ABSTRACT
The REG3β protein was identified more than 2 decades ago, but its role in PDAC development was only recently reported. In Pancreatic Ductal Adenocarcinoma (PDAC), REG3β protein is expressed and released by the far microenvironment, which is situated out of the tumor, at the periphery of the tumor mass, and is part of the healthy peri-tumoral region. This compartment is completely unrelated to the classical microenvironment that corresponds to the intra-tumoral stoma. Clinically relevant, the far microenvironment, and the factors released by it, could be novel and original therapeutic targets for treating patients with a PDAC. In this way we recently demonstrated that REG3β is an essential soluble factor necessary for PDAC development which is able to stimulate several simultaneous pro-tumoral mechanisms. We also find that secreted REG3β boosts interactions between epithelial cells and immune cells by activating the CXCL12/CXCR4 signaling cascade, which facilitates tumor escape through evasion of immune surveillance, and promotes metastasis. In addition, REG3β interfere the intercellular communication inside the tumor mediated by extracellular vesicles, resulting in relevant changes in macrophage phenotype or tumor cell migration. Therefore, we are proposing to call as near microenvironment to the classical microenvironment that is constituted by fibroblasts, inflammatory cells and fibers and located into the tumor, and as far microenvironment, which is constituted by the parenchymal non transformed cells located at the periphery of the tumor mass.
KEYWORDS: pancreas cancer, Reg3beta, microenvironment, peri-tumoral region, pancreatitis
Pancreatic Ductal Adenocarcinoma (PDAC)
Pancreatic Ductal Adenocarcinoma (PDAC) kills around of 300,000 individuals every year in the world. The median of survival time for patients with a PDAC is of 6 months and the survival rate at 5 y is of only 7%. Lamentably, using information from the Surveillance Epidemiology and End Results (SEER) database, in 2020 the number of deaths by PDAC will exceed those from breast and colorectal cancer, and be surpassed only by the loss of life from lung cancer. Moreover, in 2030 the number of patients with a PDAC will represent more than a 2-fold increase over the current rate in the occidental world.1 Unfortunately, at the moment, the only curative treatment of patients with a PDAC remains the surgery with resection in healthy margins of the tumor but only 10–15% of these patients have a localized disease at the time of the diagnosis. The vast majority of the operated patients evolve rapidly by loco-regional or metastatic progression. This is why the scientific community, advocacy groups, and government agencies around the world have recognized PDAC as an important national health priority.
PDAC classification divides pancreatic cancer into categories according to different schemes, each based on different criteria and serving a different purpose. The major categories are the histopathological type, the grade of the tumor, the stage of the tumor, and the expression of proteins and genes. As the knowledge of pancreatic cancer cell biology progresses these classifications are accordingly updated. Histopathological grading is by matching the appearance of the PDAC cells compared with the normal pancreatic tissue. Normal cells in an organ like the pancreas are highly differentiated, reflecting the sophisticated function of the exocrine pancreas. Cancerous cells lose that differentiation and consequently their function. In PDAC, the cells that would normally line up in an orderly way to make up the acini become disorganized. Cell division becomes uncontrolled. Cell nuclei become less uniform. In function of the strength of these anomalies, pathologists describe cells of the PDAC as well differentiated (low-grade), moderately differentiated (intermediate-grade), and poorly differentiated (high-grade). Poorly differentiated PDAC have a worse prognosis.
The percentage of transformed cells may vary from 85% to 15% into a PDAC whereas the stromal components such as immune, inflammatory cells, fibroblast and fibers may represent from 15% to 85% of the tumor. It is well established that the transformed cells interacting with their stromal microenvironment control the tumor behavior. In fact, stroma produces and release several active factors that control some biologic functions of the transformed cells such as growth, migration and invasion. On the other hand, the transformed cells regulate amount, quality and function of the stromal component generating a molecular dialog between them and a well-controlled feedback that favor the tumor growth and is involved in the resistance to treatments. Therefore, connection between stroma and transformed cells in PDAC is intensively studied because it may result as an efficient therapeutic target.2-4
The tumor stromal microenvironment, by its own definition, is in direct contact with the transformed cells and represents therefore a near microenvironment. Contrary to the near microenvironment, a far microenvironment is situated out of the tumor, at the periphery of the tumor mass, and is part of the healthy peri-tumoral region. Unfortunately, its functional role in PDAC development and progression was neglected until recently.
