Abstract
Purpose of Review
Our understanding of critical illness is transforming as we develop a better understanding of the impact pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) have on the pathogenesis of disease. Of the known DAMPs, there is a growing interest in mitochondrial DNA (mtDNA) as a DAMP capable of propagating the inflammatory response seen in sepsis and other conditions. In this review, we describe the varying mechanisms by which mtDNA is translocated from mitochondria into cytosol and the extracellular space where it can illicit an inflammatory response. Additionally, we present some of the most recent clinical studies to examine mtDNA in critical illness.
Recent Findings
Basic science research provides convincing data that mtDNA can influence the immune system through toll-like receptor 9 and inflammasomes. Clinical trials provide evidence that mtDNA is elevated in critically ill patients, and is associated with mortality.
Summary
While mtDNA is a DAMP shown to be elevated in numerous conditions the clinical ramifications of this finding remain elusive. Further work is needed to determine if mtDNA can be utilized as a biomarker of disease severity or mortality.
Keywords: Sepsis, mitochondria, mitochondrial DNA
Introduction
The endosymbiotic theory of mitochondria postulates that over a billion years ago energy-producing alpha-bacteria were either engulfed by or invaded archezoan cells (1). This ancient encounter resulted in a symbiotic relationship between eukaryotic cells and eubacteria (1) that facilitated the evolution of eukaryotic cells by providing them with compartmentalized bioenergetic and biosynthetic factories.
To date, much of the research on mitochondria has focused on their role as cellular organelles responsible for energy production, protein synthesis, catabolism, and cell death (2, 3). However, there has been a growing interest in mtDNA as a potential damage-associated molecular pattern (DAMP) (4, 5). The “Danger Model” stipulates that when cells are injured or dying, irrespective of the stimulus, they release their components into the extracellular space, which in turn drives an immune or inflammatory response (5). Examples of known DAMPs include DNA, high mobility group box 1, or heat shock proteins (6).
When the Danger Model is considered alongside the endosymbiotic model it raises the question of whether or not mitochondria can affect the pathogenesis of disease. Like bacteria, mitochondria possess DNA containing unmethylated CpG (4, 7) and formylated peptides (8, 9) -- molecules known to exert potent immune responses through toll-like receptor 9 (TLR9) or formyl peptide receptors, respectively. Studies of human mitochondria have illustrated polymorphonuclear leukocyte (PMN) chemotaxis can be induced by disrupting mitochondrial membranes with detergent or sonication (8). Taken together, this information suggests mitochondrial components can regulate immune responses to stressors and stimuli (5, 10).
Of the known mitochondrial DAMPs, the one that has garnered the most interest is mtDNA (4, 11). There is a growing body of literature suggesting mtDNA is not only released in the setting of critical illness (12, 13), but can also propagate the inflammatory response through its interaction with the immune system (4, 5, 11). This is of particular interest as it raises the possibility that mtDNA may function as a surrogate of disease severity or even a predictor of mortality in critically ill patients.
Herein, we will discuss the scientific evidence supporting mtDNA as a DAMP that is important in disease pathogenesis as well as well as the most recent clinical trials that investigated mtDNA in critically ill patients.
MtDNA: function and regulation
MtDNA is a circular molecule of 16,569 bp encoding for 37 genes in humans (14). Despite representing a very small portion of the DNA possessed by eukaryotic cells, mtDNA is essential as it codes for several key proteins of the oxidative phosphorylation system (2). Although, the importance of mtDNA extends beyond what is merely encoded by its nucleotide sequence as evidence suggests mtDNA is involved in cellular immune functions (4). This involvement appears to be mediated by mtDNA leaving the confines of mitochondria to enter other cellular or extracellular compartments where it can subsequently trigger or promote immune responses by being recognized as a DAMP (4).
