ABSTRACT
Background – The presence of tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment is associated with an improved prognosis and a better response to therapy in different types of cancer. In this systematic review and meta-analysis, we investigated the prognostic value of T cells in head and neck squamous cell carcinoma (HNSCC).
Methods – In a systematic review, Pubmed and Embase were searched for publications that investigated the prognostic value of T cells in HNSCC. A meta-analysis was performed including all studies assessing the association between CD3+, CD4+, CD8+, and FoxP3+ TILs and overall survival (OS), disease-free survival (DFS), or locoregional control (LRC).
Results – A pooled analysis indicated a favorable, prognostic role for CD3+ TILs (HR 0.64 (95%CI 0.47–0.85) for OS, HR 0.63 (95%CI 0.49–0.82) for DFS) and CD8+ TILs (HR 0.67 (95%CI 0.58–0.79) for OS, HR 0.50 (95%CI 0.37–0.68) for DFS, and HR 0.82 (95%CI 0.70–0.96) for LRC) in the clinical outcome of HNSCC. FoxP3+ TILs were also associated with better OS (HR 0.80 (95%CI 0.70–0.92)).
Conclusion – This systematic review and meta-analysis confirmed the favorable, prognostic role of CD3+ and CD8+ T cell infiltration in HNSCC patients and found an association between FoxP3+ TILs and improved overall survival. Future studies using homogeneous patient cohorts with regard to tumor subsite, stage and treatment are necessary to provide more insight in the predictive value of TILs in HNSCC.
KEYWORDS: head and neck squamous cell carcinoma (HNSCC), prognostic biomarkers, systematic review, T cells, tumor infiltrating lymphocytes (TILs)
Introduction
With over 600,000 new cases per year worldwide, head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy in developed countries.1 However, despite numerous developments in treatment modalities, the disease-free and overall survival of head and neck cancer patients barely improved over the last decades.2 Currently, it is not possible to accurately predict treatment outcome in HNSCC patients, because robust predictive biomarkers are lacking.
To identify new predictive biomarkers, a lot of focus has been on the biologic properties of cancer cells. However, new insights show that tumor aggressiveness and therapy resistance are also influenced by the interplay between tumor cells and their micro-environment.3,4 Therapies targeting this interaction are rapidly evolving and are already implemented in different types of cancer. Soon, immunotherapy is very likely to become implemented in the standard treatment regiments of HNSCC patients.5,6
Clarifying the prognostic value of the presence of tumor-infiltrating immune cells might lead to a better understanding of the role of different elements of the head and neck tumor microenvironment. Moreover, identifying predictive biomarkers, which specifically predict treatment outcome, is essential to direct personalized medicine.
Many studies indicated the presence of tumor-infiltrating lymphocytes (TILs) to be a favorable prognostic factor for treatment outcome in different types of cancer.7 However, different subsets of lymphocytes have different or even opposing functions in the tumor microenvironment.
Cytotoxic T-lymphocytes, characterized by the expression of CD8, have the ability to directly target and destroy tumor cells through binding to MHC class I molecules, and have therefore been studied extensively.8 The presence of CD8+ lymphocytes in the tumor microenvironment has been associated with a better prognosis in many types of cancer,9-11 which is currently also an important target of new treatment strategies.12
Regulatory T-cells (Tregs) are involved in maintaining immunological tolerance to host tissues and are therefore considered to be suppressors of the anti-tumor immune response.13 This suggests that their presence in the tumor micro-environment would predict an unfavorable prognosis. However, the prognostic value of Tregs seems to differ strongly among different types of cancer. For HNSCC, previous studies suggested that high Treg counts are associated with better prognosis.14 Tregs are most often evaluated using immunohistochemical staining for FoxP3, which is considered to be the most specific Treg marker.15
The role of CD4+ helper T-cells is unclear, because a wide range of CD4+ cell subsets with different functions exists.16 CD4+ Th1 cells promote the anti-tumor immune response by stimulating CD8+ cytotoxic T-cells, but CD4+ Th2 cells are also related to anti-tumor immunity.17 On the other hand, CD4+ regulatory T-cells are thought to inhibit an effective anti-tumor immune response.13 The exact role of the more recently described CD4+ cytotoxic T-cells and follicular helper T-cells in tumor immune surveillance has not been clarified yet.16
In this study, we systematically reviewed the literature for publications about the prognostic value of T-lymphocytes and CD4+, CD8+, and FoxP3+ T-cell subsets in HNSCC. We aimed to include all studies that assessed tumor infiltration with CD3+, CD4+, CD8+, or FoxP3+ lymphocytes as well as the ratios between these markers as a prognostic biomarker in HNSCC.
Methods
Search strategy
A broad search was conducted that included the domain (“HNSCC”), the determinant (“Tumor-infiltrating T-cells”), their synonyms, and a filter for prognostic studies.18 Pubmed/Medline and Embase were searched for publications based on title and abstract. The complete search strategy is shown in Supplementary Table 1. Two researchers (ERU and MOO) independently screened the abstracts based on predetermined in- and exclusion criteria (Supplementary Table 2). A final selection was made by full-text reading of the selected studies. Discrepancies between the two researchers were discussed and resolved by consensus. T-cell markers assessed in two or more studies qualified for inclusion.
Inclusion criteria
Studies were included in which the prognostic value of CD3+, CD4+, CD8+, and/or FoxP3+ lymphocytes was investigated in patients with oral cavity, oropharyngeal, hypopharyngeal, or laryngeal carcinoma. TILs had to be evaluated immunohistochemically; studies which only evaluated hematoxylin- and eosin-stained sections were excluded. Only publications about lymphocytes in the tumor epithelium were included. Studies that only investigated lymphocytes in the tumor stroma did not qualify. The prognostic value had to be investigated by time-to-event survival analysis with either overall survival (OS), disease-free survival (DFS) or locoregional control (LRC). Original articles published in English between January 2006 and December 2016 were eligible for inclusion. Animal studies, case reports, and commentaries were excluded.
Data extraction
The following data were obtained from each included publication: author and title, year of publication, biomarker(s), sample size, tumor subsite, tumor stage, HPV status, treatment modality, median follow-up time, scoring methods, cutoffs, and finally outcome of univariate and multivariate analysis defined by hazard ratio (HR), 95%-confidence interval (CI), and p-values. When these parameters were not mentioned in the article, we extracted the data from Kaplan-Meier curves by digitizing the curves using the open-source Engauge Digitizer software (http://digitizer.sourceforge.net/) and estimating the univariate HR.19 When HRs were not mentioned and Kaplan-Meier curves were not available, or HRs did not match the shown Kaplan-Meier curves, studies were excluded from the meta-analysis. Because HPV positive and HPV negative head and neck tumors are considered as different disease entities,20,21 we recorded data for these two subgroups separately.
