to the ranked P value, the top 20 genes that were significantly downregulated (P<1.09E-4) and the top 20 genes were significantly upregulated (P<1.26E-4) were retrieved from the Oncomine database by analyzing these 4 independent microarrays (Figure 1). Among the 40 identified dysregulated genes in PCa, 3 genes (C10orf116, AMACR, and TPM4) showed a consistent change of over 2-fold in the 4 microarrays. AMACR and TPM4 genes have been studied extensively as potential cancer drivers in PCa (Table 1). Therefore, C10orf116 gene was selected for further validation.
Figure 1.
Identification of genes expressing abnormalities in PCa by bioinformatics analysis from the Oncomine database. The top 20 candidate genes expression were found to be decreased (A) and the top 20 candidate genes expression were found to be increased in tumor samples by analyzing these 4 independent microarrays. (B): (1) Liu Prostate Statistics, 44 cases of prostate carcinoma and 13 prostate gland tissues; (2) Vanaja Prostate Statistics, 27 cases of prostate adenocarcinoma and 8 cases of prostate gland tissues; (3) Varambally Prostate Statistics, 7 cases of prostate carcinoma and 6 cases of prostate gland tissues; (4) Wallace Prostate Statistics, 69 cases of prostate adenocarcinoma and 20 cases of prostate gland tissues. The rank for a gene is the median rank among 4 microarrays.
Table 1.
Identification of genes expressing abnormalities in PCa according to four independent microarrays.
| Gene | Independent microrray data (Fold change) | No. of article* | Association with prostate cancer | ||||
|---|---|---|---|---|---|---|---|
| Direction of regulation | Liu | Vanaja | Varambally | Wallace | |||
| C10orf116 | Down | 2.08 | 2.36 | 2.37 | 2.21 | 1 | A potential prostate cancer biomarker in urinary exosomes [10] |
| AMACR | Up | 5.77 | 8.69 | 10.45 | 2.96 | 132 | Overexpression in prostate adenocarcinomas [13]; growth regulation of PCa [14]; association with biochemical recurrence and poor prognosis [15] |
| TRPM4 | Up | 2.75 | 3.94 | 3.62 | 4.54 | 4 | a novel tissue biomarker [16]; driver gene in androgen-insensitive prostate cancer [17] |
No. of articles was based on a search in the PubMed datebase.
C10orf116 mRNA expression in human prostate cancer
To confirm the significance of C10orf116 in human prostate cancer progression, we evaluated the mRNA expression of C10orf116 in human prostate cancer cells by qRT-PCR. As shown in Figure 2, the mRNA expression of C10orf116 was decreased in prostate cancer cells compared with the average expression in RWPE-1 prostate gland cells. The C10orf116 gene expression determined by qRT-PCR was consistent with the expression identified by microarrays results.
Figure 2.
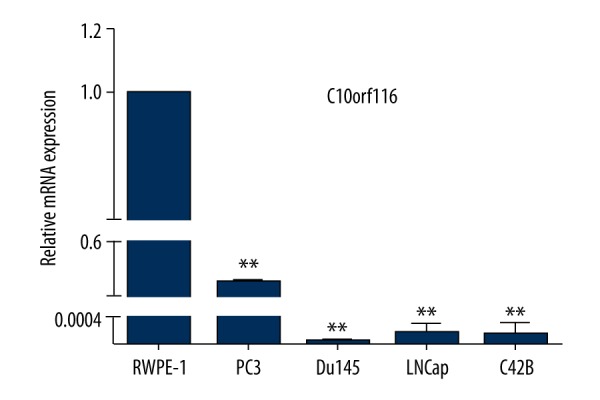
C10orf116 mRNA expression in human prostate cancer cells. RT-qPCR analysis of endogenous C10orf116 mRNA expression in 4 human prostate cancer cell lines – PC3, Du145, LNCaP, and C42B – and normal human prostate gland cell line RWPE-1. The data are expressed as means ±SD (n=3, * P<0.05, ** P<0.01, compared with control: RWPE-1).