Release of mtDNA from mitochondria to cytosolic and extracellular compartments
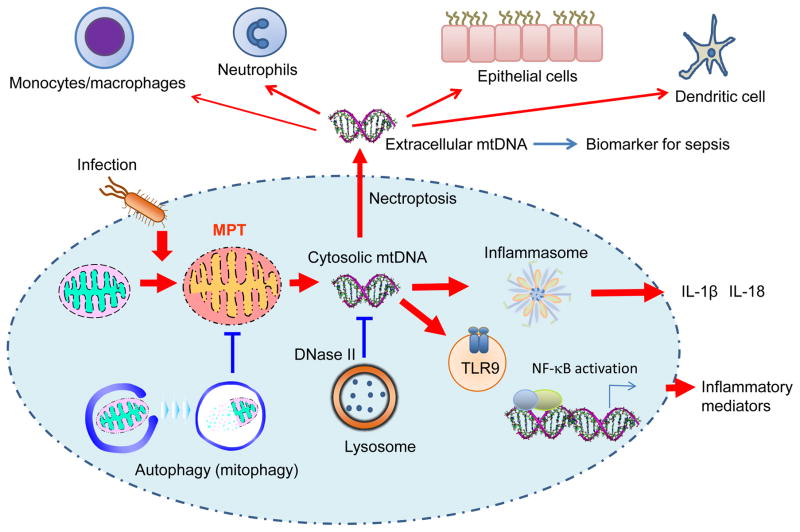
Multiple in vitro and in vivo studies have suggested mitochondrial membrane permeability transition (MPT) as the mechanism of mtDNA translocation from mitochondria to cytosol (Figure 1) (15, 16). When cells (e.g., macrophages) are exposed to pathogen associated molecular patterns (PAMPs) and/or DAMPs, the cells increase the generation of mitochondrial reactive oxygen species (ROS) (16, 17). Increased mitochondrial ROS generation can decrease mitochondrial membrane potential leading to impairment of membrane integrity (16, 18, 19). These changes could subsequently permit leakage of mtDNA into cytosol (16, 19–21). Inhibition of MPT by cyclosporine A and scavenging mitochondrial ROS by MitoTEMPO is known to prevent leakage of mtDNA into cytosol and the downstream immune response (15, 16).
Figure 1. MtDNA as a DAMP.
During infection, mitochondria cause mitochondria membrane potential transition (MPT) and ROS generation, which leads to release of mtDNA into cytosolic compartment. Cytosolic mtDNA can activate TLR9 or inflammasome-mediated innate immune responses such as the secretion of IL-1β and IL-18, and pro-inflammatory gene expression. Cytosolic mtDNA can also be secreted into the extracellular space by necroptosis-mediated programmed cell death. The extracellular mtDNA can stimulate immune cells and also epithelial cells, which may promote tissue injury and systemic inflammatory responses. Recent studies suggest that cell-free mtDNA (e.g., in plasma) can be measured and used as a biomarker to predict prognosis of patients with critically care illness.
Another possible mechanism of mtDNA translocation within cells is destabilized mtDNA packaging (22). MtDNA is packaged and stabilized by binding to Transcription Factor A, Mitochondrial (TFAM) inside the mitochondrial inner membrane. Deficiency of TFAM causes aberrant mtDNA packaging promoting escape of mtDNA into cytosol (22).
One of the proposed mechanisms by which mtDNA is translocated to the extracellular space is via necroptosis, a newly discovered form of programmed necrosis (Figure 1) (23, 24). Necroptosis is driven by defined molecular pathways and has been implicated in various immune functions and inflammatory diseases (23, 24). Evidence supporting this theory is derived from a study in mice where blood transfusions were shown to increase the levels of extracellular mtDNA by induction of endothelial cell necroptosis (25). The investigators of this study also showed an association between mtDNA and transfusion-related lung injury (25). However it remains unclear whether other types of cell death can also promote mtDNA release (26).
Platelets are another potential source of extracellular mtDNA. Platelets are activated in inflammatory conditions, including sepsis, where they secrete granules as well as thrombotic and proinflammatory membrane vesicles termed microparticles (27, 28). A recent study suggests platelets also secrete their mitochondria upon activation (29). Once in the extracellular space, mitochondrial membrane phospholipids may be hydrolyzed by serum secretory phospholipase A2-IIa (sPLA2-IIA) thereby releasing mtDNA (29).
