ABSTRACT
Background: Programmed Cell Death 1-Ligand 1 (PD-L1) and Programmed Death Protein 1 (PD-1) blocking antibodies are promising immunotherapies for malignancies. We have previously shown PD-L1 expression in 40% of malignant mesothelioma (MM); however, the temporal and spatial heterogeneity of its expression has not been thoroughly studied. We compared PD-L1 expression between paired primary and metastatic MM.
Design: Pathology files (1995–2016) were searched for MM with tissue from multiple sites and/or time points. PD-L1 (clone SP263) expression was reviewed by 2 authors. Mesothelioma cell lines (H2461, One 58, EM-MESO) were cultured with or without vinorelbine or pemetrexed. Following incubation, PD-L1 expression (clone MIH1) was analyzed by flow cytometry.
Results: 64 patients (53 men, median age, 64 years) with epithelioid (N = 50), biphasic (N = 11) or sarcomatoid (N = 2) MM or well differentiated papillary mesothelioma (N = 1) (pleural, n = 56; peritoneal, n = 8) were included. Patients had a subsequent specimen from the primary site (n = 48), from a metastasis (n = 6), or both (n = 10). Reviewers agreed on PD-L1 expression in 133 of 151 (88%) specimens. There was agreement of PD-L1 expression between paired primary lesions obtained at separate time points in 47 of 58 (81%) and between paired primary and metastatic lesions in 11 of 16 (69%) cases. A significant increase in PD-L1 expression was observed in all 3 MM cell lines (p < 0.003 each) following exposure to vinorelbine but not to pemetrexed.
Conclusion: Overall there is good agreement in PD-L1 expression between paired MM lesions; however, the 19–31% of cases with discordant PD-L1 expression, and the dynamics of PD-L1 expression may limit its use as a predictive biomarker for therapy.
KEYWORDS: malignant mesothelioma, PD-L1, immunohistochemistry
Introduction
Malignant mesothelioma (MM) is an aggressive disease of serosal surfaces including pleura, pericardium and peritoneum. The prognosis of MM in general is poor with overall survival, ranging from 14–15 months for epithelioid malignant pleural mesothelioma to less than 6 months for some patients with sarcomatoid mesothelioma despite treatment.1 While some patients with early stage and epithelioid subtype of MM might be surgical candidates2,3 the majority of patients with MM will not be considered for surgery. Moreover, the survival benefit of surgical intervention of MM remains controversial.4 Non-surgical patients are most commonly treated with chemotherapy including a Folate antagonist. However, chemotherapy typically improves survival by only about 3 months and has limited efficacy as second line treatment.5-7 Therefore, new therapeutic alternatives are urgently needed.
Recently, the engagement of programmed death protein 1 (PD-1) expressed on T cells with programmed cell death 1-ligand 1 (PD-L1) on tumor cells has been shown to hamper the immune response of the T cells against the tumor cells.8-10 Anti-PD-1 and anti-PD-L1 agents have been developed to interrupt this interaction and to boost the antitumoral immune response of T cells. Initial results are promising. For instance, patients with stage IV non-small cell lung cancer with PD-L1 expression by over 50% of tumor cells treated with anti-PD-1 as first line therapy had a better survival than patients treated with chemotherapy.11 Patients with advanced melanoma, who were treated with anti-PD-1, had a 34% response rate.12 Furthermore, we have shown that PD-L1 is expressed in 40% of MM with 94% of sarcomatoid mesotheliomas, 52% of biphasic and 21% of epithelioid MM expressing PD-L1.13 Others have since detected PD-L1 expression in MM at similar rates.14 Because of these findings, clinical trials using anti-PD-1 and anti-PD-L1 agents are underway for the treatment of MM. Preliminary reports suggest that approximately 20–30% of mesothelioma patients also respond to PD-1 targeted therapies.
Our experience and evidence in the literature suggests that PD-L1 expression can be heterogeneous within tumors.15-17 However, the temporal and spatial heterogeneity of its expression has not been thoroughly studied in MM. Specifically, it has not been shown whether PD-L1 expression varies between primary MM and metastasis or between primary MM and subsequent biopsy or resection of the tumor. Studies in non-small cell carcinoma of the lungs have revealed heterogeneity of PD-L1 expression in 12 of 32 (37.5%, κ = 0.01) cases with multifocal lung cancer18 and in 10 of 73 (14%, κ = 0.71) cases of paired primary lesions and brain metastases.17 This information is important in MM because if PD-L1 expression is heterogeneous then primary tumors, metastases and/or recurrences may not respond to anti-PD-1 or anti-PD-L1 equally. Furthermore, if PD-L1 expression is required for treatment selection, sampling error may affect treatment decisions. We therefore studied primary MM for PD-L1 expression and compared its expression with available material from metastases and/or subsequent tissue from the MM. We also assessed how PD-L1 is affected by cytotoxic chemotherapy agents since biopsies for biomarker evaluation may be obtained after failure of prior therapies.
