Abstract
Background and Aims
Antiviral therapy for patients with hepatitis B (HBV) infection is generally deferred for “immune inactive” patients, although longitudinal changes in viral load and liver fibrosis remain understudied in this population. Likewise, in treated patients, the temporal relationship between changes in viral load and liver fibrosis is not well characterized. Using data from the chronic hepatitis cohort study, the study investigated viral load and the Fibrosis-4 index (FIB4, a serum-based marker of liver fibrosis) trajectories in both untreated and treated HBV patients.
Materials and Methods
We applied a bivariate, piecewise, linear spline, mixed-effects modeling approach to data from 766 HBV patients (342 untreated, 424 treated). Treatment selection bias was adjusted using propensity scores. Multiple sensitivity analyses were used to confirm results in untreated patients.
Results
Among all untreated patients, FIB4 began to increase by 0.9% per month (11% per year; P < 0.05) at 28 months post-index date, suggesting fibrosis progression. Significant FIB4 progression was also observed in a subgroup analysis of “immune inactive” untreated patients. In treated patients, viral load declined 31.8% per month (P < 0.05) for the first 5 months after treatment initiation, and 1.4–1.7% per month (P < 0.05) thereafter. At 5 months after treatment initiation, FIB4 began to decline 0.5% per month (P < 0.05), stabilizing at 28 months.
Conclusion
Among untreated HBV patients, FIB4 gradually increases over time, suggesting fibrosis progression, even in those patients designated as immune inactive. In treated patients, antiviral therapy results in a rapid decline in viral load followed by a delayed decline in markers of liver fibrosis.
Keywords: chronic hepatitis cohort study (CHeCS), FIB4, growth curve modeling, HBV, join-point modeling
Introduction
Clinical decision-making regarding initiation of antiviral treatment for chronic hepatitis B virus (HBV) requires evaluation of the dynamic relationship between viral replication and the patient’s immune response. Antiviral therapy is not currently recommended for “immune inactive” patients without cirrhosis.1 These recommendations are largely based on data from clinical trials; the complexity of defining “immune inactive” and the level of surveillance required means that there are little long-term data regarding untreated HBV patients under routine clinical care.
Findings from several clinical trials have suggested that longterm viral suppression with antiviral therapy improves liver histology and may result in regression of fibrosis, especially in patients with more advanced liver disease2,3; however, there are no published reports describing the long-term changes in liver fibrosis among patients under routine care, particularly untreated patients. The Chronic Hepatitis Cohort Study (CHeCS) has extensively annotated longitudinal medical record data for a “real world” sample of over 3000 HBV-infected patients, including roughly 2100 untreated patients.4 Using a bivariate modeling approach, we used CHeCS data to evaluate the longitudinal evolution of viral load in both untreated and treated patients, as well as two surrogate markers of liver fibrosis. The Fibrosis-4 index (FIB4)5 and the aspartate aminotransferase (AST) to platelet ratio index (APRI) are calculated from routine laboratory assessments; both have been shown to accurately distinguish fibrosis and cirrhosis in HBV-infected patients,6,7 including patients in this cohort.8 We modeled these data over 5 years of follow-up, taking into consideration the relationship between viral load and FIB4.
Methods
Chronic Hepatitis Cohort Study
CHeCS is a retrospective/prospective observational multicenter study that includes patients from four large health systems. CHeCS follows all guidelines of the US Department of Health and Human Services regarding the protection of human subjects; protocols are reviewed annually by the institutional review board at each site —Geisinger Health System (GHS), Danville, PA; Henry Ford Health System (HFHS), Detroit, MI; Kaiser-Permanente Hawai’i (KPHI), Waipahu, HI; and Kaiser-Permanente Northwest (KPNW), Portland, OR. Written informed consent was waived because of the de-identified nature of the data. CHeCS study methods have been previously described.4
Electronic health record (EHR) data were used to identify adult patients who received services at any of the four study sites between January 2006 and December 2013; EHR data were captured through December 31, 2013. Chart abstraction was used to confirm HBV diagnoses, liver biopsy data, and antiviral treatment data (including documentation of any treatment at outside facilities). Patients were considered to have received antiviral HBV therapy if chart review indicated the patient received treatment with any of the following after HBV diagnosis: interferon α-2b; pegylated interferon α-2a or α-2b; lamivudine; entecavir; tenofovir; telbivudine; or adefovir.
“Index date” was defined as the earliest date of HBV viral load measurement after HBV-associated diagnosis (for untreated patients) or the date of first treatment initiation (for treated patients). Index dates ranged from June 2000 through May 2013. Patients were excluded if they were co-infected with chronic hepatitis C or received a liver transplant prior to their index date.
Outcomes of interest
Available viral load and liver chemistry data were collected for each patient from their index date onward. Indices were calculated using laboratory tests collected within 7 days of one another and patients’ age at the time of laboratory assessment. Hepatitis B DNA tests were collected locally at each site and analyzed with serum assays using branched DNA signal amplification assay or real-time polymerase chain reaction, with or without reflex to qualitative polymerase chain reaction. For analytic purposes, “undetectable” HBV-RNA results and HBV-DNA-quantified viral levels of less than 2000 IU/mL were analyzed as equal to 2000 IU/mL, because of varying lower levels of detection/quantification among assays.
