Abstract
Platelets, derived from megakaryocytes, are anucleate cytoplasmic discs that circulate in the blood stream and play major roles in hemostasis, inflammation, and vascular biology. Platelet transfusions are utilized in a variety of medical settings to prevent life-threatening thrombocytopenia due to cancer therapy, other causes of acquired or inherited thrombocytopenia, and trauma. Currently, platelets used for transfusion purposes are donor-derived. However, there is a drive to generate non-donor sources of platelets to help supplement donor-derived platelets. Efforts have been made by many laboratories to generate in vitro platelets and optimize their production and quality. In vitro-derived platelets have the potential to be a safer, more uniform product, and genetic manipulation could allow for better treatment of patients that become refractory to donor-derived units. This review focuses on potential clinical applications of in vitro-derived megakaryocytes and platelets, current methods to generate and expand megakaryocytes from pluripotent stem cell sources, and the use of these cells for disease modeling.
Keywords: iPSC, hematopoiesis, megakaryocyte, platelet
Graphical Abstract
Introduction: Why do we need iPSC derived megakaryocytes?
The advent of human induced pluripotent stem cell (iPSC) technology over 10 years ago provided investigators with an important tool for the field of cellular therapeutics.1 One goal in the hematopoietic field has been to generate donor-independent platelets to supplement current transfusion products. Platelets are cellular fragments derived from megakaryocytes that play a critical role in hemostasis and thrombus formation, and mediate aspects of immunity, inflammation, and angiogenesis.2–6 Although donor shortage has not been a frequent problem to date, the number of transfusions in first-world countries has steadily increased due to increased lifespan and a rise in hematologic malignancies.7 Because of this, there is a drive to develop donor-independent sources of platelets for transfusions.
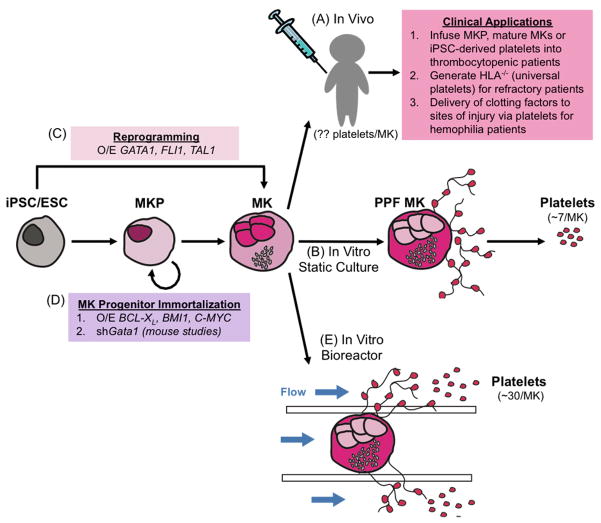
One of the advantages of using iPSCs to generate platelets is that they are amenable to genetic manipulation. Two potential applications for generating donor-independent platelets are in patients refractory to donor-derived units due to alloimmunization, and as a targeted delivery vehicle for compounds, such as clotting factors, to sites of vascular injury (Figure 1A). It is estimated that 28–44% of patients receiving long-term platelet transfusions become refractory to donor-derived units due to alloimmunization against human leukocyte antigen antigens.8–10 Generation of an human leukocyte antigen-universal iPSC line by silencing human leukocyte antigen class I epitopes would allow for a “universal” source of platelets with a low immunogenic profile.11,12 As natural killer cells are triggered in the absence of human leukocyte antigen class I expression, stable gene editing techniques such as CRISPR or zinc finger nucleases may not be appropriate for this application.13 Hemophilia A patients receive frequent plasma transfusions to compensate for factor VIII deficiency, but it is not useful in patients with high titers of anti-factor VIII antibodies.14,15 Ectopic expression of factor VIII in platelets could be used to treat Hemophilia A patients with significant inhibitors.
Figure 1.
Current Methods for Generating Stem Cell-Derived Platelets (A) Megakaryocytes infused into human patients have the potential to act as an in vivo bioreactor, as seen in mouse studies. Megakaryocytes infused into mice release ~30 platelets/megakaryocyte. (B) In static culture, megakaryocytes differentiated from iPSCs and ESCs form proplatelets. The shed platelets can be purified from the culture medium by centrifugation. This method yields ~7 platelets/megakaryocyte. (C) iPSCs and ESCs can be driven to the megakaryocyte lineage through overexpression of GATA1, FLI1, and TAL1. (D) Megakaryocyte progenitors can be expanded by overexpressing BCL-XL, BMI1, and C-MYC. In mice, these progenitors are expanded by knockdown of Gata1. (E) In vitro platelet bioreactors enhance proplatelet formation in megakaryocytes via endothelial cell contacts, extracellular matrix components, and shear stress. This method yields ~30 platelets/megakaryocyte. MKP, megakaryocyte progenitor; MK, megakaryocyte; PPF MK, proplatelet forming megakaryocyte.
