Abstract
The first highly enantioselective, diastereoselective, and regioselective [2,3]-rearrangement of iodonium ylides has been developed as a general solution to catalytic onium ylide rearrangements. In the presence of a chiral copper catalyst, substituted allylic iodides couple with α-diazoesters to generate metal-coordinated iodonium ylides, which undergo [2,3]-rearrangements with high selectivities (up to >95:5 rr, up to >95:5 dr, and up to 97% ee). The enantioenriched iodoester products can be converted stereospecifically to a variety of onium ylide rearrangement products, as well as compounds that are not accessible via classical onium ylide rearrangements.
Keywords: iodonium, ylide, [2, 3]-rearrangement, copper, iodoester
Graphical abstract
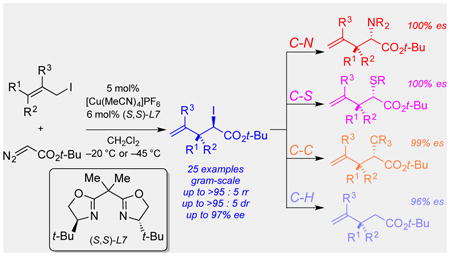
A highly enantio-, diastereo-, and regioselective [2,3]-rearrangement of iodonium ylides is reported as a general solution to catalytic onium ylide rearrangements. In the presence of a chiral copper catalyst, substituted allylic iodides couple with α-diazoesters to generate metal-coordinated iodonium ylides, which undergo [2,3]-rearrangements with high selectivities. The enantioenriched iodoester products are converted stereospecifically to a variety of useful chiral building blocks.
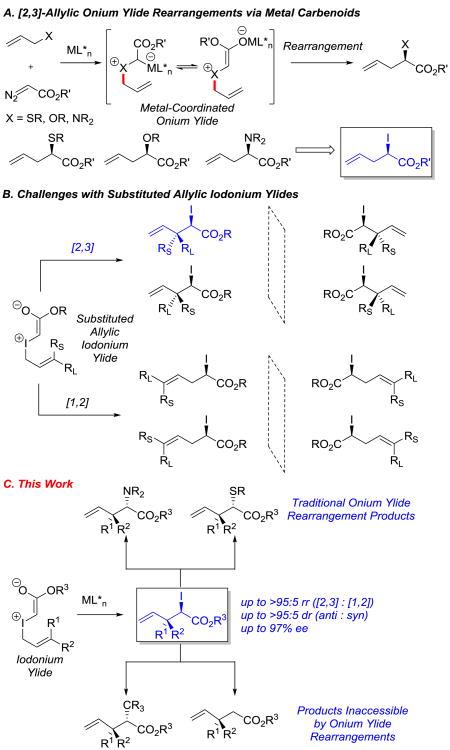
Catalytic enantioselective [2,3]-onium ylide rearrangements are historically important reactions that have been utilized for the synthesis of structurally complex chiral molecules.[1,2] The power of these transformations lies in their ability to convert achiral starting materials into products with vicinal stereocenters in high enantioselectivity and diastereoselectivity. In this context, catalytic processes via metal carbenoid intermediates have been especially useful (Scheme 1A). Traditional approaches have relied on the use of different conditions for the individual development of [2,3]-rearrangements of allylic ammonium,[3] sulfonium,[4] and oxonium[5] ylides.[6] We were interested in developing a more unified approach to [2,3]-onium ylide rearrangements. We envisioned that the discovery of an enantioselective and diastereoselective [2,3]-iodonium ylide rearrangement would provide access to chiral iodoesters that could be converted stereospecifically to products of many onium ylide rearrangements. Moreover, we could access products that are not accessible via classical onium ylide rearrangements.
Scheme 1.
Unified Approach to Enantioselective Onium Ylide Rearrangements.
