ABSTRACT
The hypoxic response underlies the pathogenesis and malignant behavior of PCC/PGL. Regulation of PD-1 receptor-ligand signaling, a therapeutically actionable driver of the anti-tumor immune response, is a hypoxic-driven trait across malignancies. We evaluated the prognostic role of PD ligands in association with biomarkers of hypoxia and angiogenesis in patients with PCC/PGL.
Tissue microarrays sections including consecutive cases diagnosed between 1983–2011 were stained for PD-L1 and 2, hypoxia inducible factor 1a (Hif-1a), Carbonic Anhydrase IX (CaIX), Vascular Endothelial Growth Factor-A (VEGF-A). We explored the biologic significance of PD ligands expression using gene set enrichment analysis (GSEA) on The Cancer Genome Atlas (TCGA) for PCC/PGL (n = 184).
In total, 100 patients, 10% malignant, 64% PCC, 29% familial with median tumor size of 4.7 cm (range 1–14) were included. Median follow-up was 4.7 y. We found PD-L1 expression in 18% of PCC/PGL, which was independent of adverse pathological features including capsular (CI), vascular invasion (VI), necrosis (N) and expression of biomarkers of hypoxia. PD-L2 expression (16%) strongly correlated with CI, VI, N and malignant behavior (p < 0.05) and was associated with stronger Hif-1a and CaIX immunolabeling (p < 0.01). PD-L2 was predictive of shorter survival (162 versus 309 months, HR 3.1 95%CI 1.1–9.2, p = 0.02). GSEA on TGCA samples confirmed enrichment of transcripts involved in hypoxia and anti-cancer immunity.
We report for the first time PD ligands expression in PCC/PGL with a distinctive prognostic, clinico-pathologic and immuno-biologic role. These findings support a potential therapeutic role for PD-1/PD-L1 targeted checkpoint inhibitors in these tumors.
KEY MESSAGE
The molecular mechanisms underlying immune evasion in malignant phaeochromocytomas and paragangliomas (PCC/PGL) are poorly understood. This study demonstrates for the first time a distinctive immune-biologic and prognostic role of programmed death ligands 1 and 2 (PD-L1, PD-L2), two actionable drivers of the anti-cancer immune response. RNA-sequencing of tumor tissues reveals enrichment of transcripts relating to hypoxia and immune-exhaustion to explain the adverse clinical course observed in PD-L2 overexpressing tumors. These findings provide a rationale for the development of anti PD-1 therapies in malignant PCC/PGL.
KEYWORDS: Phaeochromocytoma, Paraganglioma, PD-L1, PD-L2, prognosis
Introduction
Phaeochromocytomas (PCCs) and paragangliomas (PGLs) are rare catecholamine-producing neuroendocrine tumors (NETs) deriving from adrenal (PCC) or extra-adrenal (PGL) chromaffin tissue.1
Surgical resection, the mainstay of treatment in PCC/PGL, is curative in most patients. However, almost 20% of tumors are malignant with the potential to recur and spread systemically, with negative implications for prognostic outlook.2
Treatment options for metastatic PCC/PGL are limited to radionuclide3,4 and cytotoxic chemotherapy, the efficacy of which mostly relies on retrospective reports of small numbers of patients.5 Systemic chemotherapy may control disease progression6 but does not prolong survival.7 Because of these major shortcomings in managing metastatic PCC/PGL, 5-year survival rates are <50%8 and inferior to those seen in other metastatic NETs.9
Therapeutic inhibition of the programmed cell death (PD-1) receptor-ligand immune checkpoint has recently revolutionised the systemic treatment of malignancy.10
By antagonising the immune-suppressive interaction between PD-1, a T-cell co-inhibitory receptor, and its ligand PD-L1, therapeutic antibodies against this pathway can restore an efficacious anti-tumor immune response, manifest as durable clinical responses in a proportion, but not all patients.11
Identification of biologic predictors of response is therefore a priority in attempting to develop immunotherapy, more so for rare malignancies where enrichment of molecularly phenotyped patient populations with enhanced potential to respond to treatment is highly desirable in clinical trial design.
PD-L1 expression by immunohistochemistry has been advocated as a putative predictive correlate of response to anti-PD-1 therapies and incorporated as a biomarker in clinical studies since initial early-phase trials of immune-checkpoint inhibitors.12
While the stratifying potential of PD-L1 is imperfect because of significant clinical responses having been observed in PD-L1 negative tumors,13 its use has expanded in certain malignancies where prospective data has correlated high PD-L1 expression with longer progression-free and overall survival following immune-checkpoint inhibition.14
More recently, tumor cell expression of PD-L2, an important modulator of T-helper 2 cell responses15 has been independently correlated with the efficacy of PD-1 axis targeted inhibitors suggesting that the diversity in PD ligands expression might contribute to the heterogeneity of clinical responses to checkpoint inhibitors.16
Biologically, elevated PD-L1 expression within tumor cells or the surrounding microenvironment is dynamic as a result of various molecular events including hypoxia17 and similar correlations have been highlighted for PD-L2.18 We have previously shown that activation of hypoxia inducible factor 1α (Hif-1α) is a key molecular hallmark in the metastatic progression of PCC/PGL,19 a disease where constitutive Hif-1α stabilization secondary to mitochondrial dysfunction, termed “pseudo-hypoxia,” is a key pathophysiological trait.20
In a disease where the anti-cancer immune response has remained largely unexplored, we hypothesized that activation of the hypoxic response might promote cancer-specific immune-tolerance through expression of PD ligands and thus facilitate malignant progression in PCC/PGL. To test this hypothesis, and attempt to qualify novel targets for immunotherapy, we evaluated the prevalence and clinico-pathologic significance of PD-L1 and PD-L2 expression in a well-annotated series of PCC/PGL.