Assessment of study quality
This study is compliant with the PRISMA checklist.22 All relevant publications were appraised for risk of bias using the Quality and Prognosis Studies (QUIPS) criteria, a validated and useful tool for systematic reviewers for the critical appraisal of study quality.23 The risk of bias was scored as low, moderate or high for six different domains: study participation, study attrition, prognostic factor measurement, outcome measurement, study confounding and statistical analysis, and reporting. For this systematic review, baseline characteristics included HPV status, T- and N-stage, and treatment modality. Studies that used consecutive cohorts, stratified for HPV status, clearly described their methods of quantification, and included treatment in their analysis were valued highest. The risk of bias was assessed by two researchers (ERU and MOO) independently. Differences were resolved by discussion.
Statistical analysis
For the meta-analysis, HRs were used that described the risk of event for high TILs versus low TILs. If the study reported the HR for low TILs vs. high TILs, the reciprocal was taken. The meta-analysis and creation of the forest plots was performed in Review Manager version 5.0.
Results
Study selection and characteristics
The Pubmed/Medline and EMBASE search yielded 2550 hits after removing duplicates (Fig. 1). Of the 122 publications that remained after the abstract screening 48 were conference abstracts, 2 were in Polish, and 3 were not available. Therefore, 69 full-text articles were evaluated, of which 28 met our inclusion criteria.24-51 Of these, 7 could not be included because no hazard ratios or Kaplan-Meier curves were available or because the reported hazard ratios did not match their Kaplan-Meier curves; 3 studies were excluded because they reported outcome in terms of disease-specific survival; 2 studies evaluated FoxP3 expression on tumor cells instead of TILs.
Figure 1.
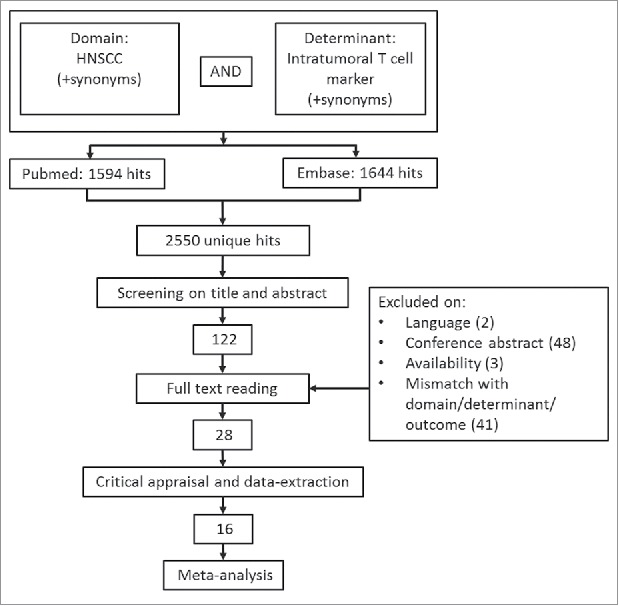
Selection process. Of the 122 articles that remained after the initial title/abstract screening, 28 remained for inclusion.
Table 1 gives an overview of the study characteristics of the remaining 19 studies that were eligible for inclusion in the meta-analysis after appraisal. The characteristics of studies that fulfilled the inclusion criteria, but could not be included in the meta-analysis are shown in Supplementary Table 3. All studies investigated TILs by immunohistochemistry. Distel et al. analyzed early stage and advanced stage cancer separately and this difference was maintained in our analysis.26 Most studies included HPV-positive as well as HPV-negative patients, multiple subsites and multiple treatment modalities.
Table 1.
Study characteristics of studies included in meta-analysis. Oral Cavity (OC), Oropharynx (OP), Hypopharynx (HP), Larynx (L), Other (O), Surgery (S), Radiotherapy (RT), Chemotherapy (CT), Chemoradiotherapy (CRT), Formalin Fixed, Paraffin Embedded material (FFPE), Fresh Frozen Tissue (FFT), Tissuemicroarray (TMA), Immunohistochemistry (IHC), Immunofluorescence Staining (IFS).
| Study | Sample size | Subsite | HPV | Stage | Treatment | Biomarkers | Material | Technique |
|---|---|---|---|---|---|---|---|---|
| Balermpas 2014 | 101 | OC, OP, HP, L | Both | All | CRT | CD3, CD4, CD8, FoxP3 | FFPE | IHC |
| Bron 2013 | 35 | OC, OP, HP, L | ? | All | S | FoxP3, (BDCA2, CD11c, CD56, COX2, BCL2, ARG2, iNOS) | FFPE | IHC |
| Distel 2009 | 115 | OP, HP | ? | All | CRT, S+RT | CD3, CD4, CD8, FoxP3, (CD20, CD79, Granzyme B) | FFPE in TMA | IHC |
| Hasmim 2013 | 83 | OC, OP, HP, L | Both | All | S, CRT, S+RT | CD8, (HLA1, EPHRI-NE, SCINDERIN) | FFT | IHC, IFS |
| Kim 2016 | 402 | OC, OP, HP, L, O | Both | All | S+CRT/RT/CT | CD3, CD8, FoxP3, (PD-L1, PD1, ICOS, LAG-1) | FFPE in TMA | IHC |
| Nasman 2012 | 83 | OP | Both | All | RT, CRT | CD8, FoxP3 | FFPE | IHC |
| Nguyen 2016 | 273 | OC, OP, HP, L | ? | All | S, RT, CRT | CD4, CD8, FoxP3, (CD68) | FFPE in TMA | IHC |
| Nordfors 2013 | 280 | OP | Both | All | RT, CRT | CD4, CD8 | FFPE | IHC |
| Oquejiofor 2015 | 139 | OP | Both | All | RT | CD3, CD4, CD8, FoxP3, (SMA) | FFPE | IHC |
| Park 2013 | 79 | OP | Both | III/IV | S, RT, S+RT | FoxP3, CD25 | FFPE in TMA | IHC |
| Pretscher 2009 | 33 | OC, OP, HP | Both | All | S+RT, S+CRT | CD3, CD8, FoxP3, (CD20, CD68, Granzyme B) | FFPE in TMA | IHC |
| Punt 2016 | 117 | OP | Both | All | ? | CD3, FoxP3, (IL-17) | FFPE | IFS |
| Van Kempen 2016 | 262 | OP | Both | All | S, S+(C)RT, RT, CRT | CD3, CD4, CD8, (SERPENB1/B4/B9, Granzyme B) | FFPE in TMA | IHC |
| Watanabe 2010 | 87 | OC | ? | All | S | CD4, CD8, CD25, FoxP3, (CD69, CCR4, Granzyme B) | FFPE | IHC |
| Wolf 2015 | 39 | OC | ? | All | S, S+RT | CD4, CD8, FoxP3, (CD104, CD68) | FFPE in TMA | IHC |
| Zancope 2010 | 70 | OC, O (lip) | ? | All | S | CD8, (CD57) | FFPE | IHC |
Quality assessment
The 28 studies remaining after full text screening were critically appraised for risk of bias using the QUIPS criteria. Almost no study mentioned study attrition or provided information about patients lost to follow-up. Most studies did not use consecutive cohorts. Several studies were unclear about their scoring methods, especially regarding TIL location (epithelial vs. stromal). This was accounted for in the prognostic factor measurement domain. Also, studies used data-dependent cut-offs, but since no consensus exists on cut-offs, this was not considered in the prognostic factor measurement domain. The complete quality assessment of the publications included in the meta-analysis is shown in Table 2. The quality assessment of the excluded articles after full text screening is shown in Supplementary Table 4.