Functions of mtDNA as a DAMP
TLR9 was initially discovered as an innate immune receptor capable of recognizing bacteria or viruses by binding unmethylated CpG motiffs within their DNA (30, 31). However, the endosymbiotic theory of mitochondria means mtDNA can also be recognized by TLR9 and trigger an innate immune response (Figure 1). This has been illustrated in vivo where the administration of mtDNA in mice can induce acute lung injury (32) or activate TLR9 leading to the development of atherosclerosis (33) and kidney injury (34). Animal models of nonalcoholic steatohepatitis have further implicated mtDNA as a DAMP by illustrating it can influence the severity of liver injury through a TLR9-mediated inflammatory response (35). Furthermore, a recent investigation into the relationship between TLR9 and mtDNA in critically ill patients found mtDNA to be significantly associated with mortality in patients with high intracellular expression of TLR9 [HR, 2.3; 95% CI, 1.1–4.8; p = 0.025)(12). These studies imply that mtDNA can be implicated in the pathogenesis of diseases via TLR9-mediated immune responses.
Another important mechanism by which mtDNA triggers an immune response is through interaction with inflammasomes, which are multi-protein complexes that modulate inflammatory responses through caspase-1 activation and secretion of pro-inflammatory cytokines (i.e., IL-1β and IL-18) (Figure 1) (36). Cytosolic mtDNA has been shown to have a synergistic effect on inflammasome activation by enhancing caspase-1 activation as well as the secretion of IL-1β and IL-18 (4, 16, 21). Interestingly, depletion of mtDNA in macrophages impairs inflammasome activation in macrophages suggesting a critical role of mtDNA in the immune response (16).
Previous studies in critically ill patients have shown elevated levels of cell-free circulating mtDNA and plasma IL-18 are associated with disease severity and mortality (13, 37). This suggests mtDNA may have a role in the pathogenesis of inflammation and tissue injury via its ability to drive IL-18 production through inflammasomes or even directly.
Elimination and degradation of mtDNA
Given the potential roles of mtDNA on inflammation, it is also important mechanisms exist to regulate or remove excess deviated mtDNA. At the cellular level autophagy, an intracellular degradation system that delivers cytoplasmic constituents to the lysosome (38), can uptake and digest damaged mitochondria before they release mtDNA (Figure 1) (16). Once mtDNA is translocated to cytosol, mtDNA can be degraded by intracellular DNase (Figure 1). Among reported isoforms of DNase, DNase II has been shown to localize in lysosome and digest cytosolic mtDNA escaped from autophagy-mediated degradation (39). Deficiency of DNase II displays accumulation of mtDNA deposits in autolysosomes and infiltration of inflammatory cells in a preclinical model of heart failure (39). Although it remains unclear how circulating cell-free mtDNA is eliminated, it has been shown that liver endothelial cells can also uptake circulating ds DNA (40). Additionally, mtDNA is detectable in urine suggesting kidneys can also regulate the elimination of mtDNA from circulating blood (41).
MtDNA in critically ill patients
Given the function of mtDNA as a DAMP, there has been a growing interest in mtDNA as a predictor of disease severity and/or mortality in critically ill patients. In 2013, Nakahira et al published one of the first clinical trials to investigate this subject (33). For their study, the investigators enrolled 200 medical ICU patients into a primary cohort. They found mtDNA to be significantly higher in patients who died within 28-days of ICU admission than in survivors (median 9,504 copies/uL versus 1,927 copies/uL, p = 2×10−8). Furthermore, the odds of dying within 28-days of ICU admission was found to increase with each log10-unit increase in mtDNA (odds ratio 1.9, 95% CI 1.5–2.4, p = 2×10−7). These findings were replicated within a validation cohort of 243 critically ill patients with ARDS. Their data also suggested a mtDNA level of 3,200 copies/uL may allow physicians to discriminate between patients who are at a high vs low of death because the addition of this value to a clinical model improved the area under the receiver operating characteristic curve for mortality from 0.76 to 0.83.
Since 2013, several studies have attempted to replicate or expanding upon the findings of Nakahira et al. Over the past two years alone, there have been five studies that examined mtDNA in critically ill adults who were admitted to a medical or cardiac ICU. The results of those studies will be discussed below.