Biological characteristics of the far microenvironment in PDAC
Between the tumor mass and the completely healthy tissue there is a region known as the peri-tumor area that is formed by not-transformed, although strongly functionally altered, cells as a consequence of its physical contact with the tumor and production of several inflammatory mediators. Morphologically and functionally, this area is distorted, inflamed and the contained cells are stressed. Therefore, gene expression of these cells is strongly modified and its profile is close to the pancreatitis-associated gene expression. It is important to note the difference with the concept of “field effect,” applied to a region of epithelium with preneoplastic changes that predisposes to tumor formation.5 In the far microenvironment, the changes observed in gene expression are unrelated to the genetic or epigenetic alterations but associated to the physiologic cell response to stress stimuli. To be noted, in the far microenvironment, the stress is triggered by the presence of neighboring tumor cells. In this regard, from several years, our laboratory has been focused on characterizing druggable pancreatitis-associated pathways and identifying genes activated in the stressed pancreas and in the far microenvironment area.6
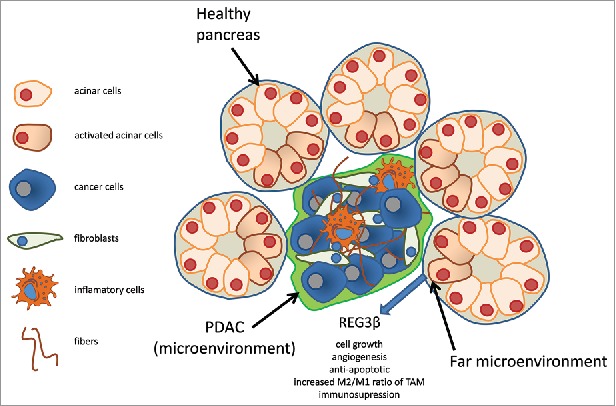
REG3β, also known as pancreatitis-associated protein (PAP), p23, or hepatocarcinoma-intestine-pancreas protein (HIP), is a 16 kDa secretory protein that was originally discovered in the pancreatic juice of animals with acute pancreatitis.7 REG3β is one of the best known pancreatitis-activated factors. We recently started a project to analyze the role of the pancreatitis-associated factors on PDAC development and progression. In this way, we demonstrated that REG3β is an essential factor for PDAC development and metastasis formation by combining a tumor environment engineered in mice for not expressing REG3β with isogenic PDAC cells orthotopically injected. In these conditions we found a dramatic reduction on PDAC growth.8 Mechanistically, this effect was mediated by several simultaneous effects such as a decreased tumor angiogenesis, increased apoptotic pictures of tumor cells, an increased CD3+ tumor-infiltrating T cells and a switch in macrophages activation reflected by a decrease in the M2/M1 ratio of tumor-associated macrophages.8 We also find that secreted REG3β boosts interactions between epithelial cells and immune cells by activating the CXCL12/CXCR4 signaling cascade,9 which sustains tumor cell growth, induces angiogenesis, facilitates tumor escape through evasion of immune surveillance, and promotes metastasis with neural invasion. Moreover, we showed that REG3β induces the expression of IL10, TGFβ and MRC-1,9 which together behave as immunomodulators and increase the synthesis of the pancreatic desmoplastic reaction (Fig. 1).
Figure 1.