Table 2.
Quality assessment of included studies. ○ = low risk of bias, ◐ = moderate risk of bias, • = high risk of bias.
| Study | Study Participation | Study Attrition | Prognostic factor | Outcome | Study con-founding | Analysis and reporting | Total Risk of Bias | |
|---|---|---|---|---|---|---|---|---|
| Balermpas 2013 | ○ | • | ◐ | ○ | ○ | ○ | 1 | Low |
| Bron 2012 | • | • | ◐ | ◐ | ◐ | ◐ | 6 | High |
| Distel 2009 | ◐ | • | ◐ | ○ | ◐ | ○ | 3 | Moderate |
| Hasmim 2013 | ◐ | • | ◐ | ○ | ◐ | ◐ | 4 | High |
| Kim 2016 | ◐ | • | ○ | ○ | ○ | ○ | 1 | Low |
| Nasman 2012 | ○ | • | ◐ | ○ | ○ | ◐ | 2 | Moderate |
| Nguyen 2016 | ○ | ◐ | ○ | ○ | ○ | ○ | 0 | Low |
| Nordfors 2013 | ○ | • | ◐ | ○ | ○ | ○ | 1 | Low |
| Oguejiofor 2015 | ◐ | • | ◐ | ◐ | ◐ | ○ | 4 | High |
| Park 2013 | ◐ | • | • | ◐ | ○ | ○ | 4 | High |
| Pretscher 2009 | ◐ | • | ○ | ○ | • | ○ | 3 | Moderate |
| Punt 2016 | ◐ | • | ○ | ○ | ◐ | ○ | 2 | Moderate |
| Van Kempen 2016 | ◐ | • | ◐ | ○ | ○ | ○ | 2 | Moderate |
| Watanabe 2010 | ◐ | • | ◐ | ◐ | ◐ | ○ | 4 | High |
| Wolf 2015 | ◐ | • | ○ | ○ | ○ | • | 3 | Moderate |
| Zancope 2010 | • | • | ◐ | ○ | ○ | • | 5 | High |
CD3+ TILs as prognostic biomarker
The prognostic value of CD3 was assessed in 9 studies, of which 6 were eligible for inclusion in the meta-analysis. The results of the meta-analysis are shown in Fig. 2. In HPV-negative patients, the pooled meta-analysis showed an advantage for high CD3+ TIL infiltration (pooled HR 0.64 (CI 0.47–0.85) for OS, pooled HR 0.63 (0.49–0.82) for DFS) (Fig. 2a). The 2 studies that reported LRC did not yield a conclusive result (pooled HR 0.72 (0.40–1.31)).
Figure 2.
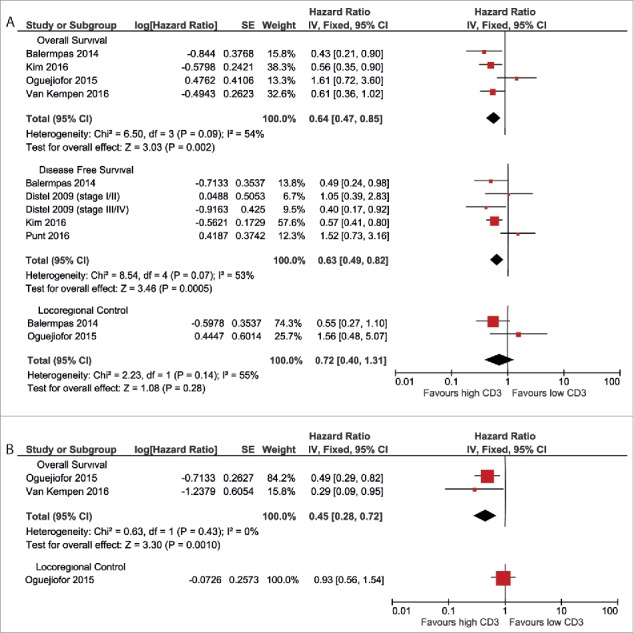
Forest plots of prognostic value of CD3+ TILs on overall survival, disease-free survival and locoregional control in HPV-negative patients (A) and HPV-positive patients (B). No data were available for disease-free survival in HPV-positive patients.
In HPV-positive patients, a similar advantage for high CD3+ cell count was observed. Two studies reported a better overall survival in patients with high CD3+ TIL infiltration (pooled HR 0.45 (0.28–0.72) (Fig. 2b). One study reported on LRC, but did not find a correlation with CD3+ TIL infiltration (HR 0.93 (0.56–1.54)). No studies reported data on disease-free survival for HPV-positive patients.
CD4+TILs as prognostic biomarker
Four studies presented data on the prognostic value of CD4+ TILs. In HPV-negative patient cohorts, high CD4+ TILs were associated with a better overall survival (pooled HR 0.76 (0.64–0.89)), as well as with a better locoregional control (pooled HR 0.81 (0.68–0.96)) (Fig. 3a). The only study that reported on disease-free survival found an advantage for patients with high CD4+ TIL count (HR 0.49 (0.24–0.98)).
