Abstract
The FDA recommends rosuvastatin dosage reductions in Asian patients because pharmacokinetic studies have demonstrated an approximate two-fold increase in median exposure to rosuvastatin in Asian subjects when compared to Caucasian controls. Yet, no explanation for this ethnic difference has been confirmed.
Here we show that rosuvastatin exposure in Asians and Whites does not differ significantly when all subjects are wildtype carriers for both Solute Carrier Organic anion transporter1B1 *1a and ATP Binding Cassette Subfamily G Member 2 c.421 transporters in a two arm, randomized, cross-over rosuvastatin pharmacokinetics study in healthy White and Asian volunteers. For single rosuvastatin doses, AUC0–48 were 92.5(±36.2) and 83.5(±32.2) ng/mL*hr and Cmax were 10.0(±4.1) and 7.6(±3.0) ng/mL for Asians and Whites, respectively. When transporters were inhibited by intravenous rifampin, rosuvastatin AUC0–48 and Cmax also showed no ethnic differences. Our study suggests that both SLCO1B1 and ABCG2 polymorphisms are better predictors of rosuvastatin exposure than ethnicity alone and could be considered in precision medicine dosing of rosuvastatin.
Keywords: clinical pharmacokinetics, drug interaction, race, organic anion-transporting polypeptide transporters, ABC transporters, efflux pumps, hepatic transport, intestinal absorption
Introduction
Statins have been utilized worldwide in millions of patients to prevent cardiovascular disease and treat lipid disorders. A number of large clinical trials and post marketing surveys have demonstrated the substantial health benefit to statin use1–3. While adherence to statin therapy is a key factor associated with improved treatment outcomes, it is concerning that as many as 50 % of patients stop treatment within one year of statin initiation4. About 62% of former statin users state the reason they stopped their statin was due to side effects, including myopathy and potentially lethal rhabdomyolysis5. Onset of side effects has been associated with elevated statin blood levels6,7. Statin-induced myalgias were reported in 10–20% of statin-treated patients and led to treatment discontinuation in 30% of the symptomatic patients8. To reduce side effects and achieve an optimal dosing regimen for better adherence to statins in each individual, a holistic understanding of the underlying mechanism is warranted.
FDA recommends that Asian patients initiate therapy at half of the normal dose for non-Asians because the rosuvastatin drug label states that “Pharmacokinetic studies have demonstrated an approximate 2-fold increase in median exposure to rosuvastatin in Asian subjects when compared to Caucasian controls”. The molecular mechanism that leads to differential drug exposure between Asians and Whites remains unknown.
Previous studies have ruled out extrinsic factors including the environment, diet and variations in body weight as causing the interethnic rosuvastatin exposure differences9,10. Rather intrinsic factors of drug absorption, distribution, metabolism and elimination are suggested to play the major roles, with hepatic clearance of unchanged drug into the bile believed to be the major route of elimination. Since rosuvastatin is poorly metabolized and mainly excreted as unchanged drug, rather than metabolism, the transporting of rosuvastatin into and out of hepatocytes by drug transporters could be playing important roles in the observed interethnic differences.
Our understanding of drug pharmacokinetics has been advanced greatly since the 1990s by recognizing the roles of drug transporters in drug disposition. Drug transporters are expressed throughout the body in different organs and facilitate uptake or efflux of drugs into or out of the body. Rosuvastatin is a hydrophilic molecule, which strongly depends on drug transporters to cross cell membranes and reach its site of action11. The effects of hepatic uptake and efflux transporters on the pharmacokinetics and pharmacodynamics of rosuvastatin have been well characterized in the literature12,13. Uptake transporters, including organic anion transporting polypeptides (OATP) 1B1 and 1B3, as well as Na+-taurocholate cotransporting polypeptide, facilitate rosuvastatin uptake into hepatocytes, where the drug inhibits HMG-CoA reductase; while efflux transporters, such as breast cancer resistance protein (BCRP), eliminate rosuvastatin into the bile. OATP1B1 is the major hepatic uptake transporter, while BCRP is the major efflux transporter, expressed on the canalicular side of the liver and at the apical border of enterocytes14,15.
Genetic polymorphisms leading to reduced function in OATP1B1 and BCRP transporters have been shown to affect rosuvastatin pharmacokinetics and its subsequent pharmacologic effects.16 Due to their abundance and important roles, SLCO1B1 (gene encoding OATP1B1)17,18 and ABCG2 (gene encoding BCRP)19,20 reduced functional polymorphisms and their minor allele frequency have previously been proposed as the cause of interethnic variations in rosuvastatin pharmacokinetics and drug-drug interactions. The reduced function SNP frequency for both SLCO1B1 *15 (defined by c.388A>G and c.521T>C) and ABCG2 c.421C>A are more prevalent in Eastern Asians (14% and 35%, respectively) compared to Whites (2.7% and 14.0%)21,22. Another two studies show that at least 2-fold higher rosuvastatin exposure was still observed in Asians compared to Whites residing in the same environment after controlling only for the SLCO1B1 wildtype9,10. Tomita et al. suggested that SLCO1B1 and ABCG2 c.421 polymorphisms could not explain the observed plasma concentration variations between Asians and Caucasians22. Tomita et al. further proposed that in addition to genetic variants, protein expression could be another contributing factor. However, a recent study showed that OATP1B1 protein expression was similar between Asians and Whites23.
None of the previous clinical studies have prospectively evaluated both wildtype OATP1B1 and BCRP transporters to explain interethnic differences in rosuvastatin systemic exposure. Thus, here we prospectively investigate if interethnic differences in rosuvastatin drug exposure could be mitigated by controlling for both SLCO1B1 *1a/ *1a or *1a/ *1b together with ABCG2 c.421 wildtype. Our results could improve treatment adherence by providing a more sound basis for determining the appropriate dosage of rosuvastatin when taken alone or combined with other medications.