FIB4 was calculated using the following formula:
We also performed a sensitivity analysis using the APRI in place of FIB4, calculated as follows:
(ALT, alanine aminotransferase; AST, aspartate aminotransferase; L, liter; U, Units; ULN, upper limit of normal (as defined by the testing laboratory).
Our outcomes of interest were FIB4 and viral load, summarized using a median smoother in monthly intervals, up to 5 years from the index date; APRI was used as an outcome in sensitivity analyses. Because of the observational nature of this study, liver biopsy data were available for fewer than 10% of patients; however, CHeCS has previously shown that FIB4 and APRI can be used to predict advanced fibrosis or cirrhosis in HBV-infected patients with available biopsy assessments (area under the receiver operator characteristic curve [AUROC] = 0.71 for advanced fibrosis, AUROC = 0.88 for cirrhosis).8 Continuous variables were used to increase statistical power in our analysis; validated FIB4/APRI cut-offs for advanced fibrosis (FIB4 = 1.58) were used for data illustration and interpretation of results. Because of a lack of normality, data were log10-transformed for analysis.
Patients with at least one FIB4 interval and one viral load interval post-index date were included in the analysis. For sensitivity analyses, we used APRI and viral load. Given that HBV treatment initiation is often informed by “infection status” (immune tolerant, immune active, immune inactive), we also performed a sub-group analysis that excluded 100 patients who were never immunologically active (no ALT measurements ≥ 2 times the upper limit of normal [ULN, 30 IU/mL for men; 19 IU/mL for women] and no HBV DNA level > 2000 IU/mL) from index date to last observation.
Covariates at index date
Demographic information included age, sex, race/ethnicity, estimated median annual household income, and insurance status at index date. Clinical data captured prior to and at index included comorbid conditions, HIV co-infection, and laboratory testing. All variables were used in the analysis to adjust for possible confounding due to treatment selection bias.
Statistical analysis
To account for confounding due to treatment selection bias, we used a propensity score modeling approach to estimate the inverse probability of treatment weight (IPTW).9 To build the propensity score model, multiple logistic regression analyses were performed using treatment as the outcome variable, and a large set of demographic variables and clinical risk factors collected at the index date as covariates. We used the strategy proposed by Ali et al.10 for selection of possible confounders and Robins’s approaches11–13 for stabilization of IPTW when propensity scores were very small. The balance of covariates at index date between untreated and treated patients was compared before and after weighting; the stabilized IPTW was then adjusted in the subsequent analysis of the treatment impact on progression of FIB4 and viral load.
Evolution of FIB4 and viral load were first estimated separately, using a linear mixed-effects model. Because of a bi-phasic evolution of the two markers in the raw data, we used a piecewise linear spline model, with one slope representing the shortterm response and a second slope representing the long-term response. The time of change of slope (knot position) was determined by the approach proposed by Fitzmaurice et al.14 Briefly, we started with high knot density and used a variable selection technique to select the best knot positions, guided by the Aiken information criteria. The linear mixed-effect model included the baseline FIB4 or viral load measures, fixed effects for the slopes, and random components for the intercept and slopes to predict the trajectory of FIB4 or viral load for each individual patient, given their treatment status.
Next, hepatitis B viral load and FIB4 trajectories were modeled jointly through the bivariate linear mixed-effects model; because FIB4 and viral load are intrinsically correlated, this type of model is not only necessary for the estimation of the correlation between the two markers but has also been shown to provide better data fit than separate modeling.15–17 This joint modeling strategy has been applied to study the longitudinal dynamics of CD4+ T-lymphocyte counts and HIV-RNA plasma concentration.15,17,18 Sensitivity analyses were conducted by the following: 1) using APRI and viral load as the outcomes of interest to determine whether the inclusion of age in the FIB4 formula effected findings; 2) excluding patients who were “persistently immunologically inactive” (no ALT result > 2*ULN or viral load > 2000 IU/mL from index date to last observation), for whom current guidelines do not recommend treatment1; and 3) analyzing only those patients who were “persistently immunologically inactive.”
Results
A total of 766 patients (342 untreated, 424 treated) had sufficient viral load and serum liver chemistry data available for analysis. Index dates ranged from years 2000 through 2013. Patient characteristics at index date are listed in Table 1. Men (59%) and Asian Americans (61%) were overrepresented in the sample. At index date, log10viral load and log10FIB4 were lower in untreated than treated patients (P < 0.001).
Table 1.
