Abstract
Background and Aim
Sulfotransferase (SULT)-mediated sulfation and steroid sulfatase (STS)-mediated desulfation represent two critical mechanisms that regulate the chemical and functional homeostasis of endogenous and exogenous molecules. STS catalyzes the hydrolysis of steroid sulfates to form hydroxysteroids. Oxygenated cholesterol derivative oxysterols are known to be endogenous ligands of the liver X receptor (LXR), a nuclear receptor with anti-cholestasis activity, whereas the sulfated oxysterols antagonize LXR signaling. The conversion of sulfated oxysterols to their non-sulfated counterparts is catalyzed by STS. The aim of this study is to determine whether STS can alleviate cholestasis by increasing the activity of LXR.
Methods
Liver-specific STS transgenic mice were created and subject to the lithocholic acid (LCA)-induced model of cholestasis.
Results
Transgenic overexpression of STS in the liver promoted bile acid elimination and alleviated LCA-induced cholestasis. The protective effect of the STS transgene was associated with the activation of LXR and induction of LXR target genes, likely because of the increased conversion of the antagonistic oxysterol sulfates to the agonistic oxysterols.
Conclusions
STS has a novel function in controlling the homeostasis of bile acids by regulating endogenous LXR ligands.
Keywords: Steroid sulfatase, Bile acid, Toxicity, Cholestasis, Liver X receptor
1. Introduction
Sulfotransferase (SULT)-mediated sulfation and steroid sulfatase (STS)-mediated desulfation represent two opposing mechanisms involved in regulating the chemical and functional homeostasis of endogenous and exogenous chemicals. STS catalyzes the hydrolysis of steroid sulfates to form hydroxysteroids and is expressed in many tissues including the liver, where the circulating steroids are extensively metabolized. By altering the levels of sulfated versus non-sulfated steroids, STS is important in the regulation of the cellular actions of steroid hormones[1].
Bile acids are the major catabolic end products of cholesterol in the liver. As a major constituent in the bile, bile acids play an important physiological role in the solubilization and absorption of lipids and other lipophilic nutrients. Bile acids also function in signaling by activating receptors such as the farnesoid X receptor (FXR) and G protein-coupled receptor 5 (TGR5, also known as G protein-coupled bile acid receptor 1, or GPBAR1) [2,3]. In addition to their beneficial functions, excessive bile acids are potentially toxic when they accumulate in the body. For example, LCA, a secondary bile acid, is a potent cholestatic agent and can induce liver injury and other pathological changes when it is not efficiently eliminated[4].
The nuclear receptor liver X receptor (LXR) represents a promising therapeutic target for bile acid detoxification by enhancing the metabolism and elimination of bile acids as well as by reducing inflammation[5]. Both pharmacological stimulation and genetic activation of LXR prevented LCA-induced hepatotoxicity. In contrast, LXRα and β double knockout mice were more sensitive to bile acid toxicity[5]. However, several existing synthetic LXR agonists not only regulate bile acid homeostasis, but also promote lipogenesis and are pro-atherogenic[6]. By contrast, oxysterols, natural LXR agonists, showed selective modulation of LXR target genes that are responsible for cholesterol elimination, while not over-activating lipogenic genes[7].
STS plays a key role in regulating the homeostasis of endogenous ligands for LXR. Oxysterols, oxygenated cholesterol derivatives, are endogenous ligands of LXR. Like many other cholesterol derivatives, oxysterols can be sulfated by sulfotransferases such as the cholesterol sulfotransferase SULT2B1b, and the sulfonated forms of oxysterols can antagonize LXR signaling[8–11]. The conversion of sulfated oxysterols to their non-sulfated counterparts is catalyzed by STS. Knowing that LXR has anti-cholestasis activity, we hypothesize that STS may attenuate cholestasis by activating LXR through increased availability of endogenous LXR agonists and reducing the level of endogenous LXR antagonists.
In this report, we show that transgenic over-expression of STS in the liver and small intestine enhanced bile acid elimination and attenuated LCA-induced liver damage in mice. The protective effect of STS was associated with the induction of LXR target genes. Our results suggest a novel function of STS in controlling the homeostasis of bile acids by regulating endogenous LXR ligands.
