Abstract
Backgrounds
Predicting the occurrence of severe postreperfusion syndrome (PRS) is clinically challenging. We investigated whether the flushed fluid potassium concentration (FFK) was associated with severe PRS in deceased donor liver transplantation (DDLT).
Material/Methods
Forty adult DDLT recipients were enrolled in this retrospective study. Effluent solution samples were collected at the end of the portal vein flush, and the FFK was determined using a point-of-care blood gas analyzer. The risk factors associated with severe PRS and the clinical outcomes in 2 groups were compared.
Results
Severe PRS occurred in 22 out of 40 patients (55.0%). The FFK of the severe PRS group was significantly higher than that of the non-severe PRS group (median, 9.6 vs. 5.8, P<0.001). Other variables associated with severe PRS included the donor risk index (DRI), Child-Turcotte-Pugh score, donor type, donor warm ischemia time, and Model for End-stage Liver Disease score. The area under the receiver operator characteristic curve for the FFK was 0.982, and the best cut-off value of the FFK for predicting severe PRS was 6.75 mmol/L (100.0% sensitivity and 88.9% specificity). A significant positive correlation was observed between the FFK and DRI (R=0.714). Patients who experienced severe PRS had a higher early allograft dysfunction rate (63.6% vs. 22.2%, P=0.019) and a longer hospital stay (median, 33.0 vs. 24.0, P=0.034).
Conclusions
Both the severity of the recipient’s liver disease and the donor graft factors play an important role in the development of severe PRS in DDLT. An FFK of more than 6.75 mmol/L was associated with severe PRS after reperfusion.
MeSH Keywords: Intraoperative Complications, Liver Transplantation, Outcome Assessment (Health Care), Risk Factors
Background
Postreperfusion syndrome (PRS) is a crucial intraoperative complication of liver transplantation (LT) and has often been associated with poor patient and liver allograft outcomes [1–4].PRS was first described by Aggarwal et al. [5] in 1987 as cardiovascular collapse and was defined as a greater than 30% decrease in the mean arterial pressure (MAP) below the baseline value within 5 min of reperfusion that persists for at least 1 min. More recently, PRS was further classified as mild or severe, with severe PRS defined as severe hemodynamic instability, including persistent hypotension, significant arrhythmias or asystole, and prolonged or recurrent fibrinolysis [1].
The causes of PRS are complex, and the syndrome has been generally attributed to the release of cold, hyperkalemic, acidotic, and vasoactive substances from the preservation solution, the donor liver, or the recipient’s ischemic intestinal system [6]. However, the exact mechanism of PRS remains to be elucidated, and there is a dearth of accurate predictors of severe PRS. During the past decade, LT from donation after circulatory death (DCD) grafts has increased dramatically worldwide due to the severe shortage of deceased liver grafts [7–9]. Unfortunately, DCD donors are always regarded as marginal donors or extended criteria donors (ECDs). Previous studies found that patients who receive DCD liver grafts experience significantly higher rates of severe PRS than those who receive donation after brain death (DBD) liver grafts [10,11]. Consequently, growing interest has developed in the relationship between liver graft factors and the development of severe PRS, including factors such as donor age [12], graft cold ischemia time (CIT) [2,4], graft warm ischemia time (WIT) [1], donor risk index (DRI) [12], and degree of steatosis [13,14].
Flushing the liver graft before reperfusion has been recommended as the most effective way to reduce the incidence of PRS [6,15–18]. A previous study found that a portal vein flush (PVF) with 500 mL of 5% albumin solution was sufficient to avoid hyperkalemia and PRS after reperfusion in the adult population [15]. However, we have encountered hyperkalemia and severe PRS more frequently in the new DCD era [10,11,14], despite the routine application of the PVF technique. We speculate that the release of intracellular potassium and other vasoactive substances from the damaged hepatocytes derived from DCD or ECD grafts may be a crucial cause of severe PRS. To investigate this hypothesis, effluent solution samples were collected, and the flushed fluid potassium concentration (FFK) was routinely monitored. The main aim of this study was to determine whether the FFK was associated with severe PRS in a cohort of adult deceased donor liver transplantation (DDLT) recipients.
Material and Methods
Patients
This retrospective study was approved by the Institutional Review Board of Beijing Friendship Hospital, Beijing, China (2017-P2-044-01). Adult patients aged 18 to 70 years who underwent DDLT at Beijing Friendship Hospital between October 2016 and June 2017 were eligible to participate. Exclusion criteria were patients who were younger than 18 years, those whose surgeries were cancelled after anesthesia induction, and those who underwent LT from liver grafts preserved with histidine-tryptophan-ketoglutarate solution.
Surgical techniques and sampling
Deceased liver grafts were procured from DBD or DCD donors, preserved with cold University of Wisconsin (UW) solution and implanted using the standard technique without venovenous bypass. After the portal vein (PV), infrahepatic vena cava (IHVC), and suprahepatic vena cava (SHVC) were clamped in turn, the native liver was removed and the new liver graft was implanted. Subsequently, the SHVC, IHVC, and PV of the donor liver graft were anastomosed in sequence. The liver graft was flushed via the PV with room temperature albumin solution when the IHVC anastomosis was nearly complete. At our center, the flush fluid contains 5% albumin, and the flush volume is 1 mL per g of the liver graft. Beginning in October 2016, effluent solution samples were routinely collected for immediate analysis at the end of the PVF, and the FFK was determined using a point-of-care blood gas analyzer (GEM Premier 3000, Instrumentation Laboratory, Bedford, MA, USA).