Differences in exposure and treatment at index date, before and after weighting, using propensity scores
| Variable | Category | Before weighting
|
After weighting
|
||||
|---|---|---|---|---|---|---|---|
| Untreated (n = 342) | Treated (n = 424) | P | Untreated (n = 342) | Treated (n = 424) | P† | ||
| Age | < 40 | 114 (33%) | 105 (25%) | 0.030 | (30%) | (28%) | 0.958 |
| 40 < 50 | 95 (28%) | 114 (27%) | (28%) | (28%) | |||
| 50 < 60 | 73 (21%) | 118 (28%) | (23%) | (25%) | |||
| ≥ 60 | 60 (18%) | 87 (21 %) | (18%) | (19%) | |||
| Sex | Female | 172 (50%) | 139 (33%) | < 0.001 | (36%) | (36%) | 0.960 |
| Male | 170 (50%) | 285 (67%) | (64%) | (64%) | |||
| Race | Asian/other | 236 (69%) | 230 (54%) | < 0.001 | (61%) | (58%) | 0.622 |
| Black | 38 (11%) | 72 (17%) | (19%) | (18%) | |||
| White | 59 (17%) | 110 (26%) | (18%) | (22%) | |||
| Unknown | 9 (3%) | 12 (3%) | (2%) | (3%) | |||
| Index year | 2000 < 2005 | 47 (14%) | 42 (10%) | 0.187 | (17%) | (10%) | .0.845 |
| 2005 < 2010 | 220 (64%) | 295 (70%) | (63%) | (65%) | |||
| ≥ 2010 | 75 (22%) | 87 (21 %) | (20%) | (25%) | |||
| ALT | < LLN and/or Normal | 282 (82%) | 215 (51%) | < 0.001 | (64%) | (64%) | 0.221 |
| ULN ≤ 2*ULN | 42 (12%) | 108 (25%) | (15%) | (20%) | |||
| > 2*ULN | 18 (5%) | 101 (24%) | (20%) | (16%) | |||
| Weighted Charlson-Deyo score (includes liver comorbidity) | 0 | 229 (67%) | 193 (46%) | < 0.001 | (54%) | (57%) | 0.202 |
| 1 | 60 (18%) | 75 (18%) | (15%) | (15%) | |||
| 2 | 13 (4%) | 44 (10%) | (5%) | (8%) | |||
| 3 | 40 (12%) | 112 (26%) | (26%) | (20%) | |||
| Decompensated cirrhosis | No | 338 (99%) | 392 (92%) | < 0.001 | (97%) | (95%) | 0.137 |
| Yes | 4 (1%) | 32 (8%) | (3%) | (5%) | |||
| HIV co-infection | No | 333 (97%) | 380 (90%) | < 0.001 | (89%) | (92%) | 0.707 |
| Yes | 9 (3%) | 44 (10%) | (11%) | (8%) | |||
| LOG10HBVQT (viralload) | 3.6 ± 1.3 | 5.5 ± 1.9 | < 0.001 | .4.9 ± 2.5 | 4.6 ± 2.3. | 0.157 | |
| FIB4‡ | 1.8 ± 4.9 | 3.0 ± 4.1 | < 0.001 | .2.1 ± 2.3 | 2.2 ± 2.4. | 0.915 | |
| APRI‡ | 0.9 ± 5.0 | 1.56 ± 2.5 | < 0.001 | 1.7 ± 5.5. | 1.1 ± 2.4. | 0.772 | |
Type III analysis using Wald Chi-square test from multiple variable logistic regression.
Log10 scale was used for the propensity score calculation. Original scale is presented for ease of interpretation.
ALT, alanine aminotransferase; APRI, AST to platelet ratio index; FIB4, Fibrosis-4; HBV, hepatitis B virus; HIV, human immunodeficiency virus; MPC, monthly percentage change; ULN, upper limit of normal.
Ninety-seven percent (97%) of those treated received nucleos(t) ide analog therapy (in isolation, or before/after interferon-based therapy); the remaining 3% received only interferon or pegylated interferon-based therapy. Median treatment duration during follow-up was 52 months (interquartile range: 22–81 months).
Following the guidance provided by Ali et al.,10 the following covariates were used in the estimation of propensity scores: sex; race; Charlson-Deyo co-morbidity score; index year; ALT at index date; FIB4 at index date; and viral load at index date, all as predictors. As shown in Table 1, after propensity score justification, patient and clinical characteristics at index date were balanced between untreated and treated groups.
Evolution of viral load and fibrosis-4
Table 2 presents estimated monthly percent change (MPC) by treatment status; Figure 1 displays the population-expected average trajectories for FIB4 and log10viral load for a given baseline value. For both untreated and treated groups, a three-knot model fitted well to both FIB4 and viral load data. This model resulted in three phases of evolution: the first slope represents the first 5 months post-index; the second slope represents 5 to 28 months; and the third slope represents from 28 months onwards. For both untreated and treated groups, the estimated time of slope change (knot) was earlier for viral load (5 months) than FIB4 (28 months). Estimates of the evolution of FIB4 and viral load are presented in Table 2 for both univariate and bivariate models. In the bivariate linear mixed model, the third slope (from 28 months onward) is close to zero for FIB4 in the treated group, but significantly different from zero for FIB4 in the untreated group. Simultaneous bivariate modeling of FIB4 and viral load demonstrated narrower confidence intervals and better goodness-of-fit (in terms of Aiken information criteria) than fitting outcomes separately.