2. Methods
2.1. Generation of STS transgenic mice
The generation of the STS transgenic mice was previously described[12]. In brief, the cDNA encoding human STS was cloned into the TRE-SV40 transgene cassette to construct the TRE-STS transgene. The TRE-STS transgenic mice were then bred with the liver and intestinal specific FABP-tetracycline-controlled transactivator (tTA) transgenic mice to generate TRE-STS/FABP-tTA double transgenic mice[12]. The mice were maintained in a C57BL/6J background strain. When necessary, doxycycline (DOX, 2 mg/ml) was given in drinking water to silence the transgene expression. All results presented are derived from male mice. The use of mice in this study complied with relevant federal guidelines and institutional policies.
2.2. Drug treatment, histology, and serum biochemistry
For LCA treatment, mice were gavaged daily with LCA (8 mg/day) or vehicle for 4 days and sacrificed 24 h after the last treatment as previously described[13]. Fecal samples were collected by using mouse metabolic cages. For histology evaluation, the liver tissues were fixed in 10% formalin, embedded in paraffin, sectioned at 6 μm, and stained with hematoxylin and eosin (H&E). The histological damage was defined by the appearance of necrotic foci and was evaluated in a blinded fashion. Serum levels of ALT, AST (Stanbio Laboratory, Boerne, TX) and total bile acids (Bio-Quant, San Diego, CA) were measured by using commercial assay kits according to the manufacturer’s instructions.
2.3. Northern blot, real-time PCR and immunohistochemistry
For Northern blot analysis, total RNA was isolated using the TRIZOL reagent from Invitrogen. Northern blot analysis was performed using 32P-labeled full-length STS cDNA to probe the transgene expression. For real-time PCR analysis, cDNA was synthesized by reverse transcription with random hexamer primers and Superscript RT III enzyme from Invitrogen. SYBR Green-based real-time PCR was performed with the ABI 7300 real-time PCR system. Data were normalized against the housekeeping gene cyclophilin. The immunohistochemical staining on paraffin sections was performed using a monoclonal anti-STS antibody (dilution 1:50) purchased from Abcam (Cambridge, MA) following heat-induced antigen retrieval procedures.
2.4. Transient transfection and luciferase reporter gene assay
COS-7 cells were seeded onto a 48-well plate and transfected using polyethyleneimine polymer transfection reagents. When necessary, cells were treated with vehicle or 22-hydroxycholesterol (10 μmol) in medium containing 10% charcoal-stripped FBS for 24 h before the luciferase assay. The luciferase activity was normalized using the β-gal activity from a co-transfected pCMX-β-gal vector to determine the transfection efficiency. The relative reporter activity was calculated by comparing with cells transfected with empty vector and vehicle-treated cells. All transfections were performed in triplicate.
2.5. Statistical analysis
Results are expressed as the mean ± SD. Statistical significance between groups was determined by an unpaired two-tailed Student t test, one-way ANOVA or two-way ANOVA. A P-value less than 0.05 is considered to be statistically significant.
3. Results
3.1. Generation of transgenic mice expressing STS in the liver and small intestine
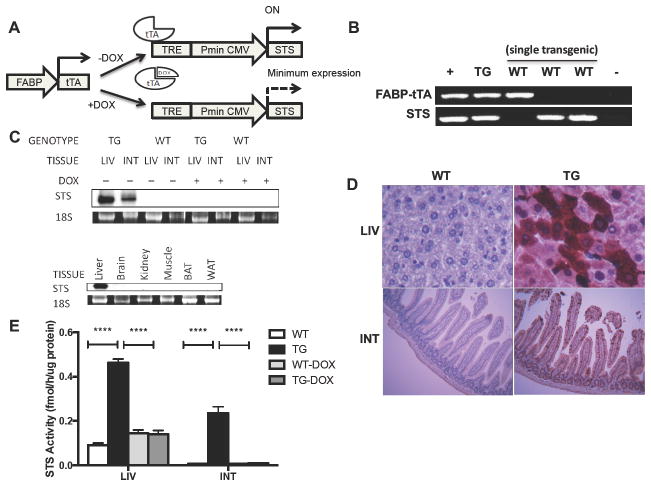
To investigate the in vivo function of STS in cholestasis, we created tetracycline-responsive STS transgenic mice (STS mice) by crossing transgenic mice expressing human STS under the control of the tetracycline response element (TRE) with transgenic mice expressing tTA in the liver and small intestine under the control of the fatty acid binding protein (L-FABP) gene promoter. In the double transgenic mice and as outlined in Fig. 1A, binding of the tTA protein to TRE induces the expression of the STS transgene, whereas administration of doxycycline (DOX) prevents the binding of tTA to TRE and thus silences the STS transgene expression. The two transgenes were independently genotyped by PCR (Fig. 1B). The expression of the STS transgene at the mRNA level in the liver and small intestine was confirmed by Northern blot analysis using a transgene-specific probe (Fig. 1C, top panel). As expected, the expression of the STS transgene was completely reversed upon DOX treatment (Fig. 1C, top panel). The transgene was not detected in a panel of non-targeting tissues including the brain, kidney, skeletal muscle, brown (BAT), and white (WAT) adipose tissues (Fig. 1C, bottom panel). To localize cells that expressed the transgene, immunohistochemistry analysis was performed on liver and small intestine sections. STS was detected both in the liver and in the epithelial cell lining of the villi of the jejunum of the STS transgenic mice (Fig. 1D), which was consistent with the tissue specificity of the FABP gene promoter[14]. The expression of the STS transgene in the liver and small intestine was also confirmed by measuring the enzymatic activity of STS. When tritium-labeled estrone sulfate was used as the substrate, the STS mice exhibited higher enzymatic activity compared with the WT mice in both the liver and small intestine, which was normalized by DOX treatment (Fig. 1E). These results demonstrated that the STS transgene was expressed and functional in the STS transgenic mice.