Materials and Methods
Study design
We conducted an investigator-initiated, prospective, two arm, crossover, randomized, controlled trial to evaluate the pharmacogenomic effect of drug transporters on rosuvastatin pharmacokinetics in Asian and White healthy volunteers. Recruitment was from the general public in the San Francisco/Bay area from November 2014 to July 2015. Each participant provided written informed consent.
Subjects were block randomly assigned to receive either an oral 20mg rosuvastatin tablet (Crestor®, AstraZeneca, Wilmington, DE) first or an oral 20mg rosuvastatin tablet immediately following a 30-min intravenous infusion of rifampin (Rifadin®, Sanofi-Aventis, Bridgewater, NJ) 600 mg in 10 ml sterile normal saline at a rate of 20 mg/min. The two periods were separated by at least a 7-day washout and all subjects completed both periods. To eliminate a food effect, subjects fasted from 8 hours prior to rosuvastatin dosing to 3 hours post dosing and standardized meals were provided. Venous blood samples (8 mL each) were collected into K3-EDTA tubes at t=0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 9, 12, 24, 32, 48 hours post dosing. Blood was centrifuged within 30 min at 4 °C and aliquot plasma samples were stored at −80 °C until bioanalysis.
Study subjects
Eight Asians and eight Whites, non-smoking, healthy volunteers, male and female, between the ages of 18–65 were enrolled. Eligibility was determined by medical history, physical examination, and clinical laboratory evaluation in a screening visit. Ethnicity was self-reported by the volunteers for both parents and all four grandparents; only European and East Asian descendants were studied. Since previous studies both in Asians and Whites showed little pharmacokinetic differences between SLCO1B1 * 1a and *1b allele9, we enrolled the volunteers carrying either SLCO1B1 *1a/*1a or *1a/ *1b allele and ABCG2 c.421CC genotype. Pre-menopausal females were tested for pregnancy before and during study enrollment, and maintained adequate birth control independent of hormonal contraceptive use during the study. Subjects with known allergies to the study medications and a history of rhabdomyolysis, gastrointestinal bleeding, peptic ulcer disease, and drug-related myalgia were excluded. Subjects abstained from caffeinated drinks, alcohol, herbal tea, and grapefruit one day prior to the study.
Genotyping of SLCO1B1 and ABCG2 polymorphisms
DNA extraction from blood samples and sequencing was conducted by the UCSF Genomics Core Lab (San Francisco, CA). All sample genotyping was carried out in a blinded fashion with use of coded ID samples. Regions containing SLCO1B1 c.388A>G, SLCO1B1 c.521T>C and ABCG2 c.421C>A were amplified using the following primers (Primer3 algorithm) on a 9700 thermal cycler (Applied Biosystems) with a touchdown PCR method:
SLCO1B1 rs2306283_F: 5’-AAACACATGCTGGGAAATTGAC-3’
SLCO1B1 rs2306283_R: 5’-TCATCCAGTTCAGATGGACAAA-3’
SLCO1B1 rs4149056_F: 5’- GCAGCATAAGAATGGACTAATACACC-3’
SLCO1B1 rs4149056_R : 5’-TCGCATGTGTGCTTAGAAAGAC-3’
ABCG2 rs2231142_F: 5’- TCATTGTTATGGAAAGCAACCA-3’
ABCG2 rs2231142_R: 5’- GGCAAATCCTTGTATGAAGCAG-3’
The PCR products were cleaned-up and sequenced with the BigDye Terminator reagent (Applied Biosystems). The sequence data were viewed and analyzed with the Sequencher program (GeneCodes).
Study end points
Primary end points were rosuvastatin systemic exposure measured as area under the curve (AUC) from 0 to 48 hours (AUC0–48) and 0 to infinity (AUC0–∞). Secondary outcomes were rosuvastatin peak plasma concentration, Cmax, time to peak concentration, Tmax, mean absorption time (MAT) and volume of distribution at steady state divided by bioavailability (Vss/F).
Study oversight
The study was approved by the Committee on Human Research of the University of California, San Francisco and conducted at the Clinical & Translational Science Institute‘s Clinical Research Center in compliance with the principles of the Declaration of Helsinki. This study was registered on the US National Institutes of Health Clinical Trials Database (NCT02215174; https://clinicaltrials.gov/ct2/show/NCT02215174.)
Plasma sample bioanalysis
Rosuvastatin concentrations were measured using a high-pressure liquid chromatography-tandem mass spectrometry method. The system consisted of QTrap 5500(AB Sciex, Redwood City, CA) with Shimadzu HPLC using electrospray ionization in the positive mode. Rosuvastatin and the internal standard, rosuvastatin-d3, were separated on a Kinetex C8 50×2.1mm column at ambient temperature. The mobile phase was a combination of (A) water and (B) acetonitrile both with 0.1% formic acid. The gradient ran from 15% to 95% for 1 minute. Ion detection was performed in the multiple reaction monitoring mode with Q1→Q3 transitions for rosuvastatin of 482.1 →258.2 m/z, and rosuvastatin-d3 of 485.1-->261.2 m/z. Plasma samples, calibration curves, and quality control (QCs) samples were prepared in the same way. The rosuvastatin method had a final LLOQ of 0.015ng/ml and ULOQ of 100ng/ml. The mean concentrations of QCs were within 15% of nominal concentrations and with coefficients of variation <15%.
Pharmacokinetic analysis
Rosuvastatin pharmacokinetic parameters were estimated from plasma concentration data by noncompartmental analysis using Phoenix® WinNonlin® (Pharsight, Mountain View, CA). The terminal rate constant (λz) was estimated by linear regression of the terminal phase of the log plasma concentration-time curve. AUC0–48 was calculated by the linear up /logarithmic down trapezoidal method. Summation of AUC0–48 and the concentration at the last measured point divided by λz yielded AUC0–∞. Rosuvastatin Tmax and Cmax were obtained directly from observed data. Oral clearance (CL/F) was calculated as dose/AUC0–∞. MAT was estimated as the reciprocal of the first-order absorption rate constant after the data were fit to a 2 compartment model with absorption from the gut compartment using Phoenix® WinNonlin®. Oral volume of distribution (Vss/F) was calculated as previously described24 as the ratio of the area under the first moment curve (AUMC0–∞) divided by AUC0–∞, multiplied by CL/F, than subtracting MAT.