Despite the potential utility of iodonium ylide rearrangements as a versatile class of reactions, selective [2,3]-rearrangements of these substrates have been rarely investigated.[7] In particular, substituted allylic iodonium ylides can generate up to 8 isomeric products via unselective [2,3]- and [1,2]-rearrangements (Scheme 1B).We recently reported the regioselective [2,3]- and [1,2]-rearrangements of iodonium ylides for the generation of racemic iodoester products.[8] Seminal studies by Doyle and co-workers reported that a single iodonium ylide substrate generated from unsubstituted allylic iodide underwent enantioselective rearrangement (69% ee), but there was no indication of the regioselectivity or diastereoselectivity.[9] To date, there are no catalytic rearrangements of these reactive substrates that can generate one product in high selectivity. In this Communication, we describe the first highly enantioselective, diastereoselective, and regioselective [2,3]-rearrangements of substituted iodonium ylides, and we disclose the synthetic versatility of the resulting iodoester products (Scheme 1C).
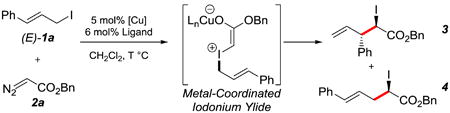
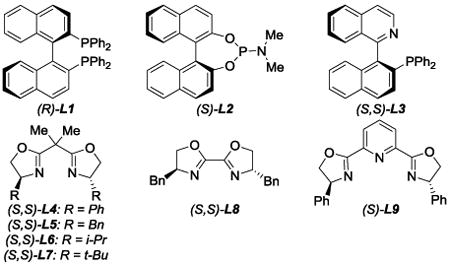
We commenced our studies with the substituted cinnamyl iodide (E)-1a and coupled it with benzyl α-diazoester 2a in the presence of 5 mol% [Cu(MeCN)4]PF6 and 6 mol% of various ligands (entries 1-9).Phosphine ligands BINAP L1, phosphoramidite L2, and QUINAP L3 favored the formation of [1,2]-rearrangement product 4 (entries 1-3).[10] Alternatively, bisoxazoline ligands L4-L9 preferentially yielded [2,3]-rearrangement product 3 with a range of regioselectivities, diastereoselectivities, and enantioselectivities (entries 4-9). t-Butyl-substituted bisoxazoline L7 provided the best regioselectivity and enantioselectivity for the [2,3]-rearrangement (entry 7, 87:13 rr, 75% and 81% ee). A screen of alternate copper sources in the presence of optimal ligand L7 did not improve the selectivities (entries 10-12). When cinnamyl iodide (E)-1a was replaced with its olefin isomer (Z)-1a, the diastereoselectivity (80:20) was drastically improved without affecting the regioselectivity (entry 13). To our delight, the bulkier t-butyl α-diazoester 2b reacted with cinnamyl iodide (Z)-1a with enhanced enantiomeric excess (entry 14, 82% ee). A screen of temperatures (entries 14-16) revealed −20 °C as the optimal temperature for this process. Under these conditions, [2,3]-iodonium ylide rearrangement product 3 was isolated in 82% yield, >95:5 rr, >95:5 dr, and 96% ee (entry 16).