Figure 3.
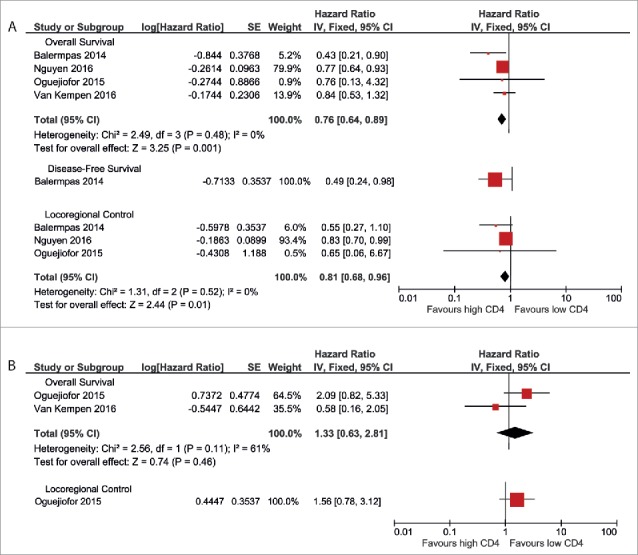
Forest plots of prognostic value of CD4+ TILs on overall survival, disease-free survival and locoregional control in HPV-negative patients (A) and HPV-positive patients (B). No data were available for disease-free survival in HPV-positive patients.
Two studies investigated CD4+ TILs in relation to the prognosis of HPV-positive patients. These showed contradictory results (pooled HR 1.33 (0.63–2.81)) (Fig. 3b). One study reported data on locoregional control and found a non-significant association between high CD4+ TIL infiltration and worse outcome (HR 1.56 (0.78–3.12)). No studies reported data on disease-free survival in HPV-positive patients.
Remarkably, 10 studies that were excluded from the meta-analysis, did assess CD4+ TILs as a prognostic marker, but a substantial part of these studies did not report HRs or Kaplan-Meier curves because they did not find a significant result.
CD8+ TILs as prognostic biomarker
CD8+ TILs were most frequently assessed in the included studies: 12 studies were eligible for inclusion in the meta-analysis. A favorable outcome for high CD8+ TIL count was observed for each outcome measurement in HPV-negative patients (pooled HR 0.67 (0.58–0.79) for OS, pooled HR 0.50 (0.37–0.68) for DFS, and pooled HR 0.82 (0.70–0.96) for LRC) (Fig. 4a).
Figure 4.
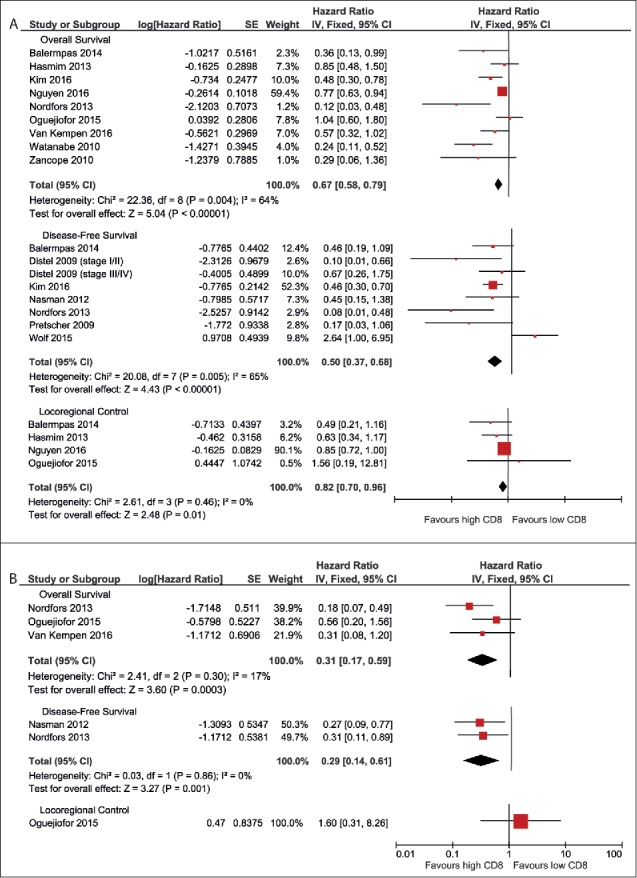
Forest plots of prognostic value of CD8+ TILs on overall survival, disease-free survival and locoregional control in HPV-negative patients (A) and HPV-positive patients (B).
A similar advantage for high CD8+ TILs was observed in HPV-positive patients for OS (pooled HR 0.31 (0.17–0.59)) and DFS (pooled HR 0.82 (0.70–0.96)) (Fig. 4b). Data on LRC were only reported in one study, which found a non-significant worse prognosis for patients with high CD8+ TIL count (HR 1.60 (0.31–8.26)).
FoxP3 as prognostic biomarker
Nine studies reported results on the prognostic value of FoxP3+ TILs. In HPV-negative patients, a better OS was observed in patients with high FoxP3+ TIL infiltration (pooled HR 0.80 (0.70–0.92)) (Fig. 5a). A comparable trend was seen for DFS (pooled HR 0.77 (0.57–1.02)) and LRC (pooled HR 0.92 (0.81–1.04)).
Figure 5.
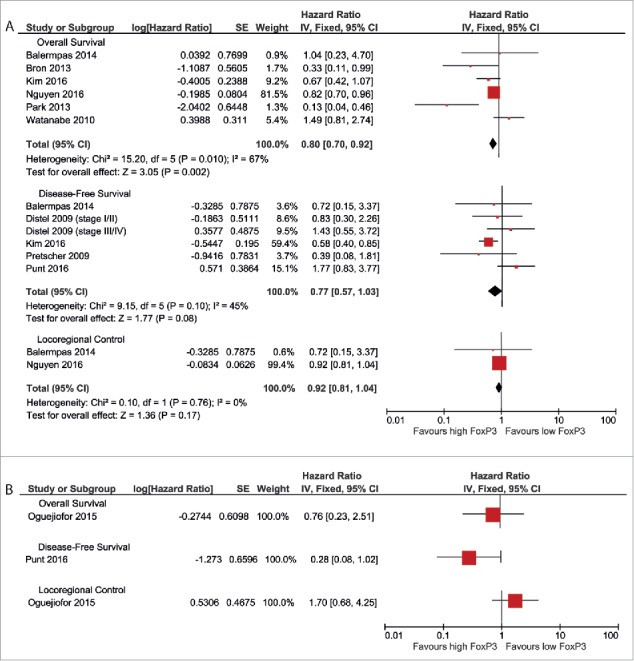
Forest plots of prognostic value of FoxP3+ TILs on overall survival, disease-free survival and locoregional control in HPV-negative patients (A) and HPV-positive patients (B).