Statistical analysis
Using a paired t-test and prior data24, the sample size was sufficient to detect a 50% difference in AUC0–48 between the two arms with a statistical power of 80%, alpha = 0.05, and standard deviation of 40%. Pharmacokinetic parameters were analyzed for differences between the two treatment periods by the paired t-test, except for Tmax where a Wilcoxon matched pair test was used. The mean ratios of all pharmacokinetic parameters from Whites over Asians were calculated.
Results
Participant demographics
During recruitment, 39 Asians and 21 Whites were screened. We found 8 eligible healthy volunteers in each ethnic group, who underwent randomization and completed the study. Asian volunteers were mainly Han-Chinese descendants (87.5%) with only one being Japanese (12.5%). All of the White volunteers were self-reported to be of European decent. The study population averaged 33.8 years old for Asians and 43.1 years old for Whites. Average weights were 63.4 kg for Asians and 68.1 kg for Whites. BMIs were similar, average of 22.3 for Asians and 23.6 for Whites (Table 1). The following results are reported based on eight Asian and seven White volunteers, because one White volunteer was mistakenly recruited rather than the identified subject with the appropriated genotype data. No statistical differences in these demographics between Asian and White volunteers were observed.
Table 1.
Demographics for all the volunteers.
| White | Asian | |
|---|---|---|
| N | 7 | 8 |
| Sex | ||
| Male | 4 | 3 |
| Female | 3 | 5 |
| Age | 43.1(14.2) | 33.8(9.3) |
| Weight | 68.1(9.7) | 63.4(14.2) |
| BMI | 23.6(2.0) | 22.3(3.4) |
| Scr | 0.82(0.11) | 0.8(0.2) |
| AST | 19.0(3.4) | 16.6(2.1) |
| ALT | 19.1(7.3) | 15.0(3.2) |
| LDL-C | 119.1(33.0) | 102.1(25.0) |
| HDL-C | 64.4(16.8) | 64.9(14.5) |
| TC | 196.3(32.5) | 184.1(33.2) |
| TG | 69.0(15.1) | 84.9(19.5) |
Mean values (±SD); Scr: Serum Creatinine; AST: Aspartate aminotransferase; ALT: alanine aminotransferase; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; TC: Total cholesterol; TG: Triglyceride
Genotype
Only volunteers with SLCO1B1*1a/*1a or *1a/*1b and ABCG2 c.421.CC wildtype were included in our analysis. In our recruitment, the frequencies of the target alleles, SLCO1B1 *1a and ABCG2 c.421CC, were different between Asians (35.9%) and Whites (52.4%). The linkage disequilibrium, reported as correlations between the two SNPs, were 0.170 in Asians and −0.25 in Whites for SLCO1B1 c.388 vs c.521.
Rosuvastatin pharmacokinetics
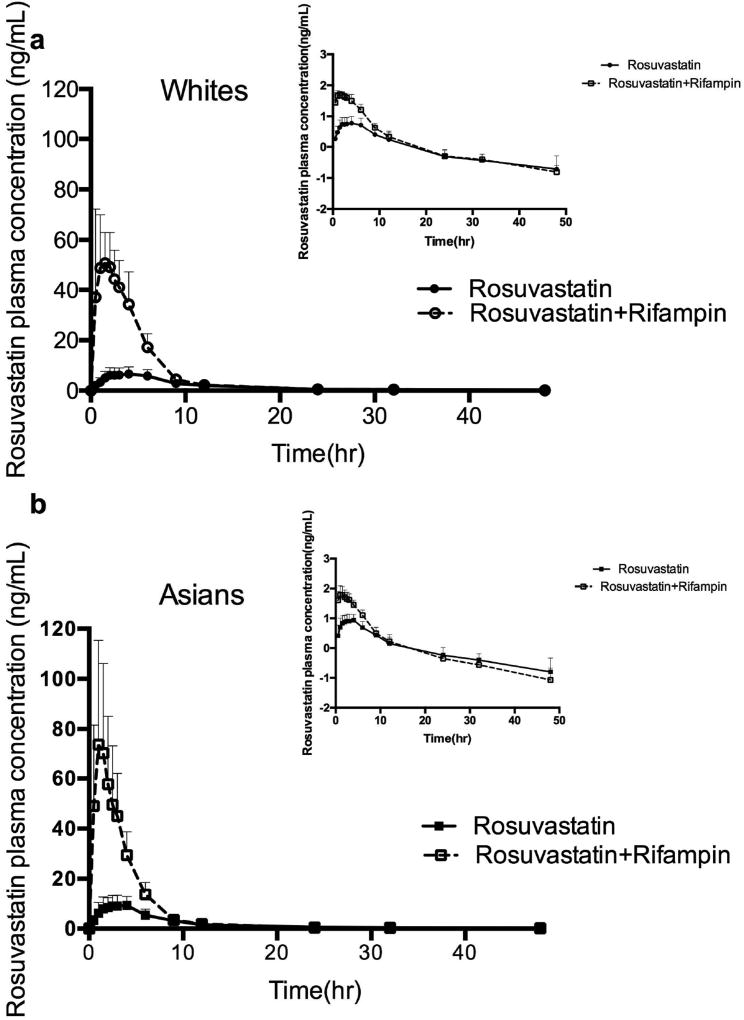
No ethnic difference in drug exposure was observed when rosuvastatin was administered alone in the control period as reported in Table 2. The concentration-time profiles of rosuvastatin alone in both ethnic groups are similar as shown in Figure 1. Total AUCs were 92.5 (± 36.2) ng*hr/mL for Asians and 83.5 (± 32.2) ng*hr/mL for Whites while the Cmax were 10.0 (± 4.1) ng/mL for Asians with a Tmax of 3.1 hours and 7.6(± 3.0) ng/mL with Tmax of 3.0 hours for Whites. The oral clearance, CL/F/kg, was calculated to be 3.90(± 1.25) L/hr/kg for Asians and 4.01(± 1.39) L/hr/kg for Whites.