Materials and methods
Patient characteristics
We included 100 consecutive PCC/PGL patients who underwent surgical treatment at Imperial College, a tertiary referral center with a specialist NET board, from 1983–2011. Haematoxylin and eosin (H&E) slides were reviewed to identify relevant pathological features and archival paraffin-embedded samples were retrieved.
Clinico-pathologic data, including tumor characteristics and survival, was gathered from medical records. Follow-up was updated prospectively and censored in November 2016. Malignant behavior was defined as detection of metastasis to lymph nodes or distant sites either at diagnosis or during subsequent review. Ethical approval was granted by the Imperial College Tissue Bank (Ref. R14066–2A).
Tissue microarrays (TMAs) and immunohistochemistry (IHC)
TMA blocks were prepared as described previously.21 All the samples utilised were surgical specimens. No biopsies were included. One mm tumor cores were sampled in triplicate using a MTA-1 Manual Tissue Microarrayer (Beecher Instruments, USA). IHC staining was performed on 5μm sections using the Leica Bond RX stainer (Leica, Buffalo, IL).
The primary antibodies anti-PD-L1 (Clone E1L3N; Cell Signaling Cat. Nr. 13684), anti-PD-L2 (Sigma Aldrich, Cat. Nr. 3500395) were incubated overnight at the concentration 1:100 and 1:300, respectively as described before.22 IHC staining for biomarkers of hypoxia/angiogenesis including VEGF-A (Cat. Nr. Sc- 152; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), Hif-1α (Cat. Nr. Ab8366 AbCam, Cambridge, UK) and CaIX (Cat. Nr. NB100–417, Novus Biologicals, Cambridge, UK) followed described previously methodology.21 Sections were incubated for 1 hour at room temperature with the secondary antibody and processed using the Polymer-HRP system (BioGenex, Fremont, CA, USA), developed in diaminobenzidine and counterstained in Mayer's Haematoxylin.
Biomarker expression was scored independently by 2 histopathologists (FAM, RED) using a semi-quantitative immuno-histoscore (IHS). For clinicopathologic correlation, continuous IHS ranging from 0–300 were categorised around the median of the distribution. Accounting for the focal pattern of PD-L1 expression cases displaying moderate intensity of signal in ≥5% of tumor cells were considered positive.22
Gene set enrichment analysis (GSEA)
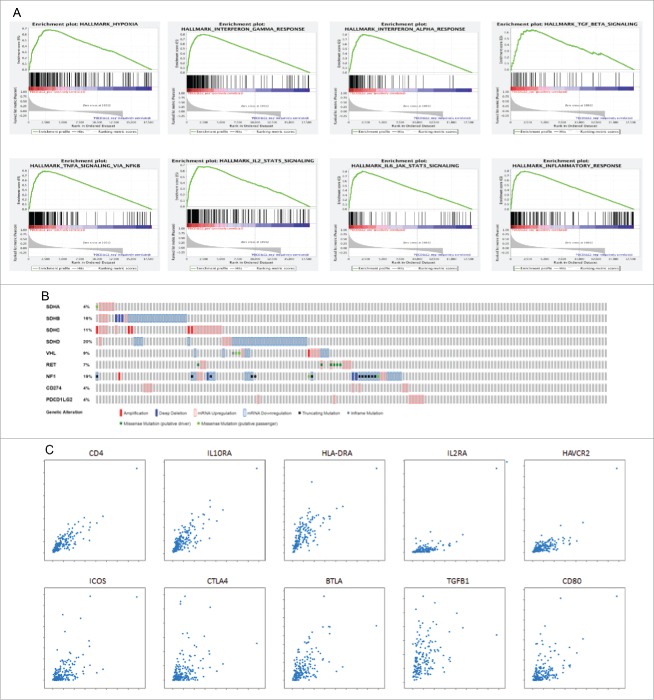
To characterize signaling pathways associated with PD-ligands expression we performed GSEA using RNA-sequencing data from the TCGA cohort of PCC/PGL (n = 184). PD-L1 (CD274) and PD-L2 (PDCD1LG2) were used as phenotypic labels. Analyzed gene sets included validated hallmark signatures derived from the MSigDB database to reflect the hypoxic response and a variety of transcriptional programs involved in anti-cancer immunity including the inflammatory response, the IL-6/JAK/STAT3 pathway, IL-2/STAT5 pathway, and signatures relating to Interferon-γ, IFN-α, Tumor Necrosis Factor a (TNF-α) and Transforming-Growth Factor β (TGF-β). We evaluated enrichment in phenotype both in positive and negative correlation with PD-L1 and PD-L2.