In 2015, Bhagirath et al published a translational study aimed at elucidating the role of nuclear DNA (nucDNA), mtDNA, and bacterial DNA (bacDNA) in sepsis (42). The clinical portion of their study consisted of measuring plasma nucDNA and plasma mtDNA in 12 consecutive patients admitted to an intensive care unit with severe sepsis. Within this cohort, the levels of plasma nucDNA and plasma mtDNA were greater than healthy controls by 200-fold and 50-fold, respectively. Recognizing the quantity and quality of a stimulus are not necessarily related, Bhagirath et al performed a follow-up in vivo experiment measuring the effect of varying levels of nucDNA, mtDNA, and bacDNA on the viability of neutrophils. Their focus on neutrophil viability stemmed from previous studies that illustrated delayed neutrophil apoptosis and their local accumulation in septic patients was associated with worse outcomes (43, 44). They conducted this experiment by incubating neutrophils for 16-hours with 1ug/mL and 15ug/mL of the varying nucleic acids to reflect systemic and local conditions. What they found was the high concentration of mtDNA and both concentrations of bacDNA increased neutrophil viability. This was in stark contrast to nucDNA where neither the low nor the high concentration had an impact on neutrophil viability.
A few months later, Krychtiuk et al published a prospective, observational study examining mtDNA in 228 critically ill patients (12); this cohort was rather heterogeneous as it enrolled critically ill surgical patients (cardiothoracic) and medical ICU patients (68% of the cohort). They found the levels of plasma mtDNA in critically ill medical patients (24.1; IQR, 10.7–42.6ng/mL) to be significantly higher than their healthy counterparts (13.8; IQR, 6.5–28.5ng/mL; p < 0.05). Furthermore, within the medical population they noted the highest levels of mtDNA were found within patients experiencing sepsis (33.2; IQR, 17.3–62.0ng/mL) or cardiogenic shock (29.8; IQR, 17.2–53.6ng/mL). Contrary to the medical patients, the levels of mtDNA in the surgical patients were virtually identical to healthy controls (p = 0.82). Despite finding a statistically significant elevation in mtDNA the investigators did not find an association between mtDNA and the use of vasopressors, the need for mechanical ventilation, renal dysfunction, or disease severity scores (APACHE II, SAPS II, SOFA). However, they did note that significantly higher median levels of mtDNA were seen in patients who died within 30-days of ICU admission independent of demographics, primary diagnosis, and APACHE II score. They also observed mtDNA was significantly associated with mortality in medical patients (27.2, IQR = 12.5–60.6ng/mL vs 21.1, IQR = 9.6–37.2ng/mL; p < 0.05), but not surgical patients (4.4, IQR = 3.3–74.5ng/mL vs 13.6, IQR = 8.8–29.9ng/mL; p = 0.20). Lastly, they calculated the area under the curve (AUC) of the receiver-operating characteristic (ROC) for mtDNA and a clinical model (involving APACHE II and gender) to be 0.6 and 0.79, respectively. When they added mtDNA to the clinical model the AUC further improved to 0.81, which was statistically significant.
In order to explore the relationship between plasma nucDNA, plasma mtDNA and various inflammatory markers (TNF-α, IL-6, IL-8, IL-10, and IL-1RA) Timmermans et al conducted a prospective observational study by collecting samples from ICU Day 1, 3, 5, 6, 9, 14, 21, and 28 in 121 patients with septic shock (45). Their data showed the levels of nucDNA, mtDNA, and inflammatory cytokines to be significantly elevated and remain elevated at all time points in septic patients relative to healthy controls; however, the fold change seen in mtDNA was far less than what was seen in nucDNA for the septic shock cohort – reminiscent of what was observed by Bhagirath et al. Their data also noted a statistically significant correlation between plasma nucDNA and inflammatory cytokines during the first few days of shock, norepinephrine dose, and markers of end-organ damage (creatinine and total bilirubin). While statistical significance could be found between plasma mtDNA and a few parameters no conclusive patterns emerged over time. Another interesting observation made by the Timmermans study is that there was no discernable difference in the levels of nucDNA and mtDNA in patients with a history of cancer or autoimmune disease relative to those without such medical conditions. This is significant because it suggests conditions with a high degree of cell turn-over do not significantly alter mtDNA levels. If true, conditions like cancer may not limit mtDNA’s promise as a biomarker as previously thought. However, further work is needed to substantiate this observation.