Table 2.
Pharmacokinetic parameters of rosuvastatin following a 20 mg oral dose of rosuvastatin alone or in combination with rifampin IV.
| White subjects (n=7) |
Asian subjects (n=8) |
Mean ratio (White to Asian) |
|
|---|---|---|---|
| Rosuvastatin | |||
| Cmax (ng/ml) | 7.6 ± 3.0 | 10.0 ± 4.1 | 0.76 |
| Tmax (h) | 3.0 (1.0–4.0) | 3.1 (1.5–4.0) | -- |
| MAT(h) | 2.70 ± 0.85 | 2.25 ± 0.74 | 1.20 |
| AUC0–∞ (ng·h/ml) | 83.5 ± 32.2 | 92.5 ± 36.2 | 0.90 |
| AUC0–48h (ng·h/ml) | 77.2 ± 31.5 | 86.2 ± 35.5 | 0.90 |
| t1/2 (h) | 16.2 ± 8.5 | 15.2 ± 10.5 | 1.07 |
| CL/F/kg (L/h/kg) | 4.01 ± 1.39 | 3.90 ± 1.25 | 1.22 |
| Vss/F/kg (L/kg) | 59.9 ± 51.7 | 48.9 ± 37.7 | 1.22 |
| Rosuvastatin + rifampin | |||
| Cmax (ng/ml) | 65.0 ± 32.2a | 78.1 ± 42.1a | 0.83 |
| Tmax (h) | 1.5 (0.5–2.5)a | 1.7 (1–3)b | -- |
| MAT(h) | 1.27 ± 0.50b | 0.77 ± 0.42a | 1.65 |
| AUC0–∞ (ng·h/ml) | 281.4 ± 73.3a | 297.2 ± 104.4a | 0.95 |
| AUC0–48h (ng·h/ml) | 278.2 ± 73.2a | 295.2 ± 102.9a | 0.94 |
| t1/2 (h) | 10.3 ± 3.0 | 9.0 ± 2.7 | 1.14 |
| CL/F/kg (L/h/kg) | 1.11 ± 0.32a | 1.21 ± 0.42a | 0.92 |
| Vss/F/kg (L/kg) | 4.90 ± 2.06c | 5.14± 3.00b | 0.95 |
Data were obtained from healthy volunteers in a crossover study design. Values are shown as arithmetic mean ± SD except for Tmax where data are given as median and range. AUC, area under the plasma concentration–time curve; Cmax, maximum plasma concentration; CL/F, oral clearance; MAT, mean absorption time; t1/2, terminal half-life; Tmax, time of observed maximal concentration; Vss/F, oral steady-state volume of distribution.
P<0.001 compared with rosuvastatin alone period.
P<0.01 compared with rosuvastatin alone period.
P<0.05 compared with rosuvastatin alone period.
Figure 1.
Rosuvastatin pharmacokinetics in 8 Asian and 7 White healthy volunteers. Mean plasma concentration of rosuvastatin (± SD) following a single oral 20mg dose of rosuvastatin. The inset depicts the same data on a semi-logarithmic scale. Similar variability in rosuvastatin AUC0–∞ between (a) White and (b) Asian subjects was noted.
Effect of rifampin on the pharmacokinetics of rosuvastatin
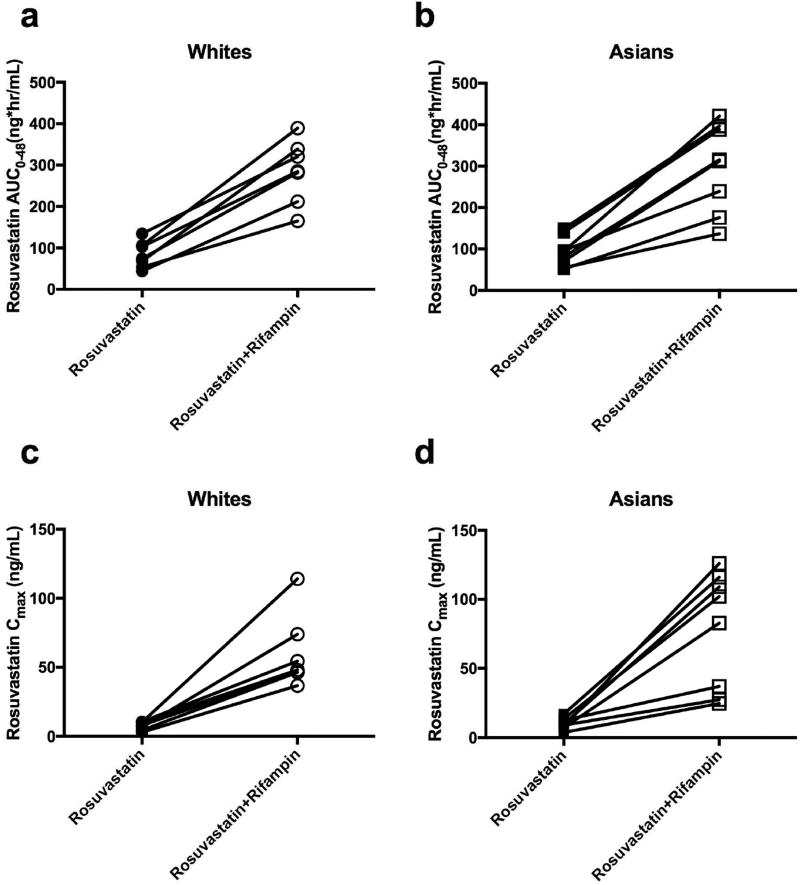
Rifampin is an inhibitor of the uptake transporter OATP1B1 and efflux transporter BCRP. When rifampin was coadministered intravenously with oral rosuvastatin, as expected, a substantial increase was seen in all of the volunteers with average AUC increasing more than three-fold (p<0.001) and Cmax increasing more than six-fold (p<0.001) compared to the control period (Fig.1 and Table 2). A single intravenous dose of rifampin had similar effects on rosuvastatin in both Asians and Whites and no significant differences were observed between Asians and Whites. The effect of rifampin on total AUC and Cmax of rosuvastatin in each individual is presented in Figure 2. Tmax values with rifampin were approximately half of that seen in the control period, a reflection of the decreased mean absorption time (MAT). There also was a very marked decrease in the volume of distribution of rosuvastatin in the presence of transporter inhibition by rifampin as reflected in the 9–12 fold decrease in Vss/F given in Table 2 in both ethnic groups.