Materials and methods
Patient population
Pathology archives were searched for cases of MM (1995–2016). After cases were identified pathology reports were explored for subsequent tissue material from metastases, recurrences and/or subsequent biopsies or resections of the MM. All cases were reviewed by a thoracic pathologist (ACR) to confirm the diagnosis. Primary MM, metastases and subsequent biopsies/resections of the MM were subtyped according to the WHO into epithelioid, biphasic and sarcomatoid subtype.19 Medical records were studied for patient demographics, treatment and outcome. The study was approved by the Institutional Review Board of Mayo Clinic Rochester (IRB#13–005053).
PD-L1 immunohistochemistry
Consecutive sections of formalin-fixed paraffin-embedded (FFPE) tissue were cut at 4 microns and stained with hematoxylin eosin and with antibody to the PD-L1 antigen (clone SP263, Ventana Medical Systems, Inc., Tucson, AZ, US). Membranous PD-L1 expression was considered positive. PD-L1 expression was scored as percent tumor cell staining of all tumor cells by 2 of the authors (SBST, ACR). PD-L1 expression was also categorized for some of the analysis as 0 [< 1% tumor cells express PD-L1 (considered negative)], 1 (1–10%), 2 (> 10–50%) and 3 (> 50%). Disagreements in scoring were solved by consensus between the 2 authors who scored the cases.
PD-L1 expression by mesothelioma cell lines
Mesothelioma cell lines [H2461, One 58, and EM-MESO (the last one was kindly provided by Dr. Edward Moon, University of Pennsylvania)] were cultured at 5 × 104/well/ml in 24-well plates with or without vinorelbine (Sargent Pharmaceuticals Schaumburg, IL, USA) at a dose of 5.5 μg/ml for 36–40 hours or pemetrexed (Lilly USA, LLC, Indianapolis, IN, USA) at 1, 10 or 50 nM for 24 and 48 hours. After incubation, cells were washed and stained with mouse anti-human PD-L1 antibody (clone MIH1, eBioscience, San Diego, CA, USA) or isotype control antibody (mouse IgG1, clone MOPC-21, BioLegend, San Diego, CA, USA). The expression of PD-L1 was analyzed by flow cytometry. Each cell line was tested 3 times in triplicate. PD-L1 expression was presented as percent of PD-L1-positive cells. The mean of each triplicate run was calculated followed by the mean of the 3 experiments.
Summary of data and statistics
Descriptive statistics were used to summarize patient characteristics. The Wald method was used to calculate confidence intervals. Agreement for PD-L1 expression (any versus none) was calculated using Cohen's kappa coefficient (κ). A circos diagram was used to visually demonstrate the agreement between cases.20 When more than 2 lesions were available for a subject, the lesions were categorized as concordant if all lesions were in agreement, and discordant if any of the lesions were not in agreement. The limits of agreement were determined with the Bland-Altman method to assess semi-quantitative PD-L1 expression between paired lesions. The paired t test was used to compare PD-L1 expression before and following exposure to vinorelbine or pemetrexed. Prism 6 for Mac OS X (GraphPad Software, Inc.) was used for analysis.
Results
Patient characteristics and specimens of malignant mesothelioma
64 patients with MM and available material from the primary tumor, and metastasis and/or subsequent biopsy or resection from the tumor were identified in the pathology archives. Patient demographics, primary tumor sites, sites of subsequent tissue and follow up are summarized in Table 1. Subsequent biopsies/resections showed different MM subtypes in 11 cases (Table 1). Patients had a subsequent specimen from the primary site [N = 48, 0.1–132 months (median, 4) after initial specimen], from a metastasis [N = 6, 7–120 months (median, 17)], or both [N = 10, 0.3–57 months (median 4.5)]. Metastatic sites from which specimens were obtained include lymph node (n = 9), skin and soft tissue (n = 3), brain (n = 2), liver, lung, peritoneum, pleura, and bone (n = 1, each). Some patients (n = 3) had biopsies from multiple metastases.
Table 1.