Megakaryocyte Ontogeny
Primitive vs definitive hematopoiesis
Two distinct hematopoietic programs, designated primitive and definitive, occur during embryonic/fetal development and both give rise to megakaryocytes. These programs can be distinguished by the subtype of globin expressed in erythroid cells.16 The first, primitive, wave of hematopoiesis develops from an extra-embryonic mesoderm population in the yolk sac.17,18 These yolk sac progenitors give rise to nucleated erythrocytes, macrophages, and primitive megakaryocytes.19,20 Towards the end of the primitive wave, definitive erythro-myeloid progenitors emerge from the yolk sac and later colonize the fetal liver, ultimately acting as the major source of hematopoiesis prior to the emergence of hematopoietic stem cells.21 Another definitive wave of hematopoiesis occurs in the aorta-gonads-mesonephros region and is responsible for the generation of the long-term hematopoietic stem cells having multi-lineage hematopoietic potential, as well as enucleated erythrocytes, myeloid cells, lymphocytes and definitive megakaryocytes.22 Following specification, these hematopoietic stem cells colonize the fetal liver before transitioning to the bone marrow, where they reside for the remainder of adult life.23
While megakaryocytes are generated in both the primitive and definitive waves of hematopoiesis, functional differences have been described.19,24,25 Primitive megakaryocytes tend to be less proliferative and display a lower ploidy, with each megakaryocyte releasing a relatively small number of platelets.26,27 Megakaryocytes generated through the definitive hematopoietic program undergo increased levels of endomitosis, where the cell does not divide after DNA duplication resulting in increased ploidy and efficient release of large numbers of higher functioning platelets, up to 103–4 platelets per megakaryocyte in the adult.27,28 Although neonatal and adult megakaryocytes are generated from the definitive program of hematopoiesis, the ploidy and functionality of megakaryocytes and their platelets do not peak until at least a year after birth.29 Distinct gene expression signatures between megakaryocytes derived from human embryonic stem cells (ESCs), fetal liver, neonate, and adult progenitors supports the finding that megakaryocyte maturation progresses during ontogeny and results in increased ploidy and platelet release.25 Recent evidence suggests IGF2BP3 as a master switch driving immature fetal/neonatal to adult megakaryopoiesis.30 Overall, a better understanding of the regulatory and functional changes that occur during megakaryocyte ontogeny are critical for generating high quality megakaryocytes and platelets for transfusion purposes.
Definitive megakaryopoiesis and thrombopoiesis
Megakaryopoiesis is the process by which a hematopoietic progenitor cell (HPC) differentiates into a large, polyploid megakaryocyte.31 This process is dependent on the growth factor TPO and transcription factors such as FLI1, GATA1, and RUNX1.32–34 In the adult, megakaryocytes are generated in the bone marrow and must undergo a series of maturation steps to release large numbers of functional platelets into the circulation. This complex maturation process involves generation of a large polyploid cell, the formation of an invaginated membrane system to serve as a cell membrane reservoir for platelet formation, and the synthesis of platelet specific granules, such as alpha and dense granules, which are distributed into platelets.28,35–37
Following maturation, megakaryocytes are equipped to release platelets into the circulation, a process termed thrombopoiesis.31 The exact mechanism of proplatelet formation and platelet release are not well understood but there are two main models: proplatelet formation and megakaryocyte rupturing. The proplatelet formation model can occur in the bone marrow and in the lung capillary beds. In the bone marrow, mature megakaryocytes exit the osteoblastic niche and enter the vasculature where they extend proplatelet extensions into the bone marrow sinusoids.38 Shear stress and high sphingosine 1-phosphate levels in the blood are proposed as mechanisms supporting the release of platelets into circulation.39 Approximately half of all platelet release in the mouse occurs when megakaryocytes exit the bone marrow and enter lung capillary beds where they release platelets into the circulation via proplatelet extensions.40–42 Although current studies examining platelet release in the lung have supported the lungs as a major site of thrombopoiesis, it is still unknown if this holds true in the human. Other data suggests megakaryocytes can rupture through an interleukin-1a-dependent pathway, thus resulting in rapid release of a large number of platelets into the bone marrow where they enter the circulation rapidly after acute injury or inflammation.43 With evidence for both models, it is possible to speculate that thrombopoiesis may occur differently depending on the physiological need of homeostasis versus acute injury.
Directed differentiation of iPSCs to megakaryocytes
The use of primary human megakaryocytes to study megakaryopoiesis and thrombopoiesis is difficult because these cells represent only ~0.01% of the nucleated cells in the bone marrow.44 Human stem cell sources, including ESCs and iPSCs, have been used successfully for the in vitro generation of megakaryocytes. However, an ongoing challenge is identifying conditions to optimize the quantity and quality of in vitro-generated megakaryocytes and released platelets.
Directed differentiation protocols have been developed for generating HPCs from ESCs/iPSCs with the overall design mimicking embryogenesis.45 Primitive streak formation is induced through BMP4 and WNT pathway activation, followed by mesoderm specification45,46. Mesoderm is further specified to hemato-endothelial mesoderm, through a still not fully understood mechanism, which ultimately gives rise to HPCs. HPCs expand upon the addition of hematopoietic cytokines such as SCF, Flt3L, and TPO.46,47,48 The primitive HPCs co-express typical adult megakaryocyte and erythrocyte markers CD41 and CD235 and give rise to primitive erythrocytes, megakaryocytes, and myeloid cells.46,49 Other widely used differentiation systems use co-culture with stromal cell lines, such as OP9, to generate HPCs.50,51 These static in vitro cultures generate approximately seven platelets per megakaryocyte (Figure 1B).
In recent years, differentiation protocols driving definitive hematopoiesis have been developed through timed modulation of the TGFβ and WNT pathways.52,53,54 Hemogenic endothelium can be purified from 3D embryoid body suspension cultures via cell sorting CD34+ cells that are CD73-CD184-.52,53 This population possesses the ability to give rise to hematopoietic progenitors with lymphoid potential, confirming its definitive nature.55
With the identification of TPO, HPCs can be differentiated in vitro to the megakaryocyte lineage.32,33 Studies comparing definitive or adult-like megakaryocytes derived from bone marrow CD34+ stem cells to primitive megakaryocytes derived from iPSCs show that the iPSC-derived primitive megakaryocytes are smaller, of lower ploidy, and release fewer platelets with a short half-life when infused into mice.25,56 With the newer protocols driving definitive hematopoiesis, further studies will define the characteristics of megakaryocytes derived from these more mature progenitor populations.