Fig. 1. Generation of transgenic mice expressing STS in the liver and small intestine.
(A) Schematic depiction of the Tet-Off STS transgenic system. (B) PCR genotyping using gene-specific primers for the detection of the FABP-tTA and TRE-STS transgenes. (C) Conditional STS transgene expression as determined by Northern blot analysis. STS full-length cDNA was used as the probe to detect transgene expression. The 18s rRNA was used as a loading control. (D) STS expression detected by immunohistochemistry in the liver and small intestine. (E) STS activity in the liver and small intestine. The enzymatic activity of STS was determined by an estrone sulfate conversion assay and was normalized to protein concentrations. Values are presented as the mean ± SD. N ≥ 4 for each group. ****, P < 0.0001. FABP, fatty acid binding protein; tTA, tetracycline-controlled transactivator; TRE, tetracycline response element; Pmin CMV, minimal human cytomegalovirus promoter. WT, wild-type mice; Tg, STS transgenic mice; LIV, liver; INT, small intestine; BAT, brown adipose tissue; WAT, white adipose tissue; DOX, doxycycline.
3.2. Over-expression of STS attenuates lithocholic acid (LCA)-induced cholestasis
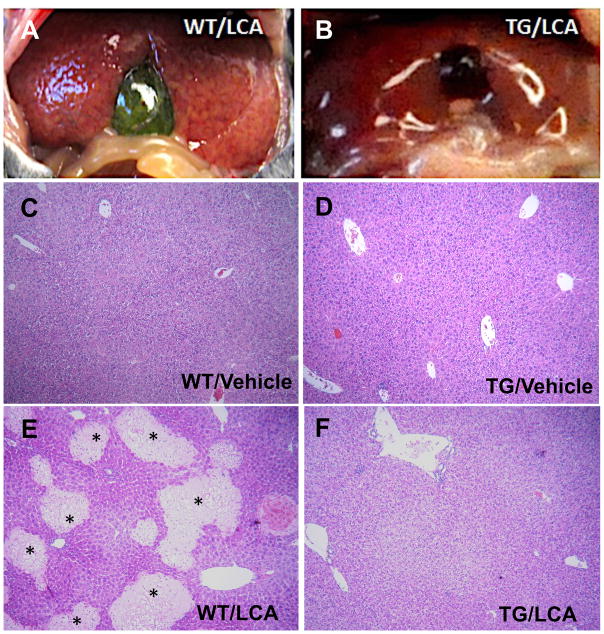
Gastric feeding of the secondary bile acid LCA to mice is an established model to study bile acid-induced cholestasis and hepatotoxicity in vivo[5, 13]. To examine the effects of STS over-expression on LCA-induced hepatotoxicity, STS transgenic mice and their WT littermates were given a daily treatment of vehicle or LCA by oral gavage for four consecutive days to induce cholestasis. Necropsy analysis showed that the livers of WT mice exhibited profound subcapsular foci that were discolored yellow (Fig. 2A), which most likely corresponded with the areas of saponification/coagulative necrosis that were observed histologically (see below). In contrast, the livers of the STS mice showed resistance to LCA (Fig. 2B). When the liver sections were examined, we found that the transgene alone had little effect on the liver histology (Fig. 2D). As expected, LCA treatment induced massive liver damage in WT mice as characterized by the appearance of necrotic foci (Fig. 2E). In contrast, STS mice had less incidence of histological liver damage as shown in Fig. 2F. Consistent with our previous report[13], 68% (15/22)of the WT mice showed liver necrosis whereas the incidence of liver necrosis decreased to 36% (9/25)in STS mice. These results suggested that the STS mice were attenuated from LCA-induced liver toxicity.