C10orf116 gene copy number loss in prostate cancer: Clinicopathological correlations and prognostic significance
CNA has been identified as an important cause of cancers and developmental abnormalities [18]. A total of 273 PCa patient samples with complete clinical data in a cohort of TCGA were analyzed. Among these, we found that more than 23% of cases had C10orf116 deletion (including homozygous deletion and hemizygous deletion), which suggests the important roles of C10orf116 loss in PCa (data no shown). We therefore assessed C10orf116 copy number status in PCa by checking data in cBioPortal.
We first evaluated their prognostic value in PCa patients from TCGA data using a Kaplan-Meier plotter. Patients were divided into 2 groups based on copy number status. We found that C10orf116 loss was associated with shorter distant relapse-free survival than C10orf116 Not-loss [HR=1.912 (1.03–3.549), P=0.0039, Figure 3]. Because there were fewer deaths in cBioPortal, we did not analyze the relationship between C10orf116 copy number status and overall survival. We also analyzed the association between C10orf116 copy number status and clinicopathological characteristics of prostate cancer. C10orf116 copy number status was significantly associated with pathological stage, clinical stage, and lymph node metastasis (Table 2, P=0.009, P=0.011, and P=0.001, respectively). There were no significant differences in C10orf116 copy number change based on age or Gleason score. In multivariable analysis, Gleason score and neoplasm status were significantly associated with DFS (Table 3). Therefore, C10orf116 loss is not an independent predictor for higher risk of recurrence.
Figure 3.
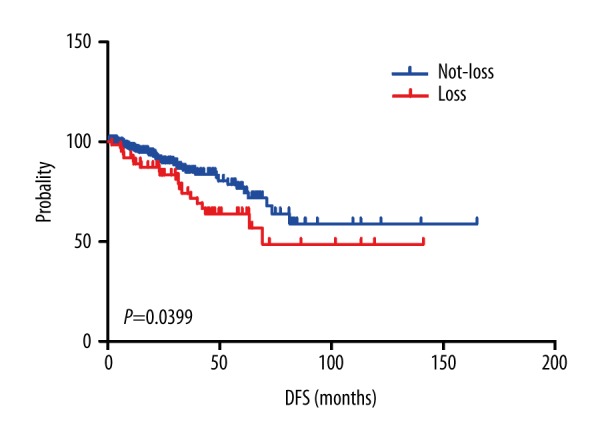
Disease-free survival analysis for prostate cancer according to C10orf116 copy number status.
Table 2.
Associations of C10orf116 copy number loss with clinicopathological characteristics of prostate cancer.
| Criteria | C10orf116 | P value* | ||
|---|---|---|---|---|
| Not-loss | Loss | Total | ||
| Agea | 0.708 | |||
| <61 | 97 (77.6%) | 28 (22.4%) | 125 | |
| ≥61 | 112 (75.7%) | 36 (24.3%) | 148 | |
| Pathological stage | 0.009 | |||
| T2 | 86 (86.9%) | 13 (13.1%) | 99 | |
| T3 | 120 (71.0%) | 49 (29.0%) | 169 | |
| T4 | 3 (60.0%) | 2 (40.0%) | 5 | |
| Stage | 0.011 | |||
| II | 159 (80.3%) | 39 (19.7%) | 198 | |
| III | 24 (77.4%) | 7 (22.6%) | 31 | |
| IV | 26 (59.1%) | 18 (40.9%) | 44 | |
| Gleason | 0.064 | |||
| <8 | 106 (81.5%) | 24 (18.5%) | 130 | |
| ≥8 | 103 (72.0%) | 40 (28.0%) | 143 | |
| Lymph node metastasis | 0.001 | |||
| Negative | 183 (80.3%) | 45 (19.7%) | 228 | |
| Positive | 26 (57.8%) | 19 (42.2%) | 45 | |
Age was divided into <61 and ≥61 using the median as a cutoff.
P value determined by Person’s χ2 test (two-sided).
Table 3.