Anesthesia protocol
All the patients were treated by the same anesthesiology team using a standardized anesthesia protocol. Anesthesia was induced with midazolam, etomidate, sufentanil, and cisatracurium. After intubation, mechanical ventilation was initiated at a tidal volume of 8–10 mL/kg, a respiratory rate of 10–12 per min, and an inspired oxygen fraction of 0.6. Anesthesia was maintained with propofol, remifentanil, and cisatracurium infusions. Monitoring for all patients included electrocardiogram, pulse oximeter, end-tidal carbon dioxide, body temperature, urine output, and invasive pressures via arterial line and pulmonary artery catheter. Intraoperative monitoring data were automatically recorded at 10-s intervals using an anesthesia information management system (AIMS.NET, Easy Monitor, Ltd., Beijing, China). Intravenous fluids (4% Gelofusine and 20% human albumin solution as colloid and lactated Ringer’s solution as crystalloid) were used for volume replacement. Packed red blood cells (RBCs) were administered to maintain a hemoglobin level ≥80 g/L. Fresh frozen plasma (FFP) was administered to treat significant coagulation disorders detected with a Sonoclot coagulation analyzer (Sienco, Inc., Arvada, CO, USA). Fibrinolysis prophylaxis with tranexamic acid was routinely administered to patients without a history of thrombosis.
Prophylaxis and management of severe PRS
The diagnosis of severe PRS was mainly based on Hilmi’s Criteria [1] when significant arrhythmia or persistent severe hypotension was present after revascularization of the liver graft. Significant arrhythmias included a decrease in heart rate (HR) exceeding 30% of the pre-reperfusion level, new-onset hemodynamically significant arrhythmias, or asystole. Persistent severe hypotension was defined as a drop in MAP exceeding 30% of the pre-reperfusion level that persisted for at least 5 min and was unresponsive to an accumulated intravenous bolus of 100 μg epinephrine. They also included postreperfusion severe hypotension requiring prolonged norepinephrine (NE) infusion until the end of surgery.
During the anhepatic period, hypothermia, acidosis, hyperkalemia, and hypocalcemia were checked and corrected in a timely manner. Before reperfusion, patients with an HR <70 bpm were given 0.5 mg atropine intravenously. If the systolic blood pressure (SAP) remained <90 mmHg despite volume loading, the patients were treated with repeated intravenous boluses of phenylephrine, followed by continuous NE infusion. If both HR and SAP were low, repeated boluses of epinephrine were administered intravenously. There was no systematic pretreatment with mannitol or nafamostat and no use of the retrograde reperfusion technique [16,17].
If severe PRS occurred with severe hypotension during the immediate postreperfusion period, epinephrine was the drug of first choice, and 10 μg of epinephrine was injected. If PRS persisted, doubled doses of epinephrine were repeatedly administered every minute for 5 min until MAP was restored to >70% of baseline. Persistent severe hypotension despite 5 doses of epinephrine was treated with titrated infusion of NE. If severe hypotension and anuria persisted when the dose of NE increased to ≥0.5 μg/kg/min, infusion of vasopressin was started in response to a suspected diagnosis of vasoplegic syndrome (VS) [18,19]. If severe PRS occurred with significant arrhythmias due to hyperkalemia during the immediate postreperfusion period, intravenous boluses of calcium chloride and epinephrine together with speed control reperfusion technique [20,21]were used.
Data collection
Recipient, donor, and graft variables were collected, including recipient age, recipient sex, recipient height, recipient weight, Child-Turcotte-Pugh (CTP) score, Model for End-stage Liver Disease (MELD) score, indications for LT, donor age, donor sex, donor body mass index, donor type (DBD or DCD), graft weight, graft-to-recipient weight ratio (GRWR), donor WIT, graft CIT, graft WIT, and DRI. Intraoperative details, including flushed fluid measurements before reperfusion, hemodynamic and blood gas parameters during the reperfusion period, and the use of vasopressor agents after reperfusion, were also recorded. Postoperative data included the highest alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TB), lactic dehydrogenase (LDH), and gamma-glutamyl transpeptidase (GGT) levels during the first week after the operation, mechanical ventilation time, intensive care unit (ICU) stay duration, hospital stay duration, and the occurrence of early allograft dysfunction (EAD) and acute kidney injury (AKI). Donor WIT was defined as the time from the withdrawal of life support in DCD donors or the occlusion of the portal vein in DBD donors to the start of cold perfusion of the donor liver. Graft CIT was defined as the time from the start of cold perfusion of the donor liver to reperfusion of the liver graft in the recipient. Graft WIT was defined as the time from the liver graft’s removal from the ice box to reperfusion in the recipient. DRI was calculated to evaluate the quality of the live grafts [12]. EAD was assessed using Olthoff’s definition [22]. AKI was defined according to the Kidney Disease: Improving Global Outcomes (KDIGO) criteria [23].
Statistical analyses
All values are expressed as the mean ± standard deviation, median (interquartile range), or number and percentage, as appropriate. Parametric and nonparametric variables were compared using independent t tests or Mann-Whitney U tests, respectively. Qualitative variables were analyzed using χ2 tests and Fisher exact tests. The performance and the best cut-off value of significant variables for predicting severe PRS were evaluated using the receiver operator characteristic (ROC) curve. The Spearman correlation coefficient was used to assess the strength of the associations of severe PRS and donor characteristics with the FFK. Two-sided p-values of less than 0.05 were considered significant. All statistical analyses were performed using SPSS software version 17 (SPSS, Inc., Chicago, IL, USA).
Results
Baseline characteristics
From October 2016 to June 2017, 49 consecutive adult patients underwent LT. Seven patients who underwent LT from living-related or domino donors and 2 for whom FFK was not measured were excluded (Figure 1). Finally, a total of 40 patients enrolled in this study were analyzed. Thirteen patients (32.5%) had hepatocellular carcinoma and 8 patients (20.0%) had hepatitis B virus cirrhosis. Twenty-eight patients (70.0%) were male, and the patients’ mean age was 52.9±10.2 years. The mean recipient body weight was 67.3±12.5 kg, mean liver graft weight was 1290.9±247.9 g, and mean GRWR was 1.97±0.53%. The median CTP score and MELD score were 7 (6–10) and 13 (9–17), respectively. No patient required pressor support, dialysis, or mechanical ventilation preoperatively. Other baseline characteristics are shown in Table 1.