It was not possible to perform a meta-analysis for HPV-positive patients, because only one study provided the required data for each outcome measurement. Oguejiofor et al. reported a slightly and non-significantly better OS for patients with high FoxP3+ TIL count (HR 0.76 (0.23–2.51)) and a non-significantly worse LRC for this patient group (HR 1.70 (0.68–4.25)).37 Punt et al. showed a trend toward a better DFS for high FoxP3+ TILs (HR 0.28 (0.08–1.02)).40 Results of these separate studies are shown in Fig. 5b.
TIL ratios as prognostic biomarker
It was not possible to perform a meta-analysis on TIL ratios, because an insufficient number of studies provided the required data. Only one study assessed the prognostic value of the CD8/FoxP3 ratio; Nasman et al. reported a significant better DFS for patients with a high CD8/FoxP3 ratio in HPV-negative patients (HR 0.28 (0.1–0.9)).34
Both CD3/FoxP3 ratios and FoxP3/CD4 ratios were also reported once; Zhou et al. (2016) found an association between a high FoxP3/CD4 ratio and a worse OS (HR 13.19 (1.96–14)).51 Distel et al. (2009) reported a trend toward better DFS in patients with a high CD3/FoxP3 ratio (HR 2.4 (0.98–7.47) for stage I/II patients, HR 1.58 (0.64–4.16) for stage III/IV patients).26
Discussion
In this systematic review and meta-analysis, we investigated the prognostic value of different subsets of T-cells for predicting HNSCC outcome.
Firstly, we investigated the prognostic value of the general T-cell marker CD3. According to our meta-analysis, high CD3+ TIL infiltration correlated with a favorable prognosis for both HPV-negative and HPV-positive head and neck tumors. This fits with the hypothesis that immune cells in the tumor micro-environment are of great importance for clinical outcome, and this is also in line with findings in other cancer types.7
The prognostic value of CD8+ TILs was most frequently assessed. Because cytotoxic T-cells belong to the subset of T-cells that directly target tumor cells, it is a more robust biomarker than CD3. Indeed, almost every study that assessed the prognostic value of CD8+ TILs found a better outcome for HPV-negative as well as HPV-positive patients with high CD8+ T cell infiltration, which was also seen in our meta-analysis.
Several studies assessed the prognostic value of CD4+ TIL infiltration in HNSCC. However, the role of CD4+ lymphocytes in the tumor micro-environment is ambiguous.16 A wide range of CD4+ cell subsets with different functions exists, ranging from the cytotoxic cell response stimulating Th1 cells, to the immune suppressing regulatory T-cells. Staining for CD4 expression alone does not distinguish between these subsets.13,17
Our meta-analysis suggested high CD4+ TIL infiltration to be a favorable, prognostic biomarker for the outcome of HNSCC. However, it was not possible to extract the required data from most studies that investigated the prognostic role of CD4+ lymphocytes. The publications did not report their results because of lack of statistical significance, possibly implicating the presence of publication bias in our analysis.
Considering the ambiguous role of CD4+ lymphocytes in the tumor micro-environment, the small number of eligible studies, and the high suspicion of publication bias for this marker, we conclude that the prognostic role of CD4 remains questionable.
Finally, we assessed the prognostic value of FoxP3. According to our meta-analysis, high tumoral infiltration with FoxP3+ lymphocytes predicts a better clinical outcome in HNSCC patients. Although this was described previously in HNSCC, colorectal cancer and esophageal cancer,14 it is unexpected that the presence of immune-suppressing T-cells in the tumor microenvironment was associated with better clinical outcome. A possible explanation for this phenomenon, is that Tregs play a role in suppressing the ongoing, ineffective inflammatory response, which is associated with promoting tumor progression by growth factor and inflammatory cytokine producing immune cells, such as macrophages and dendritic cells.14,52
Other studies suggested that the number of FoxP3+ TILs could be just a reflection of the total amount of T cells present in tumor epithelium and that the beneficial effect of CD8+ T cells outweighs the immunosuppressive effect of the Tregs.38 A way to investigate this potential phenomenon is to look at CD8/FoxP3 ratio. A high ratio, which thus reflects a relative depletion of Tregs, is a potentially robust biomarker for better clinical outcome in different tumor types.7 In HNSCC, only one study assessed this ratio, with promising results.34 Additional research is necessary to validate the prognostic value of the CD8/FoxP3 ratio in HNSCC.
A last possible explanation for the favorable role of FoxP3+ TILs that we found in our meta-analysis might be the heterogeneity of the FoxP3 expressing T-cell subset. Although considered the most specific Treg marker so far, FoxP3 is not specific for activated Tregs; additional markers, such as CD25 and the absence of CD127, might be required to discriminate Tregs with an immunosuppressive function.53
The main, general limitation of this review, is the heterogeneity within tumor subsite among and within the studies included in our meta-analysis. The prognostic value of biomarkers is likely to differ between different tumor subsites and tumor stages. However, the small number of subjects included in most studies did often not allow stratification for these different circumstances. The use of more homogeneous patient cohorts could strengthen the conclusions on prognostic biomarkers and also provide more insight into the differences between patient subgroups.
This also applies to heterogeneity in treatment modality. Since different treatment modalities have a different mechanism of action, it is likely that the prognostic value of biomarkers also depends on the given therapy. Very few studies accounted for treatment modality in their analysis. Therefore we can only conclude on the general prognostic value of T-cell markers, while the predictive value for specific treatment modalities still has to be elucidated. To incorporate prognostic T-cell markers in clinical practice, more prognostic studies with homogeneous patient cohorts with respect to tumor subsite and tumor stage as well as correction for treatment modality are needed.
Although we focused on TILs in the tumor epithelium, it should be noted that the presence of TILs in the tumor stroma has its effect on prognosis and therapy response as well. However, the prognostic significance of stromal and epithelial immune cells differs.54 To keep the data as comparable as possible we excluded data on stromal TILs from our analyses.
To incorporate TILs in clinical practice, it is necessary to determine standardized, validated cut-offs for quantification. However, since methods of quantifying TILs and patient cohorts differed strongly among the studies included in this meta-analysis, it was not possible to suggest general cut-offs yet.