Table 2.
Estimated MPC in FIB4 and HBV-DNA viral load over time, using linear mixed model separately (univariate) and jointly (bivariate).
| Cohort | Treated
|
Untreated
|
Treated vs Untreated
|
|||
|---|---|---|---|---|---|---|
| MPC (95% CI) | P | MPC (95% CI) | P | P | ||
| Univariate linear mixed model | ||||||
| Full (n = 766) | Viral load (IU/ML) | |||||
| < 5 months | −31.8% (−44.2%, −16.6%) | < 0.001 | 6.2% (−27.3%, 55.2%) | 0.756 | 0.043 | |
| 5–28 months | −1.4% (−3.0%, 0.3%) | 0.110 | −2.5% (−4.7%, −0.1%) | 0.039 | 0.448 | |
| > 28 months | −1.5% (−2.6%, −0.3%) | 0.013 | −1.3% (−2.8%, 0.3%) | 0.121 | 0.834 | |
| FIB4 | ||||||
| < 5 months | 0.2% (−2.4%, 2.8%) | 0.887 | −0.8% (−4.4%, 3.1%) | 0.696 | 0.685 | |
| 5–28 months | −0.5% (−0.9%, −0.2%) | < 0.001 | 0.3% (−0.2%, 0.7%) | 0.216 | 0.003 | |
| > 28 months | 0.1% (−0.2%, 0.3%) | 0.619 | 0.7% (0.4%, 1.0%) | < 0.001 | 0.003 | |
| Sub-cohort* (n = 666) | Viral load (IU/ML) | |||||
| < 5 months | −34.7%(−47.3%,−19.1 %) | < 0.001 | 7.6%(−29.8%, 64.9%) | 0.737 | 0.041 | |
| 5–28 months | −1.5%(−3.2%, 0.3%) | 0.098 | −2.3%(−5.1%, 0.4%) | 0.098 | 0.603 | |
| > 28 months | −1.4%(−2.6%,−0.3%) | 0.017 | −1.4%(−3.1%, 0.4%) | 0.139 | 0.949 | |
| FIB4 | ||||||
| < 5 months | −0.2%(−2.9%, 2.5%) | 0.862 | −1.6%(−5.8%, 2.7%) | 0.455 | 0.628 | |
| 5–28 months | −0.5%(−0.9%,−0.2%) | 0.002 | 0.4%(−0.2%, 0.9%) | 0.162 | 0.005 | |
| > 28 months | 0.1 %(−0.1%, 0.4%) | 0.360 | 0.8% (0.4%,1.2%) | < 0.001 | 0.005 | |
| Bivariate linear mixed model | ||||||
| Full (n = 766) | Viral load (IU/ML) | |||||
| < 5 months | −31.8% (−43.8%, −17.4%) | < 0.001 | 5.7% (−26.2%, 51.3%) | 0.762 | 0.035 | |
| 5–28 months | −1.7% (−2.8%, −0.5%) | 0.004 | −3.1% (−4.9%, −1.3%) | < 0.001 | 0.184 | |
| > 28 months | −1.4% (−2.5%, −0.2%) | 0.019 | −1.1% (−2.7%, 0.5%) | 0.162 | 0.809 | |
| FIB4 | ||||||
| < 5 months | 0.4% (−2.2%, 3.0%) | 0.784 | −0.2% (−3.8%, 3.6%) | 0.919 | 0.811 | |
| 5–28 months | −0.5% (−0.8%, −0.3%) | < 0.001 | 0.1% (−0.3%, 0.4%) | 0.668 | 0.003 | |
| > 28 months | 0.1% (−0.2%, 0.3%) | 00.616 | 0.9% (0.7%, 1.2%) | < 0.001 | < 0.001 | |
| Sub-cohort* (n = 666) | Viral load (IU/ML) | |||||
| < 5 months | 34.8% (−46.9%, −20.0%) | < 0.001 | 6.9% (−28.4%, 59.8%) | 0.743 | 0.031 | |
| 5–28 months | −1.8% (−2.9%, −0.6%) | 0.004 | −3.2% (−5.2%, −1.1%) | 0.003 | 0.244 | |
| > 28 months | −1.4% (−2.5%, −0.2%) | 0.024 | −1.2% (−3.0%, 0.6%) | 0.191 | 0.877 | |
| FIB4 | ||||||
| < 5 months | 0.0% (−2.6%, 2.8%) | 0.977 | −1.1% (−5.3%, 3.2%) | 0.604 | 0.650 | |
| 5–28 months | −0.5% (−0.8%, −0.3%) | < 0.001 | 0.1% (−0.2%, 0.5%) | 0.465 | 0.003 | |
| > 28 months | 0.1% (−0.1%, 0.4%) | 0.345 | 1.0% (0.7%, 1.4%) | < 0.001 | < 0.001 | |
Sub-cohort excludes “never immunologically active” patients (no ALT > 2*ULN or viral load > 2000 lU/mL from index date to last observation). The test for difference of MPC between treated and untreated group is reported in the last column
ALT, alanine aminotransferase; FIB4, Fibrosis-4 Index; HBV-DNA, hepatitis B virus DNA; MPC, monthly percentage change.