Figure 1.
Flowchart.
CTP – Child-Turcotte-Pugh; DCD – donation after circulatory death; FFK – flushed fluid potassium concentration; GRWR – graft-to-recipient weight ratio; HBV – hepatitis B virus; HCC – hepatocellular carcinoma; HCV – hepatitis C virus; MELD – Model for End-stage Liver Disease.
Table 1.
Baseline characteristics of patients.
| Variables | Patients (n = 40) |
|---|---|
| Recipient age: mean ±SD, y | 52.9±10.2 |
| Recipient sex: male, n (%) | 28 (70.0) |
| Underlying diseases, n (%) | |
| HCC | 13 (32.5) |
| HBV cirrhosis | 8 (20.0) |
| Primary biliary cirrhosis | 6 (15.0) |
| Alcoholic cirrhosis | 4 (10.0) |
| HCV cirrhosis | 1 (2.5) |
| Cryptogenic cirrhosis | 1 (2.5) |
| Other | 7 (17.5) |
| Recipient height: Med [IQR], cm | 170 (163.0–173.8) |
| Recipient weight: mean ±SD, kg | 67.3±12.5 |
| CTP score: Med [IQR] | 7 (6–10) |
| MELD score: Med [IQR] | 13 (9–17) |
| Donor type: DCD, n (%) | 12 (30.0) |
| Graft weight: mean ±SD, g | 1290.9±247.9 |
| GRWR: mean ±SD,% | 1.97±0.53 |
| FFK: Med [IQR], mmol/L | 7.2 (5.8–10.2) |
Incidence of severe PRS and postreperfusion complications
Twelve patients experienced persistent severe hypotension, 5 patients developed significant arrhythmias, and 5 patients experienced both persistent severe hypotension and significant arrhythmias. Thus, a total of 22 patients (55.0%) met the criteria for severe PRS. The MAP level at 5 min after reperfusion was significantly lower among severe PRS patients compared with the non-severe PRS patients (median, 61.5 vs. 79.5, P<0.001). The dose of epinephrine immediately after reperfusion was also higher among the severe PRS patients (0.73±0.55 vs. 0.22±0.22, P<0.001). The doses of NE after reperfusion and at the end of surgery in the severe PRS group were higher than that in the non-severe PRS group (median, 0.30 vs. 0.05, P<0.001; 0.10 vs. 0.00, P<0.001, respectively). Accordingly, more patients in the severe PRS group required vasopressin treatment during the reperfusion period (36.4% vs. 0.0%, P=0.005) and required NE infusion upon admission to the ICU (54.5% vs. 0.0%, P<0.001). Serum potassium and lactate levels were significantly higher at 5 min after reperfusion in the severe PRS group (median, 4.2 vs. 3.7, P=0.043; 3.2 vs. 2.8, P=0.031, respectively). Additionally, patients in the severe PRS group had more intraoperative blood loss and required higher amounts of RBCs and FFP (median, 1450.0 vs. 850.0, P=0.018; median, 7.0 vs. 3.5, P=0.017; median, 600.0 vs. 0.0, P=0.040, respectively) (Tables 2, 3). Although 10 patients in the severe PRS group had severe arrhythmias related to hyperkalemia, no patient in either group experienced cardiac arrest after pharmacological and surgical interventions.
Table 2.
Hemodynamics, electrolyte and metabolic changes during the reperfusion period.
| Variables | Severe PRS (n=22) | Non-severe PRS (n=18) | P value |
|---|---|---|---|
| One minute before reperfusion | |||
| HR: mean ±SD, bpm | 99.3±11.8 | 95.1±12.2 | 0.274 |
| MAP: mean ±SD, mmHg | 90.4±9.1 | 91.3±13.6 | 0.804 |
| CVP: mean ±SD, mmHg | 9.6±2.6 | 8.3±3.2 | 0.143 |
| K: mean ±SD, mmol/L | 4.2±0.7 | 4.2±0.6 | 0.872 |
| CA: Med [IQR], mmol/L | 1.16 (1.07–1.28) | 1.23 (1.07–1.53) | 0.283 |
| GLU: Med [IQR], mmol/L | 6.9 (5.4–7.7) | 6.7 (5.7–7.6) | 0.935 |
| LAC: Med [IQR], mmol/L* | 2.2 (2.0–2.8) | 2.0 (1.8–2.7) | 0.422 |
| One minute after reperfusion | |||
| HR: Med [IQR], bpm | 97 (81–102) | 101 (91–109) | 0.161 |
| MAP: mean ±SD, mmHg | 55.6±9.3 | 59.9±19.2 | 0.368 |
| CVP: mean ±SD, mmHg | 10.0±2.6 | 8.3±3.3 | 0.074 |
| K: mean ±SD, mmol/L** | 6.1±1.1 | 5.1±1.2 | 0.126 |
| CA: mean ±SD, mmol/L** | 1.16±0.11 | 1.15±0.16 | 0.960 |
| GLU: mean ±SD, mmol/L** | 8.6±2.2 | 9.2±3.3 | 0.660 |
| LAC: Med [IQR], mmol/L*,** | 3.6 (3.0–3.9) | 2.9 (2.7–4.8) | 0.639 |
| Five minutes after reperfusion | |||
| HR: mean ±SD, bpm | 103.0±12.1 | 101.5±15.0 | 0.736 |
| MAP: HR: mean ±SD, mmHg | 61.5 (57.1–72.6) | 79.5 (71.3–92.1) | <0.001 |
| CVP: mean ±SD, mmHg | 9.7±3.2 | 9.3±3.4 | 0.739 |
| K: mean ±SD, mmol/L | 4.2±0.8 | 3.7±0.6 | 0.043 |
| CA: Med [IQR], mmol/L | 1.18 (1.12–1.31) | 1.25 (1.13–1.35) | 0.693 |
| GLU: mean ±SD, mmol/L | 9.1±2.1 | 10.3±2.0 | 0.073 |
| LAC: Med [IQR], mmol/L* | 3.2 (2.8–3.8) | 2.8 (2.1–3.2) | 0.031 |
Measured using blood gas analysis;
Data are available from 15 out of 40 cases (severe PRS group 7 cases, non-severe PRS group 8 cases).