Figure 2.
The effect of rifampin on the pharmacokinetics of rosuvastatin in White (n=7) and Asian (n=8) healthy volunteers. Both rosuvastatin mean AUC0–48 and Cmax following a single oral dose of 20 mg rosuvastatin, with and without the administration of rifampin, in White (a and c) and Asian (b and d) healthy volunteers increased in the presence of rifampin.
Discussion
This prospective study demonstrates that the consistently observed two fold average difference in rosuvastatin drug exposure between Asian and White was mitigated after controlling for two drug transporters, SLCO1B1 *1a and ABCG2 c.421 wildtype. In our cohort, both Asian and White volunteers exhibited similar rosuvastatin AUC and Cmax, which implicates similar pharmacological effects. In addition, our study result aligns with the previous literature finding no significant difference in rosuvastatin pharmacokinetics between SLCO1B1 *1a or *1b carriers. Although the subject numbers were too small to justify statistical comparison (n=4), SLCO1B1 *1b did not affect the rosuvastatin plasma concentration in our cohort compared with wildtype.
Table 3 summarizes the interethnic rosuvastatin pharmacokinetic parameters, Cmax and AUC, for the studies of: Lee et al.9 at a 40mg rosuvastatin dose between Asians and Whites in subjects with no genotype control and subjects wild-type for SLCO1B1; Birmingham et al.10 at a 20mg rosuvastatin dose in subjects with no genotype control, subjects wild-type for SLCO1B1 and subjects wildtype for ABCG2; and our study at a 20mg rosuvastatin dose in subjects wildtype in both SLCO1B1 and ABCG2. The data for our White subjects compare favorably with the previously reported results of Birmingham et al. and dose adjusted results of Lee et al9,10. However, in Asian subjects in our study, wildtype for both SLCO1B1 and ABCG2, markedly lower levels are observed than for the two previous reports, but not different than the measurement in Whites wildtype in both transporters (Table 2). Our results differ from those of Birmingham et al.10, who briefly reported, but provided no details, that in subjects wildtype for both SLCO1B1 and ABCG2 “rosuvastatin AUC(0−t) and Cmax appeared to be, on average, higher in Japanese and Chinese compared with Caucasian subjects”. When we digitally quantified the data presented in Fig.2a for AUC(0−t) in that paper10, median values for Chinese and Japanese were 62% and 35% higher, respectively, compared to Caucasians, versus the 11% difference we observed. In a recent study, Wan et al. reported that ABCG2 c.34AA, with an allele frequency of 12.6% in Chinese, also has a significant effect on rosuvastatin pharmacokinetics in healthy Chinese subjects25, yielding a mean decrease of 34% in CL/F, although no change was observed for the heterozygous carrier of c.34GA. However, the volunteers here were not controlled for ABCG2 c.34 A>G SNP, since our clinical study preceded this publication.
Table 3.
Summary of interethnic rosuvastatin pharmacokinetic studies. Our study shows no interethnic differences in rosuvastatin PK in SLCO1B1 and ABCG2 wt carriers, while other studies showed 2-fold difference in rosuvastatin PK in either SLCO1B1 or ABCG2 wt carriers.
|
Lee et al. 2005 (40mg Rosuvastatin) |
Birmingham et al. 2015 (20mg Rosuvastatin) |
Our study (20mg rosuvastatin) |
|||||
|---|---|---|---|---|---|---|---|
| Genotype controlled for |
No control |
SLCO1B1 c.521 TT |
No control |
SLCO1B1 c.521 TT |
ABCG2 c.421 CC |
SLCO1B1 c.521 TT & ABCG2 c.421 CC |
|
| Cmax (ng/mL) | White | 25* (21.1–29.6) | 18.7* (13.9–25.2) | 8.66* (7.15–10.5) | 8.2* (6.7–10.0) | 7.9* (6.5–9.6) | 7.6 (4.6–10.6) |
| Asian | 59.1 (49.8–70.1) | 66.6 (31.8–140) | 18.7 (15.5–22.5) | 17.4 (14.3–21.0) | 15.2 (11.5–20.1) | 10.0 (5.6–14.1) | |
| AUC0–∞ (ng*hr/mL) | White | 216* (186–252) | 183* (152–221) | 95.7* (80.0–114) | 90.7* (75.8–108.7) | 88.8* (73.9–106.7) | 83.5 (51.3–115.7) |
| Asian | 500 (428–583) | 538 (260–1110) | 179 (150–212) | 167.4 (140.8–199.1) | 140.9 (108.6–182.6) | 92.5 (56.3–128.7) | |
Statistically significant when compared between Whites and Asians.