Some studies that did not meet the inclusion criteria for this systematic review were total lymphocyte counts based on hematoxyline- and eosin (H&E) stained sections of head and neck tumors.34,55-58 However, because we specifically aimed to evaluate the role of T-cells and their subsets in HNSCC prognosis, and H&E staining does not discriminate between different intratumoral immune cells, we did not include studies that quantified TILs in H&E stained sections.
Although immunohistochemistry is more distinctive than H&E staining, heterogeneity of staining for CD markers alone remains a limitation. Besides the heterogeneity in the FoxP3 expressing T-cell subset, CD4 and CD8 are also not exclusively representative for T helper cells and cytotoxic T-cells, respectively, but are also observed on macrophages and dendritic cells.59,60 Modern techniques that are able to identify different T-cell subsets more specifically might provide more robust biomarkers for HNSCC prognosis and therapy response.
In conclusion, our meta-analysis confirmed the prognostic role of both intratumoral CD3+ T-cell infiltration and CD8+ T-cell infiltration in HNSCC. High counts of CD3- and CD8-positive lymphocytes predicted a better clinical outcome. However, for incorporating different T-cell markers as predictive biomarkers in clinical practice, investigation of these biomarkers in homogeneous cohorts with regard to tumor subsite, tumor stage, and therapy with robust, modern techniques, is needed.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
E. J. de Ruiter is financially supported by the Dutch Cancer Society (project number: A6C 7072).
References
- 1.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695-709. doi: 10.1016/S0140-6736(08)60728-X. PMID:18486742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murdoch D. Standard, and novel cytotoxic and molecular-targeted, therapies for HNSCC: An evidence-based review. Curr Opin Oncol. 2007;19(3):216-21. doi: 10.1097/01.cco.0000264952.98166.99. PMID:17414639 [DOI] [PubMed] [Google Scholar]
- 3.Hadrup S, Donia M, Thor Straten P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013;6(2):123-33. doi: 10.1007/s12307-012-0127-6. PMID:23242673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Curry JM, Sprandio J, Cognetti D, Luginbuhl A, Bar-ad V, Pribitkin E, Tuluc M. Tumor microenvironment in head and neck squamous cell carcinoma. Semin Oncol. 2014;41(2):217-34. doi: 10.1053/j.seminoncol.2014.03.003. PMID:24787294 [DOI] [PubMed] [Google Scholar]
- 5.Bauml J, Seiwert TY, Pfister DG, Worden F, Liu SV, Gilbert J, Saba NF, Weiss J, Wirth L, Sukari A, et al.. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: Results from a single-arm, phase II study. J Clin Oncol. 2017;35(14):1542-9. doi: 10.1200/JCO.2016.70.1524. PMID:28328302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferris RL, Blumenschein G Jr, Fayette J, Guigay J, Colevas AD, Licitra L, Harrington K, Kasper S, Vokes EE, Even C, et al.. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-67. doi: 10.1056/NEJMoa1602252. PMID:27718784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: A systematic review with meta-analysis. Br J Cancer. 2011;105(1):93-103. doi: 10.1038/bjc.2011.189. PMID:21629244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knocke S, Fleischmann-Mundt B, Saborowski M, Manns MP, Kuhnel F, Wirth TC, Woller N. Tailored tumor immunogenicity reveals regulation of CD4 and CD8 T cell responses against cancer. Cell Rep. 2016;17(9):2234-46. doi: 10.1016/j.celrep.2016.10.086. PMID:27880900 [DOI] [PubMed] [Google Scholar]
- 9.Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, Earl HM, Poole CJ, Hiller L, Dunn JA, et al.. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann Oncol. 2014;25(8):1536-43. doi: 10.1093/annonc/mdu191. PMID:24915873 [DOI] [PubMed] [Google Scholar]
- 10.Reissfelder C, Stamova S, Gossmann C, Braun M, Bonertz A, Walliczek U, Grimm M, Rahbari NN, Koch M, Saadati M, et al.. Tumor-specific cytotoxic T lymphocyte activity determines colorectal cancer patient prognosis. J Clin Invest. 2015;125(2):739-51. doi: 10.1172/JCI74894. PMID:25562322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN, Herbst RS, Rimm DL. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst. 2015;107(3):dju435. doi: 10.1093/jnci/dju435. PMID:25650315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharpe M, Mount N. Genetically modified T cells in cancer therapy: Opportunities and challenges. Dis Model Mech. 2015;8(4):337-50. doi: 10.1242/dmm.018036. PMID:26035842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775-87. doi: 10.1016/j.cell.2008.05.009. PMID:18510923 [DOI] [PubMed] [Google Scholar]
- 14.Shang B, Liu Y, Jiang SJ, Liu Y. Prognostic value of tumor-infiltrating FoxP3+ regulatory T cells in cancers: A systematic review and meta-analysis. Sci Rep. 2015;5:15179. doi: 10.1038/srep15179. PMID:26462617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057-61. doi: 10.1126/science.1079490. PMID:12522256 [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Cantor H. CD4 T-cell subsets and tumor immunity: The helpful and the not-so-helpful. Cancer Immunol Res. 2014;2(2):91-8. doi: 10.1158/2326-6066.CIR-13-0216. PMID:24778273 [DOI] [PubMed] [Google Scholar]
- 17.Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M, et al.. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190(5):617-27. doi: 10.1084/jem.190.5.617. PMID:10477547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geersing GJ, Bouwmeester W, Zuithoff P, Spijker R, Leeflang M, Moons KG. Search filters for finding prognostic and diagnostic prediction studies in Medline to enhance systematic reviews. PLoS One. 2012;7(2):e32844. doi: 10.1371/journal.pone.0032844. PMID:22393453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. PMID:17555582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dok R, Nuyts S. HPV positive head and neck cancers: Molecular pathogenesis and evolving treatment strategies. Cancers (Basel). 2016;8(4):41. doi: 10.3390/cancers8040041. PMID:27043631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Westra WH, Lewis JS Jr. Update from the 4th edition of the World Health Organization classification of head and neck tumours: Oropharynx. Head Neck Pathol. 2017;11(1):41-7. doi: 10.1007/s12105-017-0793-2. PMID:28247229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. PMID:25554246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280-6. doi: 10.7326/0003-4819-158-4-201302190-00009. PMID:23420236 [DOI] [PubMed] [Google Scholar]