Using cBioportal (http://www.cbioportal.org, accessed 23/03/2017) we examined the 20 genes displaying the strongest positive correlation with PD ligands by Pearson's correlation index. Using oncoprint analysis we evaluated PD ligands expression in relationship to the presence of mutations in key susceptibility loci for PCC/PGL including Succinate-Dehydrogenase (SDH) subunits A-D, Von-Hippel Lindau (VHL), Neurofibromatosis-1 (NF-1) and c-RET.
Statistical analysis
Pearson's χ2 or Fisher's exact tests (2-tailed) were used to test for any significant associations between variables as indicated. A p value of <0.05 was used as a threshold for statistical significance. Kaplan-Meier curves were used for survival analyses. IBM SPSS version 23 (SPSS inc., Chicago, IL, USA) was used for all statistical analysis.
Results
Patient characteristics
The clinico-pathologic features of the patient cohort are displayed in (Table 1). Patients were followed up over a median period of 4.7 y (range 6 months – 34 years). Median age at diagnosis was 40 y. Malignant disease was identified radiologically or histologically in 10 patients as either de novo metastatic presentation (n = 3) or systemic recurrence during follow-up (n = 7). Twenty-nine patients had syndromic PCC/PGL based on germline DNA testing: the most prevalent mutation was in the RET proto-oncogene (n = 13) followed by mutation in one of the SDH subunits (n = 11), VHL (n = 3), NF-1 (n = 2). The most prevalent genotype in malignant cases was mutation in SDH-B (n = 2) followed by VHL (n = 1) and NF-1 (n = 1).
Table 1.
Clinical characteristics of patients with PCC/PGL.
| Baseline characteristic | n = 100 |
|---|---|
| Gender | |
| Male | 46 |
| Female | 54 |
| Age (years) | |
| <40 | 50 |
| ≥ 40 | 50 |
| Disease Site | |
| PCC monolateral | 59 |
| PCC bilateral | 5 |
| PGL (Extra-adrenal) | 36 |
| Maximum tumor diameter | |
| < 5 cm | 70 |
| ≥ 5 cm | 30 |
| Behavior | |
| Benign | 90 |
| Malignant | 10 |
| Catecholamine secretion | |
| Noradrenaline | 54 |
| Adrenaline | 28 |
| Dopamine | 15 |
| Silent | 20 |
| Missing | 13 |
| Tumor Necrosis | |
| Absent | 76 |
| Present | 16 |
| Capsular Invasion | |
| Absent | 79 |
| Present | 13 |
| Vascular Invasion | |
| Absent | 84 |
| Present | 8 |
| Genotype (germline mutations) | |
| SDH-B | 9 |
| SDH-D | 2 |
| NF-1 | 2 |
| VHL | 3 |
| RET | 12 |
All patients had surgery as the principal treatment of their tumor. Six patients with metastatic disease received radiotherapy, 2 received chemotherapy, and 4 received Iodine-131-meta-iodobenzylguanidine therapy.
PD ligands expression in PCCs/PGLs
In total, 18 cases stained positively for PD-L1 according to the pre-specified cut-off. PD-L1 staining was focal, with a cytoplasmic and membranous pattern of immunopositivity as shown in Fig. 1A. The prevalence of PD-L1 immunopositivity was 40% in malignant (4/10) and 15% (14/90) in benign cases (Fisher's Exact test p = 0.08). There was no significant association between PD-L1 positivity and any of the salient clinico-pathologic features including type of underlying germline mutation, tumor size, tumor localization (adrenal vs. extra-adrenal).
Figure 1.
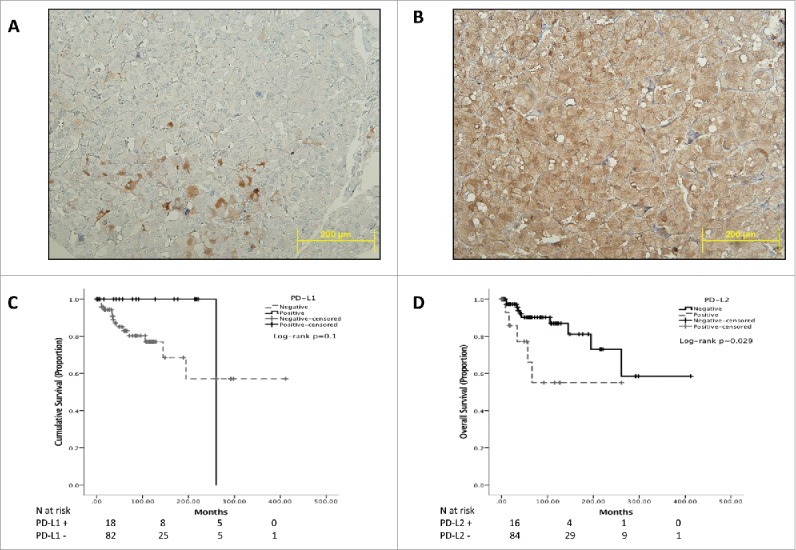
Representative sections of PCC/PGL TMA cores. Panel (A)shows focal expression of PD-L1 in a malignant case and the diffuse pattern of immunopositivity typical of PD-L2 immunostaining (Panel B). Panel (B) highlights the specificity of PD-L2 staining for PCC/PGL cells where sustentacular cells are negative for PD-L2 expression. Panels C and D show Kaplan-Meier curves describing the overall survival of patients with PCC/PGL categorized according to PD-L1 (Panel C) and PD-L2 (Panel D).