In our current study the 90% confidence intervals of AUC0–∞ were within 56.3–128.7ng*hr/mL, which was lower compared with the range found in the Birmingham and Lee et al. groups, as shown in Table 3. Although we would have expected a similar range, it was not observed in this study. We do note that at least in the Birmingham et al.10 study the lower levels of the 90%CI for the controlled genotype subjects fell below that observed in the no control group. Our results might be due to smaller sample size (8 in each group). In the Lee et al. paper, their data were reported for 21 Caucasians and 17 Chinese who were SLCO1B1 *1a carriers (ABCG2 was not reported); in the Birmingham et al. paper, the results were reported for 24 Caucasian and 12 Chinese who are SLCO1B1 *1a carriers (ABCG2 genotype was not reported in conjunction with SLCO1B1)9,10. If the diplotype/genotype of SLCO1B1 and ABCG2 can explain the racial differences of rosuvastatin exposure, the difference of AUC and Cmax between Asian and White in the Lee et al. and Birmingham et al. studies should become smaller than those in genotype No Control groups. There were slight decreases for this comparison in the Birmingham et al. data but not for the Lee et al. data. Again, this points out the variance of our results from that previously reported.
Tomita et al.22 also suggest from their retrospective analysis that SLCO1B1 c.521T>C and ABCG2 c.421 C>A polymorphisms cannot explain the observed plasma concentration variations between Asian and Whites, although SLCO1B1 *1a/*1b were not evaluated.
Previously, Tomita et al.22 reported that Vss in Asians was approximately half of that found in Whites when no allelic transporter characteristics were quantitated. Here again investigating only wildtype SLCO1B1 and ABCG2 subjects, this Vss difference between Asians and Whites was also mitigated in our cohort. Our study provides a further element to precision medicine beyond the previous finding of Lee et al.9, who demonstrated that the two fold rosuvastatin AUC difference between Asian and Whites was still observed when controlling for the SLCO1B1 allele alone. Our study shows that both SLCO1B1 *1a, ABCG2 c.421 play important roles in rosuvastatin drug disposition. This finding is in agreement with the previous pharmacogenetic and pharmacokinetic studies that rosuvastatin pharmacokinetics were susceptible to both SLCO1B1 and ABCG2 polymorphisms10. However, the results from our prospective study are not consistent with the retrospective analyses of Birmingham et al.10 and Tomita et al.22. Therefore, further studies are needed to confirm the relevance of our finding.
Interethnic differences in statin pharmacokinetics have recently been noted as a general phenomenon10. Simvastatin acid, atorvastatin, pravastatin and rosuvastatin were all shown to have higher average AUC and Cmax levels in Japanese and other Asians compared to Caucasians in healthy volunteer pharmacokinetic studies10,22,26. Since atorvastatin24,27, pravastatin28,29 and rosuvastatin10 are substrates of OATP1B1, while atorvastatin29 and rosuvastatin29 are known inhibitors of BCRP, we believe that genetic polymorphism leading to interindividual and interethnic pharmacokinetic variations in other statins exposure should be examined in addition to rosuvastatin.
As shown in Table 2, concomitant dosing of IV rifampin with oral rosuvastatin markedly increased rosuvastatin exposure, both Cmax and AUC, and markedly decreased rosuvastatin CL/F and Vss/F. Interethnic differences in drug-drug interactions with rosuvastatin were previously reported with higher AUC fold increase in non-Asians than Asians in the presence of eltromprobag, a OATP1B1 and BCRP inhibitor30. However, that study did not report SLCO1B1 and ABCG2 genotypes in their results. Here, when SLCO1B1 and ABCG2 were inhibited by rifampin, both Asians and Whites with wildtype SLCO1B1 and ABCG2 in our study experienced the same approximate fold increase in rosuvastatin exposure. The interethnic difference in drug-drug interaction was not observed in our study after controlling for SLCO1B1 and ABCG2. A recent study reported no racial difference in liver transporter protein expression23 and our study further supports the similarity of total protein expression for both OATP1B1 and BCRP between the two groups since the changes in rosuvastatin exposure in the presence of rifampin were similar between Asians (3.2 fold) and Whites (3.4 fold).
Inhibition of OATP1B1 and BCRP by rifampin also markedly affected the rate of rosuvastatin absorption in both Asians and Whites, as reflected by MAT, and resulted in shorter Tmax. Since OATP1B1 is only expressed in the liver and BCRP is found both in the gut and liver, we believe that BCRP function in the gut also affects interethnic bioavailability. A previous study showed that rifampin can inhibit OATP1B1 and BCRP and when co-dosing rifampin and rosuvastatin, oral rifampin had a bigger effect on rosuvastatin pharmacokinetics than iv rifampin; while no significant difference was noted between po and iv rifampin with pitavastatin. Pitavastatin is an in vitro substrate of OATP1B1 and BCRP31, but when pitavastatin was dosed to ABCG2 c.421 C>A subjects, the pitavastatin AUC did not differ from wildtype subjects32,33. In addition, pitavastatin exhibits high FaFg, so intestinal BCRP should have minimal effect on pitavastatin drug exposure. It was not clear as to whether rifampin could inhibit intestinal BCRP in addition to the liver. More prevalent reduced function BCRP in Asians could potentially be an explanation as to why Japanese demonstrate higher rosuvastatin bioavailability (29%) than Caucasians (20%) as cited by Tomita et al.22. We dedicate this paper to Professor Sugiyama in recognition of his outstanding contribution to the pharmaceutical sciences worldwide. Although our results here present a different outcome from that reported by Professor Sugiyama22, his earlier studies served as a beacon in the design of this work.