Methods to increase iPSC-derived progenitor and megakaryocyte numbers
With the goal of generating increased numbers of HPCs and megakaryocytes, systems utilizing the overexpression of transcription factors to drive megakaryocyte progenitor generation and expansion have been developed. The Ghevaert laboratory found that overexpression of FLI1, GATA1, and TAL1 in ESCs led to a bias for the generation of megakaryocytes (Figure 1C).57 These reprogrammed megakaryocytes had the ability to proliferate in culture for over 90 days while still maintaining megakaryocyte purity, markers of maturity (i.e.: CD41/CD42a), and platelet production. Starting with 106 iPSCs, this methodology resulted in the production of 2×1011 megakaryocytes releasing a total of 1×1012 platelets. This is equivalent to approximately three transfusion units, whereas current directed differentiation protocols would need approximately 103 more iPSCs to generate a similar number of platelets. Although this system allows for large-scale production of platelets, these platelets are still inferior to donor-derived platelets as they are less mature and have a short half-life when infused into mice.
Two approaches have been developed to overcome the asynchronous nature of cell culture while generating self-renewing megakaryocyte progenitors. The Eto Laboratory modulated the expression of transcription factors known to regulate pathways related to apoptosis, senescence, and proliferation. Doxycycline inducible expression of BCL-XL, BMI1, and C-MYC in human iPSC-derived HPCs promoted the expansion of immature megakaryocytes in culture (Figure 1D), termed imMKCLs (immortalized megakaryocyte progenitor cell lines).58 These megakaryocytes were capable of proliferating for up to five months and removal of doxycycline allowed final megakaryocyte maturation to occur. However, these immortalized megakaryocytes shed fewer platelets with a less robust response to agonist stimulation when compared to unmanipulated megakaryocytes. The Weiss laboratory manipulated the expression of GATA1, a known master regulator of megakaryopoiesis and erythropoiesis, to generate expandable megakaryocyte progenitors (Figure 1D).59,60 These studies were initiated due to the fact that patients with GATA1 mutations have increased counts of proliferative, developmentally arrested megakaryocytes.61–63 Their first system utilizing Gata1-null mice59 has been improved by enabling controlled temporal knockdown of Gata1.60 Mouse ESCs were targeted to express a doxycycline-inducible shRNA targeting Gata1. As with the original system, a biopotential progenitor expanded ~103-fold in the presence of doxycycline. Withdrawal of doxycycline allowed for re-expression of endogenous Gata1 and further differentiation into megakaryocytes or erythrocytes. The generation of human systems that display the same phenomenon will be of interest.
Although all these systems are advantageous in that they generate large populations of megakaryocytes, there are risks involved in using factors with tumorigenic abilities, for example C-MYC. Given that platelets are anucleate, the final product can be irradiated to eliminate residual live cells prior to infusing into patients which may mitigate this concern. It is also unclear if these systems are generating primitive or definitive megakaryocytes. To generate the best yield and functionality of platelets, it will be important to mimic later maturation events that occur after birth. For example, suppression of IGF2BP3 via small molecules or gene editing could allow for further maturation of megakaryocytes, or selective inhibition of the metalloproteinase ADAM17 could improve platelet functionality by preventing glycoprotein Ibα/CD42b shedding in vitro.30,64
iPSC derived megakaryocytes for disease modeling
Although animal models are invaluable for studying the biology of human diseases, there are instances where they do not recapitulate the human disease phenotype. Therefore, human iPSCs are an important tool for modeling human disease in vitro. Detailed below are a set of megakaryocyte and platelet disorders that have been studied using human iPSCs.
Familial platelet disorder with a predisposition to acute myeloid leukemia is a rare genetic disorder resulting from heterozygous mutations in the transcription factor RUNX1.65,66 As the name suggests, these patients present with thrombocytopenia and a greater than 40% chance of developing hematologic malignancies by age 35.65,66 Murine models have been used to study this disease but fail to display low platelet counts unless both alleles of Runx1 are mutated.67 With that, several laboratories have generated iPSCs from familial platelet disorder patients harboring RUNX1 mutations to further understand the mechanism of disease.68–70 A consistent finding is that iPSCs derived from these patients display a severe decrease in megakaryocyte yield.68–70 Upon lentiviral expression of wild type RUNX1 or genetic correction of the mutation, the megakaryocyte phenotype was rescued.
Paris-Trousseau syndrome is caused by a large heterozygous deletion in chromosome 11q resulting in hemizygous expression of FLI1, a critical transcription factor for megakaryocyte differentiation, as well as other hematopoietic lineages.71 Paris-Trousseau syndrome patients have macrothrombocytopenia with large platelets circulating at reduced levels. FLI1 mutations have recently been ascribed to cause thrombocytopenia, but the link between FLI1 mutations and Paris-Trousseau syndrome had not been confirmed.72,73 Upon differentiating iPSCs generated from these patients into megakaryocytes, the disease phenotype was confirmed with decreased megakaryocyte and platelet yields, and poor platelet half-life and functionality when infused into mice.74 When a wild type iPSC line heterozygous for FLI1 knockout was differentiated into megakaryocytes, it displayed the same phenotype as the megakaryocytes and platelets obtained from the Paris-Trousseau syndrome patient iPSCs.74
Glanzmann thrombasthenia is a rare autosomal recessive disease resulting from a lack of functional αIIbβ3 (GPIIb/IIIa; CD41/CD61) due to mutations in ITGA2B and ITGB3.75 This integrin complex found on platelets is a crucial receptor for fibrinogen resulting in platelet aggregation.76 Although Glanzmann thrombasthenia patients have normal platelet counts, the function of their platelets is altered resulting in increased bleeding. The iPSC lines generated from different Glanzmann thrombasthenia patients demonstrated a consistent physiological phenotype.77–79 Megakaryocytes did not express αIIbβ3 receptors due to defective αIIb/CD41 but did express a mature megakaryocyte marker, CD42b.77–79 By expressing wild type ITGA2B under a Gp1ba promoter, αIIbβ3 receptors were restored to normal as shown by PAC-1 binding following agonist stimulation.77,79
Conclusion: Are we ready for the clinic?