We identified PD-L2 expression in 16 cases, where the pattern of immunopositivity observed was cytoplasmic and diffuse (Fig. 1B). The median PD-L2 IHS was 100 (mean 146, range 60–300). PD-L2 expression was positively associated with malignant behavior. Prevalence of PD-L2 expression was 50% in malignant cases (5/10) compared with 12% in benign specimens (11/90, Pearson Chi-square p = 0.002). The proportion of PD-L2 positivity was higher in vasculo-invasive PCC/PGL (5/7 cases, 71% vs. 11/84, 13%, p < 0.001). Similarly, we found a higher rate of PD-L2 immunopositivity in cases displaying capsular invasion (6/13, 46% vs. 10/79, 13%, p = 0.003) and necrosis (7/16, 44% vs. 9/76, 12%, p = 0.002). We found evidence of PD-L1 and PD-L2 co-immuno expression in 3 cases, with a positive association with malignancy (1/89 cases vs. 2/10, Fisher Exact test p = 0.02).
A total of 26 samples showed high CaIX expression levels (median IHS 200, mean 178, range 75–275) whereas VEGF-A was overexpressed in 64 (median IHS 200, mean 178, range 75–275) and Hif-1α in 35 (median IHS 120, mean 107, range 0–300) (Supplementary Fig. S1).
We evaluated the relationship between the expression of biomarkers of hypoxia and angiogenesis and PD ligands. As shown in Fig. 2A–B, PD-L1-positive PCC/PGL were characterized by higher Hif-1α (median IHS 200 vs. 100, Mann Whitney U = 396.0, p = 0.01) but not CaIX.
Figure 2.
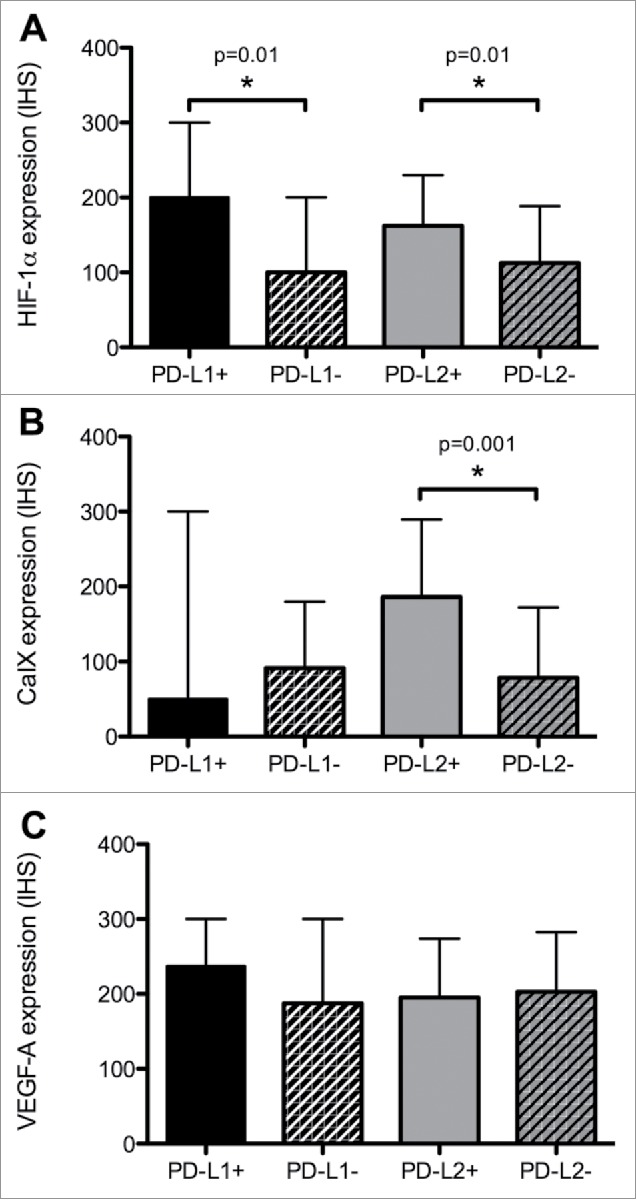
The relationship between PD ligands and the expression of Hif-1α (Panel A), CaIX (Panel B) and VEGF-A (Panel C). Expression of candidate biomarkers is quantified using an immuno-histoscore (IHS). Median values (+/− interquartile ranges) are presented. An asterisk (*) highlights a statistically significant difference in median IHS values resulting from a Mann-Whitney U test.
Similarly, PD-L2 overexpressing tumors had a higher proportion of Hif-1α (median IHS 166 vs. 100, Mann Whitney U = 407.0, p = 0.01) and CaIX overexpression (median IHS 33 vs. 200, Mann Whitney U = 280.0, p < 0.001). We found no relationship between PD ligands and VEGF-A expression (Fig. 2C).