CA – serum calcium concentration; CVP – central venous pressure; GLU – serum glucose concentration; HR – heart rate; K – serum potassium concentration; LAC – serum lactate concentration; MAP – mean arterial pressure; PRS – postreperfusion syndrome.
Table 3.
Postreperfusion and postoperative outcomes of the two study groups.
| Variables | Severe PRS (n=22) | Non-severe PRS (n=18) | P value |
|---|---|---|---|
| Dose of epinephrine after reperfusion: mean ±SD, ug/kg | 0.73±0.55 | 0.22±0.22 | <0.001 |
| Dose of NE after reperfusion: Med [IQR], ug/kg/min | 0.30 (0.19–0.50) | 0.05 (0.00–0.11) | <0.001 |
| Vasopressin use after reperfusion: yes, n (%) | 8 (36.4) | 0 (0.0) | 0.005 |
| Dose of NE at end of surgery: Med [IQR], ug/kg/min | 0.10 (0.00–0.15) | 0.00 (0.00–0.00) | <0.001 |
| Pressor use at end of surgery: yes, n (%) | 12 (54.5) | 0 (0.0) | <0.001 |
| Duration of surgery (min) | 444.1±85.9 | 437.5±109.7 | 0.832 |
| Intraoperative blood loss (mL) | 1450.0 (1000.0–2300.0) | 850.0 (500.0–1500.0) | 0.018 |
| RBC transfusion (unit) | 7.0 (4.0–10.0) | 3.5 (0.0–6.5) | 0.017 |
| FFP transfusion (mL) | 600.0 (600.0–800.0) | 0.0 (0.0–800.0) | 0.040 |
| Posttransplant peak ALT | 833.5 (510.3–1475.5) | 633.5 (323.3–1035.8) | 0.135 |
| Posttransplant peak AST | 3174.4±2090.4 | 1478.2±886.1 | 0.002 |
| Posttransplant peak TB | 96.6 (63.4–176.1) | 55.8 (35.7–76.4) | 0.002 |
| Posttransplant peak LDH | 3019.0 (1818.3–3890.5) | 1722.5 (1086.8–2663.8) | 0.007 |
| Posttransplant peak GGT | 233.5 (166.8–371.3) | 255.0 (170.3–407.5) | 0.541 |
| EAD: yes, n (%) | 14 (63.6) | 4 (22.2) | 0.019 |
| Ventilation time: Med [IQR], hours | 4.0 (2.4–7.3) | 3.4 (2.0–6.5) | 0.558 |
| AKI: yes, n (%)* | 10 (47.6) | 7 (41.2) | 0.691 |
| ICU Stay: mean ±SD, days | 3.4±1.0 | 2.8±0.9 | 0.071 |
| Hospital Stay: Med [IQR], days | 33.0 (27.5–41.5) | 24.0 (20.0–40.5) | 0.034 |
After patients with pretransplant renal dysfunction were excluded, there were 21 patients in the severe PRS group and 17 patients in the non-severe PRS group.
AKI – acute kidney injury; ALT – alanine aminotransferase; AST – aspartate aminotransferase; EAD – early allograft dysfunction; FFP – fresh frozen plasma; GGT – gamma-glutamyl transpeptidase; ICU – intensive care unit; LAC – serum lactate concentration; LDH – lactic dehydrogenase; NE – norepinephrine; PRS – postreperfusion syndrome; RBC – red blood cell; TB – total bilirubin.
Early complications and postoperative outcomes
The postoperative peak ALT and peak GGT levels during the first week were comparable between the 2 groups. The serum peak AST, peak TB, and peak LDH levels of the severe PRS group were significantly higher than those of the non-severe PRS group (3174.4±2090.4 vs. 1478.2±886.1, P=0.002; median, 96.6 vs. 35.8, P=0.002; median, 3019.0 vs. 1722.5, P=0.007, respectively). Patients who experienced severe PRS displayed EAD more frequently (63.6% vs. 22.2%, P=0.019). However, no patient in either group experienced primary allograft nonfunction or required retransplant, and there were no in-hospital deaths. Postoperative outcomes, including AKI, rejection, ventilation time, and ICU stay, did not differ significantly between the 2 groups. Additionally, the duration of hospital stay for the severe PRS group was significantly longer than that for the non-severe PRS group (median, 33.0 vs. 24.0, P=0.034) (Table 3).
Risk factors associated with severe PRS
The FFK in the severe PRS group was remarkably higher than that in the non-severe PRS group (median, 9.6 vs. 5.8, P<0.001). Additionally, significant differences were found between the non-severe PRS and severe PRS groups for the CTP score (median, 8 vs. 6, P<0.001), MELD score (median, 15 vs. 9, P=0.006), DCD donor proportion (54.5% vs. 0.0%, P<0.001), donor WIT (median, 8 vs. 3, P=0.003), and DRI (2.81±0.55 vs. 2.10±0.37, P<0.001) (Table 4).
Table 4.