Figure 1.
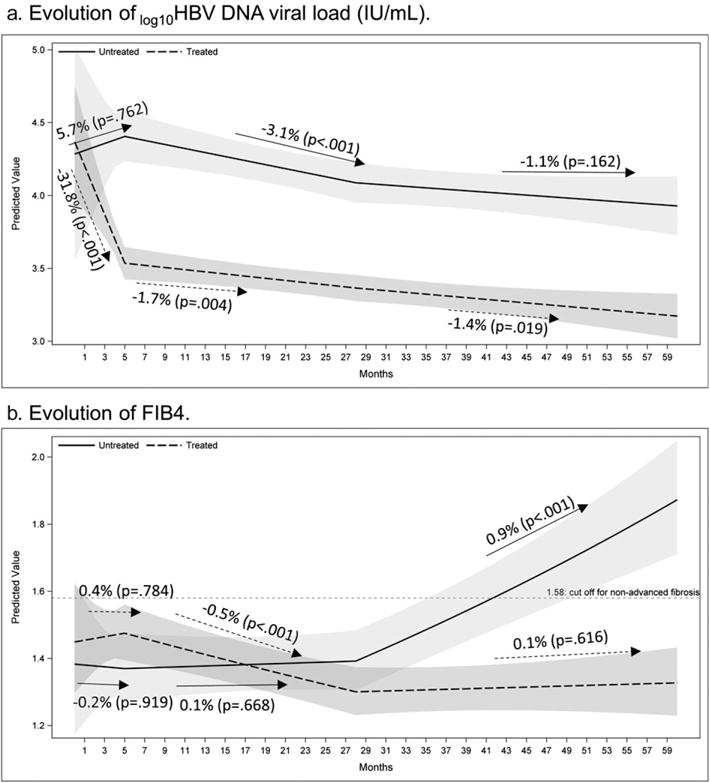
Predicted mean of trajectories of log10 viral load and fibrosis-4 (FIB4) over time by treatment groups. Shaded area: 95% confidence band.
Viral load
In untreated patients (Table 2 and Fig. 1a), the viral load slope was not significantly different from zero (P = 0.762) for the first 5 months post-index. It then decreased slightly from 5 to 28 months (−3.1% per month; P < 0.001). From 28 months onward, however, the slope was again not significantly different from zero (P = 0.162). However, in treated patients, the bivariate model showed that viral load decreased sharply for the first 5 months after index (−31.8% per month; P < 0.001) then decreased more slowly between 5 and 28 months (−1.7% per month; P = 0.004) and from 28 months onward (−1.4% per month; P = 0.019). The MPC in viral load is significantly different between treated and untreated patients for the first 5 months post-index (P = 0.035). However, after this point, there is no difference in MPC between the two groups.
Figure 1a displays the difference in viral loads between treated and untreated patients. In untreated patients, viral load became significantly higher than that of treated patients at month 4 post-index. In treated patients, viral load continued to drop and remained significantly lower in than in untreated patients through year 5.
Fibrosis-4 Index
In untreated patients, FIB4 did not change significantly from 0 to 28 months, but increased significantly thereafter (0.9% per month; P < .001, Table 2 and Fig. 1b). FIB4 in untreated patients became significantly different from that of treated patients at roughly month 33 post-index (years 3–5). The MPC of FIB4 in untreated patients is significantly higher than that of treated patients (P < 0.001). A cutoff of 1.58, previously shown to distinguish between non-advanced and advanced fibrosis in this cohort,16 is presented as a reference; untreated patients’ FIB4 passed this cutoff at roughly month 42. Given the estimated MPC, FIB4 will double within 6.5 years in patients who remain untreated—regardless of their initial FIB4 level.
In a sub-group analysis that excluded 100 patients who were persistently “immune inactive” (no ALT result > 2*ULN or viral load > 2000 IU/mL from index date to last observation), the patterns of viral load and FIB4 were similar to those of the main analysis (Table 1 and Fig. 2). An additional sub-group analysis was then restricted to only “immune inactive” untreated patients; among these patients, FIB4 varied considerably but increased significantly over 5 years’ of follow-up (annual increase = 4.7%, P = 0.002; Fig. S2a). A further sensitivity analysis using APRI (which does not include patient age) demonstrated consistent findings despite the small sample size (P = 0.07; Fig. S2b).
Figure 2.