PD-L1 and PD-L2 expression: relationship with malignancy and survival
We evaluated the prognostic value of PD-L1 and PD-L2 expression in the whole study population where mean OS was 25 y (95% CI 12–29 years) and median OS was not reached. OS was significantly shorter in malignant cases where mean OS was 7.2 y (2.2–12.3 years) and median OS was 3.6 y (95%CI 1.2–6 years, Log-rank p < 0.001).
The expression of PD-L1 was not predictive of OS in the entire patient population, with PD-L1 positive cases not differing from PD-L1 negative counterparts (Fig. 1C). When considering PD-L2 expression, we found that in patients overexpressing PD-L2 OS was significantly shorter with a median of 13.5 y (95%CI 8–19 years) compared with 25 y of patients with PD-L2 negative PCC/PGL (95%CI 20–30 years, Log-rank p = 0.029, Fig. 1D).
Other prognostic factors included presence of vascular invasion, capsular invasion and necrosis (Supplementary Fig. S2, Supplementary Table S1).
Differential relationship between PD-L1, PD-L2 and hypoxia: validation in TCGA data set
We used the PCC/PGL TCGA data set to validate the relationship between PD-L1 and 2 expression and hallmarks of hypoxia. Consistent with our immunohistochemical findings, we found enrichment of transcripts involved in the hypoxic response in relationship with PD-L2 but not PD-L1 expression (Fig. 3A, Supplementary Tables S2 and S3).
Figure 3.
Panel A illustrates the enrichment plots for 8 hallmark signatures positively correlating with PD-L2 (PDCD1LG2) expression in the PCC/PGL TCGA data set (n = 184). Panel B illustrates the relationship between PD-L1 and 2 expression and functionally relevant genomic alterations in 6 major susceptibility loci for familial PCC/PGL (SDH subunits A-D, VHL, NF-1 and RET). Panel C illustrates linear regression analysis correlating PD-L2 expression with a panel of genes reflecting immune-exhausted T cell responses including CD4, IL10RA, HLA-DRA, IL2RA, HAVCR2 (Tim-3), ICOS, CTLA-4, BTLA, TGFB1, CD80.
We addressed mutational status in key susceptibility loci for PCC/PGL as a potential confounder, demonstrating a lack of enrichment for PD-ligand overexpression in tumor samples harbouring mutations in any of the tested loci (Fig. 3B).
Immuno-biologic qualification of PD-L1 and PD-L2 in PCC/PGL
Using GSEA we found PD-L2 overexpressing PCC/PGL to be strongly enriched in transcriptional programs innate and adaptive anti-cancer immunity (Fig. 3A). Conversely, none of the gene sets were significantly enriched in PD-L1 overexpressing PCC/PGL, where we found a trend toward a positive association with a subset of 96 genes involved in protein secretion (nominal p = 0.03, FDR = 1) (Supplementary Table S1).
We confirmed differential gene expression profiles in correlation with PD ligands with a preferential co-expression of immune-inflammatory genes in relationship with PD-L2 as opposed to PD-L1 (Table 2). Given CD4 emerged as the strongest correlate to PD-L2 expression (Pearson's R 0.85, Fig. 3C) we phenotypically characterized T-cell enrichment revealing a significantly positive association in genes characterizing an exhausted immune response as shown in Fig. 3C.
Table 2.
Gene transcripts that are most strongly co-expressed in PD-L1 and PD-L2 overexpressing PCC/PGLs from the TCGA data set (n = 184).
| PD-L1 |
PD-L2 |
||||
|---|---|---|---|---|---|
| Gene Symbol | Pearson Score | Spearman Score | Gene Symbol | Pearson Score | Spearman Score |
| DNAJB14 | 0.75 | 0.75 | CD4 | 0.85 | 0.76 |
| MAN2A1 | 0.7 | 0.62 | ARHGAP30 | 0.85 | 0.74 |
| PCDHAC2 | 0.7 | 0.74 | CTSS | 0.82 | 0.75 |
| PCDHAC1 | 0.68 | 0.72 | FERMT3 | 0.82 | 0.71 |
| ATP2B4 | 0.67 | 0.59 | MS4A4A | 0.82 | 0.78 |
| IL6ST | 0.67 | 0.66 | AOAH | 0.81 | 0.73 |
| OXCT1 | 0.67 | 0.65 | CASP1 | 0.81 | 0.81 |
| G3BP2 | 0.67 | 0.71 | IL10RA | 0.81 | 0.72 |
| MID2 | 0.67 | 0.7 | PLEK | 0.81 | 0.7 |
| THSD7A | 0.67 | 0.67 | SASH3 | 0.81 | 0.72 |
| PTPRJ | 0.66 | 0.57 | CPVL | 0.81 | 0.74 |
| TBC1D9 | 0.66 | 0.6 | C1QC | 0.8 | 0.72 |
| SPOPL | 0.65 | 0.71 | IL12RB1 | 0.8 | 0.66 |
| CERS6 | 0.65 | 0.69 | MS4A6A | 0.8 | 0.74 |
| STT3B | 0.64 | 0.6 | CTSC | 0.79 | 0.73 |
| RNASEH1 | 0.64 | 0.6 | FPR3 | 0.79 | 0.66 |
| ERAP1 | 0.64 | 0.59 | HCK | 0.79 | 0.73 |
| ELMOD2 | 0.64 | 0.69 | SECTM1 | 0.79 | 0.66 |
| ZNF106 | 0.64 | 0.73 | SIGLEC1 | 0.79 | 0.67 |
Discussion
The lack of effective therapies is a highly unmet need in PCC/PGLs, a rare subtype of NETs where prediction of malignant behavior has so far been elusive and novel molecular targets are at the focus of intense research efforts.23
Previous work has highlighted several molecular differences between benign and malignant PCC/PGL. Activation of the hypoxic response mediated by stabilization of Hifs is a highly prevalent molecular mechanism24 contributing to malignant progression of PCC/PGL.25
Immune-evasion is a key hallmark of cancer progression and expression of PD ligands is a common mechanism through which tumor cells counteract immunologic clearance by negative regulation of PD-1-expressing lymphocyte populations. The presence of hypoxia-response elements (HRE) within the PD-L1 promoter region has highlighted a therapeutically attractive link between hypoxia/angiogenesis and anti-tumor immune suppression,26 with similar mechanisms being implied in the regulation of PD-L2 expression.27
In our study we document for the first time differential regulation of PD ligands in PCC/PGLs, where half of the malignant cases express at least one of the PD ligands to support their potential contribution in shaping the immune-tolerogenic environment.