Fig. 2. Over-expression of STS attenuates lithocholic acid (LCA)-induced cholestasis.
Both wild-type (WT) and STS transgenic (Tg) mice were given either vehicle (C and D) or LCA (A, B, E and F) via daily gavage for four days. (A and B) Gross appearance of representative livers. (C–F) Liver paraffin sections were histologically examined by hematoxylin and eosin (H&E) staining. Areas marked by asterisks indicate liver necrosis.
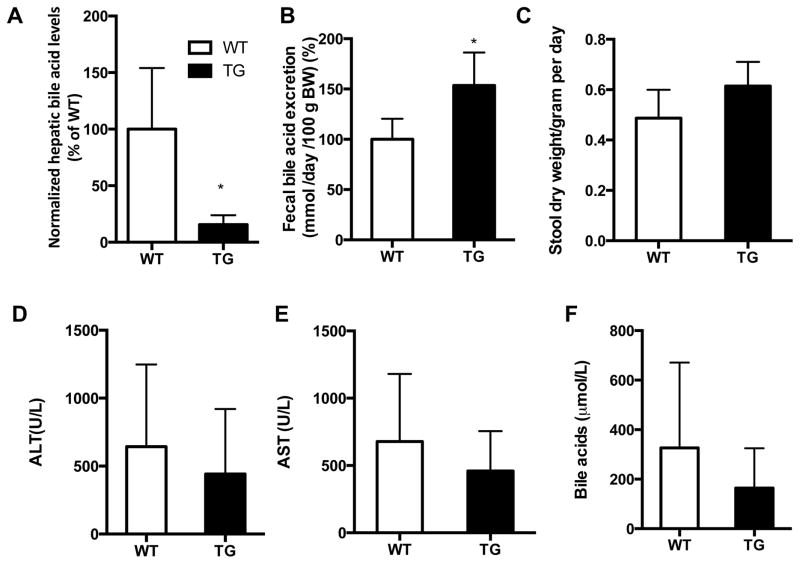
The liver toxicity associated with cholestasis often results from intrahepatic accumulation of bile acids[15]. Consistent with their attenuated cholestasis, the LCA-treated STS mice exhibited a markedly decreased hepatic concentration of bile acids compared with their WT counterparts (Fig. 3A). In contrast, the fecal elimination of bile acids was significantly increased in the LCA-treated STS mice (Fig. 3B), while the fecal output was not significantly altered (Fig. 3C). The reduced liver toxicity in LCA-treated STS mice may be related to both the decreased hepatic concentration of bile acids and increased fecal elimination of bile acids. The hepato-protective effect of the STS transgene was also supported by the trend of reduced serum levels of ALT (Fig. 3D), AST (Fig. 3E), and bile acids (Fig. 3F), although the decreases did not reach statistical significance.
Fig. 3. Biochemistry of LCA-treated WT and STS transgenic mice.
(A to F) Levels of hepatic bile acids (A), fecal bile acids (B), fecal output (C), serum ALT (D), serum AST (E), and serum total bile acids (F) in LCA-treated wild-type (WT) and STS transgenic (Tg) mice. Values are presented as the mean ± SD. N ≥ 4 for each group, *, P<0.05.