Although significant progress has been made, much work still needs to be done to bring in vitro-derived megakaryocytes and platelets to the clinic. However, efforts are being made by numerous laboratories to increase the quality and yield of iPSC-derived megakaryocytes and platelets. In vitro bioreactors are being utilized to closely mimic the in vivo environment by incorporating extracellular matrix components, endothelial cells, and applying a flow to induce shear stress (Figure 1E).80 These in vitro bioreactors yield approximately 30 platelets per megakaryocyte, which is well below the in vivo yield of >2000.81 One potential explanation for low platelet yield is that these bioreactors are mainly plastic-based; it is possible that silk bioreactors or lung mini-chip bioreactors would help to increase yields. Alternatively, iPSC-derived megakaryocytes could be infused directly into patients to obtain maximal platelet release. Although infusing megakaryocytes directly increases safety risk concerns, unpublished studies in the Poncz laboratory show that megakaryocytes can undergo irradiation without a loss in efficacy (Figure 1A). Other laboratories are trying to further mature in vitro-derived megakaryocytes to increase ploidy and ultimately increase platelet release by genetically manipulating maturation factors.30 Despite current progress, much more work remains to be done before iPSC-derived megakaryocytes and platelets make their way into the clinic.
Supplementary Material
Highlights.
Human iPSC-derived megakaryocyte development using directed differentiation
Approaches to expand human iPSC-derived megakaryocytes
Use for studying human megakaryocyte and platelet disorders
Goals for supplementing current donor-derived platelet sources
Acknowledgments
S.B. wrote and revised the manuscript, reviewed literature; X.S. designed and revised the figure using Adobe Illustrator; M.P. provided expertise in the field, scientific direction, and revised drafts; D.L.F. provided expertise in the field, scientific direction, and revised drafts; P.G. provided expertise in the field, scientific direction, and revised drafts.
Sources of Funding
Work supported by the National Institute of Health (NIH; R01 HL130689; to M. Poncz, P. Gadue, D.L. French).
Nonstandard Abbreviations and Acronyms
- iPSC
induced pluripotent stem cell
- ESC
embryonic stem cell
- HPC
hematopoietic progenitor cell
Footnotes
The authors have declared that no conflict of interest exists.
Disclosure
None.
References
- 1.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Golebiewska EM, Poole AW. Platelet secretion: From haemostasis to wound healing and beyond. Blood Rev. 2015;29:153–162. doi: 10.1016/j.blre.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ware J, Corken A, Khetpal R. Platelet function beyond hemostasis and thrombosis. Curr Opin Hematol. 2013;20:451–456. doi: 10.1097/MOH.0b013e32836344d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semple JW, Italiano JE, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 5.Jenne CN, Kubes P. Platelets in inflammation and infection. Platelets. 2015;26:286–292. doi: 10.3109/09537104.2015.1010441. [DOI] [PubMed] [Google Scholar]
- 6.Walsh TG, Metharom P, Berndt MC. The functional role of platelets in the regulation of angiogenesis. Platelets. 2015;26:199–211. doi: 10.3109/09537104.2014.909022. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker B, Rajbhandary S, Kleinman S, Harris A, Kamani N. Trends in United States blood collection and transfusion: results from the 2013 AABB blood collection, utilization, and patient blood management survey. Transfusion. 2016;56:2173–2183. doi: 10.1111/trf.13676. [DOI] [PubMed] [Google Scholar]
- 8.Legler T, Fischer I, Dittmann J, Simson G, Lynen R, Humpe A, Riggert J, Schleyer E, Kern W, Hiddemann W, Köhler M. Frequency and causes of refractoriness in multiply transfused patients. Ann Hematol. 1997;74:185–189. doi: 10.1007/s002770050280. [DOI] [PubMed] [Google Scholar]
- 9.Fasano R, Mamcarz E, Adams S, Donohue Jerussi T, Sugimoto K, Tian X, Flegel W, Childs R. Persistence of recipient human leucocyte antigen (HLA) antibodies and production of donor HLA antibodies following reduced intensity allogeneic haematopoietic stem cell transplantation. Br J Haematol. 2014;166:425–434. doi: 10.1111/bjh.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanworth SJ, Navarrete C, Estcourt L, Marsh J. Platelet refractoriness - practical approaches and ongoing dilemmas in patient management. Br J Haematol. 2015;171:297–305. doi: 10.1111/bjh.13597. [DOI] [PubMed] [Google Scholar]