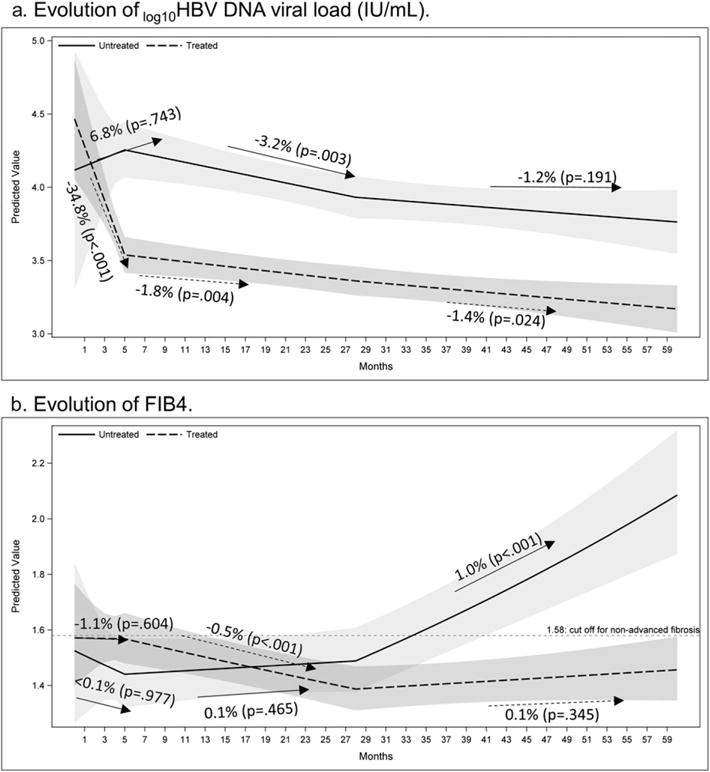
Predicted mean of trajectories of log10 viral load and fibrosis-4 (FIB4) over time by treatment groups after excluding “never immunologically active” patients (no ALT > 2*upper limit of normal or viral load > 2000 IU/mL from index date to last observation). Shaded area: 95% confidence band.
In treated patients, there was no significant change in FIB4 for the first 5 months post-index (P = 0.784). This was followed by a significant decline (−0.5% per month; P < 0.001) between 5 and 28 months, which leveled off after 28 months (P = 0.616).
Aspartate Aminotransferase-to-Platelet ratio index (sensitivity analysis)
Table S1 presents estimated intercepts and slopes for APRI and viral load by treatment status; Figure S1 displays the population-expected average trajectories for APRI and viral load for a given baseline value. The estimates of intercept and slopes for APRI were similar to those estimates obtained for FIB4. In untreated patients, viral load remains stable at a significantly higher level than in treated patients, while APRI gradually increases over time. These results are consistent with our main analysis.
Discussion
The present study is the first analysis to address longitudinal changes in fibrosis using a “real world” sample of HBV-infected patients under routine clinical care, including a large number of untreated patients. Among all such untreated patients, we found that although viral loads remained roughly stable, FIB4 increased significantly over time—11% per year. Moreover, the findings from two subgroup analyses that separated out patients who were persistently “immune inactive” suggest that fibrosis steadily increases in such untreated patients even in the absence of immunological activity. Sensitivity analyses substituting APRI for the outcome of interest (Fig. S1) generated consistent results. Our previous work has demonstrated that increasing FIB4 is associated with greater risk of HCC,19 and our current findings underscore the importance of monitoring untreated patients for progression of liver fibrosis. Current international treatment guidelines emphasize both viral load and ALT levels in the assessment of need for therapy.20–22 However, Tong et al.23 noted that 40–80% of HBV patients who developed HCC or died of liver-related conditions did not meet criteria for antiviral treatment; these authors suggested that additional parameters besides viral level and ALT should be considered.
In treated patients, viral levels declined rapidly within the first 5 months post-treatment initiation, then declined more slowly thereafter. This biphasic pattern, based on “real world” data, is consistent with findings from a clinical trial of long-term tenofovir therapy in HBV patients with baseline high viral levels.24 FIB4 also demonstrated a biphasic decay pattern; notably, this observed reduction in FIB4 lagged behind the decline in viral load and did not stabilize until more than 2 years after treatment initiation. To our knowledge, this is the first model to illustrate the temporal relationship between fibrosis regression and viral suppression.
Our findings support the role of using serum markers of liver pathology in the management of chronic HBV infection.25 Consistent results from our sensitivity analyses using APRI trajectories further validated these findings (Appendix Table S1 and Fig. S1); in fact, we observed a monthly increase of 1.2% using APRI (higher than the 0.9% increase in FIB4) in untreated patients, at 2 years’ after initial assessment. This finding confirms that the observed increase in FIB4 is not merely the result of the inclusion of age in the formula for calculating the FIB4 index.
Our study has several limitations. Despite considerable progress in the development of noninvasive methods to assess liver fibrosis, none is yet widely accepted as a substitute for liver biopsy. However, we have previously shown that FIB4 and APRI can reliably predict advanced fibrosis and cirrhosis in HBV patients (AUROC = 0.71 for advanced fibrosis, AUROC = 0.88 for cirrhosis).8 To confirm the reliability of this method in this sample, we performed a subgroup analysis using 45 patients who underwent a liver biopsy after initiation of treatment; FIB4 reliably predicted biopsy results in these patients (AUROC = 0.84 for advanced fibrosis; AUROC = 0.86 for cirrhosis; Appendix Table S2 and Fig. S3). Although these results may appear to conflict with a study reporting low correlation between serum markers of liver fibrosis and results of liver biopsy,26 that study categorized FIB4 and APRI based on binary cutoffs at a single time point during treatment, whereas our growth curve analysis uses a more sophisticated modeling approach to estimate longitudinal trends in quantitative values.