3.3 Regulation of hepatic and intestinal bile acid transporters in the STS transgenic mice
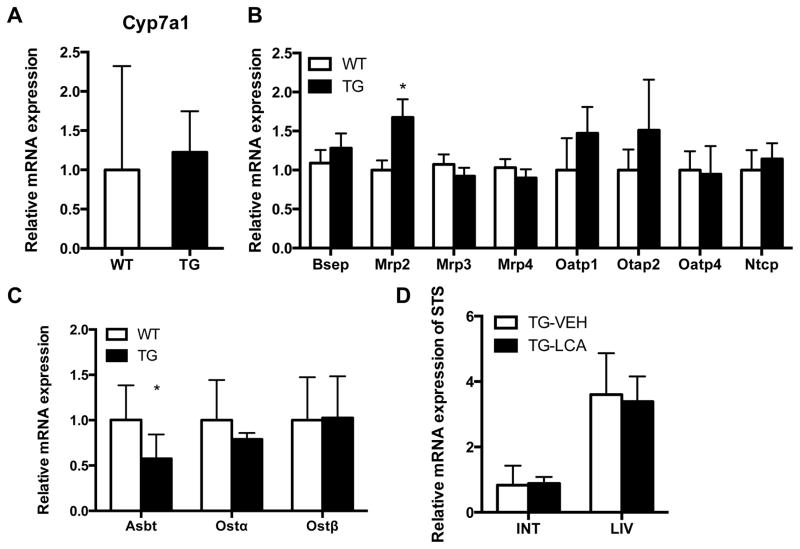
The reduced hepatic bile acid levels in STS mice prompted us to examine the expression of Cyp7a1, a key enzyme involved in the biosynthesis of bile acids from cholesterol. We found that the expression of Cyp7a1 was not different between WT and STS mice (Fig. 4A), suggesting that bile acid synthesis was not affected in STS mice. The homeostasis of bile acids is also controlled by a series of hepatic and intestinal transporters[15]. The major hepatic bile acid transporters include the uptake transporters Na+-taurocholate cotransport protein (Ntcp) and organic anion-transporting polypeptides 1, 2 and 4 (Oatps 1, 2 and 4), the canalicular efflux transporters bile salt export pump (Bsep) and multidrug resistance-associated protein 2 (Mrp2), and the basolateral efflux transporters Mrp3 and Mrp4 that excrete bile acids from the hepatocytes into the blood. Among these hepatic transporters, the expression of Mrp2 was significantly higher in LCA-treated STS mice compared with their WT counterparts (Fig. 4B).
Fig. 4. Regulation of hepatic and intestinal bile acid transporters in STS transgenic mice.
Gene expression in wild-type (WT) and STS transgenic (Tg) mice was measured by real-time PCR. (A) The expression of Cyp7a1 in LCA-treated mice. (B and C) The hepatic (B) and intestinal (C) expression of bile acid transporters in LCA-treated mice. (D) The expression of the transgene in the liver (LIV) and small intestine (INT) in Tg mice treated with vehicle (VEH) or LCA. Values are presented as the mean ± SD. N ≥ 4 for each group. *, P<0.05.
In the intestine, bile acids are initially reabsorbed from the ileum by the ileal apical sodium-dependent bile acid transporter (Asbt), followed by basolateral export via the organic solute transporters Ostα and Ostβ[16]. We found the ileal expression of Asbt was significantly decreased in LCA-treated STS mice (Fig. 4C), but the transgene had little effect on the expression of Ostα/β. The suppression of Asbt in STS mice may have contributed to the increased fecal elimination of bile acids in this genotype (Fig. 3B). The expression of the STS transgene was maintained in the liver and small intestine of the transgenic mice upon LCA treatment, suggesting that the protective phenotype was not a result of changes in transgene expression (Fig. 4D).
3.4 The cholestatic protective effect of STS is associated with the activation of LXR in vivo and in cell culture
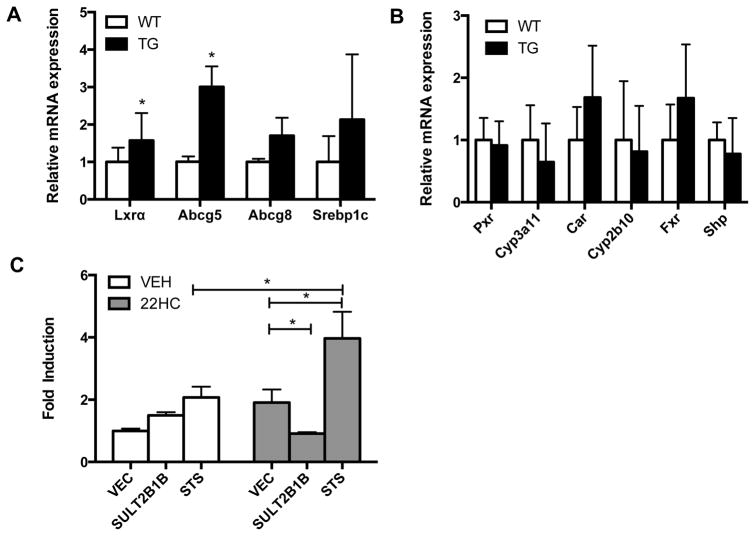
Oxysterols are endogenous LXR agonists while sulfated oxysterols antagonize LXR signaling[8–10]. The conversion of sulfated oxysterols to their non-sulfated counterparts is catalyzed by STS. Therefore, STS may function to increase the availability of endogenous LXR agonists and reduce the LXR antagonist level, which may lead to increased LXR activation. Indeed, the hepatic mRNA expression of LXRα and its target gene Abcg5[17] were significantly induced in STS mice compare with WT mice (Fig. 5A). Interestingly, the gene regulatory effect of activation of LXR in STS mice was not universal. In addition to the lack of Cyp7a1 regulation (Fig. 4A), the expression of Abcg8 and Srebp1c was also not affected in the STS mice (Fig. 5A). The expression of several other nuclear receptors known to inhibit cholestasis, PXR, CAR and FXR[13, 18, 19] and their target genes, was not affected (Fig. 5B).