Comparison of baseline characteristics between the severe PRS and non-severe PRS groups of patients.
| Variables | Severe PRS (n=22) | Non-severe PRS (n=18) | P value |
|---|---|---|---|
| Recipient age: mean ±SD, y | 54.5±8.9 | 50.8±11.6 | 0.265 |
| Recipient sex: female, n (%) | 8 (36.4) | 4 (22.2) | 0.332 |
| Recipient height: Med [IQR], cm | 170 (162–173) | 170 (163–176) | 0.548 |
| Recipient weight: mean ±SD, kg | 64.5±13.3 | 71.1±11.4 | 0.094 |
| CTP score: Med [IQR] | 8 (7–10) | 6 (5–7) | <0.001 |
| MELD score: Med [IQR] | 15.0 (11.0–21.5) | 9.0 (7.8–14.3) | 0.006 |
| Donor age: mean±SD, y | 37.8±14.1 | 39.6±13.8 | 0.698 |
| Donor sex: female, n (%) | 2 (9.1) | 4 (22.2) | 0.381 |
| Donor BMI: mean±SD, kg/cm2 | 22.06±3.56 | 21.85±2.62 | 0.841 |
| Donor type: DCD, n (%) | 12 (54.5) | 0 (0.0) | <0.001 |
| DRI: mean±SD | 2.81±0.55 | 2.10±0.37 | <0.001 |
| Donor WIT: Med [IQR], min | 8.0 (3.0–18.5) | 3.0 (3.0–5.0) | 0.003 |
| Graft CIT: mean ±SD, min | 613.0±156.8 | 595.3±142.5 | 0.713 |
| Graft WIT: mean ±SD, min | 42.1±6.0 | 40.2±9.6 | 0.433 |
| Graft weight: mean ±SD, g | 1287.6±228.3 | 1294.9±276.7 | 0.928 |
| GRWR: mean ±SD,% | 2.08±0.53 | 1.84±0.50 | 0.147 |
| Flushed fluid glucose: mean ±SD, mmol/L | 8.7±4.5 | 7.0±3.2 | 0.184 |
| Flushed fluid lactate: Med [IQR], mmol/L | 2.6 (2.1–3.1) | 2.1 (1.6–2.7) | 0.051 |
| FFK: Med [IQR], mmol/L | 9.6 (7.7–13.7) | 5.8 (5.5–6.2) | <0.001 |
BMI – body mass index; CIT – cold ischemia time; CTP – Child-Turcotte-Pugh; DCD – donation after circulatory death; DRI – donor risk index; FFK – flushed fluid potassium concentration; GRWR – graft-to-recipient weight ratio; MELD – Model for End-stage Liver Disease; PRS – postreperfusion syndrome; WIT – warm ischemia time.
Predictive value of the FFK for severe PRS and its relationship to donor characteristics
Based on the area under the ROC curve (AUC), the FFK showed the best predictive ability for the presence of severe PRS (AUC, 0.982; 95% confidence interval [95% CI] 0.000–1.000; P<0.001; sensitivity, 100.0%; specificity, 88.9%), followed by the DRI (AUC, 0.856; 95% CI, 0.737–0.975; P<0.001; sensitivity, 81.8%; specificity, 88.9%), CTP score (AUC, 0.819; 95% CI, 0.676–0.963; P=0.001; sensitivity, 90.0%; specificity, 66.7%), donor WIT (AUC, 0.770; 95% CI, 0.623–0.917; P=0.004; sensitivity, 63.6%; specificity, 88.9%), and MELD score (AUC, 0.756; 95% CI, 0.599–0.913; P=0.006; sensitivity, 90.9%; specificity, 55.6%) (Table 5, Figure 2).
Table 5.
ROC analysis to compare the predictive ability of different parameters for severe PRS.
| Variables* | AUC | Cut-off value | Sensitivity (%) | Specificity (%) | 95% CI | P value |
|---|---|---|---|---|---|---|
| FFK | 0.982±0.016 | 6.75 | 100 | 88.9 | 0.000–1.000 | <0.001 |
| DRI | 0.856±0.061 | 2.438 | 81.8 | 88.9 | 0.737–0.975 | <0.001 |
| CTP score | 0.819±0.073 | 6.5 | 90.9 | 66.7 | 0.676–0.963 | 0.001 |
| Donor WIT | 0.770±0.075 | 5.5 | 63.6 | 88.9 | 0.623–0.917 | 0.004 |
| MELD score | 0.756±0.080 | 9.5 | 90.9 | 55.6 | 0.599–0.913 | 0.006 |
The following categorical variable was not further assessed using the ROC curve but was associated with severe PRS: donor type.
AUC – area under the curve; CI – confidence interval; CTP – Child-Turcotte-Pugh; DCD – donation after circulatory death; DRI – donor risk index; FFK – flushed fluid potassium concentration; MELD – Model for End-stage Liver Disease; ROC – receiver operating characteristic; WIT – warm ischemia time.
Figure 2.
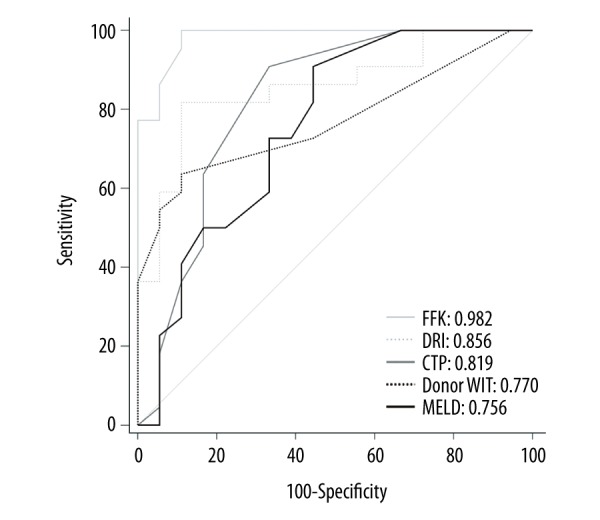
Receiver operating characteristic curves of the FFK, DRI, CTP score, donor WIT, and MELD score for predicting the presence of severe PRS in all 40 patients. FFK – flushed fluid potassium concentration; DRI – donor risk index; CTP – Child-Turcotte-Pugh; WIT – warm ischemia time; MELD – Model for End-stage Liver Disease.