Multivariate analysis of predictive factors of recurrence for 273 patients of PCa.
| Factors | B | SE | Wald | df | Sig. | Exp.(B) | 95%CI | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Gleason score (<8/≥8) | −.873 | .421 | 4.308 | 1 | .038 | .418 | .183 | .953 |
| Pathologic stage (2/3+4) | −.623 | .505 | 1.525 | 1 | .217 | .536 | .199 | 1.442 |
| Clinical stage (II/III+IV) | −.446 | .407 | 1.205 | 1 | .272 | .640 | .288 | 1.420 |
| Lymph node metastasis (negative/positive) | .464 | .417 | 1.235 | 1 | .266 | 1.590 | .702 | 3.605 |
| C10orf116 (Not-loss/Loss) | −.195 | .305 | .410 | 1 | .522 | .822 | .452 | 1.496 |
| Resection margin (R0/R1–2) | .557 | .323 | 2.984 | 1 | .084 | 1.746 | .928 | 3.286 |
| Neoplasm status (free/with) | −2.081 | .356 | 34.258 | 1 | .000 | .125 | .062 | .251 |
| Age | −.006 | .019 | .099 | 1 | .753 | .994 | .957 | 1.032 |
C10orf116 copy number is correlated with PTEN copy number and mRNA in PCa
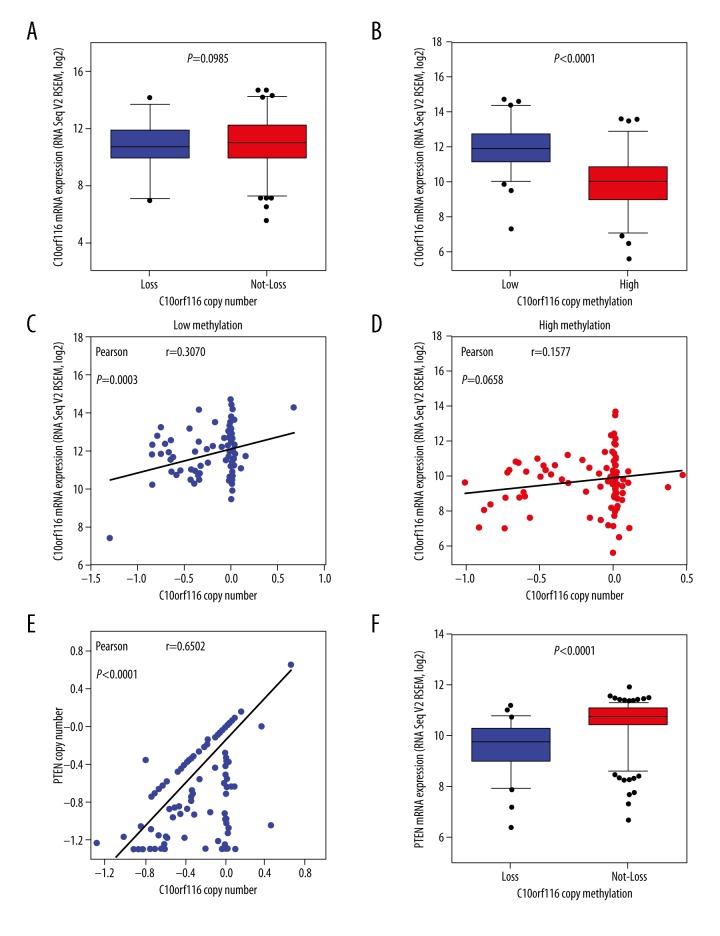
We then compared mRNA levels by C10orf116 copy number alteration in cBioPortal to elucidate the underlying mechanism of C10orf116 loss. Surprisingly, C10orf116 copy number alteration was not correlated with C10orf116 mRNA expression levels (P=0.0985, Figure 4A). We found that C10orf116 mRNA expression levels were significantly correlated with DNA methylation (P<0.0001, Figure 4B). In cBioPortal, to determine whether DNA methylation influences the association between DNA copy number and mRNA expression, the patients were divided into high-methylation and low-methylation groups using the median of DNA methylation values as a cutoff. When the Pearson correlation coefficients between the copy number and mRNA expression of C10orf116 were calculated in the high- and low-methylation groups, there was a statistically significant positive correlation between the copy number and the gene expression in the low-methylation groups (P=0.0003, Figure 4C), but there were no significant differences in the high-methylation groups (P=0.0658, Figure 4D). These findings suggest that methylation has a greater effect on gene expression of C10orf116.