Schematic representation of the far microenvironment. PDAC is composed by inflammatory cells, fibroblasts, cancer activated fibroblasts and fibers, which constitute the microenvironment and of cancer cells. The tumor mass which is in direct physical contact with the non tumoral cells induces a reaction which is like to the pancreatitis and produce factors such as REG3β. REG3β promotes cell growth, angiogenesis, protect of apoptosis, increases the M2/M1 ratio of tumoral activated macrophages (TAM) and immunosuppression. This region corresponds to the far microenvironment.
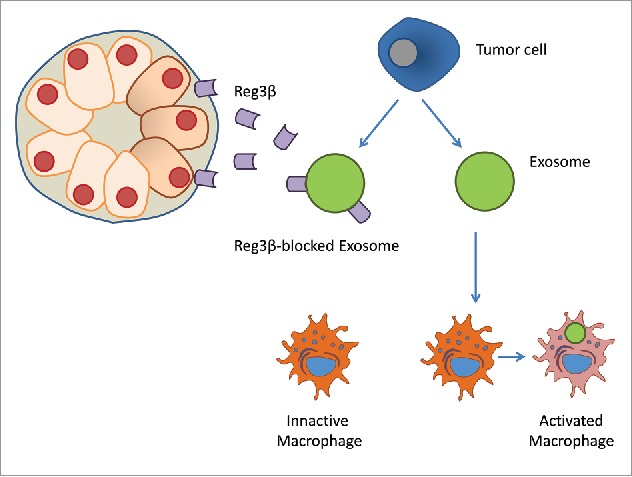
An additional mechanism through which Reg3β modulates the dialog between cells in the tumor is related with its ability to interfere in the uptake of extracellular vesicles. The lectin nature of Reg3β allows it to bind glycoproteins presents on the surface of extracellular vesicles, including exosomes, which plays a relevant role in the intercellular signaling inside the tumor.10 The interaction between exosomes and Reg3β results in a loss of the exosome-induced phenotypic switch in macrophages, the inhibition of the cell migration of cancer cells promote through exosomes and, also, in a reversion of several metabolomic changes promoted by the extracellular vesicles (Fig. 2).
Figure 2.
REG3β inhibits the intercellular exosome signaling pathway. REG3β is released by the far microenvironment, bind to the exosomes by its lectin capacity, and inhibits the function of exosomes.
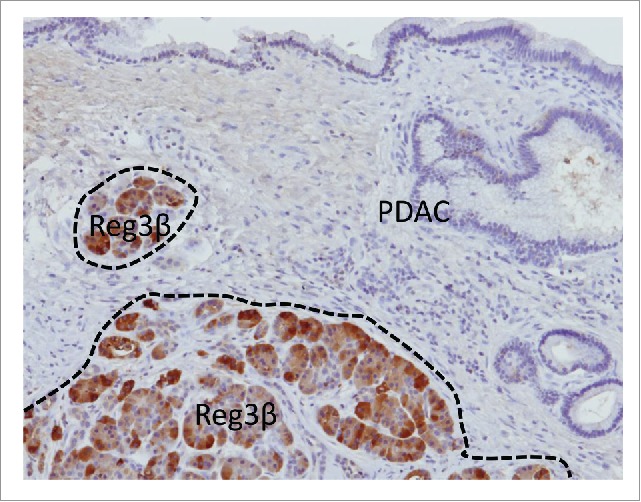
Importantly to be noted is that, in the pancreas of patients with PDAC, expression of REG3β was restricted to the far microenvironment since in transformed cells the expression of REG3β remains undetectable9 (Fig. 3). At least 3 strong and complementary arguments support the idea that the released REG3β protein mediates effect on the PDAC development: the first one is that REG3β-deficient mice does not develop PDAC in the KRASG12D genetic background contrary to the wild-type mice; the second is that neutralizing antibodies inhibit its pro-tumoral effect in vivo; and the third is that REG3β activates a gp130-, JAK2-, and STAT3-dependent signaling pathway to promote cell growth, increasing resistance to apoptosis and migration.9 Finally, other independent studies, using unrelated models and tools, confirmed that expression of REG3β promotes PDAC development11-14 supporting the concept in that the far microenvironment play a major role in PDAC.