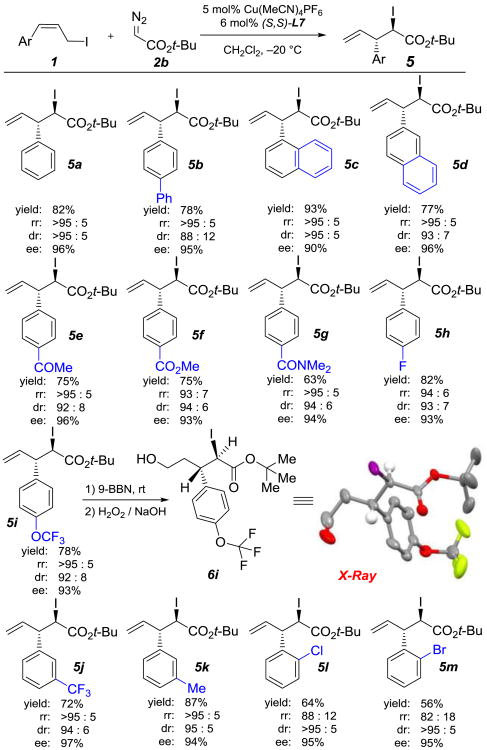
Using the optimal conditions, we evaluated the reactions of various aryl-substituted allylic iodides 1a–m with t-butyl α-diazoester 2b (Table 2). The enantioselective rearrangement exhibited good tolerance to different aromatic hydrocarbon rings (5a–5d). Substituents at the para or meta positions of the aryl ring had a negligible impact on the yield and selectivity of the reaction. Substrates with either electron-withdrawing groups (ketone, ester, amide, fluoro and trifluoromethyl) or with electron-donating groups (methyl and trifluoromethoxyl), underwent [2,3]-rearrangement smoothly and afforded the corresponding chiral α-iodoester products (5e–5k) in good yields (63–87%) with excellent selectivities (up to >95:5 rr, up to >95:5 dr, and 90–97% ee). Aryl-substituted allylic iodides with ortho substituents (5l–5m) afforded very good diasteroselectivities (>95:5 dr) and enantioselectivities (95% ee) but relatively low yields and low regioselectivities. Upon transformation of iodoester 5i to primary alcohol 6i, rearrangement product 5i was determined to have the (2R,3S)-configuration by means of X-ray diffraction analysis of a single crystal.[11]
Table 2. Scope of Aryl-Substituted Allylic Iodides[a].
Regioisomeric ratio (rr) is reported as [2,3] : [1,2]. Diastereomeric ratio (dr) is reported as anti : syn.
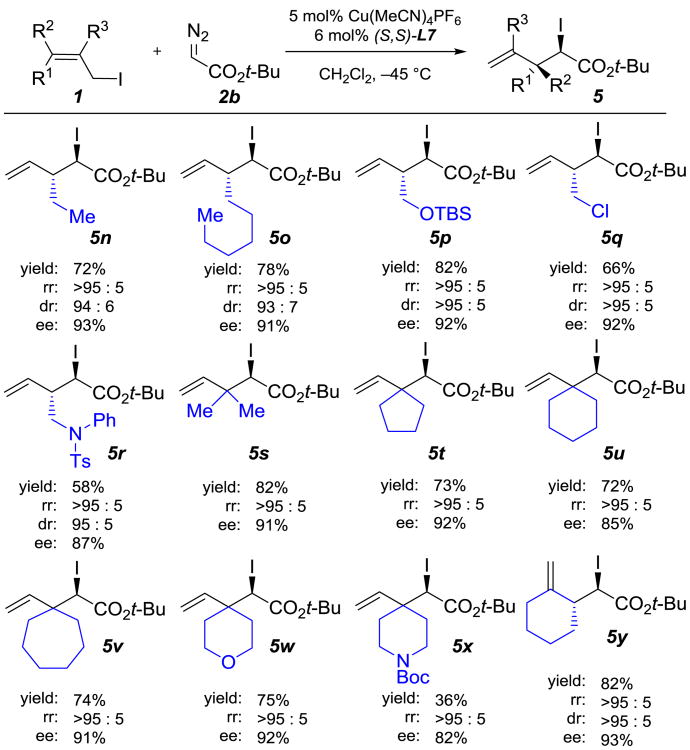
We also investigated the [2,3]-rearrangements of various alkyl-substituted allylic iodides 1n-r and poly-substituted allylic iodides 1s-y (Table 3). To obtain satisfactory enantioselectivities, the rearrangements of alkyl-substituted allylic iodides were carried out at a lower temperature (−45 °C). The [2,3]-rearrangement products were provided in good yields, regioselectivities (>95:5), diastereoselectivities (>93:7) and enantioselectivities (87–93% ee) in the presence of several functional groups, including a silyl ether, chloride, and protected amine. By using poly-substituted allylic iodides bearing 5–7 membered rings or heterocycles, structurally complex products (5t–5x) containing a quaternary carbon center adjacent to a stereocenter were obtained with good yields and selectivities.
Table 3. Scope of Alkyl and Poly-Substituted Allylic Iodides[a].