Fig. 5. The cholestatic protective effect of STS is associated with the activation of LXR in vivo and in cell culture.
(A) The hepatic expression of Lxrα and its target genes in wild-type (WT) and STS transgenic (Tg) mice. (B) The hepatic expression of Pxr, Car, Fxr, and their target genes. N ≥ 4 for each group. (C) COS-7 cells were co-transfected with Gal4-LXRα and the tk-UAS-luciferase reporter plasmid in the presence or absence of the expression vector for STS and SULT2B1b. Transfected cells were treated with vehicle (VEH) or 22-hydroxycholesterol (22HC, 10 μmol) for 24 h before the luciferase assay. *, P<0.05.
We also used a LXR reporter gene system to directly assess the regulation of LXR activity by STS through the biotransformation of LXR modulators. Treatment of COS-7 cells co-transfected with the vector expressing the Gal4 fusion of the ligand binding domain of LXRα (Gal-LXRα) and the Gal4 responsive luciferase reporter gene, tk-UAS-Luc, with 22-hydroxycholesterol (22-HC), an endogenous LXR agonist, increased the reporter activity as expected. The 22-HC responsive reporter activity was further enhanced by co-transfection of the STS expressing vector, presumably because of the STS-mediated conversion of 22-HC sulfate to 22-HC. Co-transfection of the plasmid encoding SULT2B1b, the cholesterol sulfotransferase that converts oxysterols to oxysterol sulfates, reduced the 22-HC-induced reporter activity (Fig. 5C).
4. Discussion
In this study, we uncovered a novel function of STS in the protection of LCA-induced cholestasis and liver toxicity. The cholestatic protective effect of the STS transgene was associated with reduced hepatic bile acid content and increased fecal output of bile acids. The reduction in the hepatic bile acid may have resulted from the combined effect of the increased expression of canalicular bile acid efflux transporter Mrp2 and increased expression of cholesterol efflux transporter Abcg5. Mutation of the MRP2 gene results in Dubin–Johnson syndrome and presents with cholestasis in some neonates[20]. The increased fecal output of bile acids may be explained by the suppression of ileal Asbt, a key intestinal bile acid uptake transporter responsible for the enterohepatic recirculation of bile acids. Genetic deletion of Asbt disrupted enterohepatic circulation and resulted in bile acid malabsorption in mice[21].
At the mechanistic level, our results strongly suggested that the cholestatic protective effect of STS was most likely accounted for by the activation of LXR, a nuclear receptor known for its anti-cholestatic activity[5]. The activation of LXR was supported by the induction of typical LXR target genes, such as Lxrα and Abcg5, in the liver of STS mice. The suppression of the ileal expression of Asbt in the LCA-treated STS mice (Fig. 4C) was also consistent with that observed in the LCA-treated FABP-VP-LXRα transgenic mice, in which the expression of the constitutively activated LXRα (VP-LXRα) was expressed in the liver and intestine under the control of the FABP gene promoter[5]. Since the STS transgene is expressed in both the liver and small intestine, it is likely that its expression in both tissues contributed to the protective phenotypes.