Demographics of study population (n=64).
| Characteristic | Results | ||
|---|---|---|---|
| Age, years, median (range) | 64 (37–76) | ||
| Male sex, N (%) | 53 (82.8%) | ||
| Primary tumor site, N (%) | |||
| Pleura | 56 (87.5) | ||
| Peritoneum | 8 (12.5) | ||
| Histology of malignant mesothelioma, N (%) | |||
| Epithelioid | 50 (78.1) | ||
| Biphasic | 11 (17.2) | ||
| Sarcomatoid | 2 (3.1) | ||
| Well differentiated papillary mesothelioma | 1 (1.6) | ||
| Subsequent specimen, N (%) | |||
| Primary site | 48 (75.0) | ||
| Metastasis | 6 (9.4) | ||
| Primary site and metastasis | 10 (15.6) | ||
| Subsequent specimen with different MM component (n = 11) | Morphology | N | |
| Original | Subsequent specimen | ||
| Epithelioid | Biphasic | 2 | |
| Sarcomatoid | Biphasic | 1 | |
| Epithlioid | Sarcomatoid | 1 | |
| WDPMa | Epithelioid | 1 | |
| Biphasic | Epithelioid | 5 | |
| Biphasic | Sarcomatoid | 1 | |
| Follow up, months, median (range) | |||
| Overall | 4 (0.1–132.0) | ||
| Patients with subsequent biopsy from | |||
| Primary site | 4 (0.1–132.0) | ||
| Metastasis | 17 (7.0–120.0) | ||
| Primary site and metastasis | 4.5 (0.3–57.0) | ||
| Chemotherapy between primary diagnosis and subsequent specimen, N (%) | 37 (57.8) | ||
| Carboplatin & Pemetrexet | 14 | ||
| Cisplatin & Pemetrexet | 9 | ||
| Cisplatin & Gemcitabine | 5 | ||
| Chemotherapy, agents unknown | 3 | ||
| Intraperitoneal chemotherapy | 2 | ||
| Temsirolimus CCI-779 compound | 1 | ||
| Mitomycin-C | 1 | ||
| Mitomycin-C, doxorubicin & cisplatin | 1 | ||
| Carboplatin & Gemcitabine | 1 | ||
| Additional treatment with vinorelbine | 3 | ||
WDPM, well differentiated papillary mesothelioma
Distribution of PD-L1 expression between primary malignant mesothelioma and metastasis and/or subsequent tissue of primary site
A total of 151 specimens were reviewed and stained with anti-PD-L1 antibody. Reviewers agreed on PD-L1 expression in 133 of 151 (88%) specimens. There were 8 specimens with differences greater than 10% semi-quantitative estimates between reviewers among positive cases including 1 specimen with a difference > 20%, 6 specimens with a difference of 20%, and 1 with 15% difference. An additional 10 cases had a 10% difference.
Fifty-eight cases had paired specimens from the primary lesion. Twenty-nine of 58 (50.0%) cases showed PD-L1 expression in the initial specimen; 30 of 58 (51.7%) subsequent specimens revealed PD-L1 expression (Table 2). Sixteen cases had specimens from the primary lesion and metastases. Six of 16 (37.5%) cases showed PD-L1 expression in the primary lesion; 7 of 16 (43.7%) metastases expressed PD-L1 (Table 2).
Table 2.
Differential expression of PD-L1 in primary tumor site and metastasis or subsequent specimen in malignant mesothelioma.
| PD-L1 expression | Overall* |
Epithelioid MM |
Biphasic MM |
||||
|---|---|---|---|---|---|---|---|
| Initial specimen | Subsequent specimen / metastasis | Number (%) of MM at different time points (N = 58) | Number (%) of primary MM and metastasis (N = 16) | Number (%) of MM at different time points (N = 46) | Number (%) of primary MM and metastasis (N = 14) | Number (%) of MM at different time points (N = 9) | Number (%) of primary MM and metastasis (N = 2) |
| Concordant | |||||||
| Negative | Negative | 23 (40) | 7 (44) | 20 (43) | 7 (50) | 3 (33) | 0 |
| Positive | Positive, expression score same as in initial specimen | 18 (31) | 2 (12) | 14 (30) | 1 (7) | 2 (22) | 1 (50) |
| Discordant | |||||||
| Negative | Positive | 6 (10) | 3 (19) | 5 (11) | 3 (21) | 0 | 0 |
| Positive | Negative | 5 (9) | 2 (12) | 4 (9) | 2 (14) | 1 (11) | 0 |
| Positive | Positive, expression score higher than in initial specimen | 1 (2) | 2 (12) | 1 (2) | 1 (7) | 0 | 1 (50) |
| Positive | Positive, expression score lower than in initial specimen | 5 (9) | 0 | 2 (4) | 0 | 3 (33) | 0 |
2 (of 2) sarcomatoid mesotheliomas had concordant PD-L1 expression scores in the initial specimen and subsequent specimen; 1 mesothelioma had discordant (negative to positive) PD-L1 expression scores in the initial specimen (well differentiated papillary mesothelioma) and subsequent specimen (epithelioid MM). MM, malignant mesothelioma
There was agreement of PD-L1 expression (any vs. none) between the paired primary lesions obtained at different time points in 41 of 58 cases (71%, 95% CI 58–81%; κ = 0.411, 95% CI 0.177 to 0.645) (Fig. 1A–F). There was also agreement of PD-L1 expression (any vs. none) between paired primary and metastatic lesions in 9 of 16 cases (56%, 95% CI 33–77%; κ = 0.067, 95% CI −0.386 to 0.519). In the limits of agreement analysis (Bland-Altman test) there was a bias of −0.75 (95% CI −47 to 45; Fig. 2). Table 2 shows the differential expression of PD-L1 in primary tumor site and metastasis or subsequent specimen in MM (Fig. 1G–L). Fig. 3 illustrates the aggregate agreement of PD-L1 expression between paired primary lesions, and between primary lesions and metastases.