Regioisomeric ratio (rr) is reported as [2,3] : [1,2]. Diastereomeric ratio (dr) is reported as anti : syn.
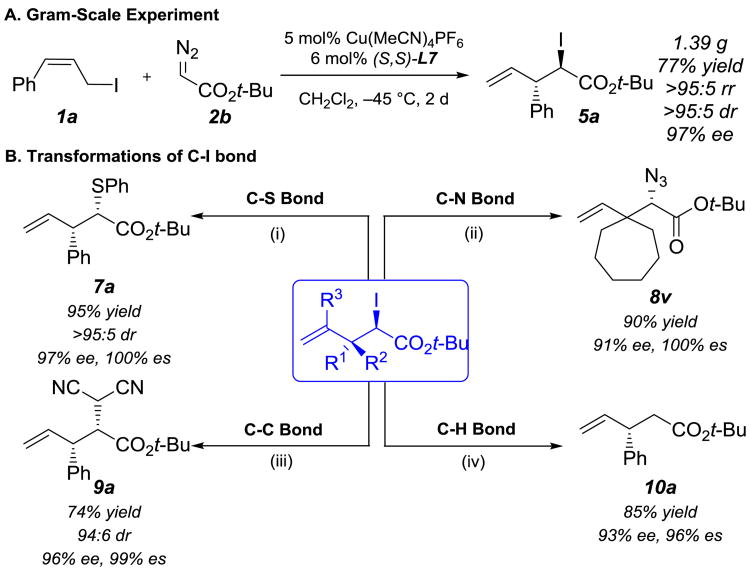
Next, we performed several experiments to demonstrate the potential utility of highly selective [2,3]-iodonium ylide rearrangements as a general solution to catalytic asymmetric onium ylide rearrangements (Scheme 2). First, the enantioselective [2,3]-rearrangement of iodonium ylides could be easily conducted on gram-scale without significant reduction of the yield or selectivities (Scheme 2A).
Scheme 2.
Synthetic Applications of Enantioselective [2,3]-Rearrangement Products. Regioisomeric ratio (rr) is reported as [2,3] : [1,2]. Diastereomeric ratio (dr) is reported as anti : syn. Conditions: (i) 5a (0.1 mmol, >95:5 rr, >95:5 dr, 97% ee), 1.5 equiv PhSH, 1.5 equiv K2CO3, Acetone, 23 °C, 12 h. (ii) 5v (0.1 mmol, >95:5 rr, 91% ee), 5 equiv NaN3, DMSO, 90 °C, 24 h. (iii) 5a (0.1 mmol, >95:5 rr, >95:5 dr, 97% ee), 1 equiv NaH, 1.5 equiv CH2(CN)2, THF, 80 °C, 12 h. (iv) 5a (0.1 mmol, >95:5 rr, >95:5 dr, 97% ee), 1.1 equiv PhCH2CH2MgCl, THF, 0 °C – 23 °C, 2 h; H2O, 23 °C.
Second, we explored stereospecific transformations of the C–I bond to other bonds (Scheme 2B). For example, the iodide in the rearrangement product 5a was displaced by thiophenol to form a new C–S bond without any diminished enantioselectivity (7a). This product resembles the α-thioesters that are generated by sulfonium ylide rearrangements. Even for the much bulkier product 5v, the C–I bond was transformed to a C–N bond with 100% es. The synthetically versatile chiral α-azidoester product (8v) successfully underwent a bioorthogonal click reaction with phenylacetylene to furnish a chiral α-triazoloester (S11).[12]
Furthermore, we converted the iodonium ylide rearrangement products to compounds that are inaccessible via traditional onium ylide rearrangements. α-Iodoester 5a was treated with sodium malononitrile, which resulted in the formation of product 9 with a new C–C bond in 99% es. Treatment of rearrangment product 5a with Grignard reagent and aqueous workup converted the C–I bond to a C–H bond via magnesium-halogen exchange.[13] The resulting chiral ester 10a was obtained with high optical purity (96% es).