Interestingly, while PD-L1 did not correlate with patients' survival, PD-L2 overexpression identified a subset of patients with poorer prognosis following radical resection. The negative impact of PD-L2 on patients' prognosis is further corroborated by the relationship between PD-L2 status and several histopathologic hallmarks of a more aggressive disease course including vascular, capsular invasion and necrosis – all of which were confirmed as predictors of poor outcome in our patient series.
Furthermore, in line with previously published studies in other malignancies, we found a positive correlation between Hif-1α and PD ligands expression, stronger for PD-L2 over PD-L1, a finding that we independently validated using the TCGA RNA-Seq data set.
Interestingly, PD ligands expression was not influenced by germline mutations neither in our case series nor in the TGCA data set to suggest that pseudo-hypoxic signals might not be the only drivers of PD ligands expression.28
This is further strengthened by the non-uniform relationship we found between PD ligands and the expression of CaIX or VEGF-A, 2 renown targets of Hif-1α, implying that the regulation of PD-ligands in the context hypoxic/pro-angiogenic signals might involve other molecular actors.29
Analysis of RNA-seq data portrayed more comprehensively the biologic heterogeneity characterizing the relationship between PD ligands and tumor hypoxia.
In fact, when we considered a broader subset of 200 genes involved in the hypoxic response, PD-L2 upregulation strikingly emerged as a stronger and more substantial determinant of tumor hypoxia than PD-L1, to suggest a potential mechanistic relationship between hypoxia and PD-L2-mediated anti-tumor immune control. When evaluating individual genes in more detail, we found that, while PD-L1 expression was predominantly associated with genes involved in endoplasmic reticulum trafficking and granule secretion such as DNAJB14, MAN2A1, PCDHAC1–2, the expression of PD-L2 was strongly and consistently associated with several transcripts involved in innate and adaptive immunity including CD4, IL10RA, IL12RB1 and C1QC to suggest inherent differences in their immunobiologic role (Table 2).
In several oncological indications where PD-1-targeted checkpoint inhibitors have been eventually proven effective in prospective trials, the clinical efficacy of these therapies was anticipated by prior clinicopathologic studies showing a relationship between PD ligands and clinical outcome.12 While the likelihood of response to immunotherapy cannot be directly inferred from our data, we provide evidence of differential regulation of PD-L1 and PD-L2 in our clinical samples, with diverse ramifications in terms of their clinico-pathologic significance and predictive role in influencing patients' survival.
Interestingly, our data suggest PD-L2 to have a more predominant role than PD-L1 in shaping the immune-tolerogenic environment, given the highly significant association with key pathways involved in innate, adaptive immunity and inflammation.
The enrichment of immunologic markers suggestive of an exhausted immune response including TGF-β-related signatures and several regulatory T-cell markers (IL10RA, IL10RB, HLA-DRA) as well as immune checkpoints (Tim-3, BTLA, CTLA-4, CD80, ICOS) provides strong immunobiologic foundations to justify the relationship between PD-L2 expression and poorer prognosis in patients with PCC/PGL.
Our results therefore provide a valuable clinical rationale for the development of immune checkpoint inhibitors in a subset PCC/PGL where anti-tumor immune exhaustion is a key prognostic trait and suggests PD-L2 expression as a putative stratifying biomarker in prospective clinical studies.
We acknowledge the relatively small sample size and the rarity of malignant cases as a limitation to our study, which is unfortunately shared with most of the published work in this field.