Figure 1.
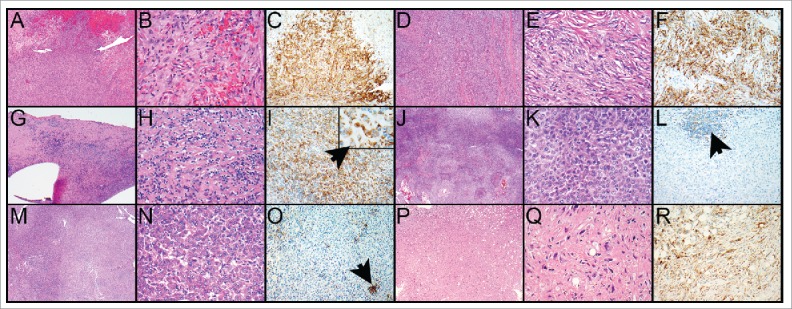
A–F. Malignant pleural mesothelioma, sarcomatoid subtype; PD-L1 expression is similar in biopsy and resection specimen. A. The biopsy specimen reveals sheets of neoplastic cells that grow in a tumefactive pattern. B. The tumor cells are spindled and arranged in a haphazard distribution; increased mitotic activity is noted. C. There is diffuse membranous staining of the tumor cells by PD-L1 (60% of tumor cells). D, E. The pneumonectomy specimen shows similar morphologic features and PD-L1 expression resembles that of the biopsy (70% of tumor cells, F). G–L. Malignant pleural mesothelioma, epithelioid subtype, resection specimen and recurrence after 4 years; PD-L1 expression was high in the primary tumor but only rare tumor cells expressed PD-L1 in the recurrent tumor. G. The primary tumor comprises tumor cells of epithelioid cytology (H) together with immune cells in a fibrotic background. I. The tumor cells diffusely express PD-L1 (70% of tumor cells; insert, arrow points toward tumor cell). J. The recurrent malignant mesothelioma shows a predominance of epithelioid tumor cells with only rare intermixed immune cells (K). L. While some of the immune cells express PD-L1, expression of PD-L1 by the tumor cells is only 1%. M–R. Malignant pleural mesothelioma, epithelioid subtype; resection specimen and biopsy of bone metastasis one year later (metastasis was of sarcomatoid subtype). While the resection specimen was negative for PD-L1 in the tumor cells the metastasis showed high expression of PD-L1. M. The resection specimen of the primary tumor comprises sheets of tumor cells that are of epithelioid cytology (N). O. PD-L1 is expressed in immune cells but not in tumor cells (arrow points toward PD-L1-positive immune cells). P. The biopsy from the bone metastasis shows also sheets of tumor cells, however, those are of spindled appearance (Q). R. Ninety % of tumor cells express PD-L1. Magnification hematoxylin and eosin, × 40 (A, D, G, J, M, P), × 400 (B, E, H, K, N, Q). PD-L1-stained slides, × 200 (C, F, I, L, O, R), × 600 (I insert).
Figure 2.
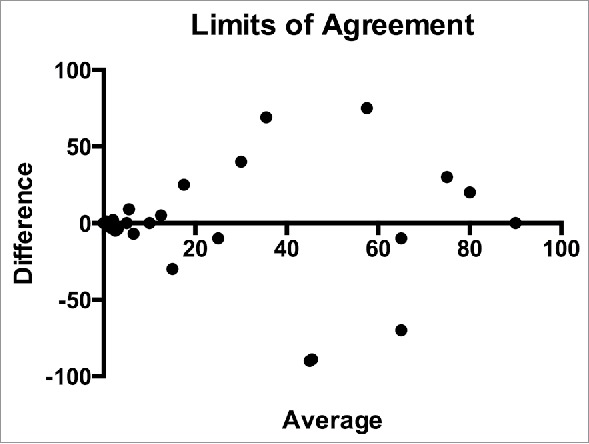
Limits of agreement between paired specimens. The difference in expression PD-L1 expression by tumor cells is plotted against the average expression.