Adding to the growing body of literature on mtDNA in sepsis, Schäfer et al collected blood from 165 patients with 24-hours of being diagnosed with severe sepsis (46). Not only was serum mtDNA significantly elevated in septic patients relative to controls (D-Loop, 76-fold increase; ATPase 6, 123-fold increase; all p < 0.0001), but they also found a significant difference in the levels of mtDNA between patients who died within 30-days of ICU admission and those who survived (D-Loop, 1.6fg/uL ± 3.6 vs. 0.4fg/uL ± 1.2; p = 0.003; ATPase 6, 1.3fg/uL ± 3.4 vs. 0.55fg/uL ± 2.3; p = 0.005). Like the Bhagirtah study, Schäfer et al were curious about the potential inflammatory effects of mtDNA, so they incubated human monocytes from the THP-1 cell line with 1 ug/mL of mtDNA. They found mRNA expression of TNF-α, IL-1β, and hypoxia-inducible factor-1α were increased after the incubation period.
While clinical investigators have primarily focused on mtDNA in patients with sepsis or trauma, Omura et al chose to study mtDNA and high-mobility group box 1 (HMGB-1) in patients with post-cardiac arrest syndrome (47). (For the sake of brevity, the results of HMGB-1 will not be discussed as it is outside the scope of this review). Their patient population consisted of adults who obtained return of spontaneous circulation (ROSC) after sustaining an out-of-hospital cardiac arrest; individuals who suffered an arrest secondary to trauma, had a DNR, a Glasgow Coma Scale score > 8 upon arrival, or a primary brain pathology were excluded. This led to the enrollment of 21 patients, out of 195 screened, over an 18-month period. Once enrolled, samples were collected at arrival in addition to day 2, 3, 5, and 7 post-ROSC. They found plasma mtDNA to be significantly higher on day 1 relative to day 2, 3, 5, and 7 (p < 0.001). Despite being able to find a measurable difference in mtDNA, they did not find a statistically significant association between mtDNA and time until ROSC (r = 0.108, p = 0.651), APACHE II (r = −0.315, p = 0.559), neurological outcome, and various other clinical parameters. To further assess the prognostic ability of mtDNA they generated a ROC curve wherein they calculated the AUC to be 0.573.
There are, however, several key points to be further addressed that may account for the variance between study results. The first is the lack of protocol standardization for the measurement of cell-free mtDNA. While most studies utilized plasma for mtDNA measurement one used serum (Table 1). Additionally, the proper preparation of plasma has yet to be established. This is significant because the presence or absence of platelets in the sample may artificially elevate the level of mtDNA if they are injured during the freeze-thaw process leading to the release of mitochondria. Another consideration is that recent studies suggest a significant portion of plasma mtDNA is fragmented and very short (less than 100 bp). It is possible that the methods used in many studies (quantitative polymerase chain reaction) may not amplify this type of mtDNA. Finally, there is also the question of the appropriate patient population – it is conceivable that the mechanism driving mtDNA release differs in patients with cellular injury from sepsis and those with a mechanical injury as seen in patients post-trauma or post-surgery.
Table 1.
Characteristics of recent clinical studies on mtDNA.
| First Author, Year of Publication | Patient Population | Population Size | Serum or Plasma | Results regarding mtDNA levels |
|---|---|---|---|---|
| Bhagirath, 2015 | Severe Sepsis | 12 | Plasma | Elevated in patients with severe sepsis versus healthy controls |
| Krychtiuk, 2015* | Critically Ill Medicine Patients | 156 | Plasma | Elevated in critically ill medicine patients versus healthy controls Associated with mortality |
| Krychtiuk, 2015* | Cardiothoracic Surgery | 72 | Plasma | Not elevated in post-surgery patients versus healthy controls Not associated with mortality |
| Timmermans, 2016 | Septic Shock | 121 | Plasma | Elevated in patients with septic shock versus healthy controls Not associated with mortality |
| Schäfer, 2016 | Sepsis | 165 | Serum | Elevated in patients with sepsis versus healthy controls Associated with mortality |
| Omura, 2016 | Post-Cardiac Arrest | 21 | Plasma | Elevated in patients post-cardiac arrest Not associated with mortality |
This paper included 228 patients; 156 medical ICU and 72 cardiothoracic patients.