Figure 4.
C10orf116 copy number was correlated with PTEN copy number and mRNA in PCa. (A) The box plots showed the relationship between mRNA expression and copy number status of C10orf116 gene in prostate tumor. Patients with PCa were divided into 2 subgroups based on their copy number status. (B) Comparison of relationships between mRNA expression and methylation values. Comparison of relationships between C10orf116 copy number and mRNA expression in low-methylation subgroups (C) or high-methylation subgroups (D). P values in the box plot were obtained by t tests (two-tailed). Two scatter plots with regression line in each picture showed an association between copy number and mRNA expression of C10orf116 gene in the low- and high-methylation subgroups. Comparison of relationships between C10orf116 copy number alteration and PTEN copy number (E) or mRNA expression (F).
PTEN is the most common tumor suppressor gene in prostate cancer (PCa) [19,20] and its loss is correlated with poor pathologic and clinical outcomes [21,22]. Therefore, we evaluated the potential correlation between C10orf116 copy number and PTEN loss. We found that C10orf116 copy number was highly correlated with PTEN copy number and mRNA expression (P<0.0001, Figure 4E, 4F). Then, we downloaded the copy number data of C10orf116 and PTEN based on the study of the Cancer Cell Line Encyclopedia. A total of 7 prostate cancer cell lines were included, and they had the same copy number of alternations in C10orf116 and PTEN. Furthermore, the copy number loss of C10orf116 and PTEN was significantly associated with lymph node metastasis in prostate cancer cells. Results in different prostate cancer cell lines are detailed in Table 4.
Table 4.
The copy number status of C10orf116 and PTEN on different prostate cancer cell lines.
| Cell line | Tumor Type | Metastastic site | C10orf116 loss | PTEN loss |
|---|---|---|---|---|
| NCI-H660 | Prostate | Lymph node | Yes | Yes |
| DU 145 | Prostate | Brain | No | No |
| LNCaP clone FGC | Prostate | Lymph node | Yes | Yes |
| 22Rv1 | Prostate | No | No | No |
| PC-3 | Prostate | Bone | Yes | Yes |
| VCaP | Prostate | Vertebral | No | No |
| MDA PCa 2b | Prostate | Bone | No | No |
Discussion
PCa is a very heterogeneous disease and has different clinical manifestations, from indolent to aggressive tumors with poor progression-free survival. Therefore, early diagnosis and identification of PCa aggressiveness are important prerequisites for efficient treatment of patients [23]. PSA, as a serum marker, is the only biomarker that is widely used for diagnosis and prognosis of PCa. However, PSA is organ-specific but not cancer-specific, and it is not able to differentiate between indolent and aggressive PCa. Many research groups are committed to investigating new molecular biomarkers of PCa to improve risk assessment and treatment strategies. Recently, biomarker research has shifted to use of “-omics” methods, including DNA, RNA, and DNA methylation. The transmembrane protease serine 2 (TMPRSS2)-ERG fusion gene is absent in approximately 50% of prostate cancer patients. Clinically, PTEN loss and MYC gain are associated with prostate cancer recurrence [5].