- 11.Börger A-K, Eicke D, Wolf C, Gras C, Aufderbeck S, Schulze K, Engels L, Eiz-Vesper B, Schambach A, Guzman C, Lachmann N, Moritz T, Martin U, Blasczyk R, Figueiredo C. Generation of HLA-Universal iPSC-Derived Megakaryocytes and Platelets for Survival Under Refractoriness Conditions. Mol Med. 2016;22:274–285. doi: 10.2119/molmed.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riolobos L, Hirata RK, Turtle CJ, Wang P-R, Gornalusse GG, Zavajlevski M, Riddell SR, Russell DW. HLA Engineering of Human Pluripotent Stem Cells. Mol Ther. 2013;21:1232–1241. doi: 10.1038/mt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabry M, Lowdell M. Tumor-primed NK cells: Waiting for the green light. Front Immunol. 2013;4:1–7. doi: 10.3389/fimmu.2013.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franchini M, Mannucci PM. Hemophilia A in the third millennium. Blood Rev. 2013;27:179–184. doi: 10.1016/j.blre.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Gewirtz J, Thornton M, Rauova L, Poncz M. Platelet-delivered factor VIII provides limited resistance to anti-factor VIII inhibitors. J Thromb Haemost. 2008;6:1160–1166. doi: 10.1111/j.1538-7836.2008.02992.x. [DOI] [PubMed] [Google Scholar]
- 16.Palis J. Primitive and definitive erythropoiesis in mammals. Front Physiol. 2014;5:1–9. doi: 10.3389/fphys.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baron MH, Fraser ST. The specification of early hematopoiesis in the mammal. Curr Opin Hematol. 2005;12:217–221. doi: 10.1097/01.moh.0000163217.14462.58. [DOI] [PubMed] [Google Scholar]
- 18.Mcgrath KE, Palis J. Hematopoiesis in the yolk sac: more than meets the eye. Exp Hematol. 2005;33:1021–1028. doi: 10.1016/j.exphem.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Xu M, Matsuoka S, Yang F, Ebihara Y, Manabe A, Tanaka R, Eguchi M, Asano S, Nakahata T, Tsuji K. Evidence for the presence of murine primitive megakarycytopoiesis in the early yolk sac. 2017;9:2016–2022. doi: 10.1182/blood.v97.7.2016. [DOI] [PubMed] [Google Scholar]
- 20.Palis J, Robertson S, Kennedy M, Wall C, Keller G. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development. 1999;126:5073–5084. doi: 10.1242/dev.126.22.5073. [DOI] [PubMed] [Google Scholar]
- 21.McGrath K, Frame J, Fegan K, Bowen J, Conway S, Catherman S, Kingsley P, Koniski A, Palis J. Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell Rep. 2015;11:1892–1904. doi: 10.1016/j.celrep.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medvinsky AL, Dzierzak EA. Development of the definitive hematopoietic hierarchy in the mouse. Dev Comp Immunol. 1998;22:289–301. doi: 10.1016/s0145-305x(98)00007-x. [DOI] [PubMed] [Google Scholar]
- 23.de Bruijn M, Speck N, Peeters M, Dzierzak E. Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J. 2000;19:2465–2474. doi: 10.1093/emboj/19.11.2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tober J, Koniski A, McGrath K, Vemishetti R, Emerson R, de Mesy-Bentley K, Waugh R, Palis J. The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood. 2007;109:1433–1441. doi: 10.1182/blood-2006-06-031898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bluteau O, Langlois T, Rivera-Munoz P, Favale F, Rameau P, Meurice G, Dessen P, Solary E, Raslova H, Mercher T, Debili N, Vainchenker W. Developmental changes in human megakaryopoiesis. J Thromb Haemost. 2013;11:1730–1741. doi: 10.1111/jth.12326. [DOI] [PubMed] [Google Scholar]
- 26.Potts K, Sargeant T, Markham J, Shi W, Biben C, Josefsson E, Whitehead L, Rogers K, Liakhovitskaia A, Smyth G, Kile B, Medvinsky A, Alexander W, Hilton D, Taoudi S. A lineage of diploid platelet-forming cells precedes polyploid megakaryocyte formation in the mouse embryo. Blood. 2014;124:2725–2729. doi: 10.1182/blood-2014-02-559468. [DOI] [PubMed] [Google Scholar]
- 27.Mattia G, Vulcano F, Milazzo L, Barca A, Macioce G, Giampaolo A, Hassan Jane. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood. 2002;99:888–897. doi: 10.1182/blood.v99.3.888. [DOI] [PubMed] [Google Scholar]
- 28.Vitrat N, Cohen-Solal K, Pique C, Le Couedic JP, Norol F, Larsen A, Katz A, Vainchenker W, Debili N. Endomitosis of human megakaryocytes are due to abortive mitosis. Blood. 1998;91:3711–3723. [PubMed] [Google Scholar]
- 29.Liu Z-J, Sola-Visner M. Neonatal and adult megakaryopoiesis. Curr Opin Hematol. 2011;18:330–337. doi: 10.1097/MOH.0b013e3283497ed5. [DOI] [PubMed] [Google Scholar]
- 30.Elagib KE, Lu C-H, Mosoyan G, Khalil S, Zasadzińska E, Foltz DR, Balogh P, Gru AA, Fuchs DA, Rimsza LM, Verhoeyen E, Sansó M, Fisher RP, Iancu-Rubin C, Goldfarb AN. Neonatal expression of RNA-binding protein IGF2BP3 regulates the human fetal-adult megakaryocyte transition. J Clin Invest. 2017;10:1–13. doi: 10.1172/JCI88936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deutsch V, Tomer A. Megakaryocyte development and platelet production. Br J Haematol. 2006;134:453–466. doi: 10.1111/j.1365-2141.2006.06215.x. [DOI] [PubMed] [Google Scholar]