In conclusion, we developed a highly enantioselective, diastereoselective, and regioselective copper-catalyzed [2,3]-rearrangement of iodonium ylides with a broad substrate scope of substituted allylic iodides. The resulting chiral α-iodoester products can be converted stereospecifically to a variety of onium ylide rearrangement products, as well as compounds that are not accessible via classical onium ylide rearrangements. We believe this transformation will benefit stereoselective C–X bond constructions in complex molecular settings.
Supplementary Material
Table 1. Optimization of Enantioselective [2,3]-Rearrangement of Iodonium Ylides[a].
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | Ligand | [Cu][b] | T (oC) | Yield (%) | 3 : 4 | dr of 3[c] | Ee of 3[d] (%, %) |
| 1[e] | (R)-L1 | CuPF6 | 25 | 19 | 11 : 89 | 48 : 52 | --, -- |
| 2 | (S)-L2 | CuPF6 | 25 | 21 | 18 : 82 | 58 : 42 | 69, 31 |
| 3 | (S)-L3 | CuPF6 | 25 | 29 | <5 : 95 | -- : -- | --, -- |
| 4 | (S)-L4 | CuPF6 | 25 | 62 | 64 : 36 | 34 : 66 | 26, 20 |
| 5 | (S)-L5 | CuPF6 | 25 | 71 | 58 : 42 | 32 : 68 | 41, 31 |
| 6 | (S)-L6 | CuPF6 | 25 | 73 | 57 : 43 | 40 : 60 | 22, 24 |
| 7 | (S)-L7 | CuPF6 | 25 | 82 | 87 : 13 | 45 : 55 | 75, 81 |
| 8 | (S)-L8 | CuPF6 | 25 | 64 | 65 : 35 | 36 : 64 | 2, 25 |
| 9 | (S)-L9 | CuPF6 | 25 | 56 | 56 : 44 | 37 : 63 | 42, 12 |
| 10 | (S)-L7 | CuCl | 25 | 63 | 60 : 40 | 42 : 58 | 10, 74 |
| 11 | (S)-L7 | CuOTf | 25 | 57 | 74 : 26 | 45 : 55 | 56, 71 |
| 12 | (S)-L7 | Cu(OTf)2 | 25 | 59 | 75 : 25 | 45 : 55 | 59, 71 |
| 13[f] | (S)-L7 | CuPF6 | 25 | 80 | 83 : 17 | 80 : 20 | 73, 70 |
| 14[f,g] | (S)-L7 | CuPF6 | 25 | 85 | 80 : 20 | 91 : 9 | 82, -- |
| 15[f,g] | (S)-L7 | CuPF6 | 0 | 84 | 90 : 10 | 94 : 6 | 90, -- |
| 16[f,g] | (S)-L7 | CuPF6 | -20 | 82 | >95 : 5 | >95 : 5 | 96, -- |
|
| |||||||
| |||||||
Reaction conditions: allylic iodide (0.4 mmol), α-diazoester (0.48 mmol), copper salt (5 mol%), ligand (6 mol%), CH2Cl2 (0.1M).
CuPF6 is an abbreviation for [Cu(MeCN)4]PF6. CuOTf is an abbreviation for (CuOTf)•½PhMe.
anti : syn ratio.
ee of anti diastereomer of 3, ee of syn diastereomer of 3.
12 mol% ligand.
(Z)-1a was utilized.
t-Butyldiazoester 2b was utilized.
Acknowledgments
Financial support was provided by W. W. Caruth, Jr. Endowed Scholarship, Welch Foundation (I-1748), National Institutes of Health (R01GM102604), National Science Foundation (1150875), and Sloan Research Fellowship. We thank Dr. Vincent Lynch for X-ray structural analysis.
Footnotes
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/anie.xxxxxxx.
Conflict of interest: The authors declare no conflict of interest.