- 24.Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rodel F, Rödel C, Fokas E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110(2):501-9. doi: 10.1038/bjc.2013.640. PMID:24129245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bron L, Jandus C, Andrejevic-Blant S, Speiser DE, Monnier P, Romero P, Rivals JP. Prognostic value of arginase-II expression and regulatory T-cell infiltration in head and neck squamous cell carcinoma. Int J Cancer. 2013;132(3):E85-93. doi: 10.1002/ijc.27728. PMID:22815199 [DOI] [PubMed] [Google Scholar]
- 26.Distel LV, Fickenscher R, Dietel K, Hung A, Iro H, Zenk J, Nkenke E, Büttner M, Niedobitek G, Grabenbauer GG. Tumour infiltrating lymphocytes in squamous cell carcinoma of the oro- and hypopharynx: Prognostic impact may depend on type of treatment and stage of disease. Oral Oncol. 2009;45(10):e167-74. doi: 10.1016/j.oraloncology.2009.05.640. PMID:19576838 [DOI] [PubMed] [Google Scholar]
- 27.Hanakawa H, Orita Y, Sato Y, Takeuchi M, Ohno K, Gion Y, Tsukahara K, Tamamura R, Ito T, Nagatsuka H, et al.. Regulatory T-cell infiltration in tongue squamous cell carcinoma. Acta Otolaryngol. 2014;134(8):859-64. doi: 10.3109/00016489.2014.918279. PMID:24921153 [DOI] [PubMed] [Google Scholar]
- 28.Hasmim M, Badoual C, Vielh P, Drusch F, Marty V, Laplanche A, de Oliveira Diniz M, Roussel H, De Guillebon E, Oudard S, et al.. Expression of EPHRIN-A1, SCINDERIN and MHC class I molecules in head and neck cancers and relationship with the prognostic value of intratumoral CD8+ T cells. BMC Cancer. 2013;13:592. doi: 10.1186/1471-2407-13-592. PMID:24330498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HR, Ha SJ, Hong MH, Heo SJ, Koh YW, Choi EC, Kim EK, Pyo KH, Jung I, Seo D, et al.. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6:36956. doi: 10.1038/srep36956. PMID:27841362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A, Pourmand N, Le QT. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74(2):553-61. doi: 10.1016/j.ijrobp.2009.02.015. PMID:19427557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee YS, Park JY, Cho KJ, Kim SB, Lee SW, Choi SH, Roh JL, Nam SY, Kim SY. Composition of inflammatory cells regulating the response to concurrent chemoradiation therapy for HPV (+) tonsil cancer. Oral Oncol. 2015;51(12):1113-9. doi: 10.1016/j.oraloncology.2015.10.001. PMID:26475063 [DOI] [PubMed] [Google Scholar]
- 32.Loose D, Signore A, Bonanno E, Vermeersch H, Dierckx R, Deron P, Van de Wiele C. Prognostic value of CD25 expression on lymphocytes and tumor cells in squamous-cell carcinoma of the head and neck. Cancer Biother Radiopharm. 2008;23(1):25-33. doi: 10.1089/cbr.2007.0373. PMID:18298326 [DOI] [PubMed] [Google Scholar]
- 33.Moreira G, Fulgencio LB, DE Mendonça EF, Leles CR, Batista AC, DA Silva TA. T regulatory cell markers in oral squamous cell carcinoma: Relationship with survival and tumor aggressiveness. Oncol Lett. 2010;1(1):127-32. doi: 10.3892/ol_00000023. PMID:22966269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nasman A, Romanitan M, Nordfors C, Grun N, Johansson H, Hammarstedt L, Marklund L, Munck-Wikland E, Dalianis T, Ramqvist T. Tumor infiltrating CD8+ and Foxp3+ lymphocytes correlate to clinical outcome and human papillomavirus (HPV) status in tonsillar cancer. PLoS One. 2012;7(6):e38711. doi: 10.1371/journal.pone.0038711. PMID:22701698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen N, Bellile E, Thomas D, McHugh J, Rozek L, Virani S, Peterson L, Carey TE, Walline H, Moyer J, et al.. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38(7):1074-84. doi: 10.1002/hed.24406. PMID:26879675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nordfors C, Grun N, Tertipis N, Ahrlund-Richter A, Haeggblom L, Sivars L, Du J, Nyberg T, Marklund L, Munck-Wikland E, et al.. CD8+ and CD4+ tumour infiltrating lymphocytes in relation to human papillomavirus status and clinical outcome in tonsillar and base of tongue squamous cell carcinoma. Eur J Cancer. 2013;49(11):2522-30. doi: 10.1016/j.ejca.2013.03.019. PMID:23571147 [DOI] [PubMed] [Google Scholar]
- 37.Oguejiofor K, Hall J, Slater C, Betts G, Hall G, Slevin N, Dovedi S, Stern PL, West CM. Stromal infiltration of CD8 T cells is associated with improved clinical outcome in HPV-positive oropharyngeal squamous carcinoma. Br J Cancer. 2015;113(6):886-93. doi: 10.1038/bjc.2015.277. PMID:26313665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park K, Cho KJ, Lee M, Yoon DH, Kim SB. Importance of FOXP3 in prognosis and its relationship with p16 in tonsillar squamous cell carcinoma. Anticancer Res. 2013;33(12):5667-73. doi: 10.1186/1471-2407-9-292. PMID:24324115 [DOI] [PubMed] [Google Scholar]