Nonetheless, taken together, our findings support the existence of a subset of PCC/PGL with biologic bases for PD-1–mediated tumor immune evasion and highlights a potential role for immunotherapy targeting the PD-1 ligand-receptor axis. The relationship between hypoxia, malignancy and anti-tumor immune control should be further explored in prospective studies with the aim of facilitating the development of immunotherapy in this otherwise neglected disease area.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments and funding
This work has been awarded the ASCO Merit Award 2017 to DJP and has been in part supported by funds from the Imperial BRC and the NIHR attributed to DJP and by the Clinical Lecturer Starter Grant awarded by the Academy of Medical Sciences (AMS Grant ID SGL013/1021) to DJP. The Starter Grant is jointly funded by the AMS, Wellcome Trust, Medical Research Council, British Heart Foundation, Arthritis Research UK, the Royal College of Physicians and Diabetes UK. The authors would like to acknowledge Dr Tricia Tan, the Imperial Center for Endocrinology and the NIHR/BRC Imperial College Tissue Bank for their support in setting up the PCC/PGL tissue microarray.
Authors contribution
Study concept and design: DJP, RSAcquisition of data: DJP, JRB, PT.Analysis and interpretation of data: DJP, JRB, RED, FAM, ST.Drafting of the manuscript: DJP, JRB.Critical revision of the manuscript for important intellectual content: All the authors.Statistical analysis: DJP, JRB, ST.Obtained funding: DJP.Administrative, technical, or material support: DJP, RED, PT, RS.Study supervision: DJP, FAM, RS.
References
- 1.Gimm O, DeMicco C, Perren A, Giammarile F, Walz MK, Brunaud L. Malignant pheochromocytomas and paragangliomas: a diagnostic challenge. Langenbecks Arch Surg. 2012;397:155-77. doi: 10.1007/s00423-011-0880-x. PMID:22124609 [DOI] [PubMed] [Google Scholar]
- 2.Manger WM, Eisenhofer G. Pheochromocytoma: diagnosis and management update. Curr Hypertens Rep. 2004;6:477-84. doi: 10.1007/s11906-004-0044-2. PMID:15527694 [DOI] [PubMed] [Google Scholar]
- 3.Gonias S, Goldsby R, Matthay KK, Hawkins R, Price D, Huberty J, Damon L, Linker C, Sznewajs A, Shiboski S et al.. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J Clin Oncol. 2009;27:4162-8. doi: 10.1200/JCO.2008.21.3496. PMID:19636009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pinato DJ, Black JR, Ramaswami R, Tan TM, Adjogatse D, Sharma R. Peptide receptor radionuclide therapy for metastatic paragangliomas. Med Oncol. 2016;33:47. doi: 10.1007/s12032-016-0737-9. PMID:27059363 [DOI] [PubMed] [Google Scholar]
- 5.Ayala-Ramirez M, Feng L, Habra MA, Rich T, Dickson PV, Perrier N, Phan A, Waguespack S, Patel S, Jimenez C. Clinical benefits of systemic chemotherapy for patients with metastatic pheochromocytomas or sympathetic extra-adrenal paragangliomas: insights from the largest single-institutional experience. Cancer. 2012;118:2804-12. doi: 10.1002/cncr.26577. PMID:22006217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niemeijer ND, Alblas G, van Hulsteijn LT, Dekkers OM, Corssmit EP. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf). 2014;81:642-51. doi: 10.1111/cen.12542. PMID:25041164 [DOI] [PubMed] [Google Scholar]
- 7.Tanabe A, Naruse M, Nomura K, Tsuiki M, Tsumagari A, Ichihara A. Combination chemotherapy with cyclophosphamide, vincristine, and dacarbazine in patients with malignant pheochromocytoma and paraganglioma. Horm Cancer. 2013;4:103-10. doi: 10.1007/s12672-013-0133-2. PMID:23361939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hescot S, Leboulleux S, Amar L, Vezzosi D, Borget I, Bournaud-Salinas C, de la Fouchardiere C, Libé R, Do Cao C, Niccoli P et al.. One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2013;98:4006-12. doi: 10.1210/jc.2013-1907. PMID:23884775 [DOI] [PubMed] [Google Scholar]
- 9.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A et al.. One hundred years after “carcinoid:” epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-72. doi: 10.1200/JCO.2007.15.4377. PMID:18565894 [DOI] [PubMed] [Google Scholar]
- 10.La-Beck NM, Jean GW, Huynh C, Alzghari SK, Lowe DB. Immune Checkpoint Inhibitors: New Insights and Current Place in Cancer Therapy. Pharmacotherapy. 2015;35:963-76. doi: 10.1002/phar.1643. PMID:26497482 [DOI] [PubMed] [Google Scholar]
- 11.Muenst S, Soysal SD, Tzankov A, Hoeller S. The PD-1/PD-L1 pathway: biological background and clinical relevance of an emerging treatment target in immunotherapy. Expert Opin Ther Targets. 2015;19:201-11. doi: 10.1517/14728222.2014.980235. PMID:25491730 [DOI] [PubMed] [Google Scholar]
- 12.Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41:868-76. doi: 10.1016/j.ctrv.2015.11.001. PMID:26589760 [DOI] [PubMed] [Google Scholar]