In this study, we found a potential PCa biomarker-C10orf116 gene by searching the Oncomine database. It was deregulated in PCa tissues and cells compared with adjacent tissues or prostate gland cells. In the TCGA prostate cancer dataset, we found that the mRNA of C10orf116 was not significantly associated with pathological stage, clinical stage, lymph node metastasis, or recurrence (data no shown). Thus, we focused on C10orf116 copy number alteration in further research. Firstly, we found C10orf116 loss was associated with poor DFS by analyzing data of 273 PCa patients, but it was not identified as an independent risk prognostic factor for DFS. Secondly, we found that C10orf116 loss was associated with higher pathological stage, higher clinical stage, and lymph node metastasis in PCa. In lymph node metastasis prostate cancer cell lines, such as NCI-H660 and LNCaP clone FGC, we also found the copy number is lost between C10orf116 and PTEN. These findings all demonstrate that the copy number loss of C10orf116 and PTEN was significantly associated with lymph node metastasis in prostate cancer. Moreover, we found that C10orf116 copy number was normal in 22Rv1 cells with relatively lower clinical stage. These findings suggest that C10orf116 copy number alteration has a role in evaluating PCa progression and risk. To the best of our knowledge, this is the first report of C10orf116 loss in PCa.
Studies confirmed that promoter region CPG island methylation-induced gene inactivation in the tumor suppressor gene is an important mechanism of tumorigenesis [24]. Intriguingly, the results of DNA methylation showed that C10orf116 mRNA expression was negatively correlated with C10orf116 methylation in PCa. However, there were no significant correlations between the copy number and the gene expression in the high-methylation groups. We speculated that the expression of C10orf116 might be regulated by both copy number loss and promoter methylation.
C10orf116, located at 10q23.2, is highly expressed in adipose tissue and is localized primarily within the nucleus. Scientists earlier found that there may be several deletions in the 10q23-26 chromosomal region [25] because of the frequent loss of PTEN and its neighboring genes in prostate cancer [26]. Due to sharing the same location at 10q23.2 with PTEN, it is possible that C10orf116 loss is critical in the pathogenesis of prostate cancer. We also found that C10orf116 copy number was highly correlated with PTEN copy number and mRNA expression. However, our study is limited by insufficient sample information to perform survival analysis.
Conclusions
We identified a PCa risk-associated gene based on copy number loss and expression of C10orf116 by analyzing a cohort of 273 PCa cases. As a predictive indicator, C10orf116 loss contributes to understating of the biology of aggressive changes in PCa and aids in evaluating patient prognosis. However, more samples need to be investigated before practical clinical use of the signature.
Acknowledgements
The authors thank Xiaolin Zhou and Xin Huang for editing.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the National Natural Science Foundation of China (NSFC) (81272415 and 81171993), the NSFC Key Project (81130046), the Guangxi Key Projects (2013GXNSFEA053004), and the Guangxi Projects (2012GXNSFCB053004)
References
- 1.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–92. doi: 10.1016/j.eururo.2012.02.054. [DOI] [PubMed] [Google Scholar]
- 2.Prensner JR, Rubin MA, Wei JT, et al. Beyond PSA: The next generation of prostate cancer biomarkers. Sci Trans Med. 2012;4:127rv3. doi: 10.1126/scitranslmed.3003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kluth M, Harasimowicz S, Burkhardt L, et al. Clinical significance of different types of P53 Gene alteration in surgically treated prostate cancer. Int J Cancer. 2014;135:1369–80. doi: 10.1002/ijc.28784. [DOI] [PubMed] [Google Scholar]