Because we used propensity scores to balance the two groups, we cannot ascertain whether patients with low levels of viremia (below the recommended cutoff for treatment) demonstrated significantly different trajectories of FIB4 over time compared with those patients for whom treatment was indicated but not received. However, even with the inclusion of these low-viremic patients in our analysis, we still observed a significant increase in FIB4 across time. In addition, our model adjusted for patients’ viral load level and fibrosis score at index date; this ensures that patients’ initial immune status did not impact the rates of change in viral load and FIB4 over time.
Because of our “real world” longitudinal study design, our data span multiple improvements in laboratory assays, with the ability to detect increasingly lower levels of virus. This truncation may have introduced a slight overestimation of viral load levels under the detection limit; as a result, our viral load trajectories may be conservative. Nevertheless, we observed a robust and significant drop in viral load in treated patients, while significant increasing markers of fibrosis over time if patients were untreated even those with viral loads persistently below 2000 IU/mL. Our analytic approach—joint modeling of two markers (FIB4 and viral load)— provides better data fit than separate modeling because it takes into account intrinsic correlation between the markers.15–17 The robust estimations we observed with this approach suggest its utility as a novel application to the field.
Our analysis illustrates the temporal relationship between viral load and changes in biomarkers for fibrosis in a “real world” cohort that includes a significant proportion of untreated patients. In our treated patient sample, we observed that antiviral treatment results in a rapid decline in viral DNA load, findings that are consistent with results from clinical trials. Our data illustrate the timing of the decline in FIB4 that follows, suggesting a delayed but significant regression of liver fibrosis. In untreated patients, it has long been recognized that the fluctuating nature of chronic HBV infection necessitates careful surveillance27; however, a recent study found that such surveillance is often inadequate.28,29 This may be cause for concern, as we observe that FIB4 increases in our untreated patient sample, suggesting fibrosis progression— even among the subset of patients who were not candidates for therapy based on current guidelines. Because of the observational nature of our study, prospective studies are needed to further characterize which patients are at highest risk for fibrosis progression.
Supplementary Material
Acknowledgments
The CHeCS Investigators include the following investigators and sites: Scott D. Holmberg, Eyasu H. Teshale, Philip R. Spradling, Anne C. Moorman, Jim Xing, and Yuna Zhong, Division of Viral Hepatitis, National Centers for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP), Centers for Disease Control and Prevention (CDC), Atlanta, Georgia; Stuart C. Gordon, David R. Nerenz, Mei Lu, Lois Lamerato, Jia Li, Loralee B. Rupp, Nonna Akkerman, Nancy Oja-Tebbe, Talan Zhang, Sheri Trudeau, and Yueren Zhou, Henry Ford Health System, Detroit, Michigan; Joseph A. Boscarino, Zahra S. Daar, and Robert E. Smith, Center for Health Research, Geisinger Health System, Danville, Pennsylvania; Yihe G. Daida, Connie M. Trinacty, and Carmen P. Wong, The Center for Health Research, Kaiser Permanente-Hawaii, Waipuha, Hawai i; Mark A. Schmidt, Judy L. Donald, and Erin M. Keast, The Center for Health Research, Kaiser Permanente-Northwest, Portland, OR.
Financial disclosure: Financial support: Henry Ford Health System receives funding for CHeCS from the Centers for Disease Control and Prevention and from Gilead Sciences. CHeCS was previously funded through May 2016 by the CDC Foundation, which received grants from AbbVie; Genentech, A Member of the Roche Group; Gilead Sciences; Janssen Pharmaceuticals, Inc. and Vertex Pharmaceuticals; past partial funders include Bristol-Myers Squibb. Granting corporations do not have access to CHeCS data and do not contribute to data analysis or writing of manuscripts.
Footnotes
Conflict of interest disclosures: Stuart C. Gordon receives grant/research support from AbbVie Pharmaceuticals, Bristol-Myers Squibb, Conatus, CymaBay, Exalenz BioScience, Gilead Pharmaceuticals, Intercept Pharmaceuticals, and Merck. He is also a consultant/advisor for Abbvie, Bristol-Myers Squibb, CVS Caremark, Gilead, Intercept, and Merck, and serves as a speaker/teacher in programs sponsored by Gilead Pharmaceuticals and Intercept Pharmaceuticals. The other authors have no potential conflicts of interest.