References
- 1.a Doyle MP. Chem Rev. 1986;86:919–939. [Google Scholar]; b) Doyle MP, Forbes DC. Chem Rev. 1998;98:911–936. doi: 10.1021/cr940066a. [DOI] [PubMed] [Google Scholar]; c) Clark JS, editor. Nitrogen, Oxygen, and Sulfur Ylide Chemistry: A Practical Approach in Chemistry. Oxford University Press; New York: 2002. pp. 1–308. [Google Scholar]; d) Jones AC, May JA, Sarpong R, Stoltz BM. Angew Chem Int Ed. 2014;53:2556–2591. doi: 10.1002/anie.201302572. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2014;126:2590–2628. [Google Scholar]
- 2.West TH, Spoehrle SSM, Kasten K, Taylor JE, Smith AD. ACS Catal. 2015;5:7446–7479. [Google Scholar]
- 3.a West TH, Daniels DSB, Slawin AMZ, Smith AD. J Am Chem Soc. 2014;136:4476–4479. doi: 10.1021/ja500758n. [DOI] [PubMed] [Google Scholar]; b) West TH, Walden DM, Taylor JE, Brueckner AC, Johnston RC, Cheong PH, Lloyd-Jones GC, Smith AD. J Am Chem Soc. 2017;139:4366–4375. doi: 10.1021/jacs.6b11851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a Nishibayashi Y, Ohe K, Uemura S. J Chem Soc, Chem Commun. 1995;31:1245–1246. [Google Scholar]; b) Fukuda T, Katsuki T. Tetrahedron Lett. 1997;38:3435–3438. [Google Scholar]; c) Itoh K, Fukuda T, Kitajima H, Katsuki T. Yuki Gosei Kagaku Kyokaishi. 1997;55:764–773. [Google Scholar]; d) Fukuda T, Irie R, Katsuki T. Tetrahedron. 1999;55:649–664. [Google Scholar]; e) McMillen DW, Varga N, Reed BA, King C. J Org Chem. 2000;65:2532–2536. doi: 10.1021/jo991842n. [DOI] [PubMed] [Google Scholar]; f) Kitagaki S, Yanamoto Y, Okubo H, Nakajima M, Hashimoto S. Heterocycles. 2001;54:623–628. [Google Scholar]; g) Zhang X, Qu Z, Ma Z, Shi W, Jin X, Wang J. J Org Chem. 2002;67:5621–5625. doi: 10.1021/jo025687f. [DOI] [PubMed] [Google Scholar]; h) Zhang X, Ma M, Wang J. Tetrahedron: Asymmetry. 2003;14:891–895. [Google Scholar]; i) Zhang XM, Ma M, Wang JB. Chin J Chem. 2003;21:878–882. [Google Scholar]; j) Ma M, Peng L, Li C, Zhang X, Wang J. J Am Chem Soc. 2005;127:15016–15017. doi: 10.1021/ja055021d. [DOI] [PubMed] [Google Scholar]; k) Liao M, Wang J. Green Chem. 2007;9:184–188. [Google Scholar]
- 5.a) McCarthy N, McKervey MA, Ye T, McCann M, Murphy E, Doyle MP. Tetrahedron Lett. 1992;33:5983–5986. [Google Scholar]; b) Ferris L, Haigh D, Moody CJ. Tetrahedron Lett. 1996;37:107–110. [Google Scholar]; c) Doyle MP, Peterson CS. Tetrahedron Lett. 1997;38:5265–5268. [Google Scholar]; d) Pierson N, Fernández-García C, McKervey MA. Tetrahedron Lett. 1997;38:4705–4708. [Google Scholar]; e) Clark JS, Fretwell M, Whitlock GA, Burns CJ, Fox DNA. Tetrahedron Lett. 1998;39:97–100. [Google Scholar]; f) Calter MA, Sugathapala PM. Tetrahedron Lett. 1998;39:8813–8816. [Google Scholar]; g) Hodgson DM, Petroliagi M. Tetrahedron: Asymmetry. 