- 32.Kaushansky K, Broudy V, Lin N, Jorgensen M, McCarty J, Fox N, Zucker-Franklin D, Lofton-Day C. Thrombopoietin, the Mpl ligand, is essential for full megakaryocyte development. Proc Natl Acad Sci. 1995;92:3234–3238. doi: 10.1073/pnas.92.8.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lok S, Kaushansky K, Holly R, Kuijper J, Lofton-Day C, Oort P, Grant F, Heipel M, Burkhead S, Kramer J, Bell A, Sprecher C, Blumberg H, Johnson R, Prunkard D, Ching A, Mathewes S, Bailey M, Forstrom J, Buddle M, Osborn S, Evans S, Sheppard P, Presnell S, O’Hara P, Hagen F, Roth G, Foster D. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994;369:565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- 34.Tijssen M, Ghevaert C. Transcription factors in late megakaryopoiesis and related platelet disorders. J Thromb Haemost. 2013;11:593–604. doi: 10.1111/jth.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulze H, Korpal M, Hurov J, Kim S-W, Zhang J, Cantley L, Graf T, Shivdasani R. Characterization of the megakaryocyte demarcation membrane system and its role in thrombopoiesis. Blood. 2006;107:3868–3875. doi: 10.1182/blood-2005-07-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heijnen H, Debili N, Vainchencker W, Breton-Gorius J, Geuze H, Sixma J. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91:2313–2325. [PubMed] [Google Scholar]
- 37.Youssefian T, Cramer E. Megakaryocyte dense granule components are sorted in multivesicular bodies. Blood. 2000;95:4004–4007. [PubMed] [Google Scholar]
- 38.Junt T, Schulze H, Chen Z, Massberg S, Goerge T, Krueger A, Wagner D, Graf T, Italiano J, Shivdasani R, von Andrian U. Dynamic Visualization of Thrombopoiesis Within Bone Marrow. Science. 2007;317:1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Orban M, Lorenz M, Barocke V, Braun D, Urtz N, Schulz C, von Brühl M-L, Tirniceriu A, Gaertner F, Proia R, Graf T, Bolz S-S, Montanez E, Prinz M, Müller A, von Baumgarten L, Billich A, Sixt M, Fässler R, von Andrian U, Junt T, Massberg S. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med. 2012;209:2165–2181. doi: 10.1084/jem.20121090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levine R, Eldor A, Shoff P, Kirwin S, Tenza D, Cramer E. Circulating megakaryocytes: delivery of large numbers of intact, mature megakaryocytes to the lungs. Eur J Haematol. 1993;51:233–246. doi: 10.1111/j.1600-0609.1993.tb00637.x. [DOI] [PubMed] [Google Scholar]
- 41.Howell W, Donahue D. The production of blood platelets in the lungs. J Exp Med. 1937;65:177–204. doi: 10.1084/jem.65.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lefrançais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah D, Thornton E, Headley M, David T, Coughlin S, Krummel M, Leavitt A, Passegué E, Looney M. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature. 2017;544:105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishimura S, Nagasaki M, Kunishima S, Sawaguchi A, Sakata A, Sakaguchi H, Ohmori T, Manabe I, Italiano J, Ryu T, Takayama N, Komuro I, Kadowaki T, Eto K, Nagai R. IL-1a induces thrombopoiesis through megakaryocyte rupture in response to acute platelet needs. J Cell Biol. 2015;209:453–466. doi: 10.1083/jcb.201410052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nakeff A, Maat B. Separation of megakaryocytes from mouse bone marrow by velocity sedimentation. Blood. 1974;43:591–595. [PubMed] [Google Scholar]
- 45.Murry C, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–680. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Mills J, Paluru P, Weiss M, Gadue P, French D. Hematopoietic differentiation of pluripotent stem cells in culture. Methods Mol Biol. 2014;1185:181–194. doi: 10.1007/978-1-4939-1133-2_12. [DOI] [PubMed] [Google Scholar]
- 47.Gori J, Butler J, Chan YY, Chandrasekaran D, Poulos M, Ginsberg M, Nolan D, Elemento O, Wood B, Adair J, Rafii S, Kiem H-P. Vascular niche promotes hematopoietic multipotent progenitor formation from pluripotent stem cells. J Clin Invest. 2015;125:1243–1254. doi: 10.1172/JCI79328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zambidis E, Peault B, Park TS, Bunz F, Civin C. Hematopoietic differentiation of human embryonic stem cells progresses through sequential hematoendothelial, primitive, and definitive stages resembling human yolk sac development. Blood. 2008;106:860–870. doi: 10.1182/blood-2004-11-4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paluru P, Hudock K, Cheng X, Mills J, Ying L, Galvao A, Tiyaboonchai A, Sim X, Sullivan S, French D, Gadue P. The negative impact of wnt signaling on megakaryocyte and primitive erythroid progenitors derived from human embryonic stem cells. Stem Cell Res. 2014;12:441–451. doi: 10.1016/j.scr.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vodyanik M, Bork J, Thomson J, Slukvin I. Human embryonic stem cell-derived CD34+ cells: efficient production in the coculture with OP9 stromal cells and analysis of lymphohematopoietic potential. Blood. 2005;105:617–626. doi: 10.1182/blood-2004-04-1649. [DOI] [PubMed] [Google Scholar]