A significant positive correlation was observed between the FFK before reperfusion and severe PRS after reperfusion (R=0.832; P<0.001). The ROC curve showed that the best cut-off value of the FFK for severe PRS was 6.75 mmol/L. Compared with patients with an FFK ≤6.75, those with elevated FFK showed a higher serum potassium concentration at 5 min after reperfusion (4.2±0.9 vs. 3.7±0.6, P=0.033) and experienced a significantly higher incidence of severe PRS (91.7% vs. 0.0%, P<0.001). The FFK before reperfusion exhibited a significant positive correlation with DRI (R=0.714; P<0.001), donor type (R=0.643; P<0.001), and donor WIT (R=0.506; P=0.001).
Discussion
The most important finding of this study was the novel observation that the FFK is strongly associated with the development of severe PRS in DDLT. Because the FFK can be easily obtained with a point-of-care blood gas analyzer, and the results can be obtained in only 2 to 3 min, this parameter appears particularly valuable for assessing the development of severe PRS before reperfusion and guiding pre-emptive interventions. The fact that the FFK is significantly correlated with the donor type, donor WIT, and DRI suggests that the FFK may serve as a useful parameter for characterizing donor graft profiles to predict severe PRS in DDLT, especially when a DCD or ECD liver graft is used. In addition, we confirmed that severe PRS is influenced not only by liver graft factors but also by the recipient’s severity of pretransplant liver disease.
Severe PRS is a dreadful intraoperative complication during LT for both transplant surgeons and anesthesiologists. The definition of PRS in LT has evolved throughout the years [1,5,10]. In our series, the incidence of severe PRS was 55.0%, which was comparable with the results reported by Hilmi et al. [1], although this value was slightly higher than that reported in another previously published investigation [3]. This difference may be attributable to the fact that a high proportion of the liver grafts in our study were procured from DCD donors. Consistently, both Blasi et al. [10] and Pan et al. [11] found that patients who received DCD grafts experienced significantly higher rates of PRS than those who received DBD grafts. Currently, donor factors have captured increasing attention in response to the increased worldwide utilization of DCD donors. Prolonged liver graft WIT [1] and CIT [2,4] have been reported as the most frequent risk factors for PRS, although this association remains somewhat controversial. Similarly, Fukazawa et al. [12] found that the DRI is a risk factor for the incidence and severity of PRS. In the DCD grafts, for example, uncontrollable longer donor WIT is unavoidable prior to organ procurement due to ethics rules; therefore, these grafts release more proinflammatory cytokines [20,24], nitric oxide [20,25], free radicals [20,26], and intracellular potassium ions [14], which may promote severe PRS after reperfusion.
Because the pathophysiologic mechanisms are not fully understood, it is still a particular challenge to predict and prevent the occurrence of severe PRS in actual clinical practice. In this study, we demonstrated that the FFK before reperfusion was significantly associated with severe PRS in patients who underwent DDLT. This association may be explained by the hyperkalemic blood from the grafts resulting from hepatocyte injury, as postreperfusion cardiac arrest or significant arrhythmia is usually attributed to hyperkalemia after reperfusion; however, the association could also be the result of higher levels of vasoactive substances released from the liver grafts. The PVF is one of the most widely used graft-washout techniques and has been reported to reduce the incidence of PRS via the clearance of the preservation solution and the necrotic elements of the damaged hepatocytes [15,27,28]. Homvises et al. [15] found that PVF with 500 mL of 5% albumin was sufficient to remove 90% of the UW solution and avoid hyperkalemia after reperfusion in the adult population with donor livers weighting approximately 1200 g to 1500 g. Although PVF using a certain volume was routinely used at our center, the FFK varied dramatically, and this might be explained by differences in liver graft hepatocyte injury. Severe PRS is usually accompanied by hyperkalemia, but whether hyperkalemia plays a leading role in the development of severe PRS remains controversial [129,30].
In addition to donor and liver graft factors, we also found that higher CTP and MELD scores were independently associated with severe PRS in DDLT. Consistent with our results, previous studies by Chung et al. [13] and Siniscalchi et al. [31] found that higher MELD scores were associated with PRS. These findings confirm that the severity of liver disease is significantly associated with severe PRS. Cases of advanced liver cirrhosis are often complicated by cardiovascular system abnormalities such as a hyperdynamic circulation state, cirrhotic cardiomyopathy, and relative vasopressin deficiency, thus increasing the risk that PRS will develop as a result of systolic and/or diastolic dysfunction, central hypovolemia, and hyporesponsiveness to vasoconstrictors [6,18]. Theoretically, the severity of a recipient’s liver disease or cardiovascular dysfunction can contribute to the development of severe PRS by inducing hemodynamic instability in DDLT. In fact, severe PRS can even occur in patients with normal liver and cardiovascular function during LT using DCD or ECD grafts. Therefore, donor and graft conditions may play a key role in the development of severe PRS and subsequently affect liver allograft and patient outcomes after DDLT, and both severe PRS and poor postoperative liver function may indicate a problem related to the graft condition.
There are several potential limitations to our study. First, this was a single-center, retrospective study with a relatively small sample size, thus leading to some confounding factors that could not be excluded by univariate analysis. Second, the management of patients with a higher FFK was not standardized. Five patients had FFK values greater than 13 mmol/L, which may even trigger cardiac arrest after reperfusion. Further research will be needed to confirm whether pre-emptive interventions should be applied to prevent the development of severe PRS, such as advanced PVF with more flushing fluids, retrograde reperfusion [16,17], vasopressor pretreatment [32,33], or the speed-controlled reperfusion technique [20,21]. Third, the definition of severe PRS in this study inevitably included a state of vasoplegia or even VS, which is usually related to advanced liver disease and ischemia-reperfusion injury of the liver graft. Admittedly, there appear to be some differences between the conventional definition of PRS [5] and VS [19], but it is exceedingly difficult to discriminate between them in a crisis situation because both PRS and vasoplegia occur within a few minutes after reperfusion, and their causes are partly attributable to the systemic inflammatory responses induced by ischemia-reperfusion injury. The definition of severe PRS [1] comprises a more serious form of PRS and vasoplegia and is more suitable for clinical use in DDLT, especially when a DCD or ECD donor liver is implanted. Fourth, we were not able to analyze the relationship between serum potassium concentration and severe PRS because the potassium concentration immediately after reperfusion was not routinely measured, and the temporarily elevated potassium level usually returns to normal in 1 to 2 min as a result of uptake by the new liver [34]. Further research will be needed to confirm whether a relationship exists between the change in serum potassium concentration and severe PRS. Lastly, some unmeasured components other than potassium ions might also be responsible for the development of severe PRS, suggesting that future studies are warranted to investigate the associations between other vasoactive substances in the effluent solution and severe PRS.