CONCLUSION
In this review, we discuss the functions and molecular regulation of mtDNA as a DAMP in addition to some of the most recent studies investigating mtDNA in critically ill patients. All of the studies presented illustrate that circulating mtDNA is elevated in critically ill patients. However, it remains unclear whether this elevation in mtDNA results from the severity of illness alone or whether mtDNA actively contributes to disease pathogenesis through interaction with TLR9 or inflammasomes (13). Additional studies are needed to explore the role of mtDNA in critically ill patients and determine if mtDNA has promise as a biomarker of disease severity or mortality in critical illness.
Key points.
mtDNA is a DAMP capable of propagating an immune response through its interaction with TLR9 and inflammasomes.
mtDNA is elevated in critically ill patients.
Clinical trials provide conflicting evidence about whether or not mtDNA is associated with mortality in critically ill patients.
Acknowledgments
We thank Dr. Ilias I Siempos who provided his valuable insight and expertise on this article.
Financial support and sponsorship: This work was supported by NIH grant P01 HL1008801, R01 HL079904 and R01HL060234 to A.M.K.C., and Department of Medicine Seed Grants for Innovative Research (Weill Cornell Medicine) to K. N.
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest.
References
- 1.Dyall SD, Brown MT, Johnson PJ. Ancient invasions: from endosymbionts to organelles. Science. 2004;304(5668):253–7. doi: 10.1126/science.1094884. Epub 2004/04/10. [DOI] [PubMed] [Google Scholar]
- 2.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012;148(6):1145–59. doi: 10.1016/j.cell.2012.02.035. Epub 2012/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G. Mitochondrial control of cellular life, stress, and death. Circulation research. 2012;111(9):1198–207. doi: 10.1161/CIRCRESAHA.112.268946. Epub 2012/10/16. [DOI] [PubMed] [Google Scholar]
- 4.West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nature reviews Immunology. 2017 doi: 10.1038/nri.2017.21. Epub 2017/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakahira K, Hisata S, Choi AM. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxidants & redox signaling. 2015;23(17):1329–50. doi: 10.1089/ars.2015.6407. Epub 2015/06/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyapati RK, Rossi AG, Satsangi J, Ho GT. Gut mucosal DAMPs in IBD: from mechanisms to therapeutic implications. Mucosal immunology. 2016;9(3):567–82. doi: 10.1038/mi.2016.14. Epub 2016/03/05. [DOI] [PubMed] [Google Scholar]
- 7.Groot GS, Kroon AM. Mitochondrial DNA from various organisms does not contain internally methylated cytosine in -CCGG- sequences. Biochimica et biophysica acta. 1979;564(2):355–7. doi: 10.1016/0005-2787(79)90233-8. Epub 1979/09/27. [DOI] [PubMed] [Google Scholar]
- 8.Le Y, Murphy PM, Wang JM. Formyl-peptide receptors revisited. Trends in immunology. 2002;23(11):541–8. doi: 10.1016/s1471-4906(02)02316-5. Epub 2002/10/29. [DOI] [PubMed] [Google Scholar]
- 9.Carp H. Mitochondrial N-formylmethionyl proteins as chemoattractants for neutrophils. The Journal of experimental medicine. 1982;155(1):264–75. doi: 10.1084/jem.155.1.264. Epub 1982/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nature reviews Immunology. 2010;10(12):826–37. doi: 10.1038/nri2873. Epub 2010/11/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyapati RKTA, Dorward DA, Ho GT. Advances in the understanding of mitochondrial DNA as a pathogenic factor in inflammatory diseases. F1000Research. 2017;6(169) doi: 10.12688/f1000research.10397.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12**.Krychtiuk KA, Ruhittel S, Hohensinner PJ, Koller L, Kaun C, Lenz M, et al. Mitochondrial DNA and Toll-Like Receptor-9 Are Associated With Mortality in Critically Ill Patients. Critical care medicine. 2015;43(12):2633–41. doi: 10.1097/CCM.0000000000001311. Epub 2015/10/09 (This is a well-written prospective observational study investigating mtDNA and Toll-Like Receptor-9 in critically ill patients. Their findings provide further evidence that plasma mtDNA is an independent predictor of mortality in critically ill patients and may contribute to the inflammatory response seen in sepsis via its interaction with TLR-9) [DOI] [PubMed] [Google Scholar]