The activation of LXR in STS mice was consistent with the notion that STS plays an important role in the control of the homeostasis of oxysterol-derived endogenous LXR ligands. Specifically, the cholesterol derivative oxysterols are endogenous LXR agonists. Oxysterols have been reported to directly bind to the LXR ligand binding domain, recruit coactivators to LXR, and activate LXR signaling in several in vitro and in vivo studies[22–26]. Oxysterols can be sulfonated by the cholesterol sulfotransferase SULT2B1b and the resultant sulfated oxysterols antagonize the LXR activity[8–10]. The sulfation of oxysterols is reversible and the desulfation is catalyzed by STS. Indeed, patients with STS deficiency showed increased serum levels of cholesterol sulfates and oxysterol sulfates[27]. Therefore, STS may function to enhance LXR agonist availability and reduce LXR antagonist levels, leading to the net gain of increased LXR activation. This notion was supported by our experimental results showing that transfection of STS and SULT2B1b increased and decreased the 22-HC induced activation of LXR in the reporter gene assay, respectively (Fig. 5C).
Interestingly and surprisingly, the activation of LXR signaling pathways in STS mice was gene selective. For example, the LXR responsive and Srebp-1c mediated lipogenic pathway was not activated and the STS mice did not exhibit spontaneous steatosis[12]. Additionally, Cyp7a1, a prototypic LXR target gene[28], was not induced in the STS mice. Although the mechanism for this gene selectivity remains to be understood, the potential therapeutic outcome of this selectivity is favorable. Although LXR has been suggested to be a promising therapeutic target for cholestasis, several existing synthetic LXR agonists such as T0901317 and GW3965 not only regulate bile acid homeostasis, but also promote lipogenesis by activating lipogenic genes, thus limiting their therapeutic applicability[6]. By contrast, the natural LXR agonist oxysterols, such as 25-hydroxycholesterol and 22-hydroxycholesterol, showed selective modulation of the LXR target gene Abca1, which is a cholesterol transporter responsible for cholesterol elimination[7].
LXR signaling has also been shown to regulate inflammation and fibrosis within the liver. We cannot exclude the possibility that the anti-inflammatory and anti-fibrotic activities of LXR may have also contributed to the protective effect of STS. Indeed, we have previously reported that the expression of pro-inflammatory genes was reduced in STS transgenic mice fed a high-fat diet or in ob/ob mice that express the STS transgene[12]. In the current study, we used an acute model of cholestasis. We plan to analyze the effects of STS on inflammation and fibrosis in chronic cholestatic models in our future studies.
In conclusion, over-expression of STS in transgenic mice mitigated bile acid-induced toxicity by enhancing bile acid elimination. This beneficial effect may have been achieved by STS mediated activation of LXR. The important function and mechanism of STS in bile acid detoxification can potentially facilitate the development of novel interventions for bile acid toxicity and cholestasis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reed MJ, Purohit A, Woo LW, Newman SP, Potter BV. Steroid sulfatase: molecular biology, regulation, and inhibition. Endocr Rev. 2005;26:171–202. doi: 10.1210/er.2004-0003. [DOI] [PubMed] [Google Scholar]
- 2.Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, et al. Bile acids: natural ligands for an orphan nuclear receptor. Science (New York, NY) 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 3.Kawamata Y, Fujii R, Hosoya M, Harada M, Yoshida H, Miwa M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 4.Radominska A, Treat S, Little J. Bile acid metabolism and the pathophysiology of cholestasis. Semin Liver Dis. 1993;13:219–234. doi: 10.1055/s-2007-1007351. [DOI] [PubMed] [Google Scholar]
- 5.Uppal H, Saini SP, Moschetta A, Mu Y, Zhou J, Gong H, et al. Activation of LXRs prevents bile acid toxicity and cholestasis in female mice. Hepatology (Baltimore, Md) 2007;45:422–432. doi: 10.1002/hep.21494. [DOI] [PubMed] [Google Scholar]
- 6.Fievet C, Staels B. Liver X receptor modulators: effects on lipid metabolism and potential use in the treatment of atherosclerosis. Biochem Pharmacol. 2009;77:1316–1327. doi: 10.1016/j.bcp.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Quinet EM, Savio DA, Halpern AR, Chen L, Miller CP, Nambi P. Gene-selective modulation by a synthetic oxysterol ligand of the liver X receptor. J Lipid Res. 2004;45:1929–1942. doi: 10.1194/jlr.M400257-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.Xu L, Bai Q, Rodriguez-Agudo D, Hylemon PB, Heuman DM, Pandak WM, et al. Regulation of hepatocyte lipid metabolism and inflammatory response by 25-hydroxycholesterol and 25-hydroxycholesterol-3-sulfate. Lipids. 2010;45:821–832. doi: 10.1007/s11745-010-3451-y. [DOI] [PubMed] [Google Scholar]