Conclusions
In conclusion, we showed that both the severity of the recipient’s liver disease and the donor graft factors were associated with the presence of severe PRS in adult DDLT recipients and that the FFK can serve as an accurate predictor of severe PRS, thus providing an early warning and allowing timely therapeutic interventions. Practically, an FFK cut-off of more than 6.75 mmol/L had a very good ability to predict severe PRS during LT from deceased donors. Close and regular monitoring of the FFK is required to identify patients at risk of severe PRS. These findings should be confirmed in additional studies.
Acknowledgments
We thank Drs. Lin Wei, Wei Qu, and Zhigui Zeng at the Liver Transplantation Center, National Clinical Research Center for Digestive Diseases, for their rewarding conversations and for providing the donor data. We thank Na Zeng in the Methodology Platform, National Clinical Research Center for Digestive Diseases, for her assistance in the statistical analysis.
Abbreviations
- AKI
acute kidney injury
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- AUC
area under the curve
- CI
confidence interval
- CIT
cold ischemia time
- CTP
Child-Turcotte-Pugh
- DBD
donation after brain death
- DCD
donation after circulatory death
- DDLT
deceased donor liver transplantation
- DRI
donor risk index
- EAD
early allograft dysfunction
- ECD
extended criteria donor
- FFK
flushed fluid potassium concentration
- GGT
gamma-glutamyl transpeptidase
- GRWR
graft-to-recipient weight ratio
- HR
heart rate
- ICU
intensive care unit
- IHVC
infrahepatic vena cava
- LDH
lactic dehydrogenase
- LT
liver transplantation
- MAP
mean arterial pressure
- MELD
Model for End-stage Liver Disease
- NE
norepinephrine
- PRS
postreperfusion syndrome
- PV
portal vein
- PVF
portal vein flush
- ROC
receiver operating characteristic
- SBP
systolic blood pressure
- SHVC
suprahepatic vena cava
- TB
total bilirubin
- UW
University of Wisconsin
- VS
vasoplegic syndrome
- WIT
warm ischemia time
Footnotes
Conflicts of interest
None.
Source of support: This study was supported by the Beijing Municipal Science and Technology Commission (Z171100001017036) and the Scientific Research Key Program of Beijing Municipal Commission of Education (201510025026)
References
- 1.Hilmi I, Horton CN, Planinsic RM, et al. The impact of postreperfusion syndrome on short-term patient and liver allograft outcome in patients undergoing orthotopic liver transplantation. Liver Transpl. 2008;14:504–8. doi: 10.1002/lt.21381. [DOI] [PubMed] [Google Scholar]
- 2.Paugam-Burtz C, Kavafyan J, Merckx P, et al. Postreperfusion syndrome during liver transplantation for cirrhosis: outcome and predictors. Liver Transpl. 2009;15:522–29. doi: 10.1002/lt.21730. [DOI] [PubMed] [Google Scholar]
- 3.Khosravi MB, Sattari H, Ghaffaripour S, et al. Post-reperfusion syndrome and outcome variables after orthotopic liver transplantation. Int J Organ Transplant Med. 2010;1:115–20. [PMC free article] [PubMed] [Google Scholar]
- 4.Xu ZD, Xu HT, Yuan HB, et al. Postreperfusion syndrome during orthotopic liver transplantation: A single-center experience. Hepatobiliary Pancreat Dis Int. 2012;11:34–39. doi: 10.1016/s1499-3872(11)60123-9. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal S, Kang Y, Freeman JA, et al. Postreperfusion syndrome: Cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc. 1987;19(4 Suppl 3):54–55. [PubMed] [Google Scholar]
- 6.Siniscalchi A, Gamberini L, Laici C, et al. Post reperfusion syndrome during liver transplantation: From pathophysiology to therapy and preventive strategies. World J Gastroenterol. 2016;22:1551–69. doi: 10.3748/wjg.v22.i4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson TA, Bekker P, Vagefi PA. Anesthetic considerations in organ procurement surgery: A narrative review. Can J Anaesth. 2015;62:529–39. doi: 10.1007/s12630-015-0345-8. [DOI] [PubMed] [Google Scholar]
- 8.Neuberger J. An update on liver transplantation: A critical review. J Autoimmun. 2016;66:51–59. doi: 10.1016/j.jaut.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Zeng L, Gao X, et al. Transformation of organ donation in China. Transpl Int. 2015;28:410–15. doi: 10.1111/tri.12467. [DOI] [PubMed] [Google Scholar]
- 10.Blasi A, Hessheimer AJ, Beltrán J, et al. Liver transplant from unexpected donation after circulatory determination of death donors: A challenge in perioperative management. Am J Transplant. 2016;16:1901–8. doi: 10.1111/ajt.13621. [DOI] [PubMed] [Google Scholar]
- 11.Pan X, Apinyachon W, Xia W, et al. Perioperative complications in liver transplantation using donation after cardiac death grafts: A propensity-matched study. Liver Transpl. 2014;20:823–30. doi: 10.1002/lt.23888. [DOI] [PubMed] [Google Scholar]