- 4.Krohn A, Diedler T, Burkhardt L, et al. Genomic deletion of PTEN is associated with tumor progression and early PSA recurrence in erg fusion-positive and fusion-negative prostate cancer. Am J Pathol. 2012;181:401–12. doi: 10.1016/j.ajpath.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Liu W, Xie CC, Thomas CY, et al. Genetic markers associated with early cancer-specific mortality following prostatectomy. Cancer. 2013;119:2405–12. doi: 10.1002/cncr.27954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hieronymus H, Schultz N, Gopalan A, et al. Copy number alteration burden predicts prostate cancer relapse. Proc Natl Acad Sci USA. 2014;111:11139–44. doi: 10.1073/pnas.1411446111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skubitz AP, Pambuccian SE, Argenta PA, et al. Differential gene expression identifies subgroups of ovarian carcinoma. Transl Res. 2006;148:223–48. doi: 10.1016/j.trsl.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Marín-Aguilera M, Mengual L, Ribal MJ, et al. Utility of urothelial mRNA markers in blood for staging and monitoring bladder cancer. Urology. 2012;79:240.e9–15. doi: 10.1016/j.urology.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Braun R, Finney R, Yan C, et al. Discovery analysis of TCGA data reveals association between germline genotype and survival in ovarian cancer patients. PLoS One. 2013;8:e55037. doi: 10.1371/journal.pone.0055037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Øverbye A, Skotland T, Koehler CJ, et al. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget. 2015;6:30357–76. doi: 10.18632/oncotarget.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: Nn open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozgur T, Atik E, Hakverdi S, et al. The expressions of AMACR and iNOS in prostate adenocarcinomas. Pak J Med Sci. 2013;29:610–13. [PMC free article] [PubMed] [Google Scholar]
- 14.Zha S, Ferdinandusse S, Denis S, et al. Alpha-methylacyl-CoA racemase as an androgen-independent growth modifier in prostate cancer. Cancer Res. 2013;63:7365–76. [PubMed] [Google Scholar]
- 15.Bishoff JT, Freedland SJ, Gerber L, et al. Prognostic utility of the cell cycle progression score generated from biopsy in men treated withprostatectomy. J Urol. 2014;192:409–14. doi: 10.1016/j.juro.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Berg KD, Soldini D, Jung M, et al. TRPM4 protein expression in prostate cancer: A novel tissue biomarker associated with risk of biochemical recurrence following radical prostatectomy. Virchows Arch. 2016;468:345–55. doi: 10.1007/s00428-015-1880-y. [DOI] [PubMed] [Google Scholar]
- 17.Holzmann C, Kappel S, Kilch T, et al. Transient receptor potential melastatin 4 channel contributes to migration of androgen-insensitive prostate cancer cells. Oncotarget. 2015;6:41783–93. doi: 10.18632/oncotarget.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beroukhim R, Mermel CH, Porter D, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor BS, Schultz N, Hieronymus H, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berger MF, Lawrence MS, Demichelis F, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–20. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmitz M, Grignard G, Margue C, et al. Complete loss of PTEN expression as a possible early prognostic marker for prostate cancer metastasis. Int J Cancer. 2007;120:1284–92. doi: 10.1002/ijc.22359. [DOI] [PubMed] [Google Scholar]
- 22.Sircar K, Yoshimoto M, Monzon FA, et al. PTEN genomic deletion is associated with p-Akt and AR signalling in poorer outcome, hormone refractory prostate cancer. J Pathol. 2009;218:505–13. doi: 10.1002/path.2559. [DOI] [PubMed] [Google Scholar]
- 23.Ziaran S, Varchulova Novakova Z, Bohmer D, et al. Biomarkers for determination prostate cancer: Implication for diagnosis and prognosis. Neoplasma. 2015;62:683–91. doi: 10.4149/neo_2015_082. [DOI] [PubMed] [Google Scholar]
- 24.Grady WM, Willis J, Guilford PJ, et al. Methylation of the CDHI promoter as the second genetic hit in hereditary diffuse gastric cancer. Nat Genet. 2000;26:16–17. doi: 10.1038/79120. [DOI] [PubMed] [Google Scholar]
- 25.Leube B, Drechsler M, Mühlmann K, et al. Refined mapping of allele loss at chromosome 10q23–26 in prostate cancer. Prostate. 2002;50:135–44. doi: 10.1002/pros.10038. [DOI] [PubMed] [Google Scholar]
- 26.Hermans KG, van Alewijk DC, Veltman JA, et al. Loss of a small region around the PTEN locus is a major chromosome 10 alteration in prostate cancer xenografts and cell lines. Genes Chromosomes Cancer. 2004;39:171–84. doi: 10.1002/gcc.10311. [DOI] [PubMed] [Google Scholar]