- 39.Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. PMID:19698134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Punt S, Dronkers EA, Welters MJ, Goedemans R, Koljenovic S, Bloemena E, Snijders PJ, Gorter A, van der Burg SH, Baatenburg de Jong RJ, et al.. A beneficial tumor microenvironment in oropharyngeal squamous cell carcinoma is characterized by a high T cell and low IL-17(+) cell frequency. Cancer Immunol Immunother. 2016;65(4):393-403. doi: 10.1007/s00262-016-1805-x. PMID:26899388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell S, Angell T, Lechner M, Liebertz D, Correa A, Sinha U, Kokot N, Epstein A. Immune cell infiltration patterns and survival in head and neck squamous cell carcinoma. Head Neck Oncol. 2013;5(3):24. doi: 10.1186/s12885-016-2419-6. PMID:24723971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song JJ, Zhao SJ, Fang J, Ma D, Liu XQ, Chen XB, Wang Y, Cheng B, Wang Z. Foxp3 overexpression in tumor cells predicts poor survival in oral squamous cell carcinoma. BMC Cancer. 2016;16:530. PMID:27457382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tertipis N, Hammar U, Nasman A, Vlastos A, Nordfors C, Grun N, Ährlund-Richter A, Sivars L, Haeggblom L, Marklund L, et al.. A model for predicting clinical outcome in patients with human papillomavirus-positive tonsillar and base of tongue cancer. Eur J Cancer. 2015;51(12):1580-7. doi: 10.1016/j.ejca.2015.04.024. PMID:26025766 [DOI] [PubMed] [Google Scholar]
- 44.van Kempen PM, Noorlag R, Swartz JE, Bovenschen N, Braunius WW, Vermeulen JF, Van-Cann EM, Grolman W, Willems SM. Oropharyngeal squamous cell carcinomas differentially express granzyme inhibitors. Cancer Immunol Immunother. 2016;65(5):575-85. doi: 10.1007/s00262-016-1819-4. PMID:26993499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wansom D, Light E, Thomas D, Worden F, Prince M, Urba S, Chepeha D, Kumar B, Cordell K, Eisbruch A, et al.. Infiltrating lymphocytes and human papillomavirus-16–associated oropharyngeal cancer. Laryngoscope. 2012;122(1):121-7. doi: 10.1002/lary.22133. PMID:22183632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watanabe Y, Katou F, Ohtani H, Nakayama T, Yoshie O, Hashimoto K. Tumor-infiltrating lymphocytes, particularly the balance between CD8(+) T cells and CCR4(+) regulatory T cells, affect the survival of patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(5):744-52. doi: 10.1016/j.tripleo.2009.12.015. PMID:20303300 [DOI] [PubMed] [Google Scholar]
- 47.Weed DT, Walker G, De La Fuente AC, Nazarian R, Vella JL, Gomez-Fernandez CR, Serafini P. FOXP3 subcellular localization predicts recurrence in oral squamous cell carcinoma. PLoS One. 2013;8(8):e71908. doi: 10.1371/journal.pone.0071908. PMID:23977174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weller P, Bankfalvi A, Gu X, Dominas N, Lehnerdt GF, Zeidler R, Lang S, Brandau S, Dumitru CA. The role of tumour FoxP3 as prognostic marker in different subtypes of head and neck cancer. Eur J Cancer. 2014;50(7):1291-300. doi: 10.1016/j.ejca.2014.02.016. PMID:24630394 [DOI] [PubMed] [Google Scholar]
- 49.Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J; University of Michigan Head and Neck SPORE Program . Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: A preliminary study. Oral Oncol. 2015;51(1):90-5. doi: 10.1016/j.oraloncology.2014.09.006. PMID:25283344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zancope E, Costa NL, Junqueira-Kipnis AP, Valadares MC, Silva TA, Leles CR, Mendonça EF, Batista AC. Differential infiltration of CD8+ and NK cells in lip and oral cavity squamous cell carcinoma. J Oral Pathol Med. 2010;39(2):162-7. doi: 10.1111/j.1600-0714.2009.00792.x. PMID:19754647 [DOI] [PubMed] [Google Scholar]
- 51.Zhou X, Su YX, Lao XM, Liang YJ, Liao GQ. CD19(+)IL-10(+) regulatory B cells affect survival of tongue squamous cell carcinoma patients and induce resting CD4(+) T cells to CD4(+)Foxp3(+) regulatory T cells. Oral Oncol. 2016;53:27-35. doi: 10.1016/j.oraloncology.2015.11.003. PMID:26631955 [DOI] [PubMed] [Google Scholar]
- 52.Finn OJ. Cancer immunology. N Engl J Med. 2008;358(25):2704-15. doi: 10.1056/NEJMra072739. PMID:18565863 [DOI] [PubMed] [Google Scholar]
- 53.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57-117. doi: 10.1016/S0065-230X(10)07003-X. PMID:20399961 [DOI] [PubMed] [Google Scholar]
- 54.Khoury T, Nagrale V, Opyrchal M, Peng X, Wang D, Yao S. Prognostic significance of stromal versus intratumoral infiltrating lymphocytes in different subtypes of breast cancer treated with cytotoxic neoadjuvant chemotherapy. Appl Immunohistochem Mol Morphol. 2017. doi: 10.1097/PAI.0000000000000466. PMID:28187033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Meulenaere A, Vermassen T, Aspeslagh S, Zwaenepoel K, Deron P, Duprez F, Ferdinande L, Rottey S. CD70 expression and its correlation with clinicopathological variables in squamous cell carcinoma of the head and neck. Pathobiology. 2016;83(6):327-33. doi: 10.1159/000446569. PMID:27389010 [DOI] [PubMed] [Google Scholar]
- 56.Vassilakopoulou M, Avgeris M, Velcheti V, Kotoula V, Rampias T, Chatzopoulos K, Perisanidis C, Kontos CK, Giotakis AI, Scorilas A, et al.. Evaluation of PD-L1 expression and associated tumor-infiltrating lymphocytes in laryngeal squamous cell carcinoma. Clin Cancer Res. 2016;22(3):704-13. doi: 10.1158/1078-0432.CCR-15-1543. PMID:26408403 [DOI] [PubMed] [Google Scholar]
- 57.Wang WL, Chang WL, Yang HB, Chang IW, Lee CT, Chang CY, Lin JT, Sheu BS. Quantification of tumor infiltrating Foxp3+ regulatory T cells enables the identification of high-risk patients for developing synchronous cancers over upper aerodigestive tract. Oral Oncol. 2015;51(7):698-703. doi: 10.1016/j.oraloncology.2015.04.015. PMID:25958829 [DOI] [PubMed] [Google Scholar]
- 58.Ward MJ, Thirdborough SM, Mellows T, Riley C, Harris S, Suchak K, Webb A, Hampton C, Patel NN, Randall CJ, et al.. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110(2):489-500. doi: 10.1038/bjc.2013.639. PMID:24169344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baba T, Ishizu A, Iwasaki S, Suzuki A, Tomaru U, Ikeda H, Yoshiki T, Kasahara M. CD4+/CD8+ macrophages infiltrating at inflammatory sites: A population of monocytes/macrophages with a cytotoxic phenotype. Blood. 2006;107(5):2004-12. doi: 10.1182/blood-2005-06-2345. PMID:16269616 [DOI] [PubMed] [Google Scholar]
- 60.Shortman K. Burnet oration: Dendritic cells: Multiple subtypes, multiple origins, multiple functions. Immunol Cell Biol. 2000;78(2):161-5. doi: 10.1046/j.1440-1711.2000.00901.x. PMID:10762417 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