2001;12:877–881. [Google Scholar]; h) Kitagaki S, Yanamoto Y, Tsutsui H, Anada M, Nakajima M, Hashimoto S. Tetrahedron Lett. 2001;42:6361–6364. [Google Scholar]; i) Shimada N, Nakamura S, Anada M, Shiro M, Hashimoto S. Chem Lett. 2009;38:488–489. [Google Scholar]; j) Li Z, Davies HML. J Am Chem Soc. 2010;132:396–401. doi: 10.1021/ja9075293. [DOI] [PubMed] [Google Scholar]; k) Li Z, Parr BT, Davies HML. J Am Chem Soc. 2012;134:10942–10946. doi: 10.1021/ja303023n. [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Li Z, Boyarskikh V, Hansen JH, Autschbach J, Musaev DG, Davies HML. J Am Chem Soc. 2012;134:15497–15504. doi: 10.1021/ja3061529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.For catalytic enantioselective onium ylide rearrangements that do not involve metal carbenoid intermediates, see: Mikami K, Takahashi O, Tabei T, Nakai T. Tetrahedron Lett. 1986;27:4511–4514.Kawasaki T, Kimachi T. Tetrahedron. 1999;55:6847–6862.Tomooka K, Komine N, Nakai T. Chirality. 2000;12:505–509. doi: 10.1002/(SICI)1520-636X(2000)12:5/6<505::AID-CHIR36>3.0.CO;2-3.McNally A, Evans B, Gaunt MJ. Angew Chem Int Ed. 2006;45:2116–2119. doi: 10.1002/anie.200504301.Angew Chem. 2006;118:2170–2173.Kitamura M, Hirokawa Y, Maezaki N. Chem Eur J. 2009;15:9911–9917. doi: 10.1002/chem.200901212.Marié JC, Xiong Y, Min GK, Yeager AR, Taniguchi T, Berova N, Schaus SE, Porco JA. J Org Chem. 2010;75:4584–4590. doi: 10.1021/jo100889c.Kondoh A, Terada M. Org Lett. 2013;15:4568–4571. doi: 10.1021/ol402144q.Denmark SE, Cullen LR. J Org Chem. 2015;80:11818–11848. doi: 10.1021/acs.joc.5b01759.; i) Reference 2, and references therein.
- 7.a) Giddings PJ, Ivor DJ, Thomas EJ. Tetrahedron Lett. 1980;21:395–398. [Google Scholar]; b) Doyle MP, Tamblyn WH, Bagheri V. J Org Chem. 1981;46:5094–5102. [Google Scholar]; c) Giddings PJ, John DI, Thomas EJ, Williams DJ. J Chem Soc, Perkin Trans. 1982;1:2757–2766. [Google Scholar]; d) Krishnamoorthy P, Browning RG, Singh S, Sivappa R, Lovely CJ, Dias HVR. Chem Commun. 2007:731–733. doi: 10.1039/b608266a. [DOI] [PubMed] [Google Scholar]; e) Deng QH, Chen J, Huang JS, Chui SSY, Zhu N, Li GY, Che CM. Chem Eur J. 2009;15:10707–10712. doi: 10.1002/chem.200901895. [DOI] [PubMed] [Google Scholar]
- 8.a) Xu B, Tambar UK. J Am Chem Soc. 2016;138:12073–12076. doi: 10.1021/jacs.6b08624. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Xu B, Gartman JA, Tambar UK. Tetrahedron. 2017 doi: 10.1016/j.tet.2017.01.048. [DOI] [Google Scholar]
- 9.a) Doyle MP, Forbes DC, Vasbinder MM, Peterson CS. J Am Chem Soc. 1998;120:7653–7654. [Google Scholar]; b) Doyle MP, Hu W, Phillips IM, Moody CJ, Pepper AG, Slawin AGZ. Adv Synth Catal. 2001;343:112–117. [Google Scholar]
- 10.The enantioselectivities for [1,2]-rearrangement product 4 were low (0% to 30% ee). For more complete optimization data, see Supporting Information.
- 11.See Supporting Information. CCDC 1550761 (6i) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 12.See Supporting Information.
- 13.Knochel P, Dohle W, Gommermann N, Kneisel FF, Kopp F, Korn T, Sapountzis I, Vu VA. Angew Chem Int Ed. 2003;42:4302–4320. doi: 10.1002/anie.200300579. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2003;115:4438–4456. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.