Figure 3.
The far microenvironment is surrounding the tumor. Reg3β expression indicates the area where induction of a stress response in induced in the vicinity of the tumor.
In addition to the pancreas, relevant changes in the areas surrounding tumor cells, that could have an impact in their status, have been reported in other organs. For instance, the outcome of hepatocellular carcinoma shows a significant association with a particular profile of gene expression in adjacent non-neoplastic liver tissue.15 Similarly, in brain, active neurons can promote high-grade glioma proliferation through the secretion of soluble proteins as neuroligin-3.16 The particular role in the progression of the tumors in each organ remains to be elucidated.
Obviously, other cytokines, chemokines and secreted biologic actives proteins are produced and released by inflamed and stressed acinar cells present at the far microenvironment area that could participate in PDAC development and progression including metastasis formation. The response of pancreatic acinar cells to stress leads to the synthesis and release of several proteins that confers protection against further damage. This protective response seems to be induced in the surrounding area of the tumor and its effects on the PDAC progression have been overlooked. However, it remains to be clarified the evolution of the far microenvironment along the progression of PDAC as well as the distance at which normal tissue shows changes in response to the presence of tumor tissue. It will also be interesting to compare the evolution of the distant microenvironment in different types of cancer. It is not unreasonable to assume that some of the specific characteristics of different types of tumor may be related to the interactions with the different surrounding tissues. Altogether these observations also points out to the potential importance of the far microenvironment in the design of novel clinical strategies and new treatments targeting on disrupting the interactions between different cell populations presents in the tumor microenvironment.
Conclusions and perspectives
The REG3β protein was identified more than 2 decades ago, but its role in PDAC development was only recently reported. More important is that, in addition to its biologic role in PDAC described above, it revealed the importance of an unexpected actor that we named far microenvironment, to be distinguished from the classical microenvironment that is in fact the tumor stoma, which play an essential role in PDAC development and progression as well as in other cancers probably. Clinically relevant, the far microenvironment, and the factors released by it, could be novel and original therapeutic targets for treating patients with a PDAC. Probably, the effect of the factors released by cells of the far microenvironment is more relevant in the periphery of the tumors, the area by which tumor progress and invades, since they are closer than the depths of the tumor. We propose to call near microenvironment to the classical microenvironment that is constituted by fibroblasts, inflammatory cells and fibers and located into the tumor, and far microenvironment, which is constituted by parenchymal non transformed cells located at the periphery of the tumor mass.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by La Ligue Contre le Cancer, INCa, Canceropole PACA, DGOS (labellisation SIRIC) and INSERM.