- 12.Fukazawa K, Yamada Y, Gologorsky E, et al. Hemodynamic recovery following postreperfusion syndrome in liver transplantation. J Cardiothorac Vasc Anesth. 2014;28:994–1002. doi: 10.1053/j.jvca.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Chung IS, Kim HY, Shin YH, et al. Incidence and predictors of post-reperfusion syndrome in living donor liver transplantation. Clin Transplant. 2012;26:539–43. doi: 10.1111/j.1399-0012.2011.01568.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang WJ, Xia WL, Pan HY, Zheng SS. Postreperfusion hyperkalemia in liver transplantation using donation after cardiac death grafts with pathological changes. Hepatobiliary Pancreat Dis Int. 2016;15:487–92. doi: 10.1016/s1499-3872(16)60116-9. [DOI] [PubMed] [Google Scholar]
- 15.Homvises B, Sirivatanauksorn Y, Limsrichamrern S, et al. The minimal flush volume for washout of preservation fluid in liver transplantation. Transplant Proc. 2008;40:2123–26. doi: 10.1016/j.transproceed.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 16.Daniela K, Michael Z, Florian I, et al. Influence of retrograde flushing via the caval vein on the post-reperfusion syndrome in liver transplantation. Clin Transplant. 2004;18:638–41. doi: 10.1111/j.1399-0012.2004.00231.x. [DOI] [PubMed] [Google Scholar]
- 17.Kniepeiss D, Iberer F, Grasser B, et al. A single-center experience with retrograde reperfusion in liver transplantation. Transpl Int. 2003;16:730–35. doi: 10.1007/s00147-003-0621-3. [DOI] [PubMed] [Google Scholar]
- 18.Valentine E, Gregorits M, Gutsche JT, et al. Clinical update in liver transplantation. J Cardiothorac Vasc Anesth. 2013;27:809–15. doi: 10.1053/j.jvca.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 19.de Armas LC, Castillo YA. Is it possible to distinguish between vasoplegic syndrome and postreperfusion syndrome during liver graft reperfusion? Anesth Analg. 2010;110:969–70. doi: 10.1213/ANE.0b013e3181c99816. [DOI] [PubMed] [Google Scholar]
- 20.Fiegel M, Cheng S, Zimmerman M, et al. Postreperfusion syndrome during liver transplantation. Semin Cardiothorac Vasc Anesth. 2012;16:106–13. doi: 10.1177/1089253212444791. [DOI] [PubMed] [Google Scholar]
- 21.Cordoví de Armas L, Jiménez Paneque RE, Gala López B, et al. Rapid and homogeneous reperfusion as a risk factor for postreperfusion syndrome during orthotopic liver transplantation. Rev Bras Anestesiol. 2010;60:154–61. 88–92. [PubMed] [Google Scholar]
- 22.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943–49. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- 23.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO clinical practice guidelines for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 24.Bezinover D, Kadry Z, McCullough P, et al. Release of cytokines and hemodynamic instability during the reperfusion of a liver graft. Liver Transpl. 2011;17:324–30. doi: 10.1002/lt.22227. [DOI] [PubMed] [Google Scholar]
- 25.Koelzow H, Gedney JA, Baumann J, et al. The effect of methylene blue on the hemodynamic changes during ischemia reperfusion injury in orthotopic liver transplantation. Anesth Analg. 2002;94:824–29. doi: 10.1097/00000539-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Sahmeddini MA, Zahiri S, Khosravi MB, et al. Effect of mannitol on postreperfusion cardiac output and central venous oxygen saturation during orthotopic liver transplant: A double-blind randomized clinical trial. Prog Transplant. 2014;24:121–25. doi: 10.7182/pit2014483. [DOI] [PubMed] [Google Scholar]
- 27.Gruttadauria S, Cintorino D, Musumeci A, et al. Comparison of two different techniques of reperfusion in adult orthotopic liver transplantation. Clin Transplant. 2006;20:159–62. doi: 10.1111/j.1399-0012.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 28.Fukazawa K, Nishida S, Hibi T, Pretto EA., Jr Crystalloid flush with backward unclamping may decrease post-reperfusion cardiac arrest and improve short-term graft function when compared to portal blood flush with forward unclamping during liver transplantation. Clin Transplant. 2013;27:492–502. doi: 10.1111/ctr.12130. [DOI] [PubMed] [Google Scholar]
- 29.Chui AK, Shi L, Tanaka K, et al. Postreperfusion syndrome in orthotopic liver transplantation. Transplant Proc. 2000;32:2116–17. doi: 10.1016/s0041-1345(00)01595-5. [DOI] [PubMed] [Google Scholar]
- 30.Acosta F, Sansano T, Contreras RF, et al. Changes in serum potassium during reperfusion in liver transplantation. Transplant Proc. 1999;31:2382–83. doi: 10.1016/s0041-1345(99)00391-7. [DOI] [PubMed] [Google Scholar]
- 31.Siniscalchi A, Dante A, Spedicato S, et al. Hyperdynamic circulation in acute liver failure: Reperfusion syndrome and outcome following liver transplantation. Transplant Proc. 2010;42:1197–99. doi: 10.1016/j.transproceed.2010.03.097. [DOI] [PubMed] [Google Scholar]
- 32.Ryu HG, Jung CW, Lee HC, Cho YJ. Epinephrine and phenylephrine pretreatments for preventing postreperfusion syndrome during adult liver transplantation. Liver Transpl. 2012;18:1430–39. doi: 10.1002/lt.23511. [DOI] [PubMed] [Google Scholar]
- 33.Fayed NA, Murad WS. Goal directed preemptive ephedrine attenuates the reperfusion syndrome during adult living donor liver transplantation. Egypt J Anaesth. 2014;30:187–95. [Google Scholar]
- 34.Scott VL, Wahl KM, Soltys K, et al. Anesthesia for organ transplantation. In: Davis PJ, Cladis FP, Motoyama EK, editors. Smith’s anesthesia for infants and children. 8th ed. Philadelphia, PA: Mosby/Elsevier; 2011. p. 924. [Google Scholar]