- 13.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS medicine. 2013;10(12):e1001577. doi: 10.1371/journal.pmed.1001577. discussion e. Epub 2014/01/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shadel GS, Clayton DA. Mitochondrial DNA maintenance in vertebrates. Annual review of biochemistry. 1997;66:409–35. doi: 10.1146/annurev.biochem.66.1.409. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 15.Patrushev M, Kasymov V, Patrusheva V, Ushakova T, Gogvadze V, Gaziev A. Mitochondrial permeability transition triggers the release of mtDNA fragments. Cellular and molecular life sciences : CMLS. 2004;61(24):3100–3. doi: 10.1007/s00018-004-4424-1. Epub 2004/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature immunology. 2011;12(3):222–30. doi: 10.1038/ni.1980. Epub 2010/12/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, et al. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472(7344):476–80. doi: 10.1038/nature09973. Epub 2011/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung SS, Moon JS, Xu JF, Ifedigbo E, Ryter SW, Choi AM, et al. Carbon monoxide negatively regulates NLRP3 inflammasome activation in macrophages. American journal of physiology Lung cellular and molecular physiology. 2015;308(10):L1058–67. doi: 10.1152/ajplung.00400.2014. Epub 2015/03/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Won JH, Park S, Hong S, Son S, Yu JW. Rotenone-induced Impairment of Mitochondrial Electron Transport Chain Confers a Selective Priming Signal for NLRP3 Inflammasome Activation. The Journal of biological chemistry. 2015;290(45):27425–37. doi: 10.1074/jbc.M115.667063. Epub 2015/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murakami T, Ockinger J, Yu J, Byles V, McColl A, Hofer AM, et al. Critical role for calcium mobilization in activation of the NLRP3 inflammasome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(28):11282–7. doi: 10.1073/pnas.1117765109. Epub 2012/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36(3):401–14. doi: 10.1016/j.immuni.2012.01.009. Epub 2012/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520(7548):553–7. doi: 10.1038/nature14156. Epub 2015/02/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38(2):209–23. doi: 10.1016/j.immuni.2013.02.003. Epub 2013/02/27. [DOI] [PubMed] [Google Scholar]
- 24.Linkermann A, Green DR. Necroptosis. The New England journal of medicine. 2014;370(5):455–65. doi: 10.1056/NEJMra1310050. Epub 2014/01/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangalmurti N, DQ, Hotz M, Siegel DL, Sondheimer N, Mangalmurti NS. Mitochondrial Dna Released Following Necroptosis Accumulates On Rbcs. American journal of respiratory and critical care medicine. 2016;193(A4309) [Google Scholar]
- 26.Maeda A, Fadeel B. Mitochondria released by cells undergoing TNF-alpha-induced necroptosis act as danger signals. Cell death & disease. 2014;5:e1312. doi: 10.1038/cddis.2014.277. Epub 2014/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boilard E, Blanco P, Nigrovic PA. Platelets: active players in the pathogenesis of arthritis and SLE. Nature reviews Rheumatology. 2012;8(9):534–42. doi: 10.1038/nrrheum.2012.118. Epub 2012/08/08. [DOI] [PubMed] [Google Scholar]
- 28.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nature reviews Immunology. 2011;11(4):264–74. doi: 10.1038/nri2956. Epub 2011/03/26. [DOI] [PubMed] [Google Scholar]
- 29.Boudreau LH, Duchez AC, Cloutier N, Soulet D, Martin N, Bollinger J, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124(14):2173–83. doi: 10.1182/blood-2014-05-573543. Epub 2014/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408(6813):740–5. doi: 10.1038/35047123. Epub 2000/12/29. [DOI] [PubMed] [Google Scholar]
- 31.Kawasaki T, Kawai T. Toll-like receptor signaling pathways. Frontiers in immunology. 2014;5:461. doi: 10.3389/fimmu.2014.00461. Epub 2014/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gan L, Chen X, Sun T, Li Q, Zhang R, Zhang J, et al. Significance of Serum mtDNA Concentration in Lung Injury Induced by Hip Fracture. Shock. 2015;44(1):52–7. doi: 10.1097/SHK.0000000000000366. Epub 2015/02/24. [DOI] [PubMed] [Google Scholar]