- 51.Vodyanik M, Thomson J, Slukvin I. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108:2095–2105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sturgeon C, Ditadi A, Awong G, Kennedy M, Keller G. Wnt signaling controls the specification of definitive and primitive hematopoiesis from human pluripotent stem cells. Nat Biotechnol. 2014;32:554–561. doi: 10.1038/nbt.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ditadi A, Sturgeon C, Tober J, Awong G, Kennedy M, Yzaguirre A, Azzola L, Ng E, Stanley E, French D, Cheng X, Gadue P, Speck N, Elefanty A, Keller G. Human definitive haemogenic endothelium and arterial vascular endothelium represent distinct lineages. Nat Cell Biol. 2015;17:580–591. doi: 10.1038/ncb3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ng ES, Azzola L, Bruveris F, Calvanese V, Phipson B, Vlahos K, Hirst C, Jokubaitis V, Yu QC, Maksimovic J, Liebscher S, Januar V, Zhang Z, Williams B, Conscience A, Durnall J, Jackson S, Costa M, Elliott D, Haylock D, Nilsson S, Saffery R, Schenke-Layland K, Oshlack A, Mikkola H, Stanley E, Elefanty A. Differentiation of human embryonic stem cells to HOXA(+) hemogenic vasculature that resembles the aorta-gonad-mesonephros. Nat Biotechnol. 2016;34:1168–1179. doi: 10.1038/nbt.3702. [DOI] [PubMed] [Google Scholar]
- 55.Kennedy M, Awong G, Sturgeon C, Ditadi A, LaMotte-Mohs R, Zuniga-Pflucker JC, Keller G. T lymphocyte potential marks the emergence of definitive hematopoietic progenitors in human pluripotent stem cell differentiation cultures. Cell Rep. 2012;2:1722–1735. doi: 10.1016/j.celrep.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Hayes V, Jarocha D, Sim X, Harper D, Fuentes R, Sullivan S, Gadue P, Chou ST, Torok-Storb B, Marks M, French D, Poncz M. Comparative analysis of human ex vivo-generated platelets vs megakaryocyte-generated platelets in mice: a cautionary tale. Blood. 2015;125:3627–3636. doi: 10.1182/blood-2014-08-593053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moreau T, Evans A, Vasquez L, Tijssen M, Yan Y, Trotter M, Howard D, Colzani M, Arumugam M, Wu WH, Dalby A, Lampela R, Bouet G, Hobbs C, Pask D, Payne H, Ponomaryov T, Brill A, Soranzo N, Ouwehand W, Pedersen R, Ghevaert C. Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat Commun. 2016;7:1–15. doi: 10.1038/ncomms11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nakamura S, Takayama N, Hirata S, Seo H, Endo H, Ochi K, Fujita KI, Koike T, Harimoto KI, Dohda T, Watanabe A, Okita K, Takahashi N, Sawaguchi A, Yamanaka S, Nakauchi H, Nishimura S, Eto K. Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell. 2014;14:535–548. doi: 10.1016/j.stem.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Stachura D, Chou S, Weiss M. Early block to erythromegakaryocytic development conferred by loss of transcription factor GATA-1. Blood. 2006;107:87–97. doi: 10.1182/blood-2005-07-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noh J-Y, Gandre-Babbe S, Wang Y, Hayes V, Yao Y, Gadue P, Sullivan S, Chou S, Machlus K, Italiano J, Kyba M, Finkelstein D, Ulirsch J, Sankaran V, French D, Poncz M, Weiss M. Inducible Gata1 suppression expands megakaryocyte- erythroid progenitors from embryonic stem cells. J Clin Invest. 2015;125:2369–2374. doi: 10.1172/JCI77670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wechsler J, Greene M, McDevitt M, Anastasi J, Karp J, Le Beau M, Crispino J. Acquired mutations in GATA1 in the megakaryoblastic leukemia of down syndrome. Nat Genet. 2002;32:148–152. doi: 10.1038/ng955. [DOI] [PubMed] [Google Scholar]
- 62.Rainis L, Bercovich D, Strehl S, Teigler-Schlegel A, Stark B, Trka J, Amariglio N, Biondi A, Muler I, Rechavi G, Kempski H, Haas O, Izraeli S. Mutations in exon 2 of GATA1 are early events in megakaryocytic malignancies associated with trisomy 21. Blood. 2003;102:981–986. doi: 10.1182/blood-2002-11-3599. [DOI] [PubMed] [Google Scholar]
- 63.Hitzler J, Cheung J, Li Y, Scherer S, Zipursky A. GATA1 mutations in transient leukemia and acute megakaryoblastic leukemia of down syndrome. Blood. 2003;101:4301–4304. doi: 10.1182/blood-2003-01-0013. [DOI] [PubMed] [Google Scholar]
- 64.Hirata S, Murata T, Suzuki D, Nakamura S, Jono-Ohnishi R, Hirose H, Sawaguchi A, Nishimura S, Sugimoto N, Eto K. Selective Inhibition of ADAM17 Efficiently Mediates Glycoprotein Ibα Retention During Ex Vivo Generation of Human Induced Pluripotent Stem Cell-Derived Platelets. Stem Cells Transl Med. 2017;6:720–730. doi: 10.5966/sctm.2016-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Song W-J, Sullivan M, Legare R, Hutchings S, Tan X, Kufrin D, Ratajczak J, Resende I, Haworth C, Hock R, Loh M, Felix C, Roy D-C, Busque L, Kurnit D, Willman C, Gewirtz A, Speck N, Bushweller J, Li F, Gardiner K, Poncz M, Maris J, Gilliland G. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23:166–175. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- 66.Glembotsky A, Bluteau D, Espasandin Y, Goette N, Marta R, Marin Oyarzun CP, Korin L, Lev P, Laguens R, Molinas F, Raslova H, Heller P. Mechanisms underlying platelet function defect in a pedigree with familial platelet disorder with a predisposition to acute myelogenous leukemia: potential role for candidate RUNX1 targets. J Thromb Haemost. 2014;12:761–772. doi: 10.1111/jth.12550. [DOI] [PubMed] [Google Scholar]