Figure 3.
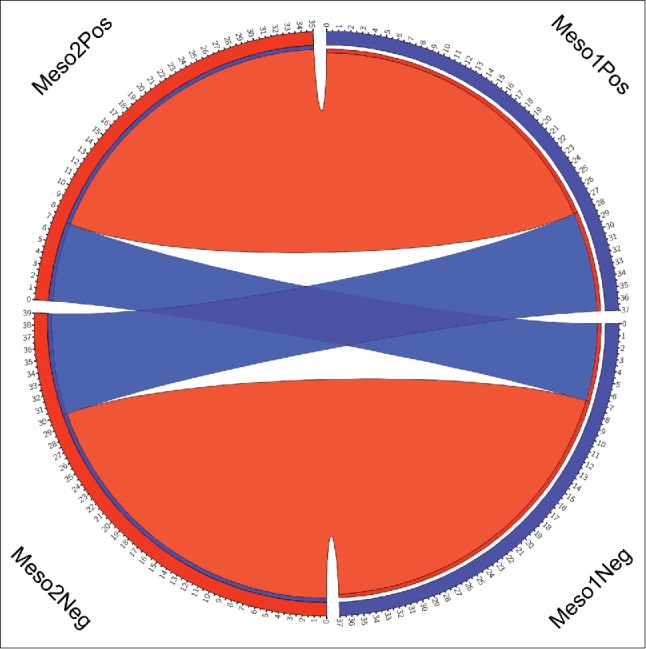
Circos diagram of agreement between paired specimens. In the figure the arbitrarily assigned first lesions are on the left side of the circle and separated by PD-L1 positive (top) and PD-L1 negative (bottom) lesions. The paired lesions are on the right side of the circos diagram and are also separated by PD-L1 positive (top) and PD-L1 negative (bottom) classification. The red ribbons connect the paired lesions with agreement in PD-L1 expression and the blue ribbons connect the paired lesions with discordant PD-L1 expression as defined in the methods section.
Thirty-seven (57.8%) patients received chemotherapy between the first and the subsequent specimen; 3 of these patients received vinorelbine. Thirty-seven of 64 (57.8%) patients were treated with chemotherapy (Table 1) between initial and subsequent specimen. In 8 of 37 (21.6%) patients PD-L1 expression increased in the subsequent specimen (Fig. 1M–R). These patients were treated with cisplatin and pemetrexed (n = 3) or cisplatin and gemcitabine, chemotherapy (agents unknown), intraperitoneal chemotherapy, mitomycin C, or carboplatin and gemcitabine (n = 1 each).
Dynamics of PD-L1 expression with chemotherapy
We analyzed 3 human MM cell lines for expression of PD-L1 with or without the treatment with pemetrexed which is often combined with a platinum agent in the frontline treatment of MM, or the commonly used, second- or third-line agent for MM, vinorelbine. We identified a significant increase in PD-L1 expression in all 3 MM cell lines including H2461 (mean difference 44.1%, 95% CI 28.9–59.4%, p = 0.0004), One 58 (mean difference 13.5%, 95% CI 6.9–20.2%, p = 0.0025) and EM-MESO (34.7%, 95% CI 21.9–47.5%, p = 0.0006; Fig. 4) following exposure to vinorelbine. There was no increase in expression of PD-L1 following treatment with pemetrexed in H2461 (mean difference 14.5%, 95% CI −18 to 47%, p = 0.20), One 58 (mean difference 4%, 95% CI −1 to 9%, p = 0.07) or EM-MESO (mean difference −12%, 95% CI −33 to 9%, p = 0.13).
Figure 4.
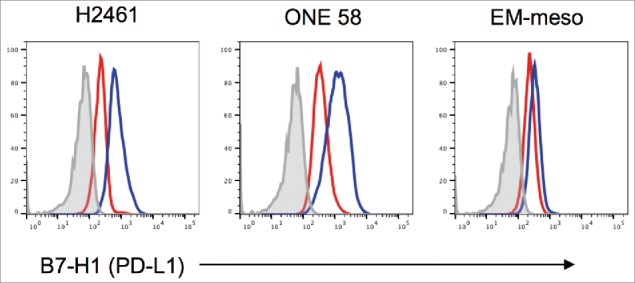
Expression of PD-L1 by human mesothelioma cell lines in the presence of vinorelbine. Histograms are the representative data of 3 independent experiments.