References
- 1.Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by race, age, and sex in the improvement of survival for major cancers: Results from the national cancer institute Surveillance, Epidemiology, and End Results (SEER) program in the united states, 1990 to 2010. JAMA Oncol. 2015;1:88-96. doi: 10.1001/jamaoncol.2014.161. PMID:26182310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neesse A, Algul H, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: A changing paradigm. Gut. 2015;64:1476-84. doi: 10.1136/gutjnl-2015-309304. PMID:25994217 [DOI] [PubMed] [Google Scholar]
- 3.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266-76. doi: 10.1158/1078-0432.CCR-11-3114. PMID:22896693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leca J, Martinez S, Lac S, Nigri J, Secq V, Rubis M, Bressy C, Sergé A, Lavaut MN, Dusetti N, et al.. Cancer-associated fibroblast-derived annexin A6+ extracellular vesicles support pancreatic cancer aggressiveness. J Clin Invest. 2016;126:4140-56. doi: 10.1172/JCI87734. PMID:27701147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai H, Brown RE. Field effect in cancer-an update. Ann Clin Lab Sci. 2009;39:331-7. PMID:19880759 [PubMed] [Google Scholar]
- 6.Dusetti NJ, Tomasini R, Azizi A, Barthet M, Vaccaro MI, Fiedler F, Dagorn JC, Iovanna JL. Expression profiling in pancreas during the acute phase of pancreatitis using cDNA microarrays. Biochem Biophys Res Commun. 2000;277:660-7. doi: 10.1006/bbrc.2000.3734. PMID:11062010 [DOI] [PubMed] [Google Scholar]
- 7.Keim V, Iovanna JL, Rohr G, Usadel KH, Dagorn JC. Characterization of a rat pancreatic secretory protein associated with pancreatitis. Gastroenterology. 1991;100:775-82. doi: 10.1016/0016-5085(91)80025-5. PMID:1704329 [DOI] [PubMed] [Google Scholar]
- 8.Gironella M, Calvo C, Fernandez A, Closa D, Iovanna JL, Rosello-Catafau J, Folch-Puy E. Reg3beta deficiency impairs pancreatic tumor growth by skewing macrophage polarization. Cancer Res. 2013;73:5682-94. doi: 10.1158/0008-5472.CAN-12-3057. PMID:23867474 [DOI] [PubMed] [Google Scholar]
- 9.Loncle C, Bonjoch L, Folch-Puy E, Lopez-Millan MB, Lac S, Molejon MI, Chuluyan E, Cordelier P, Dubus P, Lomberk G, et al.. IL17 Functions through the Novel REG3beta-JAK2-STAT3 inflammatory pathway to promote the transition from chronic pancreatitis to pancreatic cancer. Cancer Res. 2015;75:4852-62. doi: 10.1158/0008-5472.CAN-15-0896. PMID:26404002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonjoch L, Gironella M, Iovanna JL, Closa D. REG3beta modifies cell tumor function by impairing extracellular vesicle uptake. Sci Rep. 2017;7:3143. doi: 10.1038/s41598-017-03244-4. PMID:28600520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin G, Du J, Cao H, Liu X, Xu Q, Xiang M. Reg3g promotes pancreatic carcinogenesis in a murine model of chronic pancreatitis. Dig Dis Sci. 2015;60:3656-68. doi: 10.1007/s10620-015-3787-5. PMID:26182900 [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Wang J, Wang H, Yin G, Liu Y, Lei X, Xiang M. REG3A accelerates pancreatic cancer cell growth under IL-6-associated inflammatory condition: Involvement of a REG3A-JAK2/STAT3 positive feedback loop. Cancer Lett. 2015;362:45-60. doi: 10.1016/j.canlet.2015.03.014. PMID:25779676 [DOI] [PubMed] [Google Scholar]
- 13.Xu Q, Fu R, Yin G, Liu X, Liu Y, Xiang M. Microarray-based gene expression profiling reveals genes and pathways involved in the oncogenic function of REG3A on pancreatic cancer cells. Gene 2016;578(2):263-73. doi: 10.1016/j.gene.2015.12.039. [Epub 2015 Dec 21]. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Wang H, Zogopoulos G, Shao Q, Dong K, Lv F, Nwilati K, Gui XY, Cuggia A, Liu JL, et al.. Reg proteins promote acinar-to-ductal metaplasia and act as novel diagnostic and prognostic markers in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7:77838-53. doi: 10.18632/oncotarget.12834. PMID:27788482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, Gupta S, Moore J, Wrobel MJ, Lerner J, et al.. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995-2004. doi: 10.1056/NEJMoa0804525. PMID:18923165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW, Polepalli J, Mitra SS, et al.. Neuronal activity promotes glioma growth through neuroligin-3 secretion. Cell. 2015;161:803-16. doi: 10.1016/j.cell.2015.04.012. PMID:25913192 [DOI] [PMC free article] [PubMed] [Google Scholar]