Author contributions: Study concept and design: SCG, ML; Acquisition of data: SCG, LBR, EHT, JAB, MAS, CMT, ML; Analysis and interpretation of data: JL, SCG, LBR, TZ, ST, SDH, ACM, PRS, EHT, JAB, MAS, CMT, ML; Drafting of the manuscript: JL, SCG, LBR, ST, ML; Critical revision of the manuscript for important intellectual content: JL, SCG, LBR, TZ, ST, SDH, ACM, PRS, EHT, JAB, MAS, CMT, ML; Statistical analysis: JL, TZ, ML; Obtained funding: SCG, LBR, ML; Administrative, technical, or material support: JL, SCG, LBR, TZ, ST, SDH, ACM, PRS, EHT, JAB, MAS, CMT, ML; Study supervision: SCG, LBR, EHT, JAB, MAS, CMT, ML.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
Disclaimer
The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the US Department of Health and Human Services, the Centers for Disease Control and Prevention, or the authors’ affiliated institutions.
References
- 1.Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvaruso V, Craxi A. Regression of fibrosis after HBV antiviral therapy. Is cirrhosis reversible? Liver Int. 2014;34:85–90. doi: 10.1111/liv.12395. [DOI] [PubMed] [Google Scholar]
- 3.Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–75. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 4.Moorman AC, Gordon SC, Rupp LB, et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the chronic hepatitis cohort study. Clin Infect Dis. 2013;56:40–50. doi: 10.1093/cid/cis815. [DOI] [PubMed] [Google Scholar]
- 5.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 6.Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30:546–53. doi: 10.1111/j.1478-3231.2009.02192.x. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Chen Y, Zhao Y. The diagnostic value of the FIB-4 index for staging hepatitis B-related fibrosis: a meta-analysis. PLoS One. 2014;9:e105728. doi: 10.1371/journal.pone.0105728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Gordon SC, Rupp LB, et al. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepat. 2014;21:930–7. doi: 10.1111/jvh.12224. [DOI] [PubMed] [Google Scholar]
- 9.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali MS, Groenwold RH, Belitser SV, et al. Reporting of covariate selection and balance assessment in propensity score analysis is suboptimal: a systematic review. J Clin Epidemiol. 2015;68:112–21. doi: 10.1016/j.jclinepi.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Robins J. Marginal structural models versus structural nested models as tools for causal inference [Google Scholar]
- 12.Robins JM. Marginal structural models proceedings of the American Statistical Association. 1998:10. [Google Scholar]
- 13.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidimiology. 2000;11:550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Fitzmaurice G, Davidian M, Verbeke G, Molenberghs G. Longitudinal data analysis. 2008 [Google Scholar]
- 15.Boscardin WJ, Taylor JM, Law N. Longitudinal models for AIDS marker data. Stat Methods Med Res. 1998;7:13–27. doi: 10.1177/096228029800700103. [DOI] [PubMed] [Google Scholar]
- 16.Thiebaut R, Jacqmin-Gadda H, Chene G, Leport C, Commenges D. Bivariate linear mixed models using SAS proc MIXED. Comput Methods Programs Biomed. 2002;69:249–56. doi: 10.1016/s0169-2607(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 17.Thiebaut R, Jacqmin-Gadda H, Babiker A, Commenges D. Joint modelling of bivariate longitudinal data with informative dropout and left-censoring, with application to the evolution of CD4+ cell count and HIV RNA viral load in response to treatment of HIV infection. Stat Med. 2005;24:65–82. doi: 10.1002/sim.1923. [DOI] [PubMed] [Google Scholar]
- 18.Wu L, Liu W, Hu XJ. Joint inference on HIV viral dynamics and immune suppression in presence of measurement errors. Biometrics. 2010;66:327–35. doi: 10.1111/j.1541-0420.2009.01308.x. [DOI] [PubMed] [Google Scholar]
- 19.Gordon SC, Lamerato LE, Rupp LB, et al. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol. 2014;12:885–93. doi: 10.1016/j.cgh.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83. doi: 10.1002/hep.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.European Association For The Study Of The L. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tong MJ, Hsien C, Hsu L, Sun HE, Blatt LM. Treatment recommendations for chronic hepatitis B: an evaluation of current guidelines based on a natural history study in the United States. Hepatology. 2008;48:1070–8. doi: 10.1002/hep.22476. [DOI] [PubMed] [Google Scholar]
- 24.Gordon SC, Krastev Z, Horban A, et al. Efficacy of tenofovir disoproxil fumarate at 240 weeks in patients with chronic hepatitis B with high baseline viral load. Hepatology. 2013;58:505–13. doi: 10.1002/hep.26277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andersson KL, Chung RT. Monitoring during and after antiviral therapy for hepatitis B. Hepatology. 2009;49:S166–S173. doi: 10.1002/hep.22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ray Kim W, Berg T, Asselah T, et al. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64:773–80. doi: 10.1016/j.jhep.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Degertekin B, Lok AS. Do guidelines preclude hepatitis B patients from receiving treatment? Hepatology. 2009;49:700–1. doi: 10.1002/hep.22722. author reply 1–2. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109:867–75. doi: 10.1038/ajg.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spradling PR, Xing J, Rupp LB, et al. Infrequent clinical assessment of chronic hepatitis B patients in United States general healthcare settings. Clin Infect Dis. 2016;63:1205–8. doi: 10.1093/cid/ciw516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