Discussion
We have found that the detection of PD-L1 on tumor cells differs among approximately 20% of primary MM lesions sampled at multiple time points and 30% of paired primary and metastatic MM lesions. Although there was minimal bias detected between 2 authors who scored all cases overall in our limits of agreement analysis, the wide confidence interval demonstrated that expression of PD-L1 by tumor cells within the same lesion or among paired lesions can be significantly discordant. Furthermore, we have shown that PD-L1 expression is upregulated in vitro following exposure to the commonly used chemotherapeutic agent vinorelbine but not pemetrexed. With the continued development of PD-1 and PD-L1 inhibitors for MM, this degree of heterogeneity of PD-L1 expression challenges the use of PD-L1 testing for treatment selection. These data emphasize the need for better predictive biomarkers for PD-1 and PD-L1 axis inhibitors.
In our study, the agreement in PD-L1 expression between primary and metastatic lesions of MM was 69%. Our findings confirmed previous studies in non-small cell lung cancers, clear cell renal cell carcinomas and breast cancers that revealed heterogeneity of PD-L1 expression in 12 to 38%, 21% and 6% of paired primary and metastatic lesions, respectively.17,18,21-24 In addition, a study that compared PD-L1 expression in biopsies with resection specimens of non-small cell lung cancers found a discordance rate of 48%.25 In that study, biopsy specimens had underestimated PD-L1 expression in all cases. There are multiple possible reasons for this variation including sampling, treatment, variations in histologic subtype of MM, and interobserver variability. Although we used whole tissue sections (in contrast to tissue cores as used in microarrays in some studies), the majority of our specimens were biopsies given the aggressive nature of MM and only very few patients undergoing resection of the tumor. Moreover, previous studies have shown that PD-L1 expression can be heterogeneous within a tumor. In fact in a study on breast cancer PD-L1 expression varied among multiple tissue cores in 50% of tested cases.22 Variation among multiple blocks of whole tissue sections with at least 1 cm2 tumor in each block was lower with 6% in a study of non-small cell lung cancers.16 These studies show that although whole tissue sections certainly alleviate some of the variation, there is intratumoral heterogeneity of PD-L1 expression. Our own observation in daily practice confirms heterogeneous expression of PD-L1 within single tumors.
Treatment may also alter PD-L1 expression on tumor cells. Indeed, we observed a significant increased expression of PD-L1 in MM cell lines following treatment with vinorelbine. Vinorelbine is a vinca alkaloid with a potent antitumor activity, related to their ability to prevent the polymerization of microtubules and to disrupt mitotic spindles.26 While treatment with vinorelbine as first line therapy is questionable in MM, it has shown responses as second- or third-line in some patients with MM.27 Interestingly, treatment with pemetrexed which is commonly used in combination with a platinum agent in the first line setting for MM did not affect PD-L1 expression in the cell lines we tested. Although the mechanism by which PD-L1 expression is increased by MM-cells following treatment with vinorelbine is not clear, the different mechanisms of action between vinorelbine and pemetrexed are suggestive of possibilities. For example, the interference with microtubule function may affect PD-L1 expression in ways that inhibition of purine and pyrimidine synthesis does not. In addition, PD-L1 expression was higher in a subsequent MM specimen in a fifth of our patients who received chemotherapy. A study in a xenograft mouse model of MM confirmed that PD-L1 expression on MM cells might be altered following chemotherapy.28 In that model, treatment with the investigational drug trametinib reduced expression of PD-L1 while 4-MU-treatment led to an increase in PD-L1 expression. The combination of trametinib and 4-MU also resulted in higher expression of PD-L1 than 4-MU treatment alone.
PD-L1 expression differs between subtypes of MM. We have previously shown that PD-L1 expression is more common and more diffuse in sarcomatoid MM than biphasic or epithelioid MM.13 In the current study, half of primary MM was of epithelioid subtype. In addition, in some cases the MM subtype differed between paired primary lesions and primary lesions and metastases which might have contributed to the heterogeneity in PD-L1 expression.
Even though interobserver variability was low in our study and consensus was used in discordant cases, reproducibility might contribute to different results of PD-L1 expression between paired lesions. Our interobserver agreement was similar to a study in non-small cell lung cancers in which 5 pathologists scored expression of PD-L1 on tumor cells with an intraclass correlation coefficient of 94% agreement.16
Our study has some limitations. First, while we used whole tissue specimens for the analysis of PD-L1 expression, the majority of the specimens were from biopsies and therefore sampling bias cannot be excluded in some specimens specifically given the known regional heterogeneity of PD-L1 within tumors. Second, as treatment is not standardized for patients with MM and patients were collected over many years, treatments varied between individual patients.