- 33.Collins LV, Hajizadeh S, Holme E, Jonsson IM, Tarkowski A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. Journal of leukocyte biology. 2004;75(6):995–1000. doi: 10.1189/jlb.0703328. Epub 2004/02/26. [DOI] [PubMed] [Google Scholar]
- 34.Tsuji N, Tsuji T, Ohashi N, Kato A, Fujigaki Y, Yasuda H. Role of Mitochondrial DNA in Septic AKI via Toll-Like Receptor 9. Journal of the American Society of Nephrology : JASN. 2016;27(7):2009–20. doi: 10.1681/ASN.2015040376. Epub 2015/11/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Martinez I, Santoro N, Chen Y, Hoque R, Ouyang X, Caprio S, et al. Hepatocyte mitochondrial DNA drives nonalcoholic steatohepatitis by activation of TLR9. The Journal of clinical investigation. 2016;126(3):859–64. doi: 10.1172/JCI83885. Epub 2016/01/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature medicine. 2015;21(7):677–87. doi: 10.1038/nm.3893. Epub 2015/06/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. American journal of respiratory and critical care medicine. 2012;185(11):1225–34. doi: 10.1164/rccm.201201-0003OC. Epub 2012/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakahira K, Pabon Porras MA, Choi AM. Autophagy in Pulmonary Diseases. American journal of respiratory and critical care medicine. 2016;194(10):1196–207. doi: 10.1164/rccm.201512-2468SO. Epub 2016/09/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oka T, Hikoso S, Yamaguchi O, Taneike M, Takeda T, Tamai T, et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature. 2012;485(7397):251–5. doi: 10.1038/nature10992. Epub 2012/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu F, Shollenberger LM, Conwell CC, Yuan X, Huang L. Mechanism of naked DNA clearance after intravenous injection. The journal of gene medicine. 2007;9(7):613–9. doi: 10.1002/jgm.1054. Epub 2007/05/31. [DOI] [PubMed] [Google Scholar]
- 41.Whitaker RM, Stallons LJ, Kneff JE, Alge JL, Harmon JL, Rahn JJ, et al. Urinary mitochondrial DNA is a biomarker of mitochondrial disruption and renal dysfunction in acute kidney injury. Kidney international. 2015;88(6):1336–44. doi: 10.1038/ki.2015.240. Epub 2015/08/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres OV, Jayanthi S, Ladenheim B, McCoy MT, Krasnova IN, Cadet JL. Compulsive methamphetamine taking under punishment is associated with greater cue-induced drug seeking in rats. Behavioural brain research. 2017;326:265–71. doi: 10.1016/j.bbr.2017.03.009. Epub 2017/03/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keel M, Ungethum U, Steckholzer U, Niederer E, Hartung T, Trentz O, et al. Interleukin-10 counterregulates proinflammatory cytokine-induced inhibition of neutrophil apoptosis during severe sepsis. Blood. 1997;90(9):3356–63. Epub 1997/11/14. [PubMed] [Google Scholar]
- 44.Matute-Bello G, Liles WC, Radella F, 2nd, Steinberg KP, Ruzinski JT, Jonas M, et al. Neutrophil apoptosis in the acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 1997;156(6):1969–77. doi: 10.1164/ajrccm.156.6.96-12081. Epub 1997/12/31 23:52. [DOI] [PubMed] [Google Scholar]
- 45.Timmermans K, Kox M, Scheffer GJ, Pickkers P. Plasma Nuclear and Mitochondrial DNA Levels, and Markers of Inflammation, Shock, and Organ Damage in Patients with Septic Shock. Shock. 2016;45(6):607–12. doi: 10.1097/SHK.0000000000000549. Epub 2015/12/31. [DOI] [PubMed] [Google Scholar]
- 46.Schafer ST, Franken L, Adamzik M, Schumak B, Scherag A, Engler A, et al. Mitochondrial DNA: An Endogenous Trigger for Immune Paralysis. Anesthesiology. 2016;124(4):923–33. doi: 10.1097/ALN.0000000000001008. Epub 2016/01/26. [DOI] [PubMed] [Google Scholar]
- 47.Omura T, Kushimoto S, Yamanouchi S, Kudo D, Miyagawa N. High-mobility group box 1 is associated with neurological outcome in patients with post-cardiac arrest syndrome after out-of-hospital cardiac arrest. Journal of intensive care. 2016;4:37. doi: 10.1186/s40560-016-0161-4. Epub 2016/06/02. [DOI] [PMC free article] [PubMed] [Google Scholar]