- 67.Matheny C, Speck M, Cushing P, Zhou Y, Corpora T, Regan M, Newman M, Roudaia L, Speck C, Gu T-L, Griffey S, Bushweller J, Speck N. Disease mutations in RUNX1 and RUNX2 create nonfunctional, dominant-negative, or hypomorphic alleles. EMBO J. 2007;26:1163–1175. doi: 10.1038/sj.emboj.7601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakurai M, Kunimoto H, Watanabe N, Fukuchi Y, Yuasa S, Yamazaki S, Nishimura T, Sadahira K, Fukuda K, Okano H, Nakauchi H, Morita Y, Matsumura I, Kudo K, Ito E, Ebihara Y, Tsuji K, Harada Y, Harada H, Okamoto S, Nakajima H. Impaired hematopoietic differentiation of RUNX1-mutated induced pluripotent stem cells derived from FPD/AML patients. Leukemia. 2014;28:2344–2354. doi: 10.1038/leu.2014.136. [DOI] [PubMed] [Google Scholar]
- 69.Iizuka H, Kagoya Y, Kataoka K, Yoshimi A, Miyauchi M, Taoka K, Kumano K, Yamamoto T, Hotta A, Arai S, Kurokawa M. Targeted gene correction of RUNX1 in induced pluripotent stem cells derived from familial platelet disorder with propensity to myeloid malignancy restores normal megakaryopoiesis. Exp Hematol. 2015;43:849–857. doi: 10.1016/j.exphem.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 70.Connelly J, Kwon E, Gao Y, Trivedi N, Elkahloun A, Horwitz M, Cheng L, Liu P. Targeted correction of RUNX1 mutation in FPD patient-specific induced pluripotent stem cells rescues megakaryopoietic defects. Blood. 2014;124:1926–1930. doi: 10.1182/blood-2014-01-550525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Breton-Gorius J, Favier R, Guichard J, Cherif D, Berger R, Debili N, Vainchenker W, Douay L. A new congenital dysmegakaryopoietic thrombocytopenia (Paris-Trousseau) associated with giant platelet alpha-granules and chromosome 11 deletion at 11q23. Blood. 1995;85:1805–1814. [PubMed] [Google Scholar]
- 72.Stockley J, Morgan N, Bem D, Lowe G, Lordkipanidzé M, Dawood B, Simpson M, Macfarlane K, Horner K, Leo V, Talks K, Motwani J, Wilde J, Collins P, Makris M, Watson S, Daly M. Enrichment of FLI1 and RUNX1 mutations in families with excessive bleeding and platelet dense granule secretion defects. Blood. 2013;122:4090–4093. doi: 10.1182/blood-2013-06-506873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stevenson W, Rabbolini D, Beutler L, Chen Q, Gabrielli S, Mackay J, Brighton T, Ward C, Morel-Kopp M-C. Paris-Trousseau thrombocytopenia is phenocopied by the autosomal recessive inheritance of a DNA-binding domain mutation in FLI1. Blood. 2015;126:2027–2030. doi: 10.1182/blood-2015-06-650887. [DOI] [PubMed] [Google Scholar]
- 74.Vo K, Jarocha D, Lyde R, Hayes V, Thom C, Sullivan S, French D, Poncz M. FLI1 level during megakaryopoiesis affects thrombopoiesis and platelet biology. Blood. 2017;129:3486–3494. doi: 10.1182/blood-2017-02-770958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Coller B, Shattil S. The GPIIb/IIIa odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112:3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Massberg S, Schürzinger K, Lorenz M, Konrad I, Schulz C, Plesnila N, Kennerknecht E, Rudelius M, Sauer S, Braun S, Kremmer E, Emambokus NR, Frampton J, Gawaz M. Platelet adhesion via glycoprotein IIb integrin is critical for atheroprogression and focal cerebral ischemia: an in vivo study in mice lacking glycoprotein IIb. Circulation. 2005;112:1180–1188. doi: 10.1161/CIRCULATIONAHA.105.539221. [DOI] [PubMed] [Google Scholar]
- 77.Sullivan S, Mills J, Koukouritaki S, Vo K, Lyde R, Paluru P, Zhao G, Zhai L, Sullivan L, Wang Y, Kishore S, Gharaibeh E, Lambert M, Wilcox D, French D, Poncz M, Gadue P. High-level transgene expression in induced pluripotent stem cell-derived megakaryocytes : correction of Glanzmann thrombasthenia. Blood. 2014;123:753–757. doi: 10.1182/blood-2013-10-530725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Orban M, Goedel A, Haas J, Sandrock-Lang K, Gartner F, Jung C, Zieger B, Parrotta E, Kurnik K, Sinnecker D, Wanner G, Laugwitz K-L, Massberg S, Moretti A. Functional comparison of induced pluripotent stem cell- and blood-derived GPIIbIIIa deficient platelets. PLoS One. 2015;10:1–17. doi: 10.1371/journal.pone.0115978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu L, Du L, Zhao Y, Li W, Ouyang Q, Zhou D, Lu G, Lin G. Modeling Glanzmann thrombasthenia using patient specific iPSCs and restoring platelet aggregation function by CD41 overexpression. Stem Cell Res. 2017;20:14–20. doi: 10.1016/j.scr.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Thon J, Dykstra B, Beaulieu L. Platelet bioreactor: accelerated evolution of design and manufacture. Platelets. 2017;28:472–477. doi: 10.1080/09537104.2016.1265922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feng Q, Shabrani N, Thon J, Huo H, Thiel A, Machlus K, Kim K, Brooks J, Li F, Luo C, Kimbrel E, Wang J, Kim K-S, Italiano J, Cho J, Lu S-J, Lanza R. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Reports. 2014;3:817–831. doi: 10.1016/j.stemcr.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.