Conclusions
The most recent ACC/AHA Blood Cholesterol Guideline recommends rosuvastatin as one of the two most potent statins to reduce the risk of cardiovascular events in moderate and high-risk patients. And the FDA recommends beginning at a lower starting dose in patients of Asian descent. Yet, our study suggests that about one third of Asian patients (39%) exhibit wildtype genotype of the important transporters for rosuvastatin disposition. Treating these patients with lower starting doses of rosuvastatin may delay achievement of target goals by weeks to months (essentially, until the next clinic visit). We recommend that SLCO1B1 and ABCG2 polymorphism provide a better prediction for rosuvastatin dosing than ethnicity in order to meet treatment goals in a timely and effective manner. We suggest that the 39.6% of Asians who carry wild type SLCO1B1 *1a and ABCG2 c.421CC should be prescribed the same dose as Whites instead of lowering the starting dose.
In a similar manner, when treating patients of non-Asian descent with rosuvastatin, clinicians should be aware that many White patients could have reduced-function SLCO1B1 and ABCG2 alleles, leading to higher drug exposure. Given that the frequency of muscle toxicity from statin use is reported higher in real life compared to data from clinical trials these non-Asian patients may be more likely to exhibit statin toxicity and reduced adherence6. Here, we found that both SLCO1B1 *1a and ABCG2 c.421 alleles should be considered when examining interethnic rosuvastatin exposure differences. However, since our prospective study result contradict previous retrospective analyses and we did not include a No Control group for comparison, further studies are needed to confirm this finding.
Acknowledgments
This study was supported in part by National Institutes of Health (NIH) grant GM61390. It was carried out in part in the Clinical Research Center, Moffitt Hospital, University of California, San Francisco, which is supported by NIH/National Center for Research Resources (NCRR) UCSF-CTSI grant UL1RR024131. H-F.W. was supported in part by a GEMS-CTSI Clinical Research Student Award. The authors thank the nurses and staff at the Clinical Research Center, Moffitt Hospital, University of California, San Francisco, for their help with the clinical study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflict of interest.
This work was presented at the American Society for Clinical Pharmacology and Therapeutics (ASCPT) Annual Meeting, 9 March 2016, San Diego, CA by H-F.W.
References
- 1.The Long-Term Intervention with Pravastatin in Ischaemic Disease Study G. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 2.Scandinavian Simvastatin Survival Study G. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 3.Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta analysis of data from 170000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panel NCEPNE. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. 2002:3143–3421. [PubMed] [Google Scholar]
- 5.Statinusage.com. Key Findings and Implications.
- 6.Rosenson RS. Statins and the risk of rhabdomyolysis. J Endocrinol Invest. 2002;25:577. doi: 10.1007/BF03345078. [DOI] [PubMed] [Google Scholar]
- 7.Pasternak RC, Smith SC, Jr, Bairey-Merz CN, Grundy SM, Cleeman JI, Lenfant C American College of C, American Heart A, National Heart L, Blood I. ACC/AHA/NHLBI Clinical Advisory on the Use and Safety of Statins. Circulation. 2002;106:1024–1028. doi: 10.1161/01.cir.0000032466.44170.44. [DOI] [PubMed] [Google Scholar]
- 8.Rosenbaum D, Dallongeville J, Sabouret P, Bruckert E. Discontinuation of statin therapy due to muscular side effects: A survey in real life. Nutr Metab Cardiovasc Dis. 2013;23:871–875. doi: 10.1016/j.numecd.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Lee E, Ryan S, Birmingham B, Zalikowski J, March R, Ambrose H, Moore R, Lee C, Chen Y, Schneck D. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther. 2005;78:330–341. doi: 10.1016/j.clpt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Birmingham BK, Bujac SR, Elsby R, Azumaya CT, Wei C, Chen Y, Mosqueda-Garcia R, Ambrose HJ. Impact of ABCG2 and SLCO1B1 polymorphisms on pharmacokinetics of rosuvastatin, atorvastatin and simvastatin acid in Caucasian and Asian subjects: a class effect? Eur J Clin Pharmacol. 2015;71:341–355. doi: 10.1007/s00228-014-1801-z. [DOI] [PubMed] [Google Scholar]
- 11.Wu CY, Benet LZ. Predicting drug disposition via application of BCS: Transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 12.Ieiri I, Higuchi S. Pharmacogenomics: inter-ethnic and intra-ethnic differences in pharmacokinetic and pharmacodynamic profiles of clinically relevant drugs. Yakugaku Zasshi. 2009;129:231–235. doi: 10.1248/yakushi.129.231. [DOI] [PubMed] [Google Scholar]
- 13.Niemi M. Transporter pharmacogenetics and statin toxicity. Clin Pharmacol Ther. 2010;87:130–133. doi: 10.1038/clpt.2009.197. [DOI] [PubMed] [Google Scholar]
- 14.Prasad B, Evers R, Gupta A, Hop CECA, Salphati L, Shukla S, Ambudkar SV, Unadkat JD. Interindividual variability in hepatic organic anion-transporting polypeptides and P-glycoprotein (ABCB1) protein expression: quantification by liquid chromatography tandem mass spectroscopy and influence of genotype, age, and sex. Drug Metab Dispos. 2014;42:78–88. doi: 10.1124/dmd.113.053819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drozdzik M, Groer C, Penski J, Lapczuk J, Ostrowski M, Lai Y, Prasad B, Unadkat JD, Siegmund W, Oswald S. Protein abundance of clinically relevant multidrug transporters along the entire length of the human intestine. Mol Pharm. 2014;11:3547–3555. doi: 10.1021/mp500330y. [DOI] [PubMed] [Google Scholar]