- 9.Song C, Hiipakka RA, Liao S. Auto-oxidized cholesterol sulfates are antagonistic ligands of liver X receptors: implications for the development and treatment of atherosclerosis. Steroids. 2001;66:473–479. doi: 10.1016/s0039-128x(00)00239-7. [DOI] [PubMed] [Google Scholar]
- 10.Cook IT, Duniec-Dmuchowski Z, Kocarek TA, Runge-Morris M, Falany CN. 24-hydroxycholesterol sulfation by human cytosolic sulfotransferases: formation of monosulfates and disulfates, molecular modeling, sulfatase sensitivity, and inhibition of liver x receptor activation. Drug Metab Dispos. 2009;37:2069–2078. doi: 10.1124/dmd.108.025759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Y, Xu L, Rodriguez-Agudo D, Li X, Heuman DM, Hylemon PB, et al. 25-Hydroxycholesterol-3-sulfate regulates macrophage lipid metabolism via the LXR/SREBP-1 signaling pathway. Am J Physiol Endocrinol Metab. 2008;295:E1369–1379. doi: 10.1152/ajpendo.90555.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang M, He J, Kucera H, Gaikwad NW, Zhang B, Xu M, et al. Hepatic overexpression of steroid sulfatase ameliorates mouse models of obesity and type 2 diabetes through sex-specific mechanisms. J Biol Chem. 2014;289:8086–8097. doi: 10.1074/jbc.M113.535914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie W, Radominska-Pandya A, Shi Y, Simon CM, Nelson MC, Ong ES, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci U S A. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iseki S, Kondo H, Hitomi M, Ono T. Localization of liver fatty acid-binding protein and its mRNA in the liver and jejunum of rats: an immunohistochemical and in situ hybridization study. Mol Cell Biochem. 1990;98:27–33. doi: 10.1007/BF00231364. [DOI] [PubMed] [Google Scholar]
- 15.Zollner G, Trauner M. Mechanisms of cholestasis. Clin Liver Dis. 2008;12:1–26. vii. doi: 10.1016/j.cld.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 16.Dawson PA, Lan T, Rao A. Bile acid transporters. J Lipid Res. 2009;50:2340–2357. doi: 10.1194/jlr.R900012-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- 18.Saini SP, Sonoda J, Xu L, Toma D, Uppal H, Mu Y, et al. A novel constitutive androstane receptor-mediated and CYP3A-independent pathway of bile acid detoxification. Mol Pharmacol. 2004;65:292–300. doi: 10.1124/mol.65.2.292. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Binz J, Numerick MJ, Dennis S, Luo G, Desai B, et al. Hepatoprotection by the farnesoid X receptor agonist GW4064 in rat models of intra- and extrahepatic cholestasis. J Clin Invest. 2003;112:1678–1687. doi: 10.1172/JCI18945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner M, Trauner M. Transcriptional regulation of hepatobiliary transport systems in health and disease: implications for a rationale approach to the treatment of intrahepatic cholestasis. Ann Hepatol. 2005;4:77–99. [PubMed] [Google Scholar]
- 21.Dawson PA, Haywood J, Craddock AL, Wilson M, Tietjen M, Kluckman K, et al. Targeted deletion of the ileal bile acid transporter eliminates enterohepatic cycling of bile acids in mice. J Biol Chem. 2003;278:33920–33927. doi: 10.1074/jbc.M306370200. [DOI] [PubMed] [Google Scholar]
- 22.Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. An oxysterol signalling pathway mediated by the nuclear receptor LXR alpha. Nature. 1996;383:728–731. doi: 10.1038/383728a0. [DOI] [PubMed] [Google Scholar]
- 23.Forman BM, Ruan B, Chen J, Schroepfer GJ, Jr, Evans RM. The orphan nuclear receptor LXRalpha is positively and negatively regulated by distinct products of mevalonate metabolism. Proc Natl Acad Sci U S A. 1997;94:10588–10593. doi: 10.1073/pnas.94.20.10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- 25.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Chen G, Head DL, Mangelsdorf DJ, Russell DW. Enzymatic reduction of oxysterols impairs LXR signaling in cultured cells and the livers of mice. Cell Metab. 2007;5:73–79. doi: 10.1016/j.cmet.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Guijo A, Oji V, Hartmann MF, Schuppe HC, Traupe H, Wudy SA. High levels of oxysterol sulfates in serum of patients with steroid sulfatase deficiency. J Lipid Res. 2015;56:403–412. doi: 10.1194/jlr.M055608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]