- 13.Kluger HM, Zito CR, Turcu G, Baine M, Zhang H, Adeniran A, Sznol M, Rimm DL, Kluger Y, Chen L et al.. PD-L1 studies across tumor types, its differential expression and predictive value in patients treated with immune checkpoint inhibitors. Clin Cancer Res. 2017; 23(15):4270-4279. doi: 10.1158/1078-0432.CCR-16-3146. PMID:28223273. [Epub 2017, Feb 21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S et al.. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-33. doi: 10.1056/NEJMoa1606774. PMID:27718847 [DOI] [PubMed] [Google Scholar]
- 15.Rozali EN, Hato SV, Robinson BW, Lake RA, Lesterhuis WJ. Programmed death ligand 2 in cancer-induced immune suppression. Clin Dev Immunol. 2012;2012:656340. doi: 10.1155/2012/656340. PMID:22611421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yearley JH, Gibson C, Yu N, Moon C, Murphy E, Juco J, Lunceford J, Cheng J, Chow LQM, Seiwert TY et al.. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin Cancer Res. 2017;23:3158-67. doi: 10.1158/1078-0432.CCR-16-1761. PMID:28619999 [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Jiang CC, Jin L, Zhang XD. Regulation of PD-L1: a novel role of pro-survival signalling in cancer. Ann Oncol. 2016;27:409-16. doi: 10.1093/annonc/mdv615. PMID:26681673 [DOI] [PubMed] [Google Scholar]
- 18.Koh YW, Han JH, Park SY, Yoon DH, Suh C, Huh J. GLUT1 as a Prognostic Factor for Classical Hodgkin's Lymphoma: Correlation with PD-L1 and PD-L2 Expression. J Pathol Transl Med. 2017;51:152-8. doi: 10.4132/jptm.2016.11.03. PMID:28219001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pinato DJ, Ramachandran R, Toussi ST, Vergine M, Ngo N, Sharma R et al.. Immunohistochemical markers of the hypoxic response can identify malignancy in phaeochromocytomas and paragangliomas and optimize the detection of tumours with VHL germline mutations. Br J Cancer. 2013;108(2):429-37. doi: 10.1038/bjc.2012.538. [Epub 2012, Dec 20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jochmanova I, Yang C, Zhuang Z, Pacak K. Hypoxia-inducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst. 2013;105(17):1270-83. doi: 10.1093/jnci/djt201. PMID:23940289. [Epub 2013, Aug 12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinato DJ, Tan TM, Toussi ST, Ramachandran R, Martin N, Meeran K, Ngo N, Dina R, Sharma R. An expression signature of the angiogenic response in gastrointestinal neuroendocrine tumours: correlation with tumour phenotype and survival outcomes. Br J Cancer. 2014;110:115-22. doi: 10.1038/bjc.2013.682. PMID:24231952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinato DJ, Shiner RJ, White SD, Black JR, Trivedi P, Stebbing J, Sharma R, Mauri FA. Intra-tumoral heterogeneity in the expression of programmed-death (PD) ligands in isogeneic primary and metastatic lung cancer: Implications for immunotherapy. Oncoimmunology. 2016;5:e1213934. doi: 10.1080/2162402X.2016.1213934. PMID:27757309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cassol CA, Winer D, Liu W, Guo M, Ezzat S, Asa SL. Tyrosine kinase receptors as molecular targets in pheochromocytomas and paragangliomas. Mod Pathol. 2014;27:1050-62. doi: 10.1038/modpathol.2013.233. PMID:24390213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS et al.. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72-80. doi: 10.1371/journal.pgen.0010008. PMID:16103922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinato DJ, Ramachandran R, Toussi ST, Vergine M, Ngo N, Sharma R, Lloyd T, Meeran K, Palazzo F, Martin N et al.. Immunohistochemical markers of the hypoxic response can identify malignancy in phaeochromocytomas and paragangliomas and optimize the detection of tumours with VHL germline mutations. Br J Cancer. 2013;108:429-37. doi: 10.1038/bjc.2012.538. PMID:23257898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Noman MZ, Hasmim M, Messai Y, Terry S, Kieda C, Janji B, Chouaib S. Hypoxia: a key player in antitumor immune response. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol. 2015;309:C569-79. doi: 10.1152/ajpcell.00207.2015. PMID:26310815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koh YW, Han JH, Park SY, Yoon DH, Suh C, Huh J. GLUT1 as a prognostic factor for classical hodgkin's lymphoma: correlation with PD-L1 and PD-L2 expression. J Pathol Transl Med. 2017;51:152-158. doi: 10.4132/jptm.2016.11.03. PMID:28219001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fishbein L, Leshchiner I, Walter V, Danilova L, Robertson AG, Johnson AR, Lichtenberg TM, Murray BA, Ghayee HK, Else T et al.. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell. 2017;31:181-93. doi: 10.1016/j.ccell.2017.01.001. PMID:28162975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollard PJ, El-Bahrawy M, Poulsom R, Elia G, Killick P, Kelly G, Hunt T, Jeffery R, Seedhar P, Barwell J et al.. Expression of HIF-1alpha, HIF-2alpha (EPAS1), and their target genes in paraganglioma and pheochromocytoma with VHL and SDH mutations. J Clin Endocrinol Metab. 2006;91:4593-8. doi: 10.1210/jc.2006-0920. PMID:16954163 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.