- 16.Kitamura S, Maeda K, Wang Y, Sugiyama Y. Involvement of multiple transporters in the hepatobiliary transport of rosuvastatin. Drug Metab Dispos. 2008;36:2014–2023. doi: 10.1124/dmd.108.021410. [DOI] [PubMed] [Google Scholar]
- 17.Choi JH, Lee MG, Cho Jy, Lee Je, Kim KH, Park K. Influence of OATP1B1 genotype on the pharmacokinetics of rosuvastatin in Koreans. Clin Pharmacol Ther. 2008;83:251–257. doi: 10.1038/sj.clpt.6100267. [DOI] [PubMed] [Google Scholar]
- 18.Romaine SP, Bailey KM, Hall AS, Balmforth AJ. The influence of SLCO1B1 (OATP1B1) gene polymorphisms on response to statin therapy. Pharmacogenomics J. 2010;10:1–11. doi: 10.1038/tpj.2009.54. [DOI] [PubMed] [Google Scholar]
- 19.Keskitalo JE, Zolk O, Fromm MF, Kurkinen KJ, Neuvonen PJ, Niemi M. ABCG2 polymorphism markedly affects the pharmacokinetics of atorvastatin and rosuvastatin. Clin Pharmacol Ther. 2009;86:197–203. doi: 10.1038/clpt.2009.79. [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Yu BN, He YJ, Fan L, Li Q, Liu ZQ, Wang A, Liu YL, Tan ZR, Fen J, Huang YF, Zhou HH. Role of BCRP 421C>A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males. Clin Chim Acta. 2006;373:99–103. doi: 10.1016/j.cca.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Kurose K, Sugiyama E, Saito Y. Population Differences in Major Functional Polymorphisms of Pharmacokinetics/pharmacodynamics-related Genes in Eastern Asians and Europeans: Implications in the Clinical Trials for Novel Drug Development. Drug Metab Pharmacokinet. 2012;27:9–54. doi: 10.2133/dmpk.dmpk-11-rv-111. [DOI] [PubMed] [Google Scholar]
- 22.Tomita Y, Maeda K, Sugiyama Y. Ethnic variability in the plasma exposures of OATP1B1 substrates such as HMG-CoA reductase inhibitors: a kinetic consideration of its mechanism. Clin Pharmacol Ther. 2013;94:37–51. doi: 10.1038/clpt.2012.221. [DOI] [PubMed] [Google Scholar]
- 23.Peng KW, Bacon J, Zheng M, Guo Y, Wang MZ. Ethnic variability in the expression of hepatic drug transporters: Absolute quantification by an optimized targeted quantitative proteomic approach. Drug Metab Dispos. 2015;43:1045–1055. doi: 10.1124/dmd.115.063362. [DOI] [PubMed] [Google Scholar]
- 24.Lau YY, Huang Y, Frassetto L, Benet LZ. Effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther. 2007;81:194–204. doi: 10.1038/sj.clpt.6100038. [DOI] [PubMed] [Google Scholar]
- 25.Wan Z, Wang G, Li T, Xu B, Pei Q, Peng Y, Sun H, Cheng L, Zeng Y, Yang G, Zhu YS. Marked Alteration of Rosuvastatin Pharmacokinetics in Healthy Chinese with ABCG2 34G>A and 421C>A Homozygote or Compound Heterozygote. J Pharmacol Exp Ther. 2015;354:310–315. doi: 10.1124/jpet.115.225045. [DOI] [PubMed] [Google Scholar]
- 26.Ho RH, Tirona RG, Leake BF, Glaeser H, Lee W, Lemke CJ, Wang Y, Kim RB. Drug and bile acid transporters in rosuvastatin hepatic uptake: function, expression, and pharmacogenetics. Gastroenterology. 2006;130:1793–1806. doi: 10.1053/j.gastro.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 27.Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics. 2005;15:513–522. doi: 10.1097/01.fpc.0000170913.73780.5f. [DOI] [PubMed] [Google Scholar]
- 28.Deng JW, Song I-S, Shin HJ, Yeo C-W, Cho D-Y, Shon J-H, Shin J-G. The effect of SLCO1B1*15 on the disposition of pravastatin and pitavastatin is substrate dependent: the contribution of transporting activity changes by SLCO1B1*15. Pharmacogenet Genomics. 2008;18:424–433. doi: 10.1097/FPC.0b013e3282fb02a3. [DOI] [PubMed] [Google Scholar]
- 29.Hirano M, Maeda K, Matsushima S, Nozaki Y, Kusuhara H, Sugiyama Y. Involvement of BCRP (ABCG2) in the biliary excretion of pitavastatin. Mol Pharmacol. 2005;68:800–807. doi: 10.1124/mol.105.014019. [DOI] [PubMed] [Google Scholar]
- 30.Allred AJ, Bowen CJ, Park JW, Peng B, Williams DD, Wire MB, Lee E. Eltrombopag increases plasma rosuvastatin exposure in healthy volunteers. Br J Clin Pharmacol. 2011;72:321–329. doi: 10.1111/j.1365-2125.2011.03972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prueksaritanont T, Chu X, Evers R, Klopfer SO, Caro L, Kothare PA, Dempsey C, Rasmussen S, Houle R, Chan G, Cai X, Valesky R, Fraser IP, Stoch SA. Pitavastatin is a more sensitive and selective organic anion-transporting polypeptide 1B clinical probe than rosuvastatin. Br J Clin Pharmacol. 2014;78:587–598. doi: 10.1111/bcp.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ieiri I, Suwannakul S, Maeda K, Uchimaru H, Hashimoto K, Kimura M, Fujino H, Hirano M, Kusuhara H, Irie S, Higuchi S, Sugiyama Y. SLCO1B1 (OATP1B1, an uptake transporter) and ABCG2 (BCRP, an efflux transporter) variant alleles and pharmacokinetics of pitavastatin in healthy volunteers. Clin Pharmacol Ther. 2007;82:541–547. doi: 10.1038/sj.clpt.6100190. [DOI] [PubMed] [Google Scholar]
- 33.Ho RH, Choi L, Lee W, Mayo G, Schwarz UI, Tirona RG, Bailey DG, Michael Stein C, Kim RB. Effect of drug transporter genotypes on pravastatin disposition in European- and African-American participants. Pharmacogenet Genomics. 2007;17:647–656. doi: 10.1097/FPC.0b013e3280ef698f. [DOI] [PMC free article] [PubMed] [Google Scholar]