In conclusion, our findings in MM emphasize that disagreement in PD-L1 expression on tumor cells occurs between paired primary lesions or primary lesions and metastases and has to be considered by the physicians who treat patients with MM with anti-PD-1 or anti-PD-L1 inhibitors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
ASM was supported by NIH K12 CA090628.
Simone B.S.P. Terra, M.D., Aaron S. Mansfield, MD, Haidong Dong, Ph.D., Tobias Peikert, MD and Anja C. Roden, MD have no conflict of interest.
References
- 1.Borasio P, Berruti A, Bille A, Lausi P, Levra MG, Giardino R, Ardissone F. Malignant pleural mesothelioma: clinicopathologic and survival characteristics in a consecutive series of 394 patients. Eur J Cardiothorac Surg. 2008;33:307-13. doi: 10.1016/j.ejcts.2007.09.044. PMID:18164622 [DOI] [PubMed] [Google Scholar]
- 2.Spaggiari L, Marulli G, Bovolato P, Alloisio M, Pagan V, Oliaro A, Ratto GB, Facciolo F, Sacco R, Brambilla D, et al.. Extrapleural pneumonectomy for malignant mesothelioma: an Italian multicenter retrospective study. Ann Thorac Surg. 2014;97:1859-65. doi: 10.1016/j.athoracsur.2014.01.050. PMID:24726598 [DOI] [PubMed] [Google Scholar]
- 3.Sugarbaker DJ, Richards WG, Bueno R. Extrapleural pneumonectomy in the treatment of epithelioid malignant pleural mesothelioma: novel prognostic implications of combined N1 and N2 nodal involvement based on experience in 529 patients. Ann Surg. 2014;260:577-80; discussion 80–2. doi: 10.1097/SLA.0000000000000903. PMID:25203873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bovolato P, Casadio C, Bille A, Ardissone F, Santambrogio L, Ratto GB, Garofalo G, Bedini AV, Garassino M, Porcu L, Torri V, Pastorino U. Does surgery improve survival of patients with malignant pleural mesothelioma?: a multicenter retrospective analysis of 1365 consecutive patients. J Thorac Oncol. 2014;9:390-6. doi: 10.1097/JTO.0000000000000064. PMID:24518090 [DOI] [PubMed] [Google Scholar]
- 5.Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636-44. doi: 10.1200/JCO.2003.11.136. PMID:12860938 [DOI] [PubMed] [Google Scholar]
- 6.Zalcman G, Mazieres J, Margery J, Greillier L, Audigier-Valette C, Moro-Sibilot D, Molinier O, Corre R, Monnet I, Gounant V, et al.. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet. 2016;387:1405-14. doi: 10.1016/S0140-6736(15)01238-6. PMID:26719230 [DOI] [PubMed] [Google Scholar]
- 7.Mansfield AS, Symanowski JT, Peikert T. Systematic review of response rates of sarcomatoid malignant pleural mesotheliomas in clinical trials. Lung Cancer. 2014;86:133-6. doi: 10.1016/j.lungcan.2014.08.017. PMID:25217189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365-9. doi: 10.1038/70932. PMID:10581077 [DOI] [PubMed] [Google Scholar]
- 9.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800. doi: 10.1038/nm0902-1039c. PMID:12091876 [DOI] [PubMed] [Google Scholar]
- 10.Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185-92. doi: 10.1038/ni.1790. PMID:19783989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, et al.. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2016;375:1823-33. doi: 10.1056/NEJMoa1606774. PMID:27718847 [DOI] [PubMed] [Google Scholar]
- 12.Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al.. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521-32. doi: 10.1056/NEJMoa1503093. PMID:25891173 [DOI] [PubMed] [Google Scholar]
- 13.Mansfield AS, Roden AC, Peikert T, Sheinin YM, Harrington SM, Krco CJ, Dong H, Kwon ED. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol. 2014;9:1036-40. doi: 10.1097/JTO.0000000000000177. PMID:24926549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cedres S, Ponce-Aix S, Zugazagoitia J, Sansano I, Enguita A, Navarro-Mendivil A, Martinez-Marti A, Martinez P, Felip E. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). PLoS One. 2015;10:e0121071. doi: 10.1371/journal.pone.0121071. PMID:25774992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaughlin J, Han G, Schalper KA, Carvajal-Hausdorf D, Pelekanou V, Rehman J, Velcheti V, Herbst R, LoRusso P, Rimm DL. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol. 2016;2:46-54. doi: 10.1001/jamaoncol.2015.3638. PMID:26562159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehman JA, Han G, Carvajal-Hausdorf DE, Wasserman BE, Pelekanou V, Mani NL, McLaughlin J, Schalper KA, Rimm DL. Quantitative and pathologist-read comparison of the heterogeneity of programmed death-ligand 1 (PD-L1) expression in non-small cell lung cancer. Mod Pathol. 2017;30:340-9. doi: 10.1038/modpathol.2016.186. PMID:27834350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansfield AS, Aubry MC, Moser JC, Harrington SM, Dronca RS, Park SS, Dong H. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol. 2016;27:1953-8. doi: 10.1093/annonc/mdw289. PMID:27502709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansfield AS, Murphy SJ, Peikert T, Yi ES, Vasmatzis G, Wigle DA, Aubry MC. Heterogeneity of Programmed Cell Death Ligand 1 Expression in Multifocal Lung Cancer. Clin Cancer Res. 2016;22:2177-82. doi: 10.1158/1078-0432.CCR-15-2246. PMID:26667490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG. WHO Classification of tumours of the lung, pleura, thymus and heart. 4th ed. Lyon: International Agency for Research on Cancer; 2015. ISBN-13 9789283224365 ISBN-10 9283224361. [DOI] [PubMed] [Google Scholar]
- 20.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639-45. doi: 10.1101/gr.092759.109. PMID:19541911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinato DJ, Shiner RJ, White SD, Black JR, Trivedi P, Stebbing J, Sharma R, Mauri FA. Intra-tumoral heterogeneity in the expression of programmed-death (PD) ligands in isogeneic primary and metastatic lung cancer: Implications for immunotherapy. Oncoimmunology. 2016;5:e1213934. doi: 10.1080/2162402X.2016.1213934. PMID:27757309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dill EA, Gru AA, Atkins KA, Friedman LA, Moore ME, Bullock TN, Cross JV, Dillon PM, Mills AM. PD-L1 Expression and Intratumoral Heterogeneity Across Breast Cancer Subtypes and Stages: An Assessment of 245 Primary and 40 Metastatic Tumors. Am J Surg Pathol. 2017;41:334-42. doi: 10.1097/PAS.0000000000000780. PMID:28195880 [DOI] [PubMed] [Google Scholar]
- 23.Uruga H, Bozkurtlar E, Huynh TG, Muzikansky A, Goto Y, Gomez-Caraballo M, Hata AN, Gainor JF, Mark EJ, Engelman JA, et al.. Programmed Cell Death Ligand (PD-L1) Expression in Stage II and III Lung Adenocarcinomas and Nodal Metastases. J Thorac Oncol. 2017;12:458-66. doi: 10.1016/j.jtho.2016.10.015. PMID:27815126 [DOI] [PubMed] [Google Scholar]
- 24.Callea M, Albiges L, Gupta M, Cheng SC, Genega EM, Fay AP, Song J, Carvo I, Bhatt RS, Atkins MB, Hodi FS, Choueiri TK, McDermott DF, Freeman GJ, Signoretti S. Differential Expression of PD-L1 between Primary and Metastatic Sites in Clear-Cell Renal Cell Carcinoma. Cancer Immunol Res. 2015;3:1158-64. doi: 10.1158/2326-6066.CIR-15-0043. PMID:26014095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilie M, Long-Mira E, Bence C, Butori C, Lassalle S, Bouhlel L, Fazzalari L, Zahaf K, Lalvee S, Washetine K, et al.. Comparative study of the PD-L1 status between surgically resected specimens and matched biopsies of NSCLC patients reveal major discordances: a potential issue for anti-PD-L1 therapeutic strategies. Ann Oncol. 2016;27:147-53. doi: 10.1093/annonc/mdv489. PMID:26483045 [DOI] [PubMed] [Google Scholar]
- 26.Zhou XJ, Rahmani R. Preclinical and clinical pharmacology of vinca alkaloids. Drugs. 1992;44 Suppl 4:1-16; discussion 66–9. doi: 10.2165/00003495-199200444-00002. PMID:1283846 [DOI] [PubMed] [Google Scholar]
- 27.Scherpereel A, Astoul P, Baas P, Berghmans T, Clayson H, de Vuyst P, Dienemann H, Galateau-Salle F, Hennequin C, Hillerdal G, et al.. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J. 2010;35:479-95. doi: 10.1183/09031936.00063109. PMID:19717482 [DOI] [PubMed] [Google Scholar]
- 28.Cho H, Matsumoto S, Fujita Y, Kuroda A, Menju T, Sonobe M, Kondo N, Torii I, Nakano T, Lara PN, et al.. Trametinib plus 4-Methylumbelliferone Exhibits Antitumor Effects by ERK Blockade and CD44 Downregulation and Affects PD-1 and PD-L1 in Malignant Pleural Mesothelioma. J Thorac Oncol. 2017;12:477-90. doi: 10.1016/j.jtho.2016.10.023. PMID:27867002 [DOI] [PubMed] [